Abstract
Background
Anti-angiogenic treatment in recurrent glioblastoma patients suppresses contrast enhancement and reduces vasogenic edema while non-enhancing tumor progression is common. Thus, the importance of T2-weighted imaging is increasing. We therefore quantified T2 relaxation times, which are the basis for the image contrast on T2-weighted images.
Methods
Conventional and quantitative MRI procedures were performed on 18 patients with recurrent glioblastoma before treatment with bevacizumab and every 8 weeks thereafter until further tumor progression. We segmented the tumor on conventional MRI into 3 subvolumes: enhancing tumor, non-enhancing tumor, and edema. Using coregistered quantitative maps, we followed changes in T2 relaxation time in each subvolume. Moreover, we generated differential T2 maps by a voxelwise subtraction using the first T2 map under bevacizumab as reference.
Results
Visually segmented areas of tumor and edema did not differ in T2 relaxation times. Non-enhancing tumor volume did not decrease after commencement of bevacizumab treatment but strikingly increased at progression. Differential T2 maps clearly showed non-enhancing tumor progression in previously normal brain. T2 relaxation times decreased under bevacizumab without re-increasing at tumor progression. A decrease of <26 ms in the enhancing tumor following exposure to bevacizumab was associated with longer overall survival.
Conclusions
Combining quantitative MRI and tumor segmentation improves monitoring of glioblastoma patients under bevacizumab. The degree of change in T2 relaxation time under bevacizumab may be an early response parameter predictive of overall survival. The sustained decrease in T2 relaxation times toward values of healthy tissue masks progressive tumor on conventional T2-weighted images. Therefore, quantitative T2 relaxation times may detect non-enhancing progression better than conventional T2-weighted imaging.
Keywords: bevacizumab, non-enhancing tumor, quantitative MRI, T2 relaxation time
Patients with glioblastoma have a dismal prognosis even with multimodal therapy. The current standard for primary therapy consists of surgery, radiotherapy, and chemotherapy using temozolomide according to the European Organisation for Research and Treatment of Cancer trial 22981–26981.1 Recurrent glioblastoma is usually treated by second surgery, re-irradiation, and nitrosoureas. In 2009 the FDA approved bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), for the treatment of recurrent glioblastoma. This approval was based on high response rates and a progression-free survival benefit compared with historical controls. However, the results of the underlying trial were mainly based on the Macdonald criteria, which rely heavily on contrast-enhanced MRI.2,3 However, anti-VEGF strategies strongly reduce extravasation of contrast agents via their influence on vascular permeability. Therefore, impressive disappearance of contrast enhancement under bevacizumab therapy may be a “pseudoresponse,” since it indicates reduced vascular permeability but does not necessarily correspond to antitumor activity.4–6 Reduced vascular permeability can also account for the pronounced reduction of the vasogenic edema that is frequently observed on T2-weighted MRI following anti-angiogenic therapies. In addition, non-enhancing tumor progression is common. Con-sequently, updated response criteria for high-grade gliomas were formulated by the Response Assessment in Neuro-Oncology (RANO) working group.7 These updated criteria now also account for “significant changes” on T2-weighted or fluid attenuated inversion recovery (FLAIR) MRI sequences. Nevertheless, in clinical practice the evaluation of MRI scans from patients under anti-angiogenic treatment is still ambiguous. First of all, no unequivocal definition of “significant changes” is given in the RANO criteria. Furthermore, the differentiation between tumor and edema on T2-weighted images is rather challenging.
The image contrast on T2-weighted and FLAIR images is determined mainly by tissue-specific T2 relaxation times. Increased tissue water and blood volume as well as loss of brain tissue texture from gliosis or tumor infiltration are known to increase T2 relaxation time.8 Animal models showed lowest T2 relaxation times in normal brain, intermediate values in tumor tissue, and longest T2 relaxation times in edema.9,10 Therefore, a quantitative approach measuring T2 relaxation times as absolute numbers in milliseconds and depicting them in gray-scaled or color-coded T2 maps (quantitative MRI) is the most direct and objective way to determine the image contrast in T2-weighted and FLAIR images of brain tissue.
It has been shown that quantitative T2 mapping is useful to monitor initial effects of anti-angiogenic therapy, and early changes in T2 relaxation times seem to be predictive of patient outcome.11,12 Therefore, we aimed to define the value of quantitative MRI in the follow-up of patients with recurrent glioblastoma under bevacizumab therapy.
In this study we prospectively examined a cohort of patients with recurrent glioblastoma before treatment with bevacizumab and every 8 weeks during treatment until the time of further tumor progression. Besides high-resolution standard sequences, we generated quantitative maps for T2 relaxation times. On the basis of the standard sequences, we segmented the tumor into 3 subvolumes: enhancing tumor, non-enhancing tumor, and edema. Thereby we were able to follow changes in T2 relaxation times during treatment not only in the whole tumor but also in these clinically relevant subvolumes. Moreover, these quantitative data were used to generate differential T2 maps by a voxelwise subtraction of the T2 relaxation times of 2 time points.11 Finally, we asked whether these data might be predictive of the outcome of our patients.
Materials and Methods
Study Design
This prospective noninterventional study was approved by our institutional review board (the ethics committee at the University Hospital Frankfurt, reference number 4/09-SIN 01/09). Enrollment was restricted to patients with the histological diagnosis of glioblastoma or gliosarcoma, radiologically confirmed recurrence (RANO criteria), adequate laboratory values, and the recommendation of a bevacizumab-based therapy. Each participant signed written informed consent prior to inclusion. The decision on the individual treatment schedule and on any concomitant therapy (eg, chemotherapy, radiotherapy) was solely the responsibility of the treating physician. All patients were treated in our outpatient unit and were seen every other week.
