Abstract
Background
According to rodent models of postoperative cognitive decline, activation of the innate immune response following aseptic surgical trauma results in the elaboration of hippocampal proinflammatory cytokines, which are capable of disrupting long-term potentiation, the neurobiologic correlate of memory. We hypothesize that hippocampal recruitment of bone marrow-derived (BMD) macrophages plays a causal role in these processes, resulting in memory dysfunction.
Methods
Clodrolip injection (liposomal formulation of clodronate) prior to stabilized tibial fracture under general anesthesia was used to deplete BMD macrophages. Systemic and neuroinflammation were studied on postoperative day 1, and memory in a fear-trace conditioning paradigm was assessed on postoperative day 3. CX3CR1GFP/+ CCR2RFP/+ mice were used to identify BMD macrophages.
Results
Clodrolip effectively depleted splenic CCR2+ BMD macrophages. It also attenuated the surgery-induced increase of interleukin-6 in the serum and the hippocampus, and prevented hippocampal infiltration of CCR2+ cells without affecting the number of CX3CR1+ microglia. It did not alter the surgery-induced increase in hippocampal MCP-1, the recruitment signal for CCR2+ cells. Clodrolip prevented surgery-induced memory dysfunction, as evidenced by a significant increase in freezing time (29%, 95% CI: 21 to 38% vs. 48%, 95% CI: 38 to 58%, n= 20, P = 0.004), but did not affect memory in nonsurgical mice.
Conclusion
Depletion of BMD macrophages prevents hippocampal neuroinflammation and memory dysfunction after experimental tibial fracture. These data suggest that the hippocampal recruitment of BMD macrophages is a necessary mechanism in murine postoperative cognitive dysfunction. Interventions designed to prevent its activation and/or migration into the brain may represent a feasible preemptive strategy.
INTRODUCTION
Acute postsurgical memory deterioration leads to persistent cognitive decline1 that can result in considerable morbidity and increased mortality.2 The specter of memory dysfunction, including acute delirium, postoperative decline and dementia, is a source of anxiety for patients and their families.3 Knowledge about the molecular and cellular pathways involved in postoperative memory dysfunction may provide a launching pad for the development of biomarkers to identify the most vulnerable patients, as well as preventive strategies.
Using a murine model of aseptic surgical trauma with a long-bone fracture, we previously demonstrated that postoperative cognitive decline requires the engagement of the innate immune response. This engagement includes increased systemic expression of alarmins and proinflammatory cytokines such as interleukin (IL)-6 in the blood;4,5 increased ratio of CD11b+ cells corresponding to macrophages/microglia cells,4 and specifically the ratio of CCR2+ bone marrow-derived (BMD) macrophages;6 and elaboration of proinflammatory cytokines that are capable of disrupting hippocampal long-term potentiation, a neurobiologic correlate of learning and memory.3,7–10
Strategies designed to block the effect of pro-inflammatory cytokines with IL-1 receptor antagonist (anakinra) or tumor necrosis factor (TNF-α) antibody (etanercept) prevented murine postoperative memory dysfunction;4,5 these interventions also prevented inflammation-dependent wound healing.11,12
In this study, we tested the hypothesis that mediation of postoperative memory decline requires recruitment of systemic BMD macrophages into the brain, using a specific pharmacological strategy to acutely deplete systemic phagocytes prior to an aseptic surgical trauma with an experimental tibial fracture.
