Abstract
Background
Mutations in the presenilin (PSEN1, PSEN2) and amyloid precursor protein (APP) genes cause familial Alzheimer’s disease (FAD) in a nearly fully penetrant, autosomal dominant manner, providing a unique opportunity to study presymptomatic individuals who can be predicted to develop Alzheimer’s disease (AD) with essentially 100% certainty. Using tensor-based morphometry (TBM), we examined brain volume differences between presymptomatic and symptomatic FAD mutation carriers and non-carrier (NC) relatives.
Methods
Twenty-five mutation carriers and 10 NC relatives underwent brain MRI and clinical assessment. Four mutation carriers had dementia (MUT-Dem), 12 had amnestic mild cognitive impairment (MUT-aMCI) and nine were cognitively normal (MUT-Norm). TBM brain volume maps of MUT-Norm, MUT-aMCI and MUT-Dem subjects were compared to NC subjects.
Results
MUT-Norm subjects exhibited significantly smaller volumes in the thalamus, caudate and putamen. MUT-aMCI subjects had smaller volumes in the thalamus, splenium and pons, but not in the caudate or putamen. MUT-Dem subjects demonstrated smaller volumes in temporal, parietal and left frontal regions. As non-demented carriers approached the expected age of dementia diagnosis, this was associated with larger ventricular and caudate volumes and a trend towards smaller temporal lobe volume.
Conclusions
Cognitively intact FAD mutation carriers had lower thalamic, caudate and putamen volumes, and we found preliminary evidence for increasing caudate size during the predementia stage. These regions may be affected earliest during prodromal stages of FAD, while cortical atrophy may occur in later stages, when carriers show cognitive deficits. Further studies of this population will help us understand the progression of neurobiological changes in AD.
INTRODUCTION
Individuals with mild cognitive impairment (MCI) are at increased risk of developing Alzheimer’s disease (AD) compared to age-matched controls.1 However, rates of progression from MCI to AD are variable,2 and some MCI individuals revert to normal cognition. As such, many MCI individuals may not have incipient AD, reducing the utility of the MCI classification for predicting progression to dementia. This key research limitation may be circumvented by studying persons at risk of inheriting familial Alzheimer’s disease (FAD) caused by autosomal dominant mutations in the genes coding for presenilin 1 (PSEN1), presenilin 2 (PSEN2) or amyloid precursor protein (APP). These mutations are nearly fully penetrant and make it possible to examine differences in brain morphology in presymptomatic individuals who will later develop AD with essentially 100% certainty.
FAD has a generally younger age of onset and more rapid course,3 and can have distinctive clinical features such as paraparesis,4 early myoclonus and seizures.3 The neuropathological changes in FAD are generally similar to those in sporadic AD,3 but atypical neuropathology has been reported in some cases, including the presence of ‘cotton wool’ plaques in the cerebral cortex, non-neuritic amyloid plaques in the cerebellum and degeneration of corticospinal tracts and the basal ganglia.3,5,6 In-vivo neuroimaging studies of FAD mutation carriers show reduced temporal lobe and hippocampal volumes before the onset of symptoms.7 Non-demented carriers have demonstrated higher atrophy rates than healthy controls in the parietal grey matter,8 posterior cingulate gyrus8 and precuneus.9 Brain atrophy rates in FAD mutation carriers may begin to accelerate approximately 5 years before the diagnosis of AD.7,8
Earlier studies have included non-related age-matched healthy controls, but comparison of presymptomatic mutation carriers to non-carrier (NC) relatives may better control for environmental and biological factors that could confound results. We previously found that non-demented mutation carriers showed no significant differences in cortical thickness or hippocampal volume compared to NC controls, and that cortical atrophy was only observed in carriers diagnosed with dementia.10 In the current study, we separated the non-demented carriers who are cognitively intact from those who demonstrate signs of memory impairment and compared each group to NC relatives in order to examine regional volume differences associated with different stages of cognitive functioning in this population. Furthermore, we examined the whole brain using tensor-based morphometry (TBM), an approach that has been successfully used to study both cortical and subcortical brain changes in neurodegenerative disorders.11,12
METHODS
Participants
Affected persons known to have pathogenic PSEN1 or APP mutations and their at-risk first-degree relatives were approached for participation in the study. Thirty-seven individuals (eight men), aged 23–59 years (mean=37.1 years, SD=9.5) received in-depth clinical, neuropsychological and imaging assessments at the University of California, Los Angeles (UCLA). Twenty of these subjects resided in Mexico and travelled to UCLA to participate. The clinical dementia rating scale (CDR)13 was performed by authors JMR and LDM and the mini-mental state examination (MMSE)14 by LDM on all subjects; they were blind to subjects’ genetic status except in the case of demented subjects and one asymptomatic person who had previously undergone genetic testing. At-risk persons who tested negative for the FAD mutation present in their family (NC relatives) served as controls. All subjects, or their proxies, signed written informed consent. All study procedures were approved by the institutional review boards at UCLA and the National Institute of Neurology and Neurosurgery in Mexico City.
Genetic testing
Subjects underwent genetic testing for the FAD mutation for which they were known to be at risk. They were informed they would be tested, but in the context of the research protocol would not be told the result. The option of revealing test results through a genetic counsellor outside of the study was offered. Blood samples were coded according to a unique identifier and forwarded to the genetics laboratory. The presence of the A431E (number of subjects at risk=22), L235V (n=7) and S212Y (n=1) mutations in PSEN1 were assessed using restriction fragment length polymorphism analyses. The A431E PSEN1 mutation represents a founder effect originating in Jalisco State, Mexico.15 Therefore, although the 22 persons at risk for this mutation come from eight families, they are relatively closely related. The presence of the PSEN1 G206A mutation (n=1) and the APP V717I mutation (n=6) was assessed directly with bidirectional sequencing. Twenty-five of the 37 subjects were found to be mutation carriers: 21 had PSEN1 mutations and four had APP mutations (table 1). The breakdown of carriers and NC by specific mutations is not shown to maintain confidentiality regarding mutation status.
Table 1.
