Abstract
Background
The Pediatric Heart Network is conducting a large international randomized trial to compare aortic root growth and other cardiovascular outcomes in 608 subjects with Marfan syndrome randomized to receive atenolol or losartan for 3 years. The authors report here the echocardiographic methods and baseline echocardiographic characteristics of the randomized subjects, describe the interobserver agreement of aortic measurements, and identify factors influencing agreement.
Methods
Individuals aged 6 months to 25 years who met the original Ghent criteria and had body surface area–adjusted maximum aortic root diameter (ROOTmax) Z scores > 3 were eligible for inclusion. The primary outcome measure for the trial is the change over time in ROOTmax Z score. A detailed echocardiographic protocol was established and implemented across 22 centers, with an extensive training and quality review process.
Results
Interobserver agreement for the aortic measurements was excellent, with intraclass correlation coefficients ranging from 0.921 to 0.989. Lower interobserver percentage error in ROOTmax measurements was independently associated (model R2 = 0.15) with better image quality (P = .002) and later study reading date (P < .001). Echocardiographic characteristics of the randomized subjects did not differ by treatment arm. Subjects with ROOTmax Z scores ≥ 4.5 (36%) were more likely to have mitral valve prolapse and dilation of the main pulmonary artery and left ventricle, but there were no differences in aortic regurgitation, aortic stiffness indices, mitral regurgitation, or left ventricular function compared with subjects with ROOTmax Z scores < 4.5.
Conclusions
The echocardiographic methodology, training, and quality review process resulted in a robust evaluation of aortic root dimensions, with excellent reproducibility.
Keywords: Marfan syndrome, Aortic root, Echocardiography, Interobserver agreement
Marfan syndrome (MFS) is a systemic disorder of connective tissue caused by mutations in FBN1, the gene encoding fibrillin-1.1 The leading cause of mortality in MFS is cardiovascular disease, including aortic root dilation and aortic dissection. The National Heart, Lung, and Blood Institute–funded Pediatric Heart Network is conducting a randomized trial to compare the rates of aortic root enlargement and other short-term cardiovascular outcomes in children and young adults with MFS randomized to receive atenolol or losartan for 3 years. The rationale and design of this randomized trial have been reported.2
The primary outcome measure of this trial is the change over time (slope) in maximum aortic root diameter (ROOTmax) Z score measured by echocardiography at the level of the sinuses of Valsalva. Accurate and reproducible measurement of the aortic root diameter is particularly important in patients with MFS because aortic root size is one of the best predictors of cardiovascular outcome. Factors that may adversely affect intraobserver and interobserver agreement of aortic measurements in patients with MFS include increasing age and body size, pectus abnormality or scoliosis, and severity of aortic root dilation.
We report here the specifics of the echocardiographic methods used in this trial, including the training and quality review process, a detailed analysis of the interobserver and intraobserver agreement in baseline aortic measurements, and the factors influencing agreement in this cohort. The large sample size, measurement of all aortic dimensions in systole and diastole, as well as the analysis of multiple beats are distinguishing features of this trial. A detailed description of the baseline clinical characteristics of the screened population and enrolled subjects is being reported separately.3
METHODS
Patients
Subjects enrolled in this trial2 were individuals aged 6 months to 25 years who met the original Ghent criteria for MFS,4 with a body surface area (BSA)–adjusted ROOTmax Z score > 3 and absolute ROOTmax < 5 cm. Patients with prior aortic surgery were excluded. A total of 608 subjects were enrolled between February 2007 and February 2011. The study protocol was approved by the institutional review board or institutional ethics board at each participating center, and informed consent and assent were obtained, depending on age, from patients and their parents or legal guardians before trial enrollment.
Echocardiographic Protocol
The echocardiographic technical protocol and study manual were developed after consensus was reached among several experts in the field. The detailed protocol is provided in the online Appendix (available at www.onlinejase.com). Echocardiograms were performed on study subjects at baseline and at 6 months, 1 year, 2 years, and 3 years after randomization to either atenolol or losartan. Before baseline echocardiography, all patients on prophylactic therapy for aortic root dilation underwent taper and washout according to protocol. The data analyses presented here are based on the baseline echocardiographic studies only.
Patient length in centimeters and weight in kilograms were measured by an MFS trial study coordinator at the time of echocardiography and were used to calculate BSA.5 An automated vital sign device (Dinamap; GE Medical Systems, Waukesha, WI) was used to record multiple right brachial blood pressures and heart rates during echocardiographic assessment. Blood pressure and heart rate were recorded after the patient had been in a recumbent position for 5 to 10 min, during or immediately after recording of aortic images for calculation of stiffness indices. Four samples were obtained, the first of which was discarded (because it is the least reliable). The other samples were averaged.
Complete echocardiographic studies were performed using local instrumentation, transducer selection, and machine settings that provided the optimal images on the basis of the judgment of the ultrasonographer. Harmonic imaging and lateral gain were used when they helped define structures such as endocardial borders. Specific instructions for image optimization were given to the centers, including the following: use of zoom mode to optimize screen resolution when anatomic structures were to be measured, recording of transitions from full screen to zoom and from two-dimensional (2D) to spectral Doppler to enable the core laboratory readers to identify structures and sample locations, use of full-screen M mode to optimize distance-axis screen resolution, use of the highest sweep speed on M-mode and spectral Doppler recordings to optimize time-axis screen resolution, use of baseline velocity and wall-filter adjustment on spectral Doppler, adequate electrocardiographic strip for documentation of heart rate, and recording without the local measurements, if possible, to permit independent review by the core laboratory readers. For each structural measurement, 6 to 10 cardiac cycles were recorded, and for color Doppler evaluation of the valves, 10 to 15 cardiac cycles were recorded. ROOTmax was measured locally in triplicate and averaged to calculate a Z score to determine eligibility before randomization.