MATERIALS AND METHODS
Animals
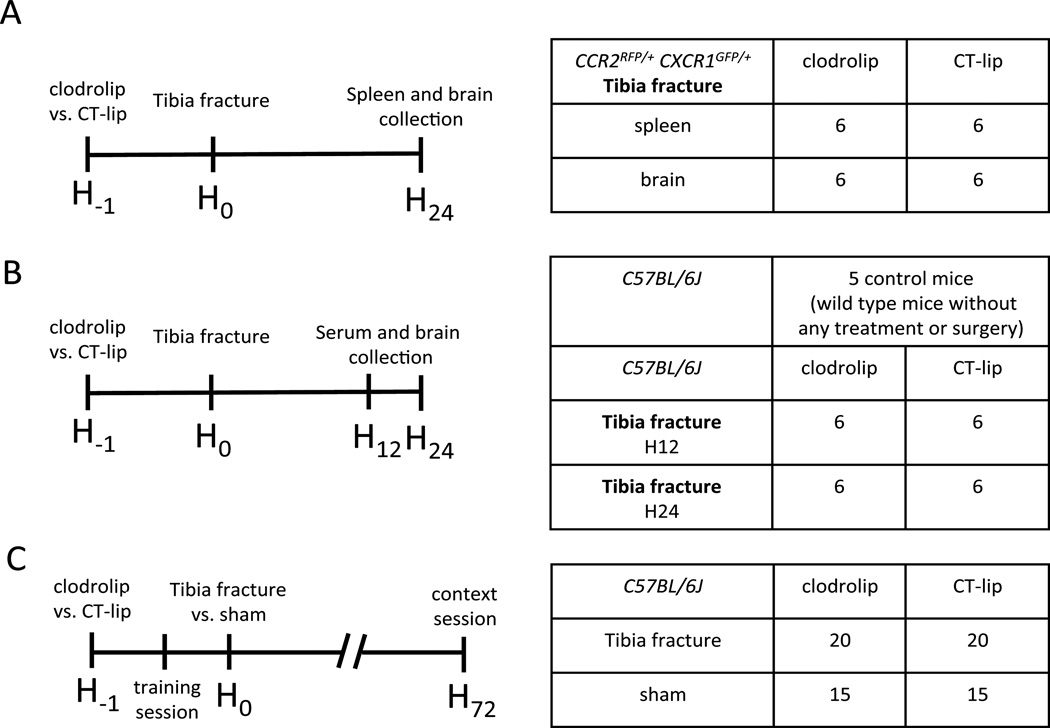
All experimental procedures involving animals were approved by the University of California, San Francisco Institutional Animal Care and Use Committee, and conformed to National Institutes of Health guidelines. Twelve 8-12-week-old CCR2RFP/+CX3CR1GFP/+ male mice6,13 (fig. 1A) were used to identify BMD macrophages. CCR2 and CX3CR1 are acronyms for chemokine (C-C motif) receptor 2 (whose cognate ligand is monocyte chemoattractant protein-1, MCP-1) that is highly expressed in BMD macrophages, and CX3C chemokine receptor 1 (CX3CR1, fractalkine receptor) that is highly expressed in resident microglia. Eighty-nine wild-type male mice (C57BL/6J, 10–12 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME): 29 for the cytokine expression (fig. 1B) and 70 for the behavior tests (fig. 1C). Mice did not experience unexpected lethality in the study and were euthanized according to our Institutional Animal Care and Use Committee guidelines.
Figure 1. Study design and splenic macrophages depletion with clodrolip.
A. First experiment with 12 CCR2RFP/+ CX3CR1GFP/+ mice divided into two groups of six mice treated with intraperitoneal (IP) injection of clodrolip versus CT-lip 1 h before the tibia fracture model and 25 h before tissue collection. B. Second experiment with 24 C57BL/6J mice divided into four groups of six mice treated with IP injection of clodrolip versus CT-lip one hour before the tibia fracture and sacrificed 12 h or 24 h after the tibia fracture C. Third experiment with 70 C57BL/6J mice into four groups treated with an IP injection of CT-lip versus clodrolip 1 h before the tibia fracture (20 mice per group) versus sham procedure (15 mice per group). The training session of the memory test was performed 30 min after the IP injection and 30 min before the surgery, and the context session was performed 72 h after the surgery. CT-lip = control liposome.