Demographic information for FAD mutation carriers separated by mutation type
| Demographic variables |
PSEN1 (n=21) Mean (SD) |
APP (n=4) Mean (SD) |
t or χ2 | p Value |
|---|---|---|---|---|
| Age, years | 35.81 (9.4) | 35.00 (10.6) | 0.14 | 0.89 |
| Relative age, years |
−8.10 (8.9) | −18.50 (10.3) | 1.88 | 0.14 |
| Education, years | 12.36 (3.6) | 8.25 (5.5) | 1.44 | 0.23 |
| Gender | 5 men/16 women | 4 women | 1.19 | 0.28 |
Group differences in age, relative age and education were assessed using independent sample t tests; differences in gender distribution were assessed using Pearson χ2 tests. PSEN1 and APP mutation carriers did not significantly differ in age, relative age, education, or gender.
FAD, familial Alzheimer’s disease.
Cognitive assessment
All non-demented subjects (with CDR < 1) underwent detailed neurocognitive assessment within a week of scan acquisition. Testing was performed in Spanish by a psychometrist (LDM) fluent in both English and Spanish in the following domains:
Memory was assessed using delayed recall trials of (1) the word list-learning test (ADP),16 a 16-word list learning and memory task, (2) memory verbal prose (MVP),16 which requires recall of a short paragraph, and (3) the Rey–Osterrieth complex figure test (ROCFT),17 a visual memory task involving reconstruction of a complex figure.
Processing speed was measured using the Digit Symbol Coding test,18 a test of graphomotor speed with rapid copying of symbols, the Stroop test,19 a measure of verbal processing speed involving rapid word reading (Stroop word) and colour naming (Stroop colour), and the Colour Trails Test (Color Trails 1),20 a measure of psychomotor speed involving rapid number sequencing.
Visuospatial skills were assessed by the ROCFT–copy,17 involving the paper-and-pencil copy of a complex figure, and block design from the Wechsler Adult Intelligence Scale–Revised,18 a measure of gross visuoconstruction using blocks to match designs.
Language was assessed using a measure of category fluency (Animals)21 and a test of object naming from the Spanish–English neuropsychological assessment scale.22
Executive functioning was assessed using the colour–word interference trial of the Stroop test,19 a measure of response inhibition, the Colour Trails Test (Colour Trails 2),20 a measure of mental flexibility, and the Wisconsin Card Sorting Test,23 a measure of novel problem-solving ability involving abstract concept formation and set-shifting.
Standardised z-scores were calculated for each test using means and SD of the 12 NC. These z-scores were averaged to calculate composite scores for each cognitive domain (memory, processing speed, visuospatial skills, language and executive functioning), which were later used to compare cognitive performance between groups.
Subjects who scored at least 1.5 SD below the established mean (≤7th percentile) on at least one of three memory tests (ADP delayed recall, MVP delayed recall, or ROCFT delayed recall) were given a diagnosis of amnestic mild cognitive impairment (aMCI), which is consistent with the Petersen criteria,1 but we did not require subjective memory complaints as a necessary criterion for defining aMCI as previous literature has suggested that some MCI patients may lack insight regarding their cognitive declines,24 and may thus fail to report memory deficits. Two of the 12 NC relatives met criteria for aMCI and reported memory complaints, and were thus excluded from our healthy control group of cognitively intact NC (n=10) in all subsequent analyses. Of the 25 mutation carriers, four subjects were diagnosed with dementia (MUT-Dem) based on CDR scores of 1 or greater, 12 met criteria for aMCI (MUT-aMCI), and nine had normal memory scores (MUT-Norm). Subjective memory complaints were reported by four (40%) NC, five (56%) MUT-Norm and seven (58%) MUT-aMCI subjects. The proportion of subjects with memory complaints did not differ between groups (χ2=0.81, p=0.67).
MRI acquisition and preprocessing
All subjects were scanned on the same 1.5T Siemens Sonata MRI scanner. Analyses of these subjects’ MRI scans have previously been published.6,10,25 High-resolution T1-weighted three-dimensional (3D) images were acquired in the sagittal plane using an MPRAGE sequence (TR=1900 ms, TE=4.38 ms, TI=1100 ms, flip angle 15°, voxel size 1×1×1 mm3). An automated brain surface algorithm from Brainsuite26 and manual editing were applied to remove scalp and other non-brain tissues. Intensity inhomogeneity caused by non-uniformities in the radio frequency receiver coils was corrected using an N3 bias field algorithm.27 To adjust for global differences in brain positioning, orientation and scaling across individuals, all scans were linearly registered to the stereotactic space defined by the International Consortium for Brain Mapping (ICBM-53)28 using a nine-parameter transformation. Globally aligned images were resampled in an isotropic space of 230 voxels along each axis (x, y and z-dimensions) with an interpolated voxel size of 1 mm3.
Three-dimensional Jacobian maps quantifying structural differences in brain volume
To quantify 3D patterns of volumetric brain differences for each subject, an individual difference map (Jacobian map) was computed by non-linearly registering each individual scan to a minimal deformation target (MDT), a customised template constructed from the voxel-wise mean of the control group. The procedure to construct the MDT is detailed in Hua et al.11 Registration to the MDT was completed using a non-linear inverse-consistent elastic intensity-based registration algorithm, with a built-in smoothing kernel, driven by a mutual information-based cost function, which has been described previously in Leow et al.29 A map of the Jacobian determinants was computed from the gradient of the deformation field to illustrate regions of relative volume differences between each individual and the MDT. All results and statistical analyses were based on the Jacobian maps.
