Abstract
Background
To determine the protective effects of memantine on cognitive function in patients receiving whole-brain radiotherapy (WBRT).
Methods
Adult patients with brain metastases received WBRT and were randomized to receive placebo or memantine (20 mg/d), within 3 days of initiating radiotherapy for 24 weeks. Serial standardized tests of cognitive function were performed.
Results
Of 554 patients who were accrued, 508 were eligible. Grade 3 or 4 toxicities and study compliance were similar in the 2 arms. There was less decline in delayed recall in the memantine arm at 24 weeks (P = .059), but the difference was not statistically significant, possibly because there were only 149 analyzable patients at 24 weeks, resulting in only 35% statistical power. The memantine arm had significantly longer time to cognitive decline (hazard ratio 0.78, 95% confidence interval 0.62–0.99, P = .01); the probability of cognitive function failure at 24 weeks was 53.8% in the memantine arm and 64.9% in the placebo arm. Superior results were seen in the memantine arm for executive function at 8 (P = .008) and 16 weeks (P = .0041) and for processing speed (P = .0137) and delayed recognition (P = .0149) at 24 weeks.
Conclusions
Memantine was well tolerated and had a toxicity profile very similar to placebo. Although there was less decline in the primary endpoint of delayed recall at 24 weeks, this lacked statistical significance possibly due to significant patient loss. Overall, patients treated with memantine had better cognitive function over time; specifically, memantine delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed in patients receiving WBRT.
RTOG 0614, ClinicalTrials.gov number CT00566852.
Keywords: radiotherapy, brain metastases, cognition, memantine, neuroprotective agents
Radiotherapy is a proven curative and palliative therapeutic tool in the treatment of a wide variety of primary and metastatic brain tumors in adults, and recent advances in multimodality therapy have led to improvements in survival. As survival has improved, more attention has been directed toward long-term treatment-related morbidity. Specifically, the effect of cerebral radiotherapy on long-term cognitive performance is a major concern.1 The vascular hypothesis of radiation injury attributes radiation-induced accelerated atherosclerosis and mineralizing microangiopathy to the vascular insufficiency and infarction that can develop after radiotherapy.2 Therefore, the mechanisms of radiation-induced injury are similar to the small vessel disease seen with vascular dementia.3,4 For this reason, there is great interest in studying vascular dementia treatments to prevent or reduce radiation-induced cognitive injury. Additionally, because treatment of cognitive decline after radiation is limited, new approaches aimed at preventing the detrimental cognitive effect of whole-brain radiotherapy (WBRT) should be developed.
Glutamate is the principal excitatory amino acid neurotransmitter in cortical and hippocampal neurons.5 One of the receptors activated by glutamate is the N-methyl-D-aspartate (NMDA) receptor, which is involved in learning and memory.6 Ischemia can induce excessive NMDA stimulation and lead to excitotoxicity, suggesting that agents that block pathologic stimulation of NMDA receptors may protect against further damage in patients with vascular dementia.7 One such agent is memantine, an NMDA receptor antagonist. Memantine is a noncompetitive, low-affinity, open-channel blocker that has been shown to be neuroprotective in preclinical models.8–10 In 2 placebo-controlled phase III trials, memantine was well tolerated and effective in treating vascular dementia, especially in patients with small vessel disease.11,12 The Radiation Therapy Oncology Group (RTOG) therefore initiated a placebo-controlled, double-blind, randomized trial to evaluate the potential protective effect of memantine on neurocognitive function in patients receiving WBRT.
Materials and Methods
Patients
Adult patients with a pathologically proven diagnosis of solid malignancy within 5 years of registration and with brain metastases visible on contrast-enhanced MRI (or a contrast-enhanced CT for patients unable to have an MRI) were eligible. Eligibility criteria included a Karnofsky performance status of ≥70, stable systemic disease in the 3 months prior to study entry, serum creatinine ≤3 mg/dL, creatinine clearance ≥30 mL/min, total bilirubin ≤2.5 mg/dL, blood urea nitrogen (BUN) <20 mg/dL, Mini Mental State Exam (MMSE) score >18, negative serum pregnancy test, no memantine allergy, no current alcohol or drug abuse, no chronic use of benzodiazepines, and no severe active comorbidity.13 Patients could have received prior therapy for brain metastasis, including radiosurgery and surgical resection (but no prior cranial external beam radiotherapy). Patients receiving systemic therapy were eligible if such therapy was given >14 days prior to study entry, and they could not receive chemotherapy for at least 14 days after completing radiotherapy. The institutional review boards of the participating institutions approved the study protocol, and all patients provided written informed consent.