In Vivo Systemic Phagocyte Depletion with Clodrolip
Clodrolip is a liposomal formulation of clodronate (dichloromethylene bisphosphonic acid), a nontoxic bisphosphonate. Liposomes are lipid vesicles consisting of concentric phospholipid bilayers surrounding aqueous compartments. In this case, liposomes are used as “Trojan horses” encapsulating clodronate, which are then ingested and digested by phagocytes, followed by an intracellular release and accumulation of clodronate. At a certain intracellular concentration, clodronate induces apoptosis of the phagocytes. Clodronate liposomes were obtained from clodronateliposomes.org (Vrije Universiteit, Amsterdam, Netherlands) at a concentration of 7 mg/ml and prepared as previously described.14,15 Clodrolip (200 microL, about 100 mg/Kg) was intraperitoneally injected 60 min before the bone fracture. Control animals received 200 microL of control liposomal solution (CT-lip). No intraperitoneal or extraperitoneal damages were observed after clodrolip intraperitoneal administration.
Long-Bone Fracture with Tibia Fracture Surgery
Anesthesia was induced and maintained with isoflurane by inhalation. We used a dedicated chamber for induction with 5% of isoflunane for 3 min, and the surgery was performed under 2% of isoflurane for 10 to 12 min. Under aseptic surgical conditions, an open tibial fracture of the right hind limb with intramedullary fixation was performed as previously described.4 Body temperature was maintained at 37 ± 0.5°C using a thermal blanket throughout the surgical procedure, and analgesia was provided by injection of buprenorphine (0.3 mg in 100 microL of saline). Sham mice for bone fracture (sham group) received the same anesthesia and analgesia as the bone fracture mice.
Measurement of IL-6 in Serum
Mouse blood was collected using cardiac puncture under general anesthesia (isoflurane, 3%) in separate cohorts 12 and 24 h after the bone fracture procedure (fig. 1B). Blood samples were centrifuged at 1300 rpm for 10 min at room temperature, and the serum was collected and frozen at −80°C. IL-6 is secreted by BMD macrophages in response to alarmins16 and IL-6 level in the serum is increased within the first 24 h after the tibia fracture.4,5 IL-6 level in the serum is also associated with the postoperative memory dysfunction phenotype5 and it is affected by clodrolip in response to lipopolysaccharide infusion.17 For these reasons, we decided to quantify IL-6 levels in the serum of mice exposed to clodrolip or CT-lip using the IL-6 ELISA kit (KMC0062, Invitrogen, Grand Island, NY). Results are expressed as fold-increase compared to that measured in five control mice that did not receive any treatment or surgery.
Measurement of Cytokines in the Hippocampus
The hippocampi of the mice were rapidly collected under a dissecting microscope, 12 and 24 h after the tibia fracture (fig. 1B), and placed in RNAlater™ solution (Qiagen, Valencia, CA). To avoid blood contamination, mice were perfused with saline for 5 minutes before sample collection. Total RNA was extracted using RNeasy Lipid tissue Kit (Qiagen) treated with recombinant DNase I using a RNase-Free Dnase set™ (Qiagen), and reverse-transcribed to complementary DNA with a High Capacity RNA to complementary DNA Kit (Applied Biosystems, Bedford, MA). TaqMan Fast Advanced Master Mix (Applied Biosystems) and gene-specific primers and probes used for qPCR are: β-actin (NM_007393.1), IL-6 (Mm00446190_m1), TNF-α (Mm00443258_m1), IL-1β (Mm01336189_m1) and MCP-1 (Mm00441242_m1). qPCR was performed using StepOnePlus™ (Applied Biosystems). Each RNA sample was run in triplicate, and relative gene expression was calculated using the comparative threshold cycle ΔCT and normalized to β-actin. Results are expressed as fold-increase compared to that observed in five control mice that did not receive any treatment or surgery.