Statistical analyses
To illustrate systematic group differences in brain volume, we constructed voxel-wise statistical maps based on the Student’s t statistic. Regions of interest (ROI) were manually hand-traced on the MDT using Brainsuite,26 and included the frontal, temporal, parietal and occipital lobes, and the caudate, putamen, thalamus, splenium and pons. The Jacobian maps of each group were compared using two-sample t tests to assess the overall significance of group differences at the whole-brain level and within each ROI, corrected for multiple comparisons using permutation tests.30 In brief, a null distribution for group differences in brain volume relative to the MDT (Jacobian values) at each voxel was constructed using 10 000 random permutations of the data. For each test, subjects’ group status (eg, NC vs MUT-Norm) was randomly permuted and voxel-wise t tests were conducted to identify voxels more significant than p=0.05. The volume of voxels inside a mask (ie, temporal lobes) more significant than p=0.05 was computed for the real experiment and for the random assignments. A ratio, describing the fraction of the time the t statistic was more extreme in the randomised tests than the original test, was calculated to yield an overall p value for the significance of the map. This approach has been used extensively in earlier work.11,12
Age of disease onset in FAD can vary between families but tends to be relatively consistent within families.15 Therefore, to compare subjects’ ages relative to the time at which they would be expected to develop dementia, we calculated subjects’ ages relative to the median age of dementia diagnosis in their families (‘relative age’). The median, rather than the mean, age of diagnosis in the family was chosen because the median is less affected by outliers, which have been observed in some FAD families.31 Pearson correlation analyses were performed to examine the relationship between regional brain volumes and relative age. Cognitive performance was compared between groups using analysis of covariance F tests with age as a covariate.
RESULTS
Demographics
The final sample consisted of 10 NC, nine MUT-Norm, 12 MUT-aMCI and four MUT-Dem subjects. Nine NC subjects came from the same family as at least one of the mutation carriers. Of the 25 mutation carriers, 20 have at least one relative in the NC group. All but one of the five mutation carriers for whom there was no NC relative have the PSEN1 A431E substitution and therefore have a genetically similar NC represented as controls. Demographic characteristics are reported in table 2. The MUT-Dem group was significantly older than the NC (p<0.05), MUT-Norm (p<0.001) and MUT-aMCI (p=0.008) groups. Relative age was significantly higher in the MUT-Dem group, who were approximately 5 years beyond their families’ median age of diagnosis, compared to the MUT-Norm (p=0.006) and MUT-aMCI (p=0.04) groups, who were on average 15 and 10 years younger than their families’ median age of diagnosis. There were no significant group differences in years of education, gender or apolipoprotein E genotype. The MUT-Dem group had significantly lower MMSE and higher CDR scores compared to the NC, MUT-Norm and MUT-aMCI groups.
Table 2.
Demographic characteristics for the NC, MUT-Norm, MUT-aMCI and MUT-Dem groups
| Demographic variables | NC (n=10) Mean (SD) |
MUT-Norm (n=9) Mean (SD) |
MUT-aMCI (n=12) Mean (SD) |
MUT-Dem (n=4) Mean (SD) |
t or χ2 | p Value |
|---|---|---|---|---|---|---|
| Age, years | 38.1 (8.2)a | 29.9 (5.5)b | 35.1 (7.5)c | 50.5 (5.1)abc | 9.22 | <0.001 |
| Relative age, years | −6.9 (11.7) | −15.4 (5.4)a | −10.4 (8.5)b | 5.0 (5.3)ab | 5.38 | 0.004 |
| Education, years | 13.7 (2.9) | 13.1 (2.5) | 10.5 (4.1) | 12.3 (6.8) | 1.50 | 0.24 |
| Gender (m/f) | 3/7 | 2/7 | 2/10 | 1/3 | 0.56 | 0.91 |
| APOE genotype (e4, %) | 2 (20%) | 1 (11%) | 1 (8%) | 0 (0%) | 1.36 | 0.72 |
| Global functioning | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F3,30 | p Value |
| MMSE | 28.9 (0.9)ab | 29.0 (0.5)c | 26.7 (2.9)ad | 11.8 (7.2)bcd | 24.42 | <0.001 |
| CDR | 0.10 (0.2)a | 0.00 (0.00)b | 0.25 (0.3)c | 2.00 (0.8)abc | 24.00 | <0.001 |
Groups denoted by same letters differ by p<0.05.
Group differences in age, relative age and education were assessed using analysis of variance. Differences in gender distribution were assessed using Pearson χ2 tests. Differences in MMSE and CDR scores were assessed using analysis of covariance, controlled for age.
APOE, apolipoprotein E; CDR,clinical dementia rating; MMSE, mini-mental state examination; MUT-aMCI, mutation carriers with amnestic mild cognitive impairment; MUT-Dem, mutation carriers with dementia; MUT-Norm, mutation carriers with normal cognition; NC, non-carriers.
Regional volume differences
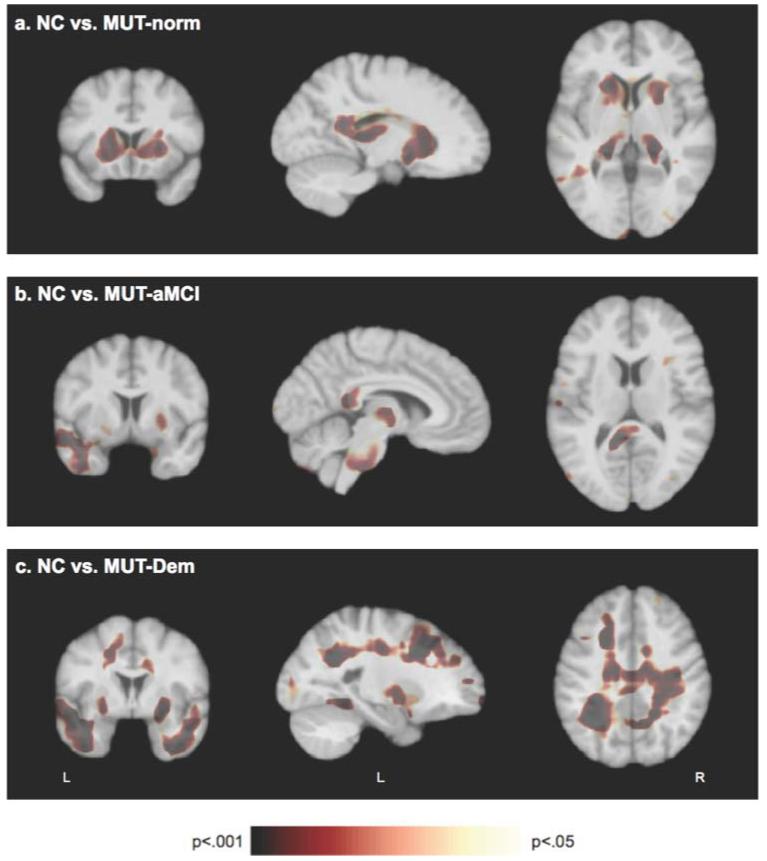
Jacobian maps of the MUT-Norm, MUT-aMCI and MUT-Dem groups were compared with the NC group to yield the statistical maps shown in figure 1, which display regions of significant volume differences between groups. Permutation tests yielded whole-brain and regional p values corrected for multiple comparisons. At the whole-brain level, only the MUT-Dem group had significantly smaller brain volume than the NC group (p<0.001); the MUT-Norm (p=0.14) and MUT-MCI (p=0.26) groups did not significantly differ from the NC group in whole-brain volume. However, permutation tests revealed significant regional differences between the groups. Compared to NC, the MUT-Norm group had significantly smaller volumes in the thalamus, (p=0.01), caudate (p=0.02) and putamen (p=0.01) (figure 1A). The MUT-aMCI group had significantly smaller volumes than the NC group (figure 1B) in the thalamus (p=0.03), splenium of the corpus callosum (p=0.02) and pons (p=0.02). The MUT-aMCI group also exhibited smaller left temporal lobe volumes than the NC group, but the difference was marginally significant (p=0.08) after correcting for multiple comparisons. The MUT-Dem group had significantly smaller volumes than the NC group (figure 1C) in the bilateral temporal (p=0.003) and parietal (p=0.001) lobes, left frontal lobe (p=0.02) and putamen (p=0.04). These differences were predominantly in white matter regions, with permutation tests significant in the temporal (p=0.005), parietal (p=0.001) and frontal (p=0.03) white matter regions.