Some of the participants (n = 11) of this noninterventional study were part of an earlier analysis with a different question and without follow-up examinations.12
MR Study Protocol
Participants underwent MR examination before treatment and every 8 weeks during therapy until radiological (RANO criteria) or clinical progression. We performed MRI of the brain on a 3T whole body system (Magnetom Trio, Siemens) with an 8-channel phased array head coil. MR protocols for quantitative mapping of relaxation times T2 and T2* were used.12 The corresponding series of measurements included high-resolution (1 × 1 × 2 mm³) T2-weighted spin-echo sequences with 5 echo times (TEs) rising from 17 to 188 ms and a series of high-resolution T2*-weighted images (8 gradient echoes per excitation with increasing TEs from 10 to 52 ms with a constant increment of 6 ms). Further, 3D T1-weighted flash sequences (resolution time = 8.2, TE = 3.62; 10° flip angle) with parallel imaging (generalized autocalibrating partially parallel acquisition) were acquired before and after application of standardized intravenous contrast agent injection (0.1 mmol/kg gadobutrol) followed by a 20-mL bolus of 0.9% saline).
Processing of MRI Data
Generation of quantitative maps
We generated quantitative maps for T2 relaxation times and T2* relaxation times from MR data by pixelwise exponential fitting of the respective image series with custom-built programs written in MATLAB.12,13 Further, we calculated the T2′ relaxation time from T2 maps and T2* maps as described before.12,14 The T2* relaxation time measures local inhomogeneities of the magnetic field B0. Paramagnetic molecules with strong effect on the local magnetic field like ferritin and deoxyhemoglobin shorten the T2* relaxation time.15 However, the T2* relaxation time is also affected by the T2 relaxation time, which is known to be increased in tumor and edema. To account for this effect, the T2′ relaxation time can be calculated by 1/T2* – 1/T2 = 1/T2′.14 Therefore, short T2′ relaxation times are found in tissue with low oxygenation and/or high oxygen consumption.
Generation of differential T2 maps
Differential maps quantitatively visualize therapy-induced T2 changes for the whole brain. According to the approach of Ellingson et al,11 these maps are generated by registering the T2 maps of 2 time points followed by a voxelwise subtraction of the T2 relaxation times.11 To ensure adequate alignment, we registered follow-up T2 maps to the T2 maps of the respective reference time points by using linear registration with FLIRT (the FMRIB Linear Image Registration Tool of the Functional Magnetic Resonance Imaging of the Brain facility)16 and final visual inspection.
Like Ellingson et al,11 we compared time point t(0) before starting bevacizumab with time point t(1) 8 weeks after starting bevacizumab, and we followed up with the patients until tumor progression. For these examinations, the best response was used as reference. At least in our cohort, the reference always was time point t(1), the first MRI 8 weeks after the start of bevacizumab, leading to differential T2 maps for each follow-up examination by subtracting the reference T2 map of time point t(1). Areas of T2 relaxation time changes related to time point t(1) should depict changes in normal brain tissue, such as tumor progression, or any changes in tumor tissue (eg, evolution of necrosis or of solid tumor). For interpretation, these differential T2 maps were compared with conventional MRI. To our knowledge, this is the first study to examine the use of differential T2 maps in the follow-up of patients with recurrent glioblastoma under anti-angiogenic therapy.
Volume definition of enhancing tumor, non-enhancing tumor, and edema
Enhancing tumor was defined at each time point by semiautomatic identification on the T1-weighted images. The enhancement was roughly delineated on the contrast-enhanced T1-weighted image to exclude other enhancing structures, like vessels and choroid plexus. Next, contrast enhancement was computed with a 20% threshold, which was applied to the image showing the ratio between the enhanced and the non-enhanced T1-weighted sequences (T1-weighted after/before contrast agent).
In contrast to the semiautomated delineation of enhancing tumor, we visually delineated volumes of interest (VOIs) on conventional T2-weighted images, identifying non-enhancing tumor, edema, and normal control tissue based on the following criteria12,17,18:
Non-enhancing tumor: Hyperintense areas less bright than CSF, more inhomogeneous than edema, and more blurred at the gray-matter junction (not respecting the cortical ribbon or the gray matter of basal ganglia). The area of coregistered enhancing tumor was not included in the VOI of non-enhancing tumor.
Edema: Areas with clear and uniformly hyperintense signal on T2-weighted images compared with gray matter and respecting anatomical borders.
Control tissue: Areas in contralateral healthy hemisphere.
These VOIs were manually drawn (K.D., A.J.) on the high-resolution T2-weighted images (TE = 103; MRICroN software)19 obtained from the measurement series of T2 maps. During VOI drawing, we paid special attention to consistency between the different time points and we avoided resection cavities. A neuroradiologist (E.H.) with more than 10 years' experience in tumor imaging controlled the quality of each VOI.
Analysis of quantitative data from tumor and edema
Mean quantitative T2 relaxation times and T2′ relaxation times were determined from the 4 VOIs (enhancing tumor, non-enhancing tumor, edema, and control tissue) by registering the VOIs to the quantitative maps of each time point.16 This analysis allowed us to compare quantitative data between different tissues and also between different time points. Further, normalized histograms of the T2 relaxation times from edema and from tumor were generated for each patient and each time point.
Statistics
We compared volumes and absolute values of relaxation times between time points with the paired Wilcoxon signed rank test and parameters within a time point with a Mann–Whitney U-test. We used R Statistics software (http://www.R-project.org/). The relation of T2 and T2′ changes upon bevacizumab (t(1)/t(0)) with overall survival was evaluated through Kaplan–Meier analysis with a log-rank test using the respective medians as cutoff values. P < .05 was considered statistically significant for all analyses.