Study Design
The Zelen14 treatment allocation scheme was used to stratify patients according to recursive partitioning analysis (RPA) class13 (class I vs class II) and prior surgical therapy (none vs radiosurgery or surgical resection within 8 wk of randomization). Within each stratum, patients were randomized in a 1:1 ratio to placebo or memantine. The RTOG performed this trial and was responsible for data collection, statistical analysis, study design, and preparation of the manuscript.
Treatment
Patients received 37.5 Gy of WBRT (15 fractions of 2.5 Gy). Study drug administration was to commence no later than the third day of WBRT. Patients were randomly assigned to receive memantine or placebo orally for 24 weeks and escalating doses over the first 4 weeks. Week 1 was a single 5-mg morning dose followed by the addition of a 5-mg dose in the evening during week 2. In week 3, the morning dose was increased to 10 mg. The target dose for weeks 4 through 24 was 10 mg in the morning and 10 mg in the evening, for a total dose of 20 mg daily. The dose was lowered to 5 mg orally twice daily if creatinine clearance fell below 30 mL/min and was held if the creatinine clearance was less than 5 mL/min with a weekly recheck of laboratory values.
Assessments
At baseline and 8, 16, 24, and 52 weeks after the start of the study drug, all patients underwent assessments including neurologic exam, history and physical examination, performance status, brain MRI or CT, creatinine, BUN, total bilirubin, translational specimen collection, and neuropsychological evaluations. The neuropsychological test battery included tests of memory (Hopkins Verbal Learning Test-Revised [HVLT-R]), processing speed (Trail Making Test Part A [TMT-A]), executive function (Trail Making Test Part B [TMT-B]), verbal fluency (Controlled Oral Word Association [COWA]),15 and the MMSE.16 Changes in cognitive function as measured by the assessments utilized in this trial have been shown in previous studies to be clinically significant and associated with quality of life, functional independence, and progression-free and overall survival.17–19 Adverse events were reported according to the Common Terminology Criteria for Adverse Events v3.0.
Endpoints
The primary trial endpoint was whether the addition of memantine preserved cognitive function, specifically memory, as measured by the HVLT-R for Delayed Recall (HVLT-R DR) compared with placebo at 24 weeks from the start of drug treatment. Secondary endpoints included time to cognitive failure, overall survival, progression-free survival, and assessment of adverse events. Time to cognitive failure was defined as the first cognitive failure on any of the neurocognitive tests.
Statistical Analysis
In a previous trial of patients treated with WBRT, there was a decline in the mean score in the HVLT-R DR by 0.87 from 7.04 at baseline to 6.17 at 24 weeks and an SD of 3.19.20 It was expected that patients receiving placebo would experience a similar decline in cognitive function, while patients receiving memantine would experience a smaller decline in cognitive function over time. On the basis of a one-sided Wilcoxon rank-sum test with α = 0.025, we calculated that 221 patients per arm were required to have 80% statistical power to detect a mean difference of 0.87 in the HVLT-R DR change scores between the 2 treatment arms.21 Assuming that 20% of patients might be ineligible or nonevaluable (eg, death, progression) at the 24-week assessment, the target sample size was set at 536. All eligible patients randomized to the study were included (intent-to-treat analysis). Patients missing assessments due to neurologic disability were assigned the worst score. The multiple imputation procedure employing the Markov chain Monte Carlo method was also used to determine values for all remaining living patients missing assessments.22