Quantification of BMD Macrophages and Microglia
Twenty-four hours after the tibia fracture surgery, the brain and spleen of the CCR2RFP/+CX3CR1GFP/+ mice were collected after intracardiac perfusion with paraformaldehyde 4% (fig. 1A). Spleen and brain (bregma −1.0 to −1.4 mm, corresponding to interaural 2.7 to 2.3mm in coronal orientation) were sectioned into 20-microm thick slices and mounted with Vectashield DAPI (Vector Laboratories, Burlingame, CA). The expression of CCR2-RFP and CX3CR1-GFP cells was assessed using confocal images, performed with a Spectral Confocal microscope (Nikon, Melville, NY) using three laser lines (405nm, 488nm, 561nm). Z-stacks were rendered into a three-dimensional image using the NIS-Elements AR 3.0 software (Nikon), and the expression of CCR2-RFP and CX3CR1-GFP cells was quantified using image J (National Institutes of Health, Bethesda, MA), with 3 different pictures/mice taken with a 20X objective. Data are expressed as relative cell percentages, normalized to the average value of the CT-lip group.
Behavioral Test for Hippocampal-dependent Memory with Trace Fear Conditioning
Fear conditioning is used to assess memory in rodents, which are trained to associate a conditional stimulus, such as a conditioning chamber, with an aversive, unconditional stimulus, such as a foot-shock. Freezing behavior is an indicator of aversive memory that is measured when subjects are reexposed to the conditional stimulus. With this model, lesions of the hippocampus disrupt recall of fear responses to the presentation of the context, resulting in a diminution in freezing.18,19
For this study, we used a previously published paradigm.4–6,20 Briefly, the behavioral study was conducted using a conditioning chamber (Med. Associates Inc., St. Albans, VT) and an unconditional stimulus (two periods of foot-shocks of 0.75 mAmp during 2 s). An infrared video camera, mounted in front of the chamber, captured motion speed (Video Freeze, Med. Associates Inc., St. Albans, VT).
All the animals underwent the same training session, regardless of the specific intervention, and received their training 30 to 40 min after the liposomal intraperitoneal injections (whether clodrolipid or CT-lip) that occurred 30 min before surgery (fig. 1C). Three days after conditioning, mice were returned to the same chamber where training had occurred for a context test. During the context test, mice were exposed just to the context and no tones or foot-shocks were delivered. Freezing was recognized by the software as a total lack of movement, excluding breathing and movement of vibrissae (linear detection with a minimal freeze duration of 20 frames corresponding to 0.7 seconds and a motion threshold of 20 arbitrary units).4–6,20 Decrease in the percentage of time spent freezing indicated impairment of memory.
Body Weight and Maximal Motion Speed
The body weight of the animals was measured three days after the surgery following assessment of freezing behavior. An infrared video camera (Video Freeze, Med. Associates Inc.) captured and quantified motion speed during the context test, and the maximal motion speed was recorded for each mouse.
Statistical Analyses
Data are presented as a mean ± 95% confidence interval (95%CI). Normality was tested with the d’Agostino and Pearson omnibus normality test. Equality of variances was tested with the F-test. For two-sample comparisons, t-tests were used (using the Welch correction if necessary); Mann-Whitney U-tests were used if data were not normally distributed. For comparisons of more than two groups, means were compared using one-way analysis of variance (ANOVA) followed by t-tests with a Bonferroni-corrected alpha level.
We used the two-way ANOVA procedure to determine whether or not time and treatment were significant factors in predicting IL-6 concentration in the serum, and IL-6, IL-1β, TNF-α, and MCP-1 mRNA (messenger RNA) expression in the hippocampi. Given the highly skewed nature of the mRNA expression, we checked the distribution of the residuals. We applied a log transformation (In(X)) to the response of the mRNA expression before performing analysis to better adhere to ANOVA model’s assumptions of normally distributed residuals and homoscedascity of residuals.
For the behavior tests, animals were tagged and randomly allocated to each group before any treatment, and researchers were blinded to the group assignment that was revealed only after the analysis phase. A repeated measure ANOVA was performed to determine whether treatment (CT-lip and clodrolip) and the three time periods (baseline, first shock, and second shock) were significant predictors of percentage freezing time during the training session.