Results
Study Subjects
Twenty-two consecutive patients (5 female, 17 male) with recurrent glioblastoma were included. We excluded 1 patient due to severe movement artifacts and 1 patient due to asymptomatic intratumoral hemorrhage that falsified the tumor measurements. Two patients were lost to follow-up. Therefore, this analysis includes 18 evaluable patients.
The median age of the patients was 52 years (range, 31–67 y). All patients had at least primary therapy with irradiation and temozolomide before starting bevacizumab. The median number of chemotherapies prior to bevacizumab was 2 (range, 1–4). The median number of recurrences prior to bevacizumab was 3 (range, 2–5). Fourteen patients received bevacizumab (10 mg/kg body weight) intravenously every other week. Four patients received bevacizumab and irinotecan (125 mg/m² body surface). At the time of data analysis, all but 2 patients had died. The median survival time was 235 days from the start of bevacizumab treatment (mean, 264 d; range, 152–767). Of the 2 surviving patients, one was censored with 463 days and the other with 960 days of survival at the time of data analysis.
In summary, the patient characteristics of our cohort matches the results of the hitherto largest trial on the use of bevacizumab in patients with recurrent glioblastoma.2
MR Examinations
Table 1 shows the number of follow-up MR examinations for each patient. The following time points were considered for further data analysis:
t(0): before start of treatment
t(1): 8 weeks after start of treatment
t(n − 1): 8 weeks before progression
t(n): progression
Some patients with poor clinical status either moved during the contrast-enhanced sequence or prematurely ended the scanning, which resulted in a lack of these sequences. In summary, 6 contrast agent examinations could not be performed across all time points and subjects.
Table 1.
Follow-up MR examinations for each patient
| Patient | Time Points |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | t(0) | t(1) | t(2) | t(3) | t(4) | t(5) | t(6) | t(7) | t(8) | t(9) | t(10) | t(11) | t(12) |
| 1 | • | • | • | ||||||||||
| 2 | • | • | • | ||||||||||
| 3 | • | • | • | ||||||||||
| 4 | • | • | • | • | • | • | |||||||
| 5 | • | • | • | • | |||||||||
| 6 | • | • | • | ||||||||||
| 7 | • | • | • | ||||||||||
| 8 | • | • | • | ||||||||||
| 9 | • | • | • | • | • | • | |||||||
| 10 | • | • | • | • | • | ||||||||
| 11 | • | • | • | • | • | • | |||||||
| 12 | • | • | • | ||||||||||
| 13 | • | • | • | ||||||||||
| 14 | • | • | • | • | • | • | • | • | • | • | • | • | |
| 15 | • | • | • | • | • | • | |||||||
| 16 | • | • | • | • | |||||||||
| 17 | • | • | • | • | |||||||||
| 18 | • | • | • | • | • | • | • | • | • | • | • | • | • |
Time intervals between the examinations were 8 weeks. Time point t(0) determines the MR examination immediately before start of bevacizumab therapy; MR examination at time point t(1) was performed 8 weeks after start of treatment; and the last MR examination, t(n), was performed at progression.
Differential T2 Maps for Early Bevacizumab Effects
Differential T2 maps between pretreatment t(0) and the first scan on bevacizumab t(1) allowed us to visualize areas with a drop in T2 relaxation times upon starting therapy (Fig. 1). Comparison with conventional T2-weighted images revealed that this decrease was mainly due to decreased edema, whereas non-enhancing tumor areas changed to a lesser extent.
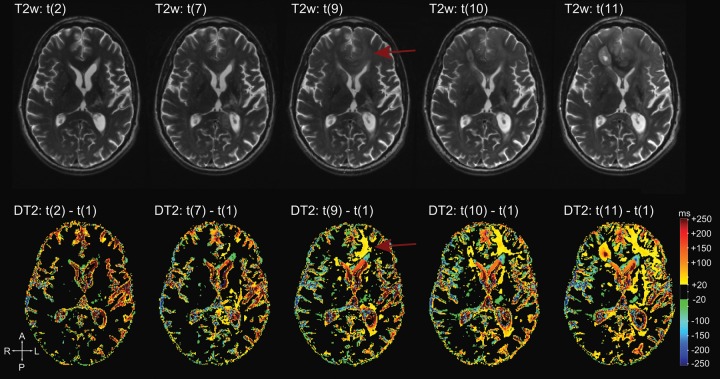
Fig. 1.
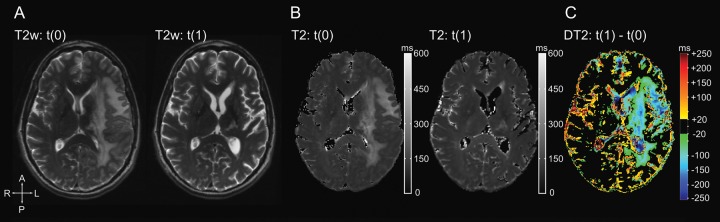
Differential quantitative T2 maps (in milliseconds) at various time points from subject 14. (A) Pre- and post-bevacizumab conventional T2-weighted images (T2w), (B) T2 maps (T2) pre- and post-bevacizumab (t(0) and t(1)), and (C) the differential T2 maps (DT2) of t(1) – t(0).
Differential T2 Maps for Detection of Progressive Disease
Differential T2 maps between the reference time point t(1) and each follow-up time point t(x) allowed us to visualize the evolution of T2 relaxation times during therapy with bevacizumab (Figs 2 and 3). In general, increased T2 relaxation times in brain areas that were initially unaffected corresponded to progressive tumor. A decrease of T2 relaxation times in remaining edema or non-enhancing tumor reflected mainly ongoing tumor decrease.