Cognitive decline on any of the measures was analyzed using both the raw and the standardized scores. For the standardized score, the Clinical Trial Battery Composite was calculated by averaging across all standardized z scores for the HVLT-R, TMT-A and TMT-B, and COWA tests combined.18 The raw score was also evaluated using the reliable change index23 (RCI) criteria and the standardized score using 2 SD criteria to determine decline, stability, and improvement. Cognitive failure for each test was defined as a posttreatment score that met one of the following criteria: follow-up score that was at least 2 SD worse than the patient's personal baseline score or the patient's raw score change greater than the RCI. The cumulative incidence approach was used to estimate the time to cognitive failure to account for the competing risks of disease progression and death. Gray's test was used to test for a statistically significant difference in the distribution of cognitive failure times at the α = 0.025 level.24 In addition, the Cox proportional hazards regression model was used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) for the treatment difference.25
Disease progression in the brain included an increase of at least 50% for lesions ≤1 cm, an increase of at least 25% for lesions >1 cm, or the appearance of any new brain metastases. Death was also considered a failure. The median progression-free survival and overall survival times were estimated using the Kaplan–Meier approach.26 The stratified log-rank test was used to test for a statistically significant difference in survival distributions with α = 0.025.27
Results
Patients
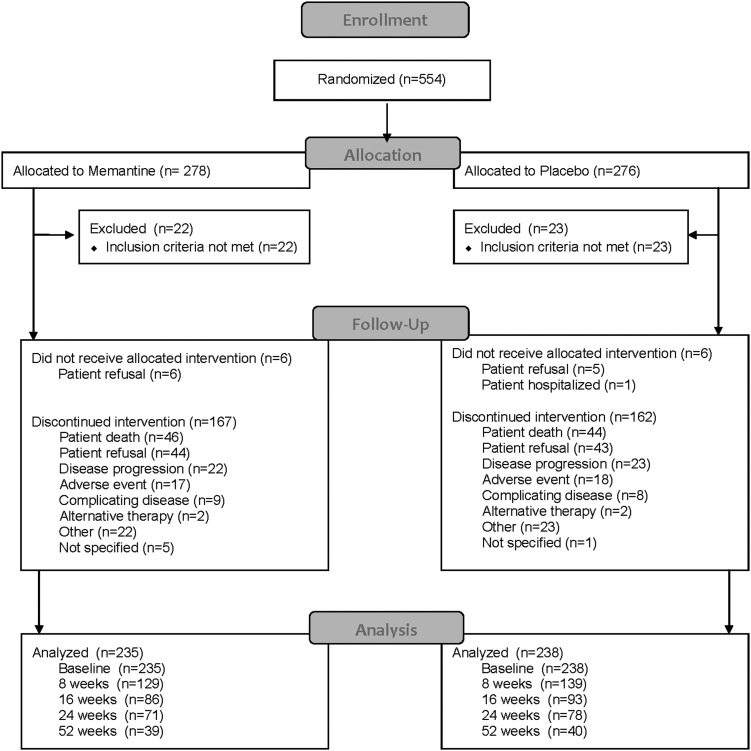
Between March 2008 and July 2010, a total of 554 patients from 143 centers in the United States and Canada were randomly assigned to WBRT and memantine or to WBRT and placebo (Fig. 1). Forty-six patients were ineligible primarily due to elevated creatinine or BUN. The treatment groups were well balanced and had no significant differences in demographic, baseline neurologic function, or tumor-related characteristics (Table 1), except that more patients in the memantine arm were receiving steroids at study entry (P = .05). For the 508 eligible patients, the median age was 59 years with the majority female (56%) and with primary lung cancer (70%). There were no differences in steroid or chemotherapy use over time between the study arms. During the study period, there was little change in chemotherapy use (29%, 33%, and 29% at 3, 6, and 12 months, respectively), while in contrast there was a gradual decline in steroid use over time (42%, 28%, and 24% at 3, 6, and 12 months, respectively).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 1.
Baseline demographic and clinical characteristics of the eligible patients
| Characteristic | Memantine (n = 256) | Placebo (n = 252) |
|---|---|---|
| Age (y) | ||
| Median | 60 | 59 |
| Range | 31–84 | 29–86 |
| Sex, n (%) | ||
| Male | 115 (44.9) | 107 (42.5) |
| Female | 141 (55.1) | 145 (57.5) |
| Race, n (%) | ||
| American Indian/Alaska Native | 2 (0.8) | 2 (0.8) |
| Asian | 5 (2.0) | 5 (2.0) |
| Black or African American | 27 (10.5) | 28 (11.1) |
| White | 215 (84.0) | 210 (83.3) |
| Other or unknown | 7 (2.7) | 7 (2.8) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 13 (5.1) | 11 (4.4) |
| Not Hispanic or Latino | 239 (93.4) | 234 (92.9) |
| Unknown (individuals not reporting ethnicity) | 4 (1.6) | 7 (2.8) |
| Education, n (%) | ||
| Grade 0–12 | 164 (64.1) | 165 (65.5) |
| Some college/vocational/technical school | 49 (19.1) | 44 (17.5) |
| Bachelor's degree | 43 (16.8) | 43 (17.1) |
| Neurologic function status, n (%) | ||
| No symptoms, fully active | 101 (39.5) | 105 (41.7) |
| Minor symptoms, fully active | 115 (44.9) | 98 (38.9) |
| Moderate symptoms, fully active | 26 (10.2) | 29 (11.5) |
| Moderate symptoms, not active | 14 (5.5) | 19 (7.5) |
| Severe symptoms | 0 (0.0) | 1 (0.4) |
| Primary disease site, n (%) | ||
| Lung | 181 (70.7) | 174 (69.0) |
| Breast | 32 (12.5) | 43 (17.1) |