For this study, our primary outcome was percentage of freezing time during the context session. Based on previous freezing time data,4 we estimated that a sample of 18 C57BL/6J surgical mice per group was necessary to demonstrate a 20% increase in percentage freezing time, with 80% power at the 0.017 alpha level (after adjusting for three comparisons) to find a significant difference between clodrolip and CT-lip.
A two-tailed P value < 0.05 was considered statistically significant for 2-group comparisons and the significance threshold was adjusted for multiple comparisons with a Bonferroni correction. Prism 5 (GraphPad Software, Inc., La Jolla, CA) was used to conduct the statistical analyses.
RESULTS
Clodrolip Depletes Splenic BMD Macrophages and Prevents Hippocampal BMD Macrophage Infiltration
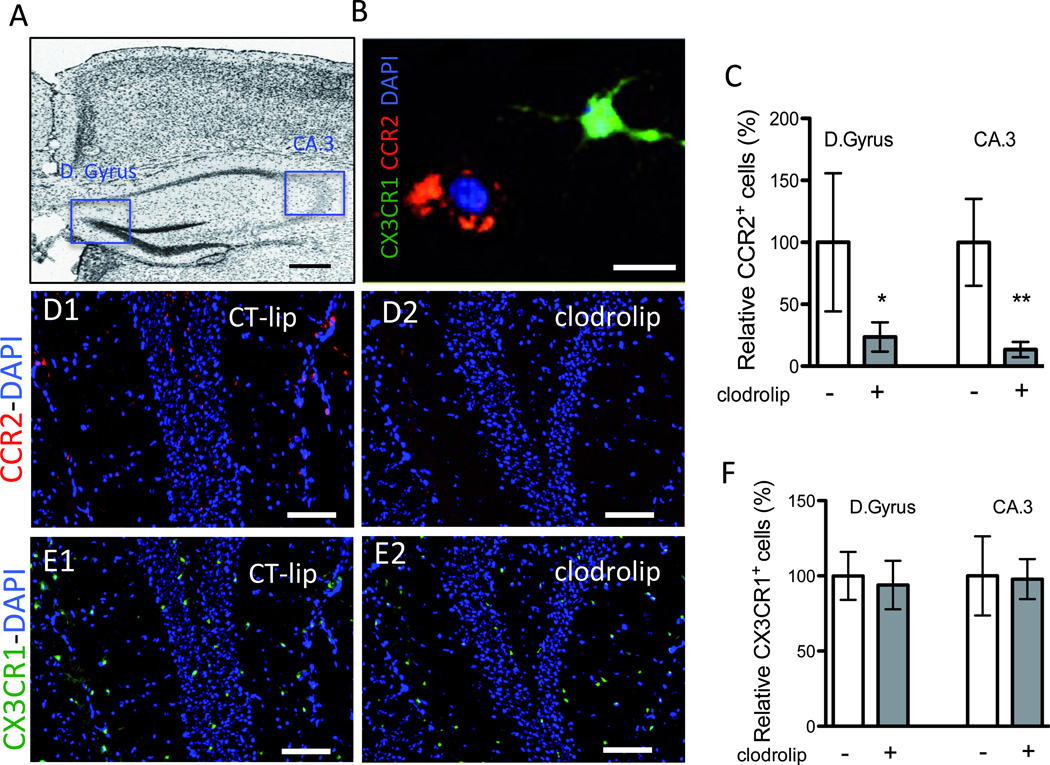
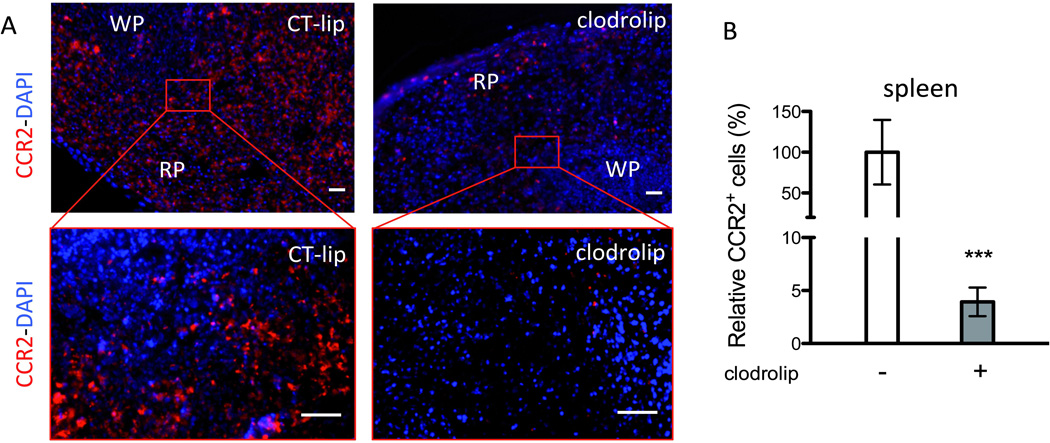
Using CCR2RFP/+CX3CR1GFP/+ mice (fig. 1A), in which RFP+ BMD macrophages and GFP+ resident microglia can be tracked,6,13 we found that clodrolip depleted splenic macrophages and surgery-induced BMD macrophage infiltration into the hippocampus. The CCR2+ cells, which are mainly present in the splenic red pulp (fig. 2A), decreased by 96% in the clodrolip-exposed mice (fig. 2B, 95% CI: 95 to 97%, P < 0.001). As shown in figure 3, the number of CCR2+ cells was also significantly reduced in the hippocampi of clodrolip-treated mice compared to control liposome-treated mice 24 h after surgery (decrease of 76% for the Dentate Gyrus and 87% in the CA 3). However, clodrolip treatment did not change the number of CX3CR1+ cells in the Dentate Gyrus and CA 3 hippocampal regions (fig. 3).
Figure 2. Effects of systemic macrophage depletion with clodrolip on the CCR2+ splenic cells.