Fig. 2.
Differential quantitative T2 maps (in milliseconds) during follow-up from subject 14. First row: T2-weighted images (T2w) of time points t(1), t(7), t(9), t(10), and t(11) at tumor progression. Second row: differential T2 maps (DT2) showing the evolution of T2 differences to t(1) from corresponding time points. Note the obvious changes on the differential T2 map at time point t(9) in the left frontal lobe. These changes are hardly visible on conventional T2-weighted images (arrows).
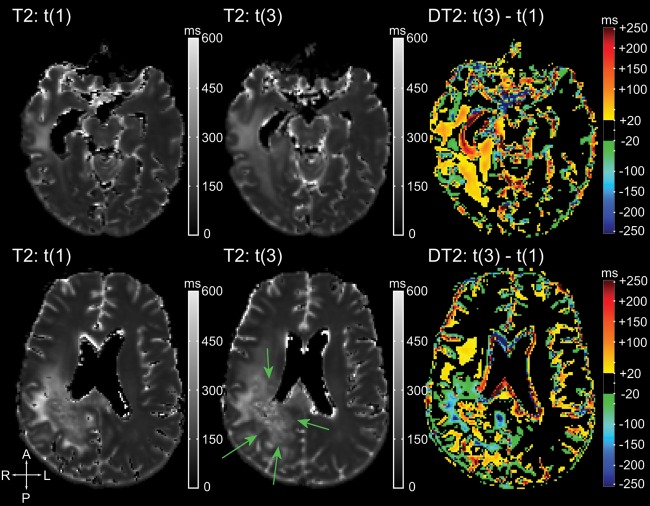
Fig. 3.
A caveat for the interpretation of differential T2 maps at the last time point is shown for subject 16. Each row represents one axial slice and contains (from left to right) the T2 map at the first time point under bevacizumab (t(1)), the T2 map at tumor progression (t(3)), and the differential T2 maps between the 2. Arrows indicate the progressive tumor growing partly in the preexisting edema. T2 relaxation times of this tumor are lower than the preexisting edema, which appears as blue areas in the differential T2 maps (negative ΔT2). This should not be misinterpreted as treatment response.
Pitfalls of Differential T2 Maps
In areas with augmented T2 relaxation times at time point t(1), changes could have various causes. In 2 patients, areas of increased T2 relaxation times in preexisting non-enhancing tumor were caused by the development of necrotic areas. In some areas, decreased T2 relaxation times in preexisting edema were attributed to growing tumor that replaced edema (Fig. 3). This is explained by a lower T2 relaxation time in progressive tumor compared with the T2 relaxation times of preexisting edema.
Changes in Volume of Enhancing Tumor, Non-enhancing Tumor, and Edema
Volume changes over time of enhancing tumor, non-enhancing tumor, and edema are shown in Fig. 4 for the representative time points mentioned.
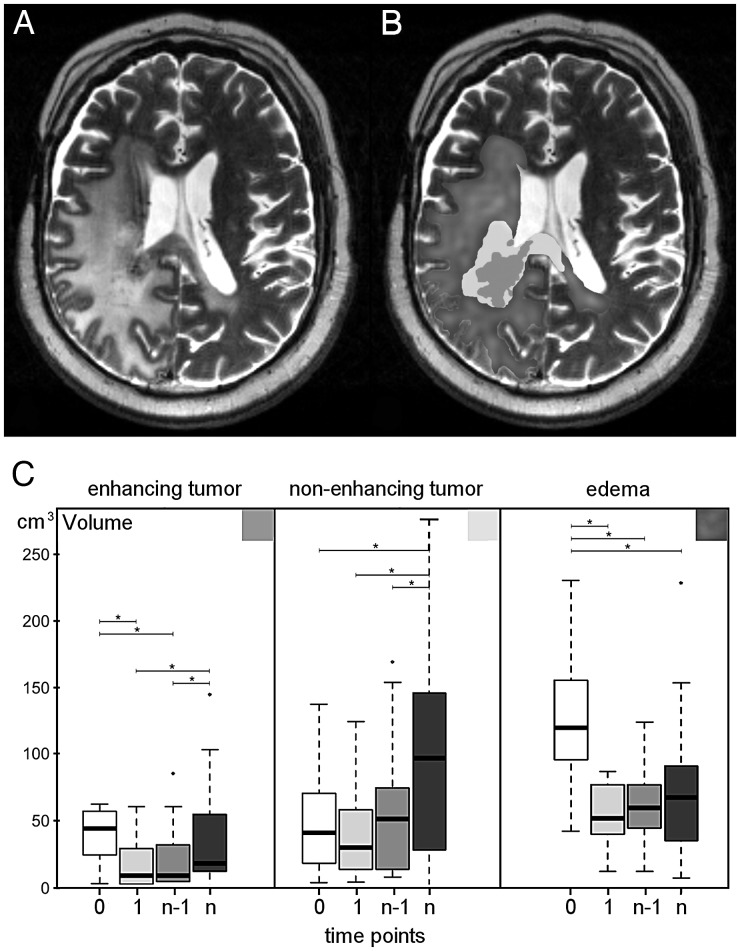
Fig. 4.