| Colon | 3 (1.2) | 2 (0.8) |
| Other | 40 (15.6) | 33 (13.1) |
| RPA class, n (%) | ||
| Class I | 114 (44.5) | 112 (44.4) |
| Class II | 142 (55.5) | 140 (55.6) |
| Prior radiosurgery/surgical resection, n (%) | ||
| No | 178 (69.5) | 180 (71.4) |
| Yes | 78 (30.5) | 72 (28.6) |
| Receiving WBRT at study entry, n (%) | ||
| No | 189 (73.8) | 184 (73.0) |
| Yes | 67 (26.2) | 68 (27.0) |
| Prior chemotherapy, n (%) | ||
| No | 149 (58.2) | 132 (52.4) |
| Yes | 107 (41.8) | 120 (47.6) |
| Receiving steroids at study entry, n (%) | ||
| No | 81 (31.6) | 93 (36.9) |
| Yes | 175 (68.4) | 155 (61.5) |
| Unknown | 0 (0.0) | 4 (1.6) |
| HVLT-R Total Recall* | (n = 235) | (n = 238) |
| Median | –1.5 | –1.7 |
| HVLT-R Delayed Recall* | (n = 234) | (n = 236) |
| Median | –1.5 | –1.6 |
| HVLT-R Delayed Recognition* | (n = 234) | (n = 235) |
| Median | –0.6 | –0.6 |
| TMT-A (sec)* | (n = 233) | (n = 236) |
| Median | –1.3 | –1.1 |
| TMT-B (sec)* | (n = 226) | (n = 226) |
| Median | –2.0 | –1.5 |
| COWA* | (n = 235) | (n = 238) |
| Median | –1.0 | –1.0 |
| CTB Composite* | (n = 230) | (n = 235) |
| Median | –1.5 | –1.4 |
Abbreviations: HVLT-R, Hopkins Verbal Learning Test-Revised; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B; COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery.
*Standardized.
Compliance
WBRT delivery was comparable between treatment arms, and 93% of patients completed WBRT per protocol. Both arms had similar percentages of patients who completed all 6 months of the study drug per protocol with no modifications or delays (31% for the memantine arm and 33% for the placebo arm). The primary reasons for not completing therapy included disease progression (9%), adverse events (7%), death (18%), and patient refusal to complete treatment (18%); there were no differences in reasons for not completing treatment between the study arms. Central reviews were completed for all evaluable patients, and patients receiving memantine were just as likely to complete treatment per protocol as patients receiving placebo (47% vs 53%, P = .291).
Of the 508 clinically eligible patients, 173 (34%) died prior to completing the 24-week assessment and 55 (11%) withdrew consent. Evaluable patients (n = 280) included analyzable patients completing cognitive assessments (n = 149; 53%) and patients alive at time of missed assessment (n = 131; 47%). The percentage of analyzable patients completing follow-up assessments was consistent over time at 59%, 52%, 53%, and 43% for 8, 16, 24, and 52 weeks, respectively (Table 2). Reasons for noncompliance for cognitive testing were similar between treatment arms and most commonly were either not reported or reported as reason unspecified. To identify possible biases introduced because of missing cognitive assessments, the baseline characteristics of patients who had no tumor progression and no HVLT-R DR (ie, primary endpoint) scores were compared with those who had HVLT-R DR scores. There were no significant differences in baseline characteristics between the groups except that the patients who completed the cognitive evaluations were more likely to be RPA class I (P = .0210 for the 8-wk evaluation), to have better neurologic function (P = .0003 and .0441 for 8- and 16-wk evaluations, respectively), and to have undergone prior radiosurgery or surgical resection (P = .0272 and .0040 for 8- and 24-wk evaluations, respectively). In addition, patients who completed the cognitive evaluations at all time points had significantly longer survival times than patients who did not complete the tests and had a median overall survival of 12.4 versus 2.7 months for the 8-week evaluation (P < .0001), 17.0 versus 3.7 months for the 16-week evaluation (P < .0001), and 19.7 versus 4.1 months for the 24-week evaluation (P < .0001). These results suggest that patients with a better prognosis and longer survival were more likely to complete the cognitive assessments.28
Table 2.