Core Laboratory Analysis
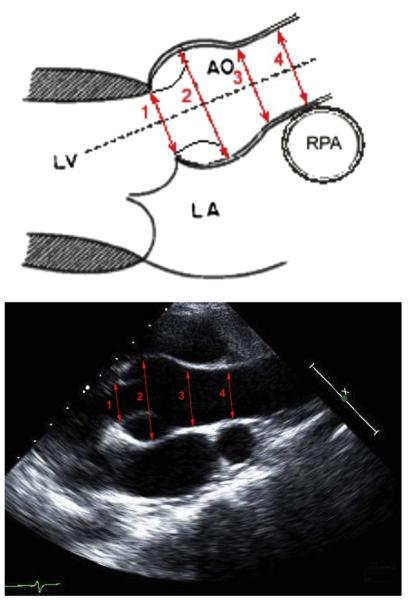
Measurements were performed in the core laboratory on a microcomputer-based workstation custom programmed for electronic caliper overlay of captured digital images for recording (EchoTrace; Marcus Laboratories, Boston, MA). The aortic diameters were measured at their maximum and minimum dimensions in systole and diastole from inner edge to inner edge at the aortic valve annulus, aortic root at the sinuses of Valsalva, sinotubular junction, and ascending aorta (Figure 1). Each of these eight measurements was performed in triplicate by two independent observers.
Figure 1.
Proximal aortic measurements in the parasternal long-axis view. The maximum and minimum measurements were taken from inner edge to inner edge: (1) aortic valve “annulus” at the hinge points of the leaflets; (2) aortic root at the largest diameter within the sinuses of Valsalva; (3) sinotubular junction at the transition point from sinus to tubular aorta; (4) ascending aorta at the level of the right pulmonary artery (RPA). AO, Aorta; LA, left atrium; LV, left ventricle.
The anteroposterior diameter of the distal thoracic aorta was measured at the level of the diaphragm. The lateral diameter of the main pulmonary artery (MPA)6 was measured from a parasternal imaging window. The durations of diastolic antegrade and retrograde flow in the proximal and distal descending thoracic aorta were measured. Left ventricular (LV) size and functional parameters were calculated according to American Society of Echocardiography pediatric guidelines in the apical and parasternal short-axis views.6
Mitral valve prolapse (MVP) or tricuspid valve prolapse (TVP) was classified as present if all or part of a valve leaflet was observed to pass through the plane of the valve annulus in the parasternal long-axis imaging plane. Prolapse was classified as borderline if one of the valve leaflets was observed to manifest posterior motion relative to the other leaflet without passing through the plane of the valve annulus. The degree of mitral regurgitation (MR) and aortic regurgitation (AR) were categorized qualitatively as mild or more, trivial, or none.
The eight averaged aortic measurements were reported for each of the two independent observers. Z scores were available for the maximum dimensions only.7 Raw values and Z scores were calculated for LV dimensions, volumes, mass, shortening fraction, and ejection fraction.8,9
Percentage duration of diastolic flow reversal in the proximal and distal thoracic aorta was calculated. The arterial pressure-strain elastic modulus10 and stiffness index11 were calculated for the aortic root at the sinuses of Valsalva and the ascending aorta. The averages of the systolic, diastolic, and mean blood pressures along with corresponding Z scores (Boston Children’s Hospital normative database) were calculated.
Training and Quality Control
The training process for site investigators included a face-to-face session with site investigators and a webinar session during which samples of each recording were reviewed in detail. An instructional CD-ROM was also distributed to all sites. As part of quality control, each site went through a certification process whereby three local aortic root measurements in addition to three full study protocol echocardiograms were reviewed at the core laboratory. A detailed review letter with constructive feedback was provided to each site.
Two core laboratory readers blinded to treatment assignment independently reviewed all echocardiograms. Each study was graded for image quality (excellent, good, fair, or unacceptable) and variation from protocol. A random subset (8%) of the studies was reviewed twice by each core laboratory reader to assess intrareader agreement.
Statistical Methods
Summary statistics are presented as mean ± SD or as median (interquartile range [IQR]). Continuous variables were compared by treatment assignment and other characteristics (e.g., gender, family history of MFS) using Student’s t tests or Wilcoxon’s rank-sum tests for approximately normally distributed and skewed variables, respectively. Categorical variables were compared by treatment assignment and other subgroup factors using χ2 or Fisher’s exact tests. To examine the associations of age and most echocardiographic indices, we compared growing children (male < 16.0 years of age, female < 15.0 years of age) with all others (male ≥ 16.0 years of age, female ≥ 15.0 years of age).12 For aortic elastic modulus and stiffness index, we examined differences in outcomes by age using age quartiles. Group comparisons that required age adjustment used continuous age and were performed using analysis of covariance and logistic regression for continuous and dichotomous measures, respectively. Echocardiographic and blood pressure Z scores for BSA and/or age were derived from the Boston Children’s Hospital normative database and compared with a healthy population mean of zero using a one-sample t test or Wilcoxon’s signed-rank test. Interobserver and intraobserver agreement was estimated using intraclass correlation coefficients (ICCs) and 95% confidence intervals. Agreement was also estimated by a percentage error measurement, defined as the absolute value of the difference in the two measurements divided by the mean of the two measurements multiplied by 100. To identify factors that might influence interobserver agreement, a multivariate model using percentage error was constructed (defined as the absolute difference between raters divided by the mean across raters multiplied by 100). To assess the robustness of the findings, a secondary analysis of group differences and ICC estimates was conducted using weighting according to image quality, with higher quality images having greater weight. This secondary analysis had little impact on statistical inferences. Because of the large number of comparisons, P values ≤ .01 were considered statistically significant. Analyses were conducted using R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Imaging of the aortic root was graded as excellent in 203 (33%), good in 357 (59%), and fair in 47 (8%) patients. ROOTmax was measurable at least once in all echocardiograms and in triplicate in 95%.