A. Representative photos of spleen section showing CCR2+ cell repartition mainly in the red pulp (RP) and less in the white pulp (WP), 24 h after tibia fracture. Top photos are of low magnification (scale bar: 100 microm) and bottom photos are highly magnified images (scale bare: 50 microm) in the CT-lip and the clodrolip mice; B. Quantification relative percentage of CCR2+ cells in the spleen after clodrolip (n = 6, ***: P < 0.001 with unpaired t-test). CCR2 = chemokine (C-C motif) receptor 2; CT-lip = control liposome; DAPI = 4',6'-diamidino-2-phenylindole; IP = intraperitoneal. Bars are means ± 95% confidence interval.
Figure 3. Effects of systemic macrophage depletion with clodrolip on the hippocampal CCR2+ and CX3CR1+ cells.
A. Representative photo of the section of interest corresponding to bregma −1.2mm (scale bar: 500 microm), showing the Dentate Gyrus (D.Gyrus) and the Cornu Ammonis subdivision 3 (CA.3). B. Representative high-magnified photo (scale bar: 20 microm) of a ramified CX3CR1+ green cells and an amoeboid CCR2+ red cell in the hippocampus. C. Bar graph shows quantification of the relative percentage of CCR2+ cells in the D Gyrus and the CA.3 regions after clodrolip treatment (n = 6, significant F test for both comparisons, * P = 0.02 ** P=0.002 with unpaired t-tests with Welch’s correction) D. Representative photos of the D.Gyrus hippocampal sections in the CT-lip (D1) and the clodrolip (D2) mice (scale bar: 100 microm) showing the decrease of CCR2+ cells after clodrolip treatment. E. Representative photos of the D.Gyrus hippocampal section in the CT-lip (E1) and the clodrolip (E2) mice (scale bar: 100 microm) showing the absence of CX3CR1+ cells depletion after clodrolip treatment. F. Bar graph shows quantification of the relative percentage of CCR2+ cells in the D Gyrus and the CA.3 regions after clodrolip (n = 6, P = 0.51 for D.Gyrus and P = 0.51 for CA.3). CT-lip = control-liposome; CCR2 = chemokine (C-C motif) receptor 2; CT-lip = control liposome; DAPI = 4',6'-diamidino-2-phenylindole; Bars are means ± 95% confidence interval.