Figure 1.
Statistical p-maps show significant differences in brain volumes between the non-carrier (NC) group and mutation carriers with normal cognition (MUT-Norm), amnestic mild cognitive impairment (MUT-aMCI) and dementia (MUT-Dem) groups. (A) The MUT-Norm group demonstrated significantly smaller volumes (as indicated in brown colour) than the NC group in the caudate, putamen and thalamus. (B) The MUT-aMCI group exhibited significantly smaller volumes than the NC group in the thalamus, splenium and pons, and a trend for smaller volumes in the left temporal lobe. (C) The MUT-Dem group had significantly smaller volumes than the NC group in the bilateral temporal and parietal lobes, the left frontal lobe and putamen.
PSEN1 mutation carriers
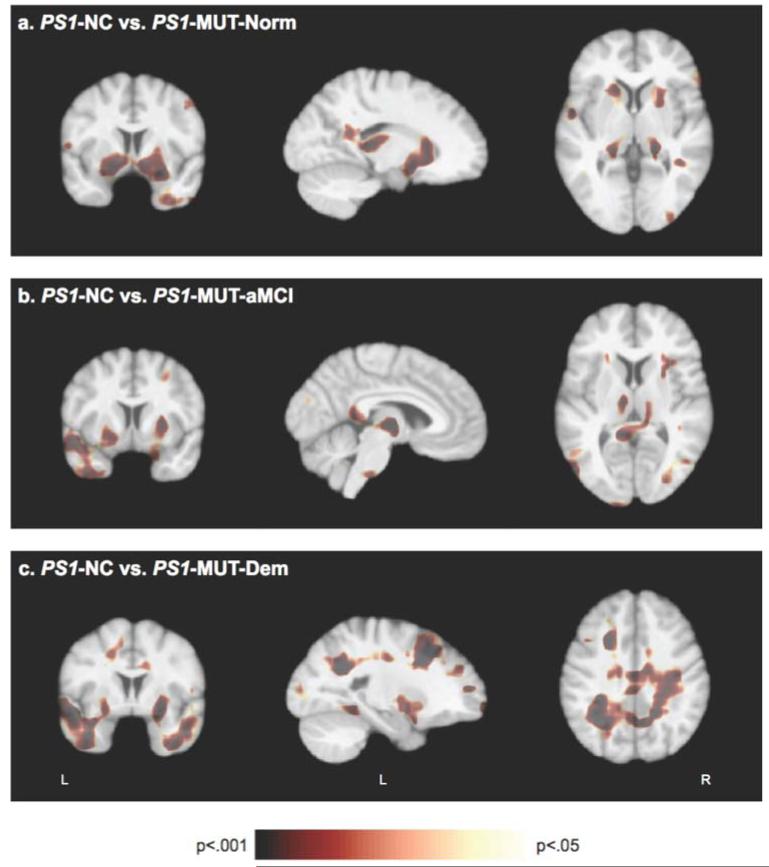
We repeated the above analysis in a subset of participants who were from families with PSEN1 mutations, which included seven mutation carriers with normal cognition (PS1-MUT-Norm), 10 with aMCI (PS1-MUT-aMCI), four with dementia (PS1-MUT-Dem) and eight NC (PS1-NC). Statistical p-maps of differences between the PS1-NC group and each PS1-MUT group are presented in figure 2. Permutation tests, correcting for multiple comparisons, were significant at the whole-brain level for differences between the PS1-NC and PS1-MUT-Dem groups (p<0.001), but did not reach significance for differences between the PS1-NC group and PS1-MUT-Norm (p=0.25) or PS1-MUT-aMCI (p=0.17) groups. Region-specific permutation tests revealed that the PS1-MUT-Norm group had significantly smaller volumes of the thalamus (p=0.02), caudate (p=0.04), putamen (p=0.02) and right temporal lobe (p=0.04) compared to the PS1-NC group (figure 2A). The PS1-MUT-aMCI group had significantly smaller volumes of the thalamus (p=0.02) and putamen (p=0.03) compared to the PS1-NC group, but permutation tests in the splenium (p=0.11), pons (p=0.053) and left temporal lobe (p=0.29) yielded non-significant results (figure 2B). The PS1-MUT-Dem group exhibited smaller volumes of the bilateral temporal (p=0.002) and parietal (p=0.003) lobes, putamen (p=0.03) and splenium (p=0.01) compared to the PS1-NC group (figure 2C). A region in the left frontal lobe was also smaller in the PS1-MUT-Dem group, but the difference did not reach significance (p=0.10) on permutation tests. Like the overall sample, these differences were predominantly in white matter regions, particularly in the parietal (p=0.003) and temporal (p=0.006) white matter. Permutation tests in the left frontal white matter were marginally significant (p=0.052).