Agreement of the Echocardiographic Measurements
Interobserver Agreement
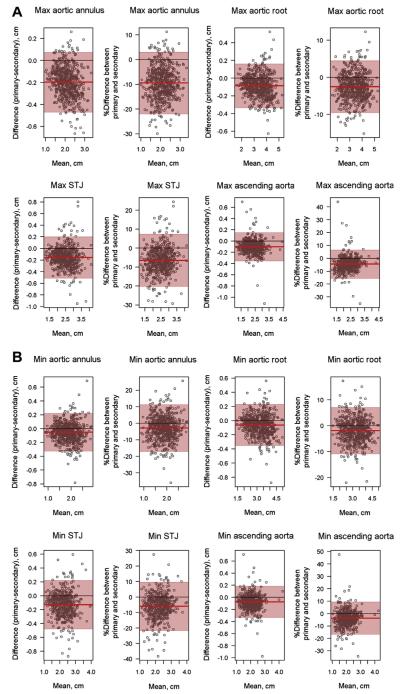
Interobserver agreement for the aortic measurements was excellent, with ICCs between the two core laboratory readers ranging from 0.921 to 0.989 (Table 1). Bland-Altman plots for interobserver agreement on all aortic measurements depict excellent agreement, with a systematic bias wherein the primary reader obtained lower values than the secondary reader (Figures 2A and 2B). This difference was slight, with mean interobserver differences ranging from 0.5 to 2.0 mm. However, the plots do not reveal any other systematic trends; specifically, the degree of agreement does not depend on the magnitude of the measurement. The Bland-Altman plots were similar when absolute dimensions, percentage error, and Z scores (not shown) were used.
Table 1.
ICCs between primary and secondary readers
| Variable | n | ICC (95% CI) |
|---|---|---|
| Minimum aortic annular diameter | 592 | 0.962 (0.955–0.967) |
| Maximum aortic annular diameter | 589 | 0.921 (0.907–0.933) |
| Minimum aortic root diameter | 600 | 0.987 (0.985–0.989) |
| ROOTmax | 607 | 0.989 (0.987–0.990) |
| Minimum sinotubular junction diameter | 487 | 0.952 (0.942–0.960) |
| Maximum sinotubular junction diameter | 538 | 0.948 (0.939–0.956) |
| Minimum ascending aortic diameter | 483 | 0.972 (0.966–0.976) |
| Maximum ascending aortic diameter | 525 | 0.971 (0.966–0.976) |
| Ascending aorta elastic modulus* | 480 | 0.626 (0.533–0.688) |
| Ascending aorta stiffness index* | 480 | 0.589 (0.509–0.657) |
| Aortic root elastic modulus* | 598 | 0.708 (0.658–0.752) |
| Aortic root stiffness index* | 598 | 0.676 (0.619–0.724) |
These indices were log transformed for this analysis.
Figure 2.
Bland-Altman plots for interobserver agreement for all maximum (A) and minimum (B) aortic root measurements using absolute dimensions and percentage error. The Bland-Altman plots graph the difference in absolute dimension or the percentage difference between primary and secondary readers (primary minus secondary reader) versus the mean across readers. The shaded 95% confidence bands are typically accepted as the range of “clinical equivalence.” Agreement is considered very good if all data points are within these bands. These plots emphasize the systematic bias, with values obtained from the primary reader consistently lower than those from the secondary reader. STJ, Sinotubular junction.
The univariate analysis showed that the interobserver percentage error in ROOTmax measurements was significantly lower by later read date and differed by center (P = .003 and P < .001, respectively). The other variables examined include image quality, age at echocardiography, BSA, absolute aortic root dimension, presence of pectus deformity or scoliosis, number of Ghent criteria met, family history of MFS, and presence of FBN1 mutation. The multivariate model showed that lower interobserver percentage error in ROOTmax measurements was only independently associated (model R2 = 0.15) with better image quality (P = .002) and later study reading date (P < .001).
Stiffness Indices
Interobserver agreement for aortic stiffness indices was moderate, with ICCs between the two core laboratory readers ranging from 0.59 to 0.71 for the ascending aorta and aortic root (Table 1).
One Beat versus Three Beats
In 95% of the echocardiographic examinations, the primary reader obtained three measurements of the primary outcome (ROOTmax). Percentage error was significantly lower for all averaged aortic measurements compared with single-beat measurements, except for maximum aortic annulus and maximum sinotubular junction (P ≤ .01; data not shown). For example, median percentage error for three-beat versus single-beat ROOTmax dimension was 3.6 ± 2.6% versus 3.9 ± 3.0% (P = .0002).
Intraobserver Agreement of the Aortic Root and MPA Measurements
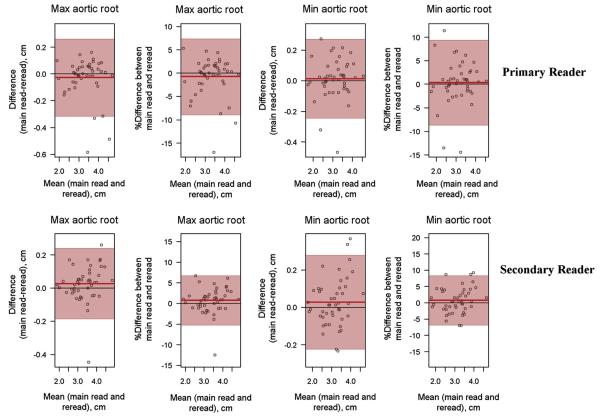
Intraobserver agreement for aortic root (Figure 3) and MPA measurements was excellent for both primary and secondary readers, with all ICCs > 0.90.
Figure 3.
Bland-Altman plots for intraobserver agreement for minimum and maximum aortic root measurements at the level of the sinuses of Valsalva.