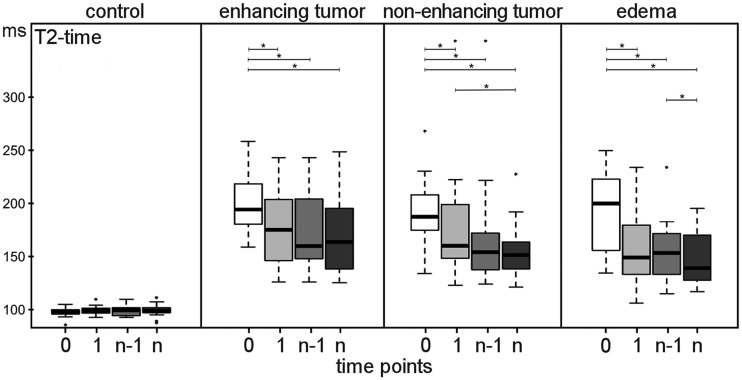
MRI shows (A) a T2-weighted image of a patient with recurrent glioblastoma and (B) the corresponding volume segmentations of enhancing tumor, non-enhancing tumor, and edema. (C) Volume change over time for enhancing tumor, non-enhancing tumor, and edema. Four time points are compared: t(0), before start of treatment (white boxes); t(1), 8 weeks after start of treatment (light gray boxes); t(n − 1), 8 weeks before progression (gray boxes); and t(n) at progression (dark gray boxes).
The volume of enhancing tumor decreased significantly (P < .0001) after start of bevacizumab therapy and remained low until progression (P = .003). In 15 of the 18 patients, the initial decrease was >20%, and in 10 of the 14 patients who received contrast agent at the last time point, the final increase was >20%.
Non-enhancing tumor was visible and definable in all patients. The volume of non-enhancing tumor did not decrease significantly after start of bevacizumab therapy (P = .07), and under therapy, it remained relatively constant until the time point of progression (P < .0001). Subject-wise, the volume of non-enhancing tumor increased >20% in all but 2 patients (16/18) at progression. One of these 2 patients had no tumor progression, while the other showed only an increase of the enhancing tumor volume. On the other hand, 2 patients (also receiving contrast agent) had a growth of non-enhancing tumor only.
The volume of edema decreased significantly (P < .0001) after start of bevacizumab therapy and remained unchanged during follow-up with a trend to increase (P = .06) at progression. Subject-wise, in 13/18 patients, the pretreatment edema volume decreased >20% upon therapy, and in 11/18 patients, it increased at the last time point.
Differences of T2 Relaxation Time in Enhancing Tumor, Non-enhancing Tumor, and Edema
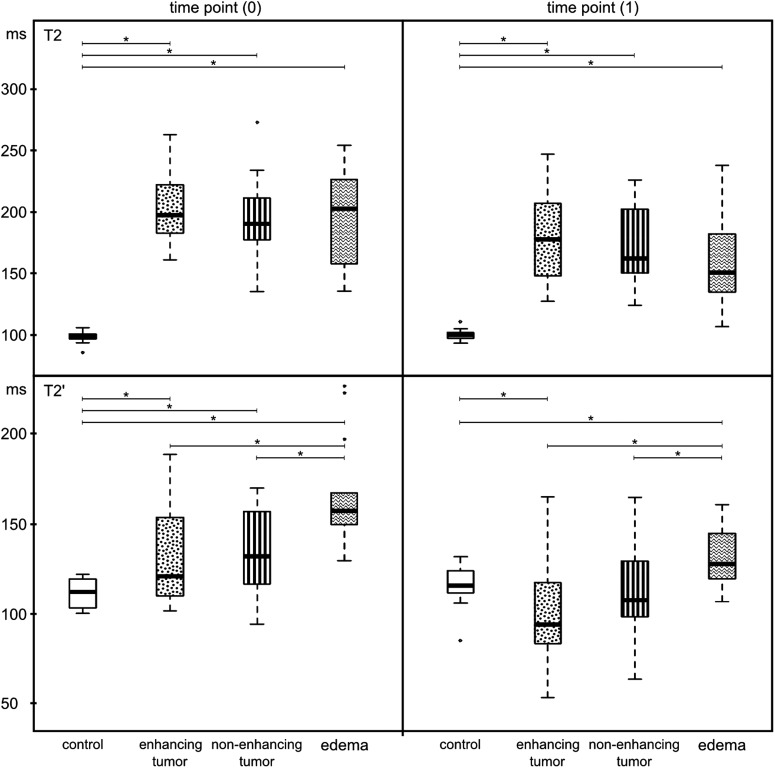
All quantitative values of tumor and edema differed at every time point from the values of control tissue (Figs 5 and 6). T2 relaxation time did not differ significantly between non-enhancing tumor and edema, between enhancing tumor and edema, or between non-enhancing tumor and enhancing tumor (Fig. 5). This negative result was found at all 4 time points (Supplementary data, Fig. S1). We further analyzed histograms of the T2 relaxation times because differences might not be reflected by the mean values. Figure S2 in the Supplementary data shows that there was no consistent pattern in the forms or shapes of T2 relaxation times comparing edema with non-enhancing tumor.
Fig. 5.
Comparison of T2 relaxation times and T2′ relaxation times among the 4 volumes of interest (control, contrast-enhancing tumor, edema, and non-enhancing tumor) before (time point 0) and 8 weeks after starting bevacizumab therapy (time point 1).
Fig. 6.
Changes in T2 relaxation times before and under bevacizumab therapy for control, contrast-enhancing tumor, edema, and non-enhancing tumor. Four time points are compared: t(0), before start of treatment (white boxes); t(1), 8 weeks after start of treatment (light gray boxes); t(n − 1), 8 weeks before progression (gray boxes); and t(n), at progression (dark gray boxes).