Neurocognitive evaluation completion
| Baseline | 8 Weeks | 16 Weeks | 24 Weeks | 52 Weeks | |
|---|---|---|---|---|---|
| Clinically eligible patients | 508 | 508 | 508 | 508 | 508 |
| Withdrawn consent (entire protocol) | 9 (2%) | 43 (8%) | 53 (10%) | 55 (11%) | 55 (11%) |
| Inevaluable, patient death | 2 (0.4%) | 14 (3%) | 109 (21%) | 173 (34%) | 271 (53%) |
| Evaluable patients | 497 | 451 | 346 | 280 | 182 |
| Analyzable | 470 (95%) | 266 (59%) | 179 (52%) | 149 (53%) | 79 (43%) |
| Not analyzable | 27 (5%) | 185 (41%) | 167 (48%) | 131 (47%) | 103 (57%) |
Cognitive Outcomes
There was less decline in HVLT-R DR in the memantine arm (median decline of 0) compared with the placebo arm (median decline of –0.90) at 24 weeks, but the difference did not reach statistical significance (P = .059) possibly because there were only 149 analyzable patients at 24 weeks compared with an expected 442 evaluable cases in the protocol, resulting in only 35% statistical power to detect the absolute 0.87 difference in HVLT-R DR decline hypothesized in the protocol. There was also a trend to benefit for the memantine arm at 8 weeks (median decline –0.36 in the memantine arm vs –0.72 in the placebo arm, P = .069). However, there were statistically significant differences favoring the memantine arm in other cognitive tests, including HVLT-R Delayed Recognition (median decline 0 vs –1, P = .0149) and MMSE (median decline 0 vs –1, P = .0093) scores at 24 weeks using raw scores, HVLT-R Delayed Recognition scores (median decline 0 vs –0.715, P = .0115) at 24 weeks using standardized scores, and COWA scores (2% deterioration vs 13% deterioration, P = .0015) at 8 weeks using 2 SD criteria. Although differences were not statistically significant in the majority of cognitive tests, many of the cognitive outcomes again favored the memantine arm; Table 3 reports the standardized scores over time and is also reflective of the trends noted with the other methods of analysis (eg, raw scores, RCI, 2 SD).
Table 3.
Median cognitive decline: standardized scores
| Memantine | Placebo | P* | |
|---|---|---|---|
| Week 8 | |||
| HVLT-R Total Recall | –0.465 | –0.62 | .3805 |
| HVLT-R Delayed Recall | –0.36 | –0.72 | .0692 |
| HVLT-R Delayed recognition | 0 | –0.71 | .0762 |
| TMT-A | 0 | –0.1 | .0848 |
| TMT-B | 0 | –0.35 | .2886 |
| COWA | –0.11 | –0.31 | .0513 |
| CTB Composite | –0.29 | –0.48 | .2157 |
| Week 16 | |||
| HVLT-R Total Recall | –0.62 | –0.615 | .3854 |
| HVLT-R Delayed Recall | –0.915 | –0.71 | .4109 |
| HVLT-R Delayed Recognition | 0 | 0 | .4541 |
| TMT-A | –0.2 | –0.285 | .4375 |
| TMT-B | –0.39 | –0.59 | .2470 |
| COWA | –0.05 | –0.42 | .0380 |
| CTB Composite | –0.335 | –0.45 | .1926 |
| Week 24 | |||
| HVLT-R Total Recall | –0.23 | –0.415 | .2093 |
| HVLT-R Delayed Recall | 0 | –0.895 | .0587 |
| HVLT-R Delayed Recognition | 0 | –0.715 | .0115 |
| TMT-A | 0.075 | –0.365 | .0237 |
| TMT-B | –0.45 | –0.49 | .2966 |
| COWA | –0.1 | –0.16 | .3080 |
| CTB Composite | –0.03 | –0.41 | .0212 |
Abbreviations: HVLT-R, Hopkins Verbal Learning Test-Revised; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B; COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery.
*Wilcoxon rank-sum test (one-sided).
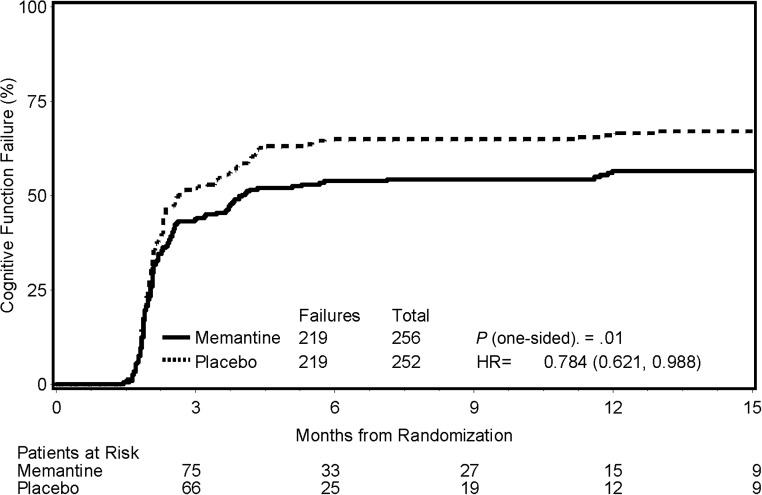
Time to cognitive failure, defined as the first cognitive failure on any of the neurocognitive tests, was found to significantly favor the memantine arm (HR, 0.78; 95% CI, 0.62−0.99; P = .01; Fig. 2). The probabilities of cognitive function failure at 24 weeks were 53.8% and 64.9% in the memantine and placebo arms, respectively (Table 4), a 21% relative reduction.