Interobserver Agreement between the Local Echocardiographic Laboratory and the Core Laboratory
The only reported measurement from the local laboratory was the ROOTmax used to determine eligibility. Interobserver agreement for the ROOTmax measurement between the local and core laboratories was near perfect (ICC, 0.988; 95% confidence interval, 0.986–0.990; 3.36 ± 0.71 vs 3.35 ± 0.70; P = .55).
Echocardiographic Characteristics of the Randomized Cohort
The echocardiographic characteristics of the randomized subjects did not differ by treatment arm (Table 2). By design, randomized subjects had larger aortic dimensions (Z score = 4.04; IQR, 3.41 to 4.92) than healthy children (mean Z score = 0). MPA dimension Z scores were also larger but to a lesser degree (mean Z score = 2.22 ± 1.34). All median and mean values for LV size and function were within 1 standard deviation of mean values for healthy children, except for diastolic septal and posterior wall thickness Z scores (−1.20 [IQR, −1.83 to 0.45] and −1.22 ± 1.02, respectively).
Table 2.
Echocardiographic characteristics of randomized subjects in aggregate and by treatment arm
| Aggregate (n = 608) |
Treatment A (n = 303) |
Treatment B (n = 305) |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | Value | n | Value | n | Value | P |
| Aortic annular maximum diameter Z score | 589 | 1.76 ± 1.30† | 296 | 1.71 ± 1.24 | 293 | 1.81 ± 1.36 | .32 |
| Aortic annular maximum diameter (cm) | 589 | 2.01 ± 0.42 | 296 | 2.01 ± 0.42 | 293 | 2.00 ± 0.41 | .79 |
| ROOTmax Z score (sinuses of Valsalva) | 607 | 4.04 (3.41 to 4.92)† | 303 | 4.04 (3.47 to 4.82) | 304 | 4.04 (3.34 to 5.01) | .68* |
| ROOTmax (cm) (sinuses of Valsalva) | 607 | 3.36 ± 0.71 | 303 | 3.37 ± 0.72 | 304 | 3.36 ± 0.71 | .96 |
| Aortic sinotubular junction maximum diameter Z score |
552 | 1.98 (1.32 to 2.72)† | 276 | 1.94 (1.35 to 2.58) | 276 | 2.04 (1.30 to 2.85) | .14* |
| Aortic sinotubular junction maximum diameter (cm) | 552 | 2.41 ± 0.53 | 276 | 2.40 ± 0.54 | 276 | 2.43 ± 0.52 | .51 |
| Ascending aortic maximum diameter Z score | 543 | 0.88 (0.38 to 1.47)† | 263 | 0.80 (0.37 to 1.38) | 280 | 0.91 (0.40 to 1.56) | .13* |
| Ascending aortic maximum diameter (cm) | 543 | 2.30 (1.94 to 2.60) | 263 | 2.30 (1.93 to 2.56) | 280 | 2.30 (1.97 to 2.63) | .62* |
| Descending aortic maximum diameter (cm) | 592 | 1.31 ± 0.33 | 296 | 1.32 ± 0.34 | 296 | 1.31 ± 0.32 | .92 |
| Percentage duration of diastolic flow reversal in PTA |
319 | 17.8 (10.3 to 23.4) | 158 | 17.3 (4.7 to 23.2) | 161 | 18.3 (11.7 to 23.4) | .28* |
| Percentage duration of diastolic flow reversal in DTA |
513 | 0.0 (0.0 to 9.4) | 259 | 0.0 (0.0 to 9.0) | 254 | 0.0 (0.0 to 10.6) | .58* |
| MPA maximum diameter (cm) | 512 | 2.36 ± 0.46 | 261 | 2.37 ± 0.47 | 251 | 2.34 ± 0.45 | .47 |
| MPA maximum diameter Z score | 512 | 2.22 ± 1.34† | 261 | 2.19 ± 1.36 | 251 | 2.26 ± 1.32 | .55 |
| Presence of AR | 607 | 67 (11%) | 302 | 35 (12%) | 305 | 32 (11%) | .70 |
| Among subjects with AR | 67 | 35 | 32 | .29 | |||
| Trivial | 47 (70%) | 27 (77%) | 20 (62%) | MH = .19 | |||
| Mild or more | 20 (30%) | 8 (23%) | 12 (38%) | ||||
| Presence of MR | 597 | 384 (64%) | 298 | 195 (65%) | 299 | 189 (63%) | .61 |
| Among subjects with MR | 384 | 195 | 189 | .09 | |||
| Trivial | 278 (72%) | 149 (76%) | 129 (68%) | MH = .07 | |||
| Mild or more | 106 (28%) | 46 (24%) | 60 (32%) | ||||
| MVP | 596 | 298 | 298 | .38 | |||
| None | 230 (39%) | 114 (38%) | 116 (39%) | MH = .45 | |||
| Borderline | 140 (24%) | 64 (22%) | 76 (25%) | ||||
| Present | 226 (38%) | 120 (40%) | 106 (36%) | ||||
| TVP | 551 | 276 | 275 | .18 | |||
| None | 281 (51%) | 143 (52%) | 138 (50%) | MH = .66 | |||
| Borderline | 149 (27%) | 66 (24%) | 83 (30%) | ||||
| Present | 121 (22%) | 67 (24%) | 54 (20%) | ||||
| LV size and function | |||||||
| LV end-diastolic volume Z score | 521 | −0.23 ± 1.38† | 260 | −0.25 ± 1.36 | 261 | −0.21 ± 1.39 | .69 |
| LV end-systolic volume Z score | 521 | −0.42 ± 1.36† | 259 | −0.42 ± 1.40 | 262 | − 0.43 ± 1.31 | .93 |
| LV ejection fraction (%) | 521 | 64.6 ± 6.4 | 260 | 64.4 ± 6.1 | 261 | 64.9 ± 6.6 | .44 |
| LV mass Z score | 518 | −0.25 (−0.90 to 0.68)† | 259 | −0.22 (−0.99 to 0.66) | 259 | −0.27 (−0.87 to 0.71) | .79* |
| LV mass/volume ratio (g/mL) | 519 | 0.92 ± 0.15 | 260 | 0.91 ± 0.15 | 259 | 0.92 ± 0.15 | .83 |
| LV end-diastolic dimension Z score | 585 | 0.70 (−0.14 to 1.65)† | 291 | 0.71 (−0.30 to 1.63) | 294 | 0.70 (0.01 to 1.72) | .28* |