However, T2′ relaxation times confirmed a difference between visually segmented non-enhancing tumor and edema (Fig. 5). As stated in the Materials and Methods section, T2′ relaxation times were independent of T2, showing mainly the abundance of paramagnetic substances like deoxyhemoglobin. Further, the T2′ relaxation times of enhancing and non-enhancing tumor were similar. The differences between edema and tumor tissue were maintained during treatment (Supplementary data, Fig. S1).
Evolution of Quantitative T2 Relaxation Time During Bevacizumab Therapy
Compared with pretreatment, T2 relaxation times significantly decreased after start of bevacizumab therapy in enhancing tumor, non-enhancing tumor, and edema, but not in the control tissue (Fig. 6). After this first decrease, quantitative values stayed relatively constant during the follow-up without re-increasing at tumor progression. At progression the T2 relaxation time of non-enhancing tumor was significantly (P < .05) lower compared with the values at time point t(1).
T2 Relaxation Time as a Predictive Marker for Overall Survival Under Bevacizumab
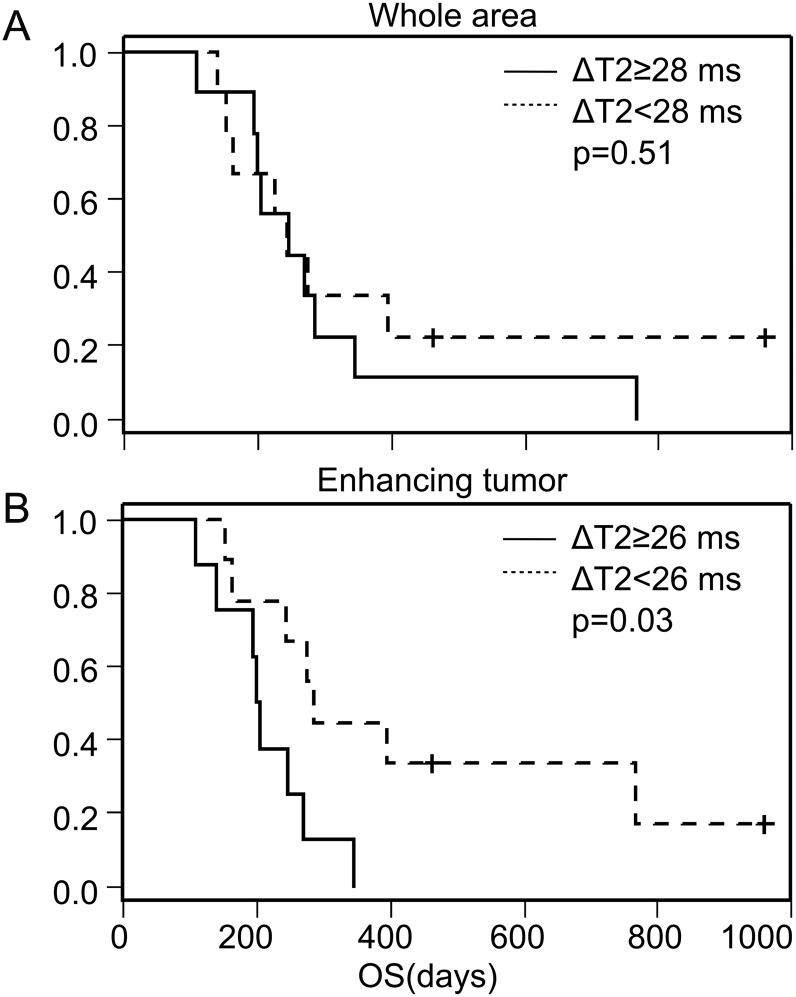
Changes in T2 relaxation times (Fig. 7) in the entire volume of T2 alterations (whole tumor and edema) were not predictive of outcome under bevacizumab therapy.
Fig. 7.
Kaplan–Meier curves for overall survival (OS) stratifying patients by the reduction of T2 relaxation time 8 weeks after start of bevacizumab using the median as a cutoff value. No difference is observed for the whole tumor (A), whereas patients with a T2 relaxation time decrease in the enhancing tumor of <26 ms had longer OS than those with ≥26 ms (B). Tick marks indicate the time of patient censoring. Changes in T2 relaxation time in edema and non-enhancing tumor volume were not predictive of survival (data not shown).
Patients with a decrease in T2 relaxation time (ΔT2 = T2 at t(1) – T2 at t(0)) of <26 ms (median) in the contrast-enhancing tumor had longer overall survival times (P = .03; Fig. 7). Changes in T2 relaxation time in edema and non-enhancing tumor volumes were not predictive of survival.
Discussion
This is the first study to investigate differential T2 maps in the follow-up of 18 patients with recurrent glioblastoma under anti-angiogenic therapy with bevacizumab. In this small but representative cohort, we prospectively evaluated absolute values for T2 relaxation times in combination with visual inspection/segmentation of conventional MRI.
We were able to confirm previous results from Ellingson et al11 showing that differential T2 maps convincingly visualize initial treatment effects of bevacizumab (Fig. 1). We extended this technique to objectively monitor tumor evolution during therapy with bevacizumab. As shown in Fig. 3, the use of quantitative values allows the visual detection of any tissue change during treatment by calculating color-coded differential T2 maps.