Fig. 2.
Cumulative incidence of cognitive function failure according to treatment arm.
Table 4.
Cognitive function failure by treatment arm
| Month | Memantine (n = 256) |
Placebo (n = 252) |
||
|---|---|---|---|---|
| Estimate (%) | At risk | Estimate (%) | At risk | |
| 0 | 0.0 | 256 | 0.0 | 252 |
| 3 | 43.7 | 75 | 51.9 | 66 |
| 6 | 53.8 | 33 | 64.9 | 25 |
| 9 | 54.3 | 27 | 64.9 | 19 |
| 12 | 56.4 | 15 | 65.9 | 12 |
| 15 | 56.4 | 9 | 67.1 | 9 |
| Total failures | 219 | 219 | ||
Linear regression models were used to determine the memantine treatment effect on a single 8-, 16-, or 24-week follow-up assessment, adjusted for baseline assessment score and intracranial progression. Results from complete case and imputed case analyses were consistent. There were no significant treatment differences in HVLT-R DR scores. For the complete case data, significant differences were found favoring the memantine arm for COWA scores at 8 (P = .008) and 16 weeks (P = .0041) and for TMT-A and MMSE at 24 weeks (P = .0137 and .0038, respectively). Using the imputed data, a significant difference was found for COWA scores at 8 weeks (P = .0103) favoring the memantine arm.
To determine the influence of steroids on cognitive function, and in particular the effect on HVLT-R scores, analyses were conducted both with the study arms combined and with each study arm separately. The only significant difference was in HVLT-R DR at 8 weeks; patients treated with steroids had more decline (median decline –2 vs –0.5, P = .0350).
Survival and Progression
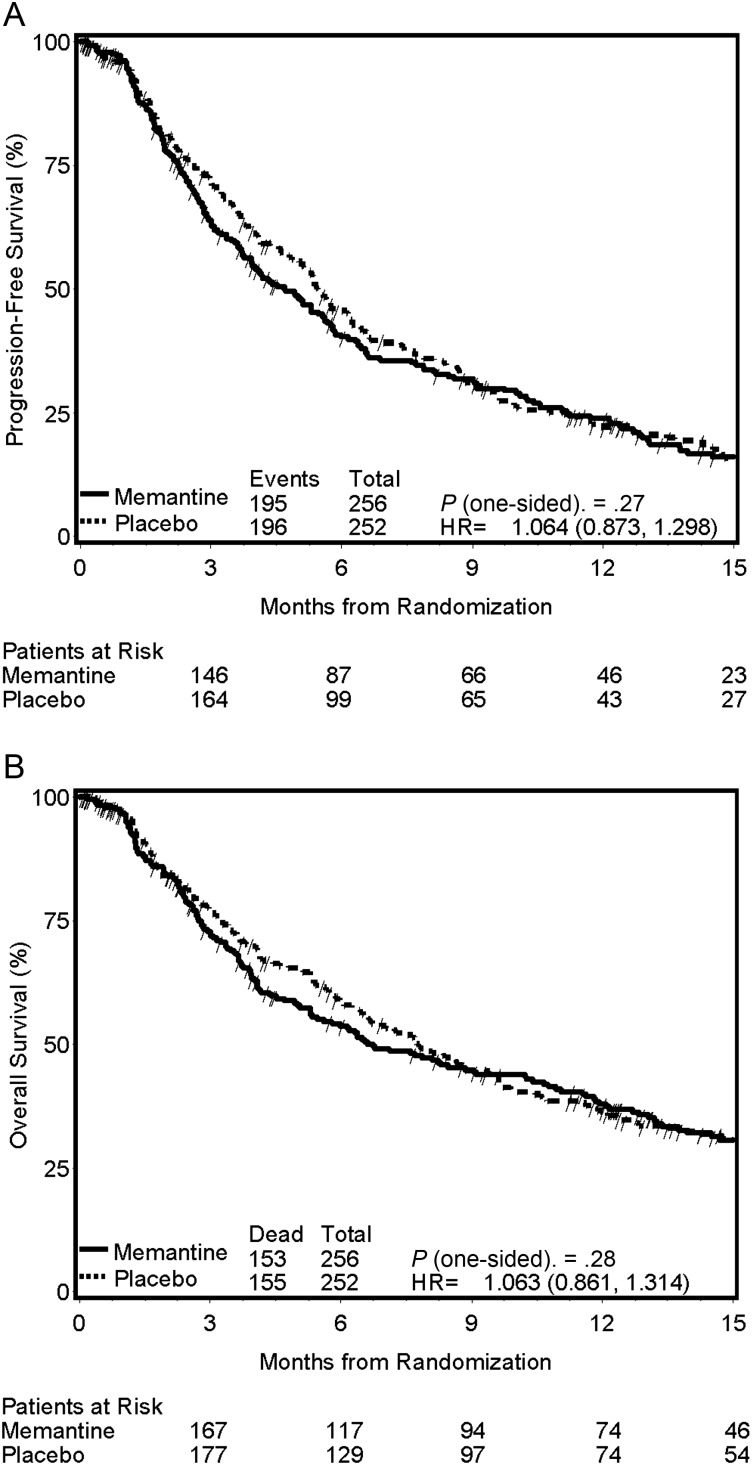
The median range of follow-up for all censored patients was 12.4 months. There were no differences in progression-free survival (median 4.7 vs 5.5 mo; HR, 1.06; 95% CI, 0.87–1.30; P = .27; Fig. 3A) or overall survival (median 6.7 vs 7.8 mo; HR, 1.06; 95% CI, 0.86–1.31; P = .28; Fig. 3B) between the memantine arm and the placebo arm, respectively.