| LV end-systolic dimension Z score | 585 | 0.35 (−0.53 to 1.22)† | 291 | 0.31 (−0.71 to 1.28) | 294 | 0.39 ( −0.44 to 1.19) | .33* |
| LV mass Z score (by M-mode echocardiography) | 585 | −0.65 (−1.52 to 0.34)† | 291 | −0.74 (−1.51 to 0.27) | 294 | − 0.59 (−1.56 to 0.39) | .46* |
| LV shortening fraction (%) | 587 | 37.2 ± 5.1 | 292 | 37.2 ± 5.3 | 295 | 37.2 ± 5.0 | .98 |
| LV diastolic septal thickness Z score | 585 | −1.20 (−1.83 to −0.45)† | 291 | −1.15 (−1.79 to −0.54) | 294 | −1.23 (−1.87 to −0.37) | .78* |
| LV diastolic posterior wall thickness Z score | 585 | −1.22 ± 1.02† | 291 | −1.23 ± 1.01 | 294 | −1.21 ± 1.03 | .84 |
| Stiffness indices | |||||||
| Ascending aortic elastic modulus (mm Hg) | 492 | 318 (231 to 447) | 237 | 319 (231 to 430) | 255 | 317 (231 to 463) | .60* |
| Ascending aortic stiffness index | 492 | 4.95 ± 2.78 | 237 | 4.81 ± 2.62 | 255 | 5.08 ± 2.92 | .29 |
| Aortic root elastic modulus (mm Hg) | 598 | 618 (407 to 914) | 299 | 602 (386 to 887) | 299 | 644 (431 to 970) | .112* |
| Aortic root stiffness index | 598 | 8.2 (5.5 to 12.2) | 299 | 7.9 (5.2 to 11.8) | 299 | 8.5 (5.9 to 12.4) | .110* |
| Systolic blood pressure (mm Hg) | 606 | 97.2 ± 14.3 | 303 | 97.2 ± 13.4 | 303 | 97.2 ± 15.1 | .98 |
| Systolic blood pressure Z score | 604 | −0.65 ± 0.98† | 303 | −0.69 ± 0.99 | 301 | −0.61 ± 0.97 | .30 |
| Diastolic blood pressure (mm Hg) | 606 | 58.6 ± 9.9 | 303 | 59.1 ± 9.9 | 303 | 58.1 ± 9.9 | .22 |
| Diastolic blood pressure Z score | 604 | 0.32 ± 0.94† | 303 | 0.34 ± 0.97 | 301 | 0.29 ± 0.91 | .57 |
| Mean blood pressure (mm Hg) | 606 | 69.9 ± 15.4 | 303 | 70.5 ± 15.1 | 303 | 69.3 ± 15.8 | .33 |
| Mean blood pressure Z score | 593 | −0.25 ± 0.96† | 298 | −0.24 ± 1.01 | 295 | −0.26 ± 0.92 | .81 |
Data are expressed as mean ± SD or as median (IQR). All echocardiographic measurements are based on core laboratory interpretations. All aortic measures reported are maximal dimensions.
DTA, Descending thoracic aorta; MH, Mantel-Haenszel; PTA, proximal thoracic aorta.
Nonparametric P value.
Aggregate values compared with norms: statistically different from healthy normal (Z = 0) at P < .05.
MVP or borderline MVP was present in 366 of the subjects (61%). TVP or borderline TVP was present in 270 of the subjects (49%). When MVP was present, there was more than a twofold risk that TVP was also present (67% of those with MVP had TVP vs 28% of those without MVP having TVP; relative risk, 2.37; P < .001). Mild or greater MR was present in 106 of the patients (18%). Mild or greater AR was uncommon, present in only 20 subjects (3%). There were only four subjects with both mild or greater AR and MR.
Gender Differences
There were no significant gender differences in age in years (11.21 ± 6.02 in male subjects vs 11.19 ± 6.77 in female subjects, P = .97) or BSA (1.31 ± 0.49 in male subjects vs 1.24 ± 0.47 in female subjects, P = .10). MVP and mild or greater MR were more common in female subjects (45% vs 33%, P < .01, and 25% vs 13%, P < .001, respectively). Male subjects had larger aortic annular Z scores (1.89 ± 1.39 vs 1.57 ± 1.14, P = .005). Aortic root elastic modulus and stiffness indices were higher in male subjects (685 [IQR, 449–1015] vs 551 [IQR, 359–774] and 8.6 [IQR, 6.1–13.4] vs 7.4 [IQR, 4.8–10.3], respectively, P < .001 for both) but ascending aortic elastic modulus and stiffness index did not vary by gender. There were no other significant gender differences in echocardiographic characteristics of this cohort.
Age at Randomization
The mean age at randomization was 11.2 ± 6.3 years. Older teenagers and adults had lower Z scores compared with growing children (male < 16.0 years of age, female < 15.0 years of age) for the aortic annulus (1.5 ± 1.1 vs 1.9 ± 1.3, P = .003), sinotubular junction (1.7 [IQR, 1.0 to 2.5] vs 2.1 [IQR, 1.4 to 2.8], P = .003), ascending aorta (0.5 [IQR, −0.1 to 1.1] vs 1.0 [IQR, 0.5 to 1.5], P < .001), and MPA (1.4 ± 1.3 vs 2.4 ± 1.3, P < .001). However there was no significant difference in ROOTmax Z scores between children and adults. Systolic blood pressure Z scores were lower in children (−0.72 ± 0.99 vs −0.42 ± 0.94 in adults, P = .001), but there was no difference in mean arterial blood pressure or diastolic blood pressure Z scores. When analyzed by age quartiles, the elastic modulus and stiffness index for both the ascending aorta and aortic root increased significantly with age (Table 3).