Figure 2.
Jacobian maps of PSEN1 mutation carriers with normal cognition (PS1-MUT-Norm), amnestic MCI (PS1-MUT-aMCI) and dementia (PS1-MUT-Dem) were compared to that of non-carrier relatives of PSEN1 mutation carriers (PS1-NC). Significance p-maps show that (A) PS1-MUT-Norm subjects exhibited smaller volumes in the thalamus, striatum and right temporal region; (B) PS1-MUT-aMCI subjects exhibited smaller volumes in the thalamus, putamen, splenium and left temporal lobe; and (C) PS1-MUT-Dem groups exhibited smaller volumes in the putamen and temporal, parietal and left frontal regions.
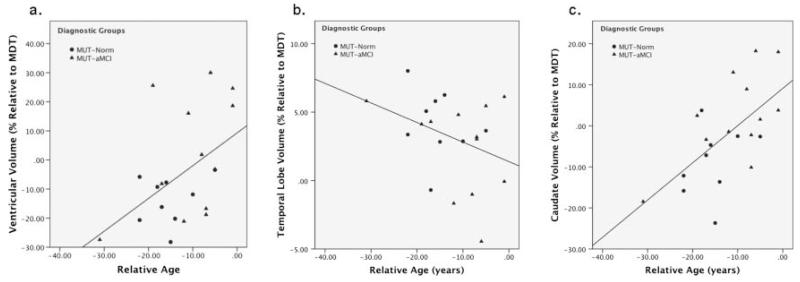
Brain volume and relative age
Mean Jacobian values within select ROI (temporal lobe, parietal lobe, frontal lobe, caudate, putamen, thalamus, ventricles) were computed for each subject to provide a numeric summary of regional volume. The ROI volumes of all non-demented mutation carriers (MUT-Norm, MUT-aMCI) were correlated with relative age to estimate the progression of brain atrophy in predementia stages as individuals approached the expected age of dementia diagnosis. In these two groups, higher relative age was associated with larger ventricular volume (r=0.48, p=0.03, figure 3A; MUT-Norm r=0.24, p=0.54; MUT-aMCI r=0.43, p=0.16), and smaller temporal lobe volume (r=−0.35, p=0.12, figure 3B; MUT-Norm r=−0.24, p=0.54; MUT-aMCI r=−0.30, p=0.34), although the latter relationship did not reach significance. Interestingly, the MUT-Norm and MUT-aMCI groups demonstrated a positive correlation between caudate volume and relative age (r=0.62, p=0.003, figure 3C; MUT-Norm r=0.32, p=0.40; MUT-aMCI r=0.63, p=0.03). Examination of the group-specific correlations indicate that this association is largely driven by the MUT-aMCI group, although the MUT-Norm group generally shows the same trend, thus contributing to the strength of the overall correlation in non-demented carriers. No other ROI volumes were significantly correlated (p>0.30) with relative age.
Figure 3.
Scatterplots and trend lines show the association between relative age (in years) and ROI volumes (% relative to the minimal deformation target; MDT) of the (A) ventricles, (B) temporal lobes and (C) caudate in non-demented mutation carriers (cognitively normal (MUT-Norm), with amnestic mild cognitive impairment MUT-aMCI).
We further examined the relationship between relative age and temporal lobe, caudate and ventricular volume separately in non-demented mutation carriers who reported subjective memory complaints (n=12) and in those who did not report memory complaints (n=9). The association between relative age and caudate volume was driven largely by those with memory complaints (r=0.85, p<0.001); non-significant trends were observed in those without memory complaints (r=0.32, p=0.41). Larger ventricular volume associated with relative age was seen only in subjects with memory complaints (r=0.77, p=0.003) but not in those without memory complaints (r=0.08, p=0.83). The correlation between temporal lobe volume and relative age was non-significant in subjects with and without memory complaints (r=−0.39, p=0.22 and r= −0.28, p=0.46, respectively). Further stratifying the groups based on cognitive status (MUT-aMCI vs MUT-norm) makes the sample size too small for any meaningful statistical analyses, but these individuals are identified in supplementary figure S1 (available online only).
Cognitive functioning
Means and SD of the five composite cognitive domain scores and individual neurocognitive tests are presented in table 3. The MUT-aMCI group demonstrated lower memory scores than the NC (p<0.001) and MUT-Norm (p<0.001) groups. As performance on memory tests was used to determine group membership, memory scores were excluded from subsequent analyses. An analysis of covariance, controlling for age, revealed significant group differences in visuospatial skills, language and executive functioning, but not in processing speed. The MUT-aMCI group had lower language scores than both the NC (p=0.01) and MUT-Norm (p<0.05) groups. The MUT-aMCI group also had lower visuospatial (p=0.002) and executive functioning (p<0.001) scores than the NC group. The MUT-Norm group did not differ from the NC group in any cognitive domain.
Table 3.