As expected, T2 relaxation times of tumor tissue and edema were significantly higher than the T2 relaxation times of control tissue. Worth mentioning, our mean values of T2 relaxation time matched the values of previous studies, which emphasizes the interinstitutional reproducibility of quantitative MRI methods.11,20 The increase in T2 relaxation times in initially nonaffected tissue detects tumor progression with high sensitivity. This may alleviate the main problems in monitoring brain tumors under bevacizumab as proposed by RANO.21 Quantitative MRI seems appropriate to diminish low interobserver reliability and to increase sensitivity in detecting tumor-related signal changes.17
Reliable registration as a prerequisite for good differential maps may be difficult under bevacizumab due to the impressive withdrawal of edema and the consequent anatomical changes. However, in the follow-up, such anatomical changes were negligible so that our proposed monitoring, which compares time points during therapy, may suffer less from registration errors. Further, the interpretation of differential T2 maps has some caveats. Several other tissue alterations (necrosis, gliosis, and bleeding) may also result in changed T2 relaxation times (Table 2); thus, T2 relaxation times of a progressive tumor can be lower, similar, or higher compared with the T2 relaxation times of previously affected tissue. Tumor progression inside already altered areas might result in decreased, equal, or increased values on differential T2 maps. Therefore, changes in T2 relaxation time in previously altered tissue require interpretation by referring to conventional MRI. But in general, a decrease in T2 relaxation time corresponds to treatment response and an increased T2 relaxation time to tumor progression.
Table 2.
Interpretations of changes of T2 relaxation times under bevacizumab therapy as shown in the color-coded differential T2 maps
| Normal Tissue | Tissue With Changes in T2 Relaxation Times | |
|---|---|---|
| Decrease in T2 relaxation times (green to blue) | Not observed* | Tumor response Rarely tumor progression |
| Increase in T2 relaxation times (yellow to red) | Tumor progression | Tumor progression Rarely conversion of tumor to necrosis or gliosis |
*Dilatation of ventricles or sulci due to the loss of brain volume under bevacizumab appears as blue areas.
These maps voxelwise subtract T2 relaxation times of any follow-up time point t(x) from T2 relaxation times 8 weeks after start of bevacizumab therapy (t(1), representing the time point of best response). A decrease in T2 relaxation times is indicated with a color range from green to blue, whereas an increase in T2 relaxation times is depicted with the range from yellow to red (see also Figs 1–3).
The disadvantage of differential T2 maps is the lack of discrimination between anti-edema and antitumor effects in the first weeks of bevacizumab treatment. To follow changes in T2 relaxation time more thoroughly, we segmented the tumor into 3 subvolumes: enhancing tumor, non-enhancing tumor, and edema. Enhancing tumor was semiautomatically defined as described in Materials and Methods. Non-enhancing tumor and edema were visually delineated on conventional T2-weighted images using previously described criteria.
There are no unequivocal criteria to differentiate tumor from edema. FLAIR sequences are often preferred due to their high sensitivity in detecting any pathology. However, FLAIR signals are the result of complex interactions between the relaxation times T1 and T2, which also depend on vendor-specific pulse sequences, impairing the reproducibility of signal intensities. In addition, the low gray/white matter contrast makes the reliable differentiation between tumor infiltration and edema difficult.22 For T2-weighted images, different patterns of tumor infiltration and edema were previously described.17 Non-enhancing tumor was defined as a region with a hyperintensity less than the intensity of CSF, showing mass effect and architectural distortion, including blurring of the gray/white interface and cortical thickening. In contrast, edema is confined mostly to the white matter, forming fingerlike extensions with pronounced gray/white matter interfaces.17,18 Pope et al17 reported that these specific imaging features confidently distinguished non-enhancing tumor from edema with a high interobserver reliability.
To our knowledge, this is the first study that separates T2 hyperintensities into edema and non-enhancing tumor to monitor bevacizumab therapy. As expected, bevacizumab resulted in an early and impressive reduction of edema and enhancing tumor (Fig. 4). In contrast, no significant reduction of non-enhancing tumor volume was observed. Without challenging a real antiglioma activity of bevacizumab, one could at least discuss the high response rates reported for the anti-angiogenic treatments. In our cohort, the progressive tumor under bevacizumab was rather non-enhancing, showing an impressive increase of non-enhancing tumor volume but only a small increase of edema and enhancing tumor. Therefore, differential T2 mapping would be the method of choice to indicate early and subtle tumor progression that might be missed on conventional MRI.
With absolute values for T2 relaxation times available, we wanted to know whether the 3 subvolumes—enhancing tumor, non-enhancing tumor, and edema—differed in those. The mean values did not reveal significant differences among the 3 subvolumes. This may be attributed to the inhomogeneity of tumor tissue. Non-enhancing tumor delineated visually by the methods described might be a mixture of gliosis, necrosis, and solid tumor tissue that average to a T2 relaxation time similar to that of edema. On the other hand, the histograms of T2 relaxation times also did not reveal a clear pattern to discriminate non-enhancing tumor and edema (Supplementary data, Fig. S2). With no obvious differences between the subvolumes, we raised the question of whether our visual definition of tumor and edema really discriminated different tumor-related tissues. In this context, the T2′ maps provide important information, showing significantly higher T2′ relaxation times of edema compared with enhancing and non-enhancing tumor tissue (Fig. 5). Both edema and tumor are under the influence of VEGF secreted by the tumor, while higher oxygen consumption might result in lower T2′ relaxation times in the tumor compared with edema.12,14,15 These findings confirm the validity of visually discriminating different tumor-related tissues.