Table 3.
Ascending aortic and aortic root elastic modulus and stiffness index by quartiles of age
| Characteristic | n | Age < 6.0 y | n | Age 6.0–10.8 y | n | Age 10.8–15.5 y | n | Age > 15.5 y | P |
|---|---|---|---|---|---|---|---|---|---|
| Ascending aortic elastic modulus (mm Hg) |
133 | 257 (197–338) | 134 | 285 (221–389) | 129 | 356 (264–454) | 96 | 426 (324–582) | <.0001*† |
| Ascending aortic stiffness index | 133 | 4.48 ± 2.85 | 134 | 4.61 ± 2.89 | 129 | 5.10 ± 2.57 | 96 | 5.86 ± 2.60 | <.001† |
| Aortic root elastic modulus (mm Hg) | 152 | 430 (312–614) | 150 | 581 (389–867) | 147 | 719 (469–1040) | 149 | 821 (537–1224) | <.0001*† |
| Aortic root stiffness index | 152 | 6.13 (4.54–8.78) | 150 | 7.88 (5.45–11.53) | 147 | 8.90 (6.21–13.74) | 149 | 9.75 (6.57–14.92) | <.0001*† |
Data are expressed as median (IQR) or as mean ± SD.
Nonparametric P value.
Statistically significant (P < .01).
Aortic Root Diameter Z Score
Table 4 shows the echocardiographic characteristics by ROOTmax Z score dichotomized at 4.5. More than one third of the subjects (36%) had ROOTmax Z scores ≥ 4.5. In these subjects, aortic annular, sinotubular junction, ascending aortic, and MPA Z scores were higher than those in subjects with ROOTmax Z scores < 4.5. Subjects with ROOTmax Z scores ≥ 4.5 were also more likely to have MVP and TVP. The prevalence of MR and AR did not vary with ROOTmax Z score. LV end-diastolic and end-systolic volume Z scores were higher in subjects with ROOTmax Z scores ≥ 4.5, but LV shortening and ejection fractions were not different from those with smaller aortic root Z scores. Results were similar for LV dimensions determined by M-mode imaging (data not shown). There were no differences in stiffness indices and blood pressure measurements between the two groups.
Table 4.
Echocardiographic outcomes by ROOTmax Z score
| ROOTmax
Z score < 4.5 (n = 387) |
ROOTmax
Z score ≥ 4.5 (n = 220) |
||||
|---|---|---|---|---|---|
| Characteristic | n | Value | n | Value | P |
| Aortic annular maximum diameter Z score | 376 | 1.41 ± 1.07 | 213 | 2.39 ± 1.44 | <.0001† |
| ROOTmax Z score (sinuses of Valsalva) | 387 | 3.55 (3.14 to 4.00) | 220 | 5.32 (4.82 to 6.12) | <.0001*,† |
| Aortic sinotubular junction maximum diameter Z score | 357 | 1.65 (1.01 to 2.21) | 195 | 2.79 (2.11 to 3.77) | <.0001*,† |
| Ascending aortic maximum diameter Z score | 349 | 0.79 (0.30 to 1.26) | 194 | 1.19 (0.59 to 1.84) | <.0001*,† |
| Descending aorta maximum diameter (cm) | 377 | 1.33 ± 0.34 | 214 | 1.29 ± 0.31 | .27 |
| MPA maximum diameter Z score | 323 | 2.01 ± 1.24 | 188 | 2.58 ± 1.43 | <.0001† |
| Presence of AR | 386 | 33 (9%) | 220 | 34 (16%) | .011 |
| Presence of MR | 383 | 235 (61%) | 213 | 148 (70%) | .05 |
| MVP | 380 | 215 | <.001 | ||
| None | 163 (43%) | 67 (31%) | MH < .001† | ||
| Borderline | 94 (25%) | 45 (21%) | |||
| Present | 123 (32%) | 103 (48%) | |||
| TVP | 348 | 203 | .003† | ||
| None | 192 (55%) | 89 (44%) | MH = .001† | ||
| Borderline | 95 (27%) | 54 (27%) | |||
| Present | 61 (18%) | 60 (30%) | |||
| LV size and function | |||||
| LV end-diastolic volume Z score | 339 | −0.47 ± 1.18 | 181 | 0.22 ± 1.60 | <.0001† |
| LV end-systolic volume Z score | 339 | −0.61 ± 1.32 | 181 | −0.08 ± 1.37 | <.0001† |
| LV ejection fraction (%) | 339 | 64.5 ± 6.2 | 181 | 64.9 ± 6.8 | .56 |
| LV mass Z score | 337 | −0.43 (−1.00 to 0.42) | 180 | 0.005 (−0.77 to 1.01) | <.0001*,† |
| LV mass/volume ratio (g/mL) | 338 | 0.92 ± 0.15 | 180 | 0.90 ± 0.16 | .23 |
| LV shortening fraction (%) | 375 | 37.2 ± 5.2 | 211 | 37.3 ± 5.1 | .79 |
| Stiffness indices | |||||
| Ascending aortic elastic modulus (mm Hg) | 315 | 310.5 (222.8 to 453.8) | 177 | 336.8 (249.0 to 442.5) | .12* |
| Ascending aortic stiffness index | 315 | 4.84 ± 2.74 | 177 | 5.15 ± 2.85 | .23 |
| Aortic root elastic modulus (mm Hg) | 380 | 610.6 (403.2 to 882.1) | 218 | 684.8 (410.3 to 1014.8) | .16* |
| Aortic root stiffness index | 380 | 8.0 (5.5 to 11.2) | 218 | 8.8 (5.4 to 13.3) | .144* |
Data are expressed as mean ± SD or as median (IQR). All echocardiographic measurements are based on core laboratory interpretations.