Performance on neuropsychological measures: composite scores and individual test scores for each cognitive domain
| Cognitive domains/tests |
NC (n=10) Mean (SD) |
MUT-Norm (n=9) Mean (SD) |
MUT-aMCI (n=12) Mean (SD) |
F2,27 | p Value |
|---|---|---|---|---|---|
| Memory | 0.25 (0.5)a | 0.11 (0.3)b | −1.78 (1.2)ab | 25.81 | <0.001 |
| MVP–delayed recall |
16.80 (1.8)a | 16.17 (1.9)b | 10.42 (3.2)ab | 22.56 | <0.001 |
| ADP–delayed free recall |
13.30 (1.8)a | 13.89 (1.2)b | 10.50 (3.2)ab | 7.04 | 0.003 |
| ROCFT− delayed recall |
21.60 (3.7)a | 19.78 (6.1) | 14.63 (5.4)a | 7.50 | 0.003 |
|
Processing
speed |
0.09 (0.7) | 0.56 (0.8) | −0.28 (0.9) | 1.98 | 0.16 |
| Digit symbol | 56.70 (7.3) | 70.67 (12.9)a | 53.17 (13.9)a | 3.98 | 0.03 |
| Stroop word | 96.40 (16.4) | 95.78 (15.7) | 87.25 (16.3) | 1.06 | 0.36 |
| Stroop colour | 65.70 (13.4) | 75.22 (1 1.9) | 67.67 (7.9) | 1.53 | 0.24 |
| Colour trails 1 (time) |
39.00 (10.4) | 41.78 (10.1) | 46.67 (20.1) | 1.02 | 0.38 |
|
Visuospatial
skills |
0.27 (0.7)a | −0.08 (0.7) | −0.82 (1.3)a | 5.99 | 0.007 |
| ROCFT–copy | 33.50 (2.7)a | 32.67 (3.4) | 30.21 (5.8)a | 3.50 | 0.04 |
| Block design | 33.80 (6.7)a | 29.44 (7.0) | 22.42 (9.4)a | 6.64 | 0.005 |
| Language | 0.22 (0.7)a | 0.16 (0.7)b | −0.48 (0.5)ab | 4.35 | 0.02 |
| Category fluency |
24.00 (7.2) | 25.78 (3.9) | 22.25 (3.2) | 1.38 | 0.27 |
| Object naming | 27.70 (4.1)a | 26.11 (4.9) | 22.50 (4.2)a | 4.43 | 0.02 |
|
Executive
functioning |
0.16 (0.7)a | −0.42 (0.7) | −1.40 (1.5) a | 6.45 | 0.005 |
| Stroop colour– word |
41.60 (10.0) | 40.44 (8.2) | 35.00 (9.6) | 1.82 | 0.18 |
| Colour trails 2 (time) |
76.30 (15.8) | 87.44 (29.6) | 96.58 (22.6) | 2.05 | 0.15 |
| WCST perseverative errors |
7.30 (2.8)a | 10.56 (4.1) | 16.73 (9.9)a | 6.30 | 0.006 |
Between-group differences in composite domain scores were assessed using analysis of covariance, controlling for age.
Groups denoted by same letters differ by p<0.05.
ADP, aprendizaje de palabras; MUT-aMCI, mutation carriers with amnestic mild cognitive impairment; MUT-Norm, mutation carriers with normal cognition; MVP, memory verbal prose; NC, non-carrier; ROCFT, Rey–Osterrieth complex figure test; WCST, Wisconsin card sorting test.
DISCUSSION
We demonstrated that cognitively intact FAD mutation carriers (who were, on average, 15 years younger than the expected age of dementia diagnosis) already exhibit differences in brain volume compared to NC relatives. In our sample, MUT-Norm subjects had significantly smaller thalamus, caudate and putamen volumes compared to NC subjects. The thalamus and striatum may be affected early during the prodromal/presymptomatic stages of FAD, before any detectable changes in cognitive functioning. Postmortem examination of FAD patients has revealed extensive amyloid deposition in the caudate and putamen,32,33 and in-vivo molecular imaging studies of PSEN1 and APP mutation carriers using Pittsburgh compound B have reported increased uptake in both the striatum and thalamus.32,34 Although pathological involvement of the striatum has also been documented in sporadic AD patients,35 some studies suggest that FAD mutation carriers exhibit relatively more striatal pathology than typically observed in sporadic AD.32,36
In our sample, mutation carriers with aMCI exhibited lower volumes in the thalamus, splenium, pons and left temporal lobe compared to NC. Reduced temporal lobe volume has been well documented in patients with MCI.11 Pontine changes, however, have been less clearly established in the literature, although some studies have reported degeneration of the locus coeruleus37 and tegmentopontine reticular nucleus38 in sporadic AD. The pons is often used as a reference region in amyloid imaging as previous studies have suggested it remains relatively free of AD pathology.32,36 Our finding of pontine atrophy in FAD indicates that such atrophy may occur via mechanisms other than the direct local effects of amyloid deposition.
Interestingly, the smaller caudate and putamen volumes seen in MUT-Norm subjects were not evident in the MUT-aMCI group, suggesting a possible volume increase in these regions as disease pathology progresses from presymptomatic stages to MCI. Moreover, caudate volume was found to increase with relative age in mutation carriers before dementia diagnosis. This association was driven largely by the MUT-aMCI group, suggesting that caudate size may increase more as the disease advances closer to the dementia stage. This finding was independently verified using an alternative method of volumetric analysis with manually delineated dorsal caudate volumes in the ICBM space (see supplementary table S1, available online only). Similar to the TBM results, this independent analysis also showed smaller mean caudate volumes in MUT-Norm compared to NC subjects, and a significant positive correlation (r=0.54, p=0.01) between caudate volume and relative age in non-demented mutation carriers. Fortea et al39 also reported increased caudate volume and cortical thickness in the precuneus and parietotemporal areas in a group of PSEN1 carriers approximately 9.9 years younger than the predicted age of disease onset, similar to the mean relative age (10.4 years) of MUT-aMCI subjects in our sample. This increase in volume may be due to reactive neuronal hypertrophy and/or inflammatory processes.39 Another possibility is that increased brain volume may be associated with amyloid burden, as individuals with high Pittsburgh compound B retention have exhibited larger temporal lobe volumes.40 Neuropathological examination of persons with PSEN1 mutations has shown atypical ‘cotton wool’ plaques, which may be space occupying41 and may provide another explanation for increased brain volume in select regions.
Although the presymptomatic mutation carriers exhibited early changes in the thalamus and striatum, they did not perform any worse than NC controls on cognitive testing. Cognitive decline was only observed in MUT-aMCI subjects, who also showed slightly reduced temporal lobe volume. Therefore, significant cognitive changes may not be observable in FAD mutation carriers until neuropathology begins to affect the cortical regions, which is consistent with our previous finding that mutation carriers and NC show no significant differences in cortical thickness before significant cognitive impairment.10 Atrophy of cortical regions probably begins in the temporal lobes, and eventually progresses to frontal and parietal regions as cognitive and functional impairments develop into dementia. Accordingly, our sample of mutation carriers demonstrated decreasing temporal lobe volume and increasing ventricular volume (a gross indication of global brain atrophy) as they approached the age of dementia diagnosis, and those with dementia demonstrated significant cortical atrophy in the temporal, parietal and frontal regions, particularly in white matter regions. Earlier studies have shown evidence of white matter pathology in sporadic AD,42,43 and at the MCI stage.44 Therefore, although the neuroanatomical regions affected in the early stages of FAD may differ from sporadic AD, the pattern of cortical changes observed in the later stages seems to converge with that of sporadic AD.