Clodrolip Reduces Systemic and Hippocampal Pro-inflammatory Cytokines
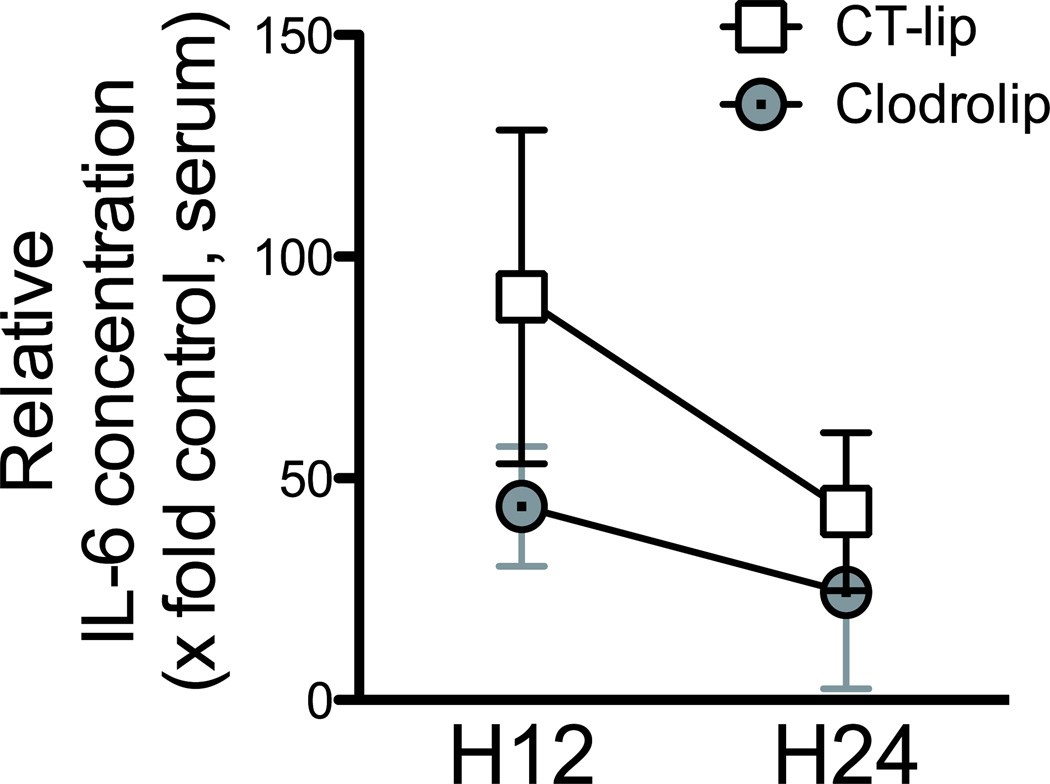
We previously showed that proinflammatory cytokines in the blood and hippocampus increased within the first day after surgery.4 To test if clodrolip treatment would reduce the proinflammatory cytokines, we studied serum and hippocampal expression 12 and 24 h after surgery (fig. 1B). Twelve hours after surgery, the rise in IL-6 in the serum was significantly attenuated in mice exposed to clodrolip (two-way ANOVA, P = 0.004 for the treatment, P = 0.003 for the time effect, P = 0.19 for interaction, fig. 4).
Figure 4. Effects of systemic macrophage depletion with clodrolip on the IL-6 serum concentration after the tibia fracture.
Serum IL-6 concentration 12 h and 24 h after tibia fracture in the