MH, Mantel-Haenszel.
Nonparametric P value.
Statistically significant (P < .01).
DISCUSSION
Aortic root size is a major determinant in the clinical diagnosis of MFS13 and is the best predictor of cardiovascular outcome.14 Varying methods to assess aortic root size by echocardiography have been reported, including M-mode versus 2D imaging, systolic versus diastolic measurements, and leading edge–to–leading edge versus inner edge–to–inner edge measurements.6,15-17 Most adult echocardiography laboratories use the leading edge–to–leading edge method in diastole, as recommended by the guidelines developed by the American Society of Echocardiography in conjunction with the European Association of Echocardiography.15 Similarly, a commonly used 2D pediatric nomogram (n = 52) published by Roman et al.16 uses the leading edge–to–leading edge method in diastole. However, recently published pediatric guidelines recommend inner edge–to–inner edge measurements in systole.6 In addition, recently published pediatric normative databases based on 2D echocardiography use inner edge–to–inner edge measurements of vessel diameters.7,18,19 Our protocol included both systolic and diastolic measurements and used the inner edge–to–inner edge 2D method and allometrically adjusted the systolic measurements to BSA.7 Our results demonstrate that this is a feasible and highly reproducible method to be used in a clinical trial involving pediatric and young adult patients. The use of a common measurement technique for pediatric and adult patients would be ideal for longitudinal studies over a wider age range; however, none of the currently available normative databases cover the full age span. Previous investigators have favored diastolic measurements for better reproducibility, but our study found excellent agreement for both systolic and diastolic measurements of the aorta.
Despite the difficulties inherent in multicenter data acquisition due to differing local styles, as well as a patient population often challenging to image with echocardiography, a reliable system was put into place with comprehensive training and quality review consistent with the American Society of Echocardiography’s consensus statement on standards on echocardiographic core laboratories,20 which resulted in excellent interobserver and intraobserver agreement on the aortic measurements performed at the core laboratory. This study was not designed to assess the overall interobserver variability between study centers. Although prior studies have reported excellent agreement for aortic root diameter using M-mode echocardiography in relatively small cohorts of healthy children,21–23 our study is by far the largest to report agreement, particularly in the MFS population. As expected, higher image quality was associated with better agreement (smaller percentage error). Later reading date was also associated with better agreement, which could be due to improved imaging acquisition at the local sites over time and/or improved standardization of measurement at the core laboratory over time. In the present study, two independent observers performed the described eight measurements of the aorta on all echocardiograms obtained during the course of the trial. A similar method was used by Selamet Tierney et al.24 to measure ROOTmax in a much smaller MFS cohort (n = 63), but the measurements were made only in systole, and the ascending aorta was not measured. Furthermore, interobserver agreement improved when three beats were averaged compared with one or two beats, consistent with the Pediatric Health Network Ventricular Volume Variability study on the variability of echocardiographic indices of LV size and function.25 Improving the reproducibility of outcome measures improves the likelihood of detecting treatment effects; therefore, we recommend multiple-beat averaging whenever feasible in research settings, although the impact in the clinical setting is not known.
Although the maximum and minimum aortic root and ascending aortic measurements by 2D imaging were highly reproducible, the stiffness indices showed only moderate reproducibility. This finding is in contrast to prior reports of excellent reproducibility of stiffness indices of the ascending aorta using M-mode imaging of the aortic wall over five cardiac cycles and using auto-detection software.26,27 However, this observation is in alignment with the reported observation in the Ventricular Volume Variability study25 that derived parameters on the basis of two or more measurements are invariably less reproducible than the measurements themselves because of propagation of error. Assessment of aortic root stiffness measured by M-mode echocardiography has been reported in patients with bicuspid aortic valves, with a percentage of mean measurements between two observers of 3.5% for stiffness index (n = 35) and variability of 14% in aortic root stiffness index when measured at different times (n = 6).28 However, aortic root stiffness indices have not been reported previously in patients with MFS and are therefore unique to our study.
We report the echocardiographic characteristics of a large cohort of children and young adults with MFS and moderate aortic root dilation enrolled in a clinical trial of atenolol versus losartan. Consistent with successful randomization, there were no differences in these characteristics between the two treatment arms. Not surprisingly, subjects with more severe aortic root dilation had a more severe echocardiographic profile overall, with greater likelihood of having MVP, TVP, and dilation of the MPA and left ventricle.
Our finding that MVP and mild or greater MR were more common in female patients is in contrast to a prior study by Detaint et al.29; however, their cohort had a median age at diagnosis of 22 years, twice that of our cohort. Our analysis suggests a higher prevalence for mitral valve disease in young girls with MFS. Aortic annular dilation has been reported in patients with MFS,30 but gender differences in the degree of dilation have not been reported previously. Detaint et al. also reported that men presented earlier and with more severe aortic dilation and related complications compared with women. Our finding that male subjects had significantly stiffer aortic roots despite similar ROOTmax Z scores might explain this difference in reported aortic outcomes in male subjects.
Limitations
By trial design, our cohort excluded patients with lower ROOTmax Z scores and with prior or impending aortic surgery; therefore, the associations reported here are representative of patients with MFS and moderate aortic root dilation and may not reflect the full spectrum of the disorder. In addition, a large number of comparisons were conducted, and some findings may be due to chance, even with our use of the more stringent significance level of .01.