In the current study, diagnosis of aMCI did not require the presence of subjective memory complaint, which was reported by 58% of the MUT-aMCI subjects. Earlier literature has suggested that some MCI patients may experience anosognosia, or lack of awareness of declining cognition,24 which may be a predictor of progression to AD.45 However, other studies have reported that subjective memory decline was a significant predictor of future cognitive decline.46 In our analyses, the increase in ventricular and caudate volumes observed in the non-demented mutation carriers was driven largely by those with subjective memory complaints. Therefore, the presence of memory complaints may indicate greater disease severity. Memory complaints were also reported by 40% of NC, which were probably associated with non-AD-related aetiologies, including anxiety regarding genetic status, depression, or sleep problems, as they were cognitively intact on objective testing.
The clinical heterogeneity of different mutation types has been well documented33,47 and may reflect underlying differences in neuropathological effects. Twenty-one of the 35 subjects in this study came from families with the PSEN1 A431E substitution, therefore biasing our results. Subsequent analysis of the PSEN1 mutation carriers yielded results similar to the overall sample, with cognitively normal and aMCI carriers exhibiting smaller volumes in the thalamus and striatum, while the demented carriers demonstrated reduced cortical volumes compared to NC. Due to the small sample size and limited number of individuals with APP mutations, we were unable to investigate potential differences between PSEN1 and APP mutation carriers in our sample.
Unlike other whole-brain volumetric methods, TBM does not require a segmentation step, thus avoiding potential errors in accurate tissue classification. The use of a customised template, such as the MDT, also facilitates more accurate registration. Alternative approaches, including unified segmentation with diffeomorphic registration, may hold mathematical and theoretical advantages.48 However, the approach used in the current study has been found to be robust and is successfully used to study both cortical and subcortical brain changes in a variety of disorders.11,12
Several other limitations should be acknowledged. Group differences in brain volume were unadjusted for age. In age-associated diseases such as AD, in which age is strongly correlated to disease severity, it is difficult to isolate age effects without removing real disease effects. Notably, the MUT-Norm and MUT-aMCI groups, which were both on average younger than the NC group, would be expected to have relatively larger brain volumes. However, our findings were in the opposite direction and it is unlikely that regions of relatively smaller brain volumes found in these younger mutation carriers would be solely an effect of age. Moreover, age-adjusted group comparisons (see supplementary figure S2, available online only) did not meaningfully change the results, and a subanalysis comparing a small subgroup of six MUT-norm and six age-matched NC participants (see supplementary figure S3, available online only) also yielded similar findings, albeit with smaller effects due to the limited sample size. In addition, the cross-sectional design of our study limits our ability to draw conclusions regarding the longitudinal progression of neuroanatomical changes, and the relatively small sample size of our study may have resulted in inadequate statistical power to detect additional group differences in brain volume and cognitive performance. Even so, FAD is a rare disorder and our sample compares favourably to previous FAD studies.7,39 Still, future studies with larger samples and age-matched controls are needed to replicate and confirm the findings presented here. Finally, our FAD findings may not fully generalise to sporadic AD, as there are both clinical and pathological differences between the two forms of the disease.49,50 For instance, subcortical changes in the thalamus and striatum, which are not evident until more severe stages of sporadic AD, may be seen earlier in presymptomatic stages of FAD, while cortical changes such as temporal lobe atrophy and ventricular expansion generalise to both sporadic AD and FAD by the MCI stage.
While there may be differences between FAD and sporadic AD, this and future studies of FAD at different stages of disease severity offer a model for better understanding the pathophysiological process in sporadic AD. Furthermore, as these individuals are relatively young, they are less likely to have comorbid illnesses (eg, hypertension) that might contribute to confounding cerebral pathology (eg, ischaemic changes). In addition, the use of a control group comprised mostly of NC relatives may limit environmental and biological factors that might otherwise confound the results. The current findings suggest that FAD mutation carriers may begin to experience visible neuroanatomical changes even in presymptomatic stages several years before the onset of cognitive decline. The ability to study FAD mutation carriers years before the clinical manifestation of symptoms can provide valuable insight into the earliest detectable changes in neuropathology and cognition, which in turn can help improve the early detection and development of targeted therapies.
Supplementary Material
Acknowledgments
Funding
This study was supported by UC MEXUS grant 05123901, PHS K08 AG-22228, California DHS 04-35522 and the Shirley and Jack Goldberg Trust. This research was also supported by NIH grants P30 AG010129, K23-AG028727, a grant from the Alzheimer’s Association (NIRG-07-60424), Alzheimer’s Disease Research Center grant P50 AG-16570 from the National Institute on Aging, general clinical research centers programme M01-RR00865, a California Alzheimer’s Disease Research Center grant, the Sidell Kagan Foundation and the Easton Consortium for Biomarkers and Drug Discovery. Algorithm development for this study was funded by the NIA, NIBIB, the National Library of Medicine and the National Center for Research Resources (AG016570, EB01651, LM05639, RR019771 to PT). This work was presented in part at the 2010 Alzheimer’s Association International Conference on Alzheimer’s Disease, in Honolulu, Hawaii, USA.
Footnotes
Contributors JMR designed the study, supervised data collection, interpreted the results and revised the draft paper. He is the guarantor, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. GJL and PHL designed the study, analysed the data, interpreted the results, drafted and revised the paper. YRA and LDM participated in the study design, data collection and revision of the draft paper. SM assisted with data acquisition and analysis and revised the draft paper. GC conducted the genetic analysis, contributed to the interpretation of results and revised the draft paper. MNB, XH and LGA contributed to the interpretation of results and revision of the draft paper. ADL and PMT designed the imaging analysis tools, contributed to the interpretation of results and revised the draft paper.
Competing interests None.