Fig. 3.
Kaplan–Meier estimates of progression-free survival (A) and overall survival (B) according to treatment arm.
Toxic Effects
Grade 3–4 events were reported for 28% of patients on each of the 2 treatment arms. Grade 3–4 events that were attributable to treatment were reported for 14% of patients on each treatment arm, with the most common side effects being fatigue, alopecia, nausea, and headache, but there were no statistically significant differences between the treatment arms. No grade 5 treatment-related events were reported; however, grade 5 events not attributable to treatment were reported for 2% and 1% of patients on the memantine and placebo arms, respectively.
Discussion
Cognitive deterioration after WBRT can be clinically devastating.1 With neurocognitive testing, significant abnormalities can be seen in nearly half of patients after WBRT.29 Treatment of cognitive sequelae after cerebral radiation remains very limited. The majority of trials have been small studies that have found limited benefit from symptomatic treatments such as methylphenidate30 and donepezil.31 Because of this limited efficacy, there has been great interest in prophylactic approaches to diminish the neurotoxicity of radiation. In the current trial, even though the primary endpoint did not reach statistical significance (possibly due to sample size diminution with time), many of the secondary endpoints showed that memantine in patients receiving WBRT delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared with placebo.
Although there was less decline in the HVLT-R DR in the memantine arm at 24 weeks, the difference did not reach statistical significance possibly due to the limited statistical power at that time point. In hindsight, the assumptions made for the sample size estimation were much too optimistic: in reality, only 149 (29%) of 508 eligible patients completed the 24-week assessment, in contrast to the 80% completion rate assumed in the protocol. Because patients who did not complete cognitive assessment were more likely to have worse prognostic factors (eg, neurologic function) and shorter survival at every time point, this poor compliance was likely due primarily to tumor progression and death. The neurocognitive testing battery used in this trial is the same battery utilized in many cooperative group trials, and although the battery is only ∼20 min in length, poor compliance has been seen in many other trials, and completion rates were under 40% at 6 months.29,32 There are probably many causes for this poor compliance, but it is likely not due to complexity or length of the neurocognitive battery or even disease progression. A review of 8 brain cancer trials with 1957 patients found completion rates of the MMSE to be frequently less than 50% at 6 months; even for patients with tumors having a favorable prognosis—such as low-grade glioma and oligodendroglioma and more than 98% alive at 12 months—completion rates were less than 50%.33 For future trials the RTOG has addressed this issue with educational sessions to emphasize the importance of collecting the neurocognitive data, sending automated reminders to research assistants prior to and within a couple of weeks of when assessments are due, using reimbursement as staged payments tied to completion of evaluations at certain time points, and doing monthly monitoring of compliance.
Time to cognitive failure was found to significantly favor the memantine arm. Of interest, in early follow-up a high rate of cognitive failure was seen. Other studies of patients with brain metastases treated with WBRT34 have had similar findings and have noted the cause of cognitive decline to be due primarily to progressive disease,28 although there are likely many other possible causes, such as generalized deterioration, systemic therapies, and the WBRT itself. In the current study, the rate of cognitive decline over time slowed by 4 months after WBRT in both arms, but more so in the memantine arm. This meant that the benefit from memantine was due mainly to a difference in the hazard ratios of the 2 trial arms between 3 and 6 months after WBRT. This is consistent with prior studies34 suggesting that cognitive function at the shorter follow-up times is affected by (subclinical) progressive disease in the brain. In addition, at all time points patients with better prognostic factors and better survival were more likely to complete the cognitive assessments. Therefore, the potential benefit of memantine is more likely to be realized in prognostically favorable patients13 and in patients with a good response to radiation.