In the follow-up of our cohort, we observed that T2 relaxation times decreased in all subvolumes (Fig. 6). T2 relaxation time is influenced mainly by the extracellular water content of brain tissue. Thus, the reduction of T2 relaxation time under anti-VEGF therapy may reflect lower water content due to normalized vessel permeability. Intriguingly, at the time point of tumor progression, T2 relaxation time did not increase in any of the 3 subvolumes. Furthermore, increased T2 relaxation times were not observed in the newly emerging non-enhancing tumor areas (data not shown). These findings suggest that the progressive tumor may contain a lower number of vessels with increased permeability. A potential rationale would be the ongoing anti-VEGF effects of bevacizumab even at the time point of progression. Alternatively, it might indicate a change in the pattern of tumor growth. Some previous preclinical and clinical studies reported on a sustained reduction of tumor blood vessels under anti-VEGF therapy.23–25 The difference of T2 relaxation times between tumor and normal tissue decreased from about 100 ms before bevacizumab to 50 ms at progression. The resulting lower contrast between tumor and healthy brain tissue on conventional T2-weighted images might lead to a delayed recognition of tumor progression under bevacizumab therapy, which could be a confounding factor for progression-free survival in clinical trials, falsely prolonging the progression-free survival of patients treated with bevacizumab.21 Therefore, more sophisticated methods to recognize progressive tumor are required. Quantitative T2 relaxation time measurements enable the calculation of differential T2 maps depicting even minor local changes in T2 relaxation time.
While differential T2 maps can depict tumor progression, averaged T2 relaxation times can also predict outcome of patients with recurrent glioblastoma under bevacizumab therapy. Ellingson et al11 averaged the T2 relaxation times from the entire area of signal abnormality containing both non-enhancing tumor and vasogenic edema.7 This approach revealed that values below 160 ms 4–6 weeks after start of bevacizumab therapy were associated with longer overall survival.11 In this study we segmented the tumor and found that the change in T2 relaxation time of the enhancing tumor before/after start of treatment is predictive of overall survival. Like Ellingson et al, we found no significant results for the relative change in T2 relaxation times of the entire area with signal abnormality.
In conclusion, quantitative mapping of T2 relaxation times improves the monitoring of patients with glioblastoma under bevacizumab therapy. Differential T2 maps depict even minor tissue changes, such as those caused by diffuse tumor infiltration, which might be missed on conventional MRI. However, conventional MRI is still important for the interpretation of differential T2 maps. Finally, changes in T2 relaxation times in the enhancing tumor were predictive of overall survival.
Supplementary Material
Funding
There was no funding for the present study.
Conflict of interest statement. J. P. S. has served as a consultant for Roche, the European distributor of bevacizumab (Avastin). The other authors have no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Stefanie Pellikan and Maurice Harter of the Institute of Neuroradiology and the staff and nurses of the Dr Senckenberg Institute of Neurooncology who supported this study. Excellent technical assistance was provided by Dr Jörg Magerkurth from the Institute of Neuroradiology and Professor Ralf Deichmann from the Brain Imaging Center Frankfurt. The Dr Senckenberg Institute of Neurooncology is supported by the Dr Senckenberg Foundation and the Hertie Foundation. J. P. S. is a Hertie Professor of Neurooncology.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald D, Cascino T, Schold SJ, Cairncross J. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 4.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol. 2009;27(18):2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. doi: 10.1097/WCO.0b013e328332363e. Review. [DOI] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Oh J, Cha S, Aiken, et al. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging. 2005;21(6):701–708. doi: 10.1002/jmri.20335. [DOI] [PubMed] [Google Scholar]
- 9.Hoehn-Berlage M, Tolxdorff T, Bockhorst K, Okada Y, Ernestus RI. In vivo NMR T2 relaxation of experimental brain tumors in the cat: a multiparameter tissue characterization. Magn Reson Imaging. 1992;10(6):935–947. doi: 10.1016/0730-725x(92)90448-9. [DOI] [PubMed] [Google Scholar]
- 10.Eis M, Els T, Hoehn-Berlage M. High resolution quantitative relaxation and diffusion MRI of three different experimental brain tumors in rat. Magn Reson Med. 1995;34(6):835–844. doi: 10.1002/mrm.1910340608. [DOI] [PubMed] [Google Scholar]
- 11.Ellingson BM, Cloughesy TF, Lai A, et al. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2012;106(1):111–119. doi: 10.1007/s11060-011-0638-x. [DOI] [PubMed] [Google Scholar]
- 12.Hattingen E, Jurcoane A, Bähr O, et al. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol. 2011;13(12):1349–1363. doi: 10.1093/neuonc/nor132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magerkurth J, Volz S, Wagner M, et al. Quantitative T*2-mapping based on multi-slice multiple gradient echo flash imaging: retrospective correction for subject motion effects. Magn Reson Med. 2011;66(4):989–997. doi: 10.1002/mrm.22878. [DOI] [PubMed] [Google Scholar]
- 14.Saitta L, Heese O, Förster AF, Matschke J, et al. Signal intensity in T2′ magnetic resonance imaging is related to brain glioma grade. Eur Radiol. 2011;21(5):1068–1076. doi: 10.1007/s00330-010-2004-3. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 18.Pope WB, Chen JH, Dong J, et al. Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology. 2008;249(1):268–277. doi: 10.1148/radiol.2491072000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 20.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;4:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Pope WB, Hessel C. Response assessment in neuro-oncology criteria: implementation challenges in multicenter neuro-oncology trials. AJNR Am J Neuroradiol. 2011;32(5):794–797. doi: 10.3174/ajnr.A2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melhem ER, Itoh R. Effect of T1 relaxation time on lesion contrast enhancement in FLAIR MR imaging: a study using computer-generated brain maps. AJR Am J Roentgenol. 2001;176(2):537–539. doi: 10.2214/ajr.176.2.1760537. [DOI] [PubMed] [Google Scholar]
- 23.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of ‘rebound’ revascularization as mode of escape. Cancer Res. 2011;71(1):19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabatabai G, Felsberg J, Sabel M, et al. Bevacizumab failure in glioblastomas [Meeting Abstracts] J Clin Oncol. 2012;30(15):2067. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.