Ethics approval All study procedures were approved by the institutional review boards at UCLA and the National Institute of Neurology and Neurosurgery in Mexico City.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional supplementary files are published online only. To view these files please visit the journal online (http://dx.doi.org/10.1136/jnnp-2011-302087).
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatrics. 2004;16:129–40. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 3.Lampe TH, Bird TD, Nochlin D, et al. Phenotype of chromosome 14-linked familial Alzheimer’s disease in a large kindred. Ann Neurol. 1994;36:368–78. doi: 10.1002/ana.410360308. [DOI] [PubMed] [Google Scholar]
- 4.Verkkoniemi A, Somer M, Rinne JO, et al. Variant Alzheimer’s disease with spastic paraparesis: clinical characterization. Neurology. 2000;54:1103–9. doi: 10.1212/wnl.54.5.1103. [DOI] [PubMed] [Google Scholar]
- 5.Verkkoniemi A, Kalimo H, Paetau A, et al. Variant Alzheimer disease with spastic paraparesis: neuropathological phenotype. J Neuropathol Exp Neurol. 2001;60:483–92. doi: 10.1093/jnen/60.5.483. [DOI] [PubMed] [Google Scholar]
- 6.Ringman JM, Gylys KH, Medina LD, et al. Biochemical, neuropathological, and neuroimaging characteristics of early-onset Alzheimer’s disease due to a novel PSEN1 mutation. Neurosci Lett. 2011;487:287–92. doi: 10.1016/j.neulet.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006;5:828–34. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 8.Fox NC, Crum WR, Scahill RI, et al. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5. doi: 10.1016/S0140-6736(01)05408-3. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 9.Scahill RI, Schott JM, Stevens JM, et al. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Nat Acad Sci U S A. 2002;99:4135–7. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolova LG, Hwang KS, Medina LD, et al. Cortical and hippocampal atrophy in patients with autosomal dominant familial Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;32:118–25. doi: 10.1159/000330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua X, Leow AD, Parikshak N, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage. 2008;43:458–69. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu PH, Thompson PM, Leow A, et al. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. J Alzheimers Dis. 2011;23:433–42. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–14. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Murrell J, Ghetti B, Cochran E, et al. The A431E mutation in PSEN1 causing familial Alzheimer’s disease originating in Jalisco State, Mexico: an additional fifteen families. Neurogenetics. 2006;7:277–9. doi: 10.1007/s10048-006-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artiola-i-Fortuny L, Hermosillo D, Heaton RK, et al. Manual de Normas y Procedimientos para la Bateria Neuropsicologica en Espanol. m Press; Tucson, AZ: 1999. [Google Scholar]
- 17.Rey A. L’examen clinique en psychologie. Presses Universitaires de France; Paris, France: 1964. [Google Scholar]
- 18.Wechsler D. Wechsler adult intelligence scale, revised: manual. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- 19.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 20.D’Elia LF, Satz P, Uchiyama CL, et al. Color trails test: professional manual. Psychological Assessment Resources; Odessa, FL: 1996. [Google Scholar]
- 21.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2nd edn. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 22.Mungas D, Reed BR, Crane PK, et al. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16:347–59. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- 23.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test Manual: Revised and Expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- 24.Vogel A, Stokholm J, Gade A, et al. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17:181–7. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 25.Ringman JM, O’Neill J, Geschwind D, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain. 2007;130:1767–76. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 26.Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6:129–42. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 27.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 28.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leow A, Huang SC, Geng A, et al. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Inf Process Med Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- 30.Chiang MC, Reiss AL, Lee AD, et al. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage. 2007;36:1096–109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopera F, Ardilla A, Martinez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–9. [PubMed] [Google Scholar]
- 32.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–84. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J Neurol. 2006;253:139–58. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 34.Remes AM, Laru L, Tuominen H, et al. Carbon 11-labeled Pittsburgh compound B positron emission tomographic amyloid imaging in patients with APP locus duplication. Arch Neurol. 2008;65:540–4. doi: 10.1001/archneur.65.4.540. [DOI] [PubMed] [Google Scholar]
- 35.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 36.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 37.Haglund M, Sjobeck M, Englund E. Locus ceruleus degeneration is ubiquitous in Alzheimer’s disease: possible implications for diagnosis and treatment. Neuropathology. 2006;26:528–32. doi: 10.1111/j.1440-1789.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 38.Rub U, Schultz C, Del Tredici K, et al. Early involvement of the tegmentopontine reticular nucleus during the evolution of Alzheimer’s disease-related cytoskeletal pathology. Brain Res. 2001;908:107–12. doi: 10.1016/s0006-8993(01)02598-7. [DOI] [PubMed] [Google Scholar]
- 39.Fortea J, Sala-Llonch R, Bartres-Faz D, et al. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J Alzheimers Dis. 2010;22:909–22. doi: 10.3233/JAD-2010-100678. [DOI] [PubMed] [Google Scholar]
- 40.Chetelat G, Villemagne VL, Pike KE, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–58. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- 41.Takao M, Ghetti B, Hayakawa I, et al. A novel mutation (G217D) in the Presenilin 1 gene (PSEN1) in a Japanese family: presenile dementia and Parkinsonism are associated with cotton wool plaques in the cortex and striatum. Acta Neuropathol. 2002;104:155–70. doi: 10.1007/s00401-002-0536-6. [DOI] [PubMed] [Google Scholar]
- 42.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. AnnNeurol. 1986;19:253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 43.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32:1341–71. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scola E, Bozzali M, Agosta F, et al. A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. J Neurol, Neurosurg Psychiatry. 2010;81:798–805. doi: 10.1136/jnnp.2009.189639. [DOI] [PubMed] [Google Scholar]
- 45.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–64. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 46.Crowe M, Andel R, Wadley V, et al. Subjective cognitive function and decline among older adults with psychometrically defined amnestic MCI. Int J Geriatr Psychiatry. 2006;21:1187–92. doi: 10.1002/gps.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippa CF, Swearer JM, Kane KJ, et al. Familial Alzheimer’s disease: site of mutation influences clinical phenotype. Ann Neurol. 2000;48:376–9. [PubMed] [Google Scholar]
- 48.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan NS, Rossor MN. Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomark Med. 2010;4:99–112. doi: 10.2217/bmm.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.