Memantine was well tolerated in this population of patients with brain metastases and had a side effect profile essentially equivalent to that for placebo. Other trials have also found memantine to be well tolerated even in elderly dementia patients with multiple comorbidities and polypharmacy. In these trials, adverse event rates with memantine were similar to rates for placebo, and more patients taking placebo than memantine discontinued the study medication.11,12
In conclusion, the use of memantine during and after WBRT resulted in better cognitive function over time, specifically delaying time to cognitive decline and reducing the rates of decline in memory, executive function, and processing speed. No statistically significant difference was seen in the HVLT-R DR; however, because the toxicity and tolerance of memantine are essentially equivalent to those for placebo, treatment of patients receiving WBRT with memantine to maintain cognitive function should be considered. Many issues remain to be considered, such as the role of hippocampal sparing WBRT, the existence of biologic-based subsets of patients more susceptible to the detrimental effects of WBRT, and the determination of subsets of patients more responsive to treatment with memantine. These questions will be further explored in ongoing trials such as RTOG 0933 and future translational analyses of the current trial.
Disclosure
D.K. is a consultant for Varian and has received speaker's honoraria from Accuray. M.P.M. has or has had consulting (including speaking and advisory) relationships with Abbott, Genentech, Elekta, Merck, Novocure, Schering-Plough, and Tomotherapy; he serves currently on the board of directors of Pharmacyclics (with stock options in Pharmacyclics) and served previously on the board for Tomotherapy.
Funding
This trial was conducted by the Radiation Therapy Oncology Group (RTOG) and was supported by RTOG grant U10 CA21661 and Community Clinical Oncology Program grant U10 CA37422 from the National Cancer Institute (NCI) and by Forest Pharmaceuticals. This manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Acknowledgments
The authors acknowledge the efforts of Sandrine Geinoz, PhD, and Wendy Bergantz, RN, for expert data management assistance during the conduct of this trial.
References
- 1.Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702–713. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 3.Belka C, Budach W, Kortmann RD, et al. Radiation induced CNS toxicity—molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 5.Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56:539–555. doi: 10.1016/0306-4522(93)90355-j. [DOI] [PubMed] [Google Scholar]
- 6.Danysz W, Parsons CG, Karcz-Kubicha M, et al. GlycineB antagonists as potential therapeutic agents. Previous hopes and present reality. Amino Acids. 1998;14:235–239. doi: 10.1007/BF01345268. [DOI] [PubMed] [Google Scholar]
- 7.Lancelot E, Beal MF. Glutamate toxicity in chronic neurodegenerative disease. Prog Brain Res. 1998;116:331–347. doi: 10.1016/s0079-6123(08)60446-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen HS, Pellegrini JW, Aggarwal SK, et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrini JW, Lipton SA. Delayed administration of memantine prevents N-methyl-D-aspartate receptor-mediated neurotoxicity. Ann Neurol. 1993;33:403–407. doi: 10.1002/ana.410330414. [DOI] [PubMed] [Google Scholar]
- 11.Orgogozo JM, Rigaud AS, Stoffler A, et al. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33:1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 12.Wilcock G, Mobius HJ, Stoffler A, et al. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17:297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 14.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Diseases. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 15.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Bentzen SM, Renschler M, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DR, Sawyer AM, Meyers CA, et al. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14:808–816. doi: 10.1093/neuonc/nos082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers CA, Hess KR, Yung WK, et al. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 20.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 21.Randles RH, Wolfe DA. Introduction to the Theory of Nonparametric Statistics. New York, NY: John Wiley & Sons; 1979. [Google Scholar]
- 22.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Developments. Rockville, MD: SAS Institute Inc.; 2002. [Google Scholar]
- 23.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 25.Cox DR. Regression models and life tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports—Part 1. 1966;50:163–170. [PubMed] [Google Scholar]
- 28.Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24:5427–5433. doi: 10.1200/JCO.2006.08.5605. [DOI] [PubMed] [Google Scholar]
- 29.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyers CA, Weitzner MA, Valentine AD, et al. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998;16:2522–2527. doi: 10.1200/JCO.1998.16.7.2522. [DOI] [PubMed] [Google Scholar]
- 31.Shaw EG, Rosdhal R, D'Agostino RB, Jr, et al. Phase II study of donepezil in irradiated brain tumor patients. Effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 32.Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae K, Bruner DW, Baek S, et al. Patterns of missing Mini Mental Status Exam (MMSE) in Radiation Therapy Oncology Group (RTOG) brain cancer trials. J Neurooncol. 2011;105:383–395. doi: 10.1007/s11060-011-0603-8. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Neurooncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]