CONCLUSIONS
The echocardiographic methodology, training, and quality review process used in this multicenter randomized clinical trial resulted in a robust evaluation of aortic root dimensions with excellent reproducibility despite the challenges of a multicenter study design and the imaging difficulties common in patients with MFS. This will optimize our ability to detect differences in treatment effects between atenolol and losartan in children and young adults with MFS.
Supplementary Material
Acknowledgments
This study was supported by U01 grants HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, and HL085057 from the National Heart, Lung, and Blood Institute (Bethesda, MD) and the US Food and Drug Administration Office of Orphan Products Development (Silver Spring, MD). Additional support was provided by the National Marfan Foundation (Port Washington, NY), Merck & Co., Inc. (Whitehouse Station, NJ), and Teva Canada Limited (Mirabel, QC, Canada). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations
- AR
Aortic regurgitation
- BSA
Body surface area
- ICC
Intraclass correlation coefficient
- IQR
Interquartile range
- LV
Left ventricular
- MFS
Marfan syndrome
- MPA
Main pulmonary artery
- MR
Mitral regurgitation
- MVP
Mitral valve prolapse
- ROOTmax
Maximum aortic root diameter
- TVP
Tricuspid valve prolapse
- 2D
Two-dimensional
Footnotes
The authors have no conflict of interest to disclose.
Benjamin W. Eidem, MD, FASE served as Guest Editor on this article.
REFERENCES
- 1.Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–76. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacro RV, Dietz HC, Wruck LM, Bradley TJ, Colan SD, Devereux RB, et al. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J. 2007;154:624–31. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacro RV, Guey LT, Dietz HC, Pearson GD, Yetman AT, Gelb BD, et al. Characteristics of children and young adults with Marfan syndrome and aortic root dilation in arandomized trial comparing atenolol and losartan therapy. Am Heart J. 2013 doi: 10.1016/j.ahj.2013.02.019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 6.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 8.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 9.Matitiau A, Perez-Atayde A, Sanders SP, Sluysmans T, Parness IA, Spevak PJ, et al. Infantile dilated cardiomyopathy. Relation of outcome to left ventricular mechanics, hemodynamics, and histology at the time of presentation. Circulation. 1994;90:1310–8. doi: 10.1161/01.cir.90.3.1310. [DOI] [PubMed] [Google Scholar]
- 10.Merillon JP, Motte G, Fruchaud J, Masquet C, Gourgon R. Evaluation of the elasticity and characteristic impedance of the ascending aorta in man. Cardiovasc Res. 1978;12:401–6. doi: 10.1093/cvr/12.7.401. [DOI] [PubMed] [Google Scholar]
- 11.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80:78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- 12.Erkula G, Jones KB, Sponseller PD, Dietz HC, Pyeritz RE. Growth and maturation in Marfan syndrome. Am J Med Genet. 2002;109:100–15. doi: 10.1002/ajmg.10312. [DOI] [PubMed] [Google Scholar]
- 13.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 14.Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–13. doi: 10.1056/NEJM199904293401702. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–12. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 17.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–61. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 18.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of Z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Zilberman MV, Khoury PR, Kimball RT. Two-dimensional echocardiographic valve measurements in healthy children: gender-specific differences. Pediatr Cardiol. 2005;26:356–60. doi: 10.1007/s00246-004-0736-z. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS, DeCara JM, Devereux RB, Duckworth S, Gardin JM, Jaber WA, et al. Echocardiographic imaging in clinical trials: American Society of Echocardiography Standards for echocardiography core laboratories: endorsed by the American College of Cardiology Foundation. J Am Soc Echocardiogr. 2009;22:755–65. doi: 10.1016/j.echo.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Dai S, Ayres NA, Harrist RB, Bricker JT, Labarthe DR. Validity of echocardiographic measurement in an epidemiological study. Project HeartBeat! Hypertension. 1999;34:236–41. doi: 10.1161/01.hyp.34.2.236. [DOI] [PubMed] [Google Scholar]
- 22.Geelhoed MJ, Snijders SP, Kleyburg-Linkers VE, Steegers EA, van Osch-Gevers L, Jaddoe VW. Reliability of echocardiographic measurements of left cardiac structures in healthy children. Cardiol Young. 2009;19:494–500. doi: 10.1017/S1047951109990862. [DOI] [PubMed] [Google Scholar]
- 23.Schieken RM, Clarke WR, Mahoney LT, Lauer RM. Measurement criteria for group echocardiographic studies. Am J Epidemiol. 1979;110:504–14. doi: 10.1093/oxfordjournals.aje.a112831. [DOI] [PubMed] [Google Scholar]
- 24.Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr. 2007;150:77–82. doi: 10.1016/j.jpeds.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, et al. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–54. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgartner D, Baumgartner C, Matyas G, Steinmann B, Loffler-Ragg J, Schermer E, et al. Diagnostic power of aortic elastic properties in young patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2005;129:730–9. doi: 10.1016/j.jtcvs.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Bradley TJ, Potts JE, Potts MT, DeSouza AM, Sandor GG. Echocardiographic Doppler assessment of the biophysical properties of the aorta in pediatric patients with the Marfan syndrome. Am J Cardiol. 2005;96:1317–21. doi: 10.1016/j.amjcard.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 28.Pees C, Michel-Behnke I. Morphology of the bicuspid aortic valve and elasticity of the adjacent aorta in children. Am J Cardiol. 2012;110:1354–60. doi: 10.1016/j.amjcard.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Detaint D, Faivre L, Collod-Beroud G, Child AH, Loeys BL, Binquet C, et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur Heart J. 2010;31:2223–9. doi: 10.1093/eurheartj/ehq258. [DOI] [PubMed] [Google Scholar]
- 30.Gautier M, Detaint D, Fermanian C, Aegerter P, Delorme G, Arnoult F, et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am J Cardiol. 2010;105:888–94. doi: 10.1016/j.amjcard.2009.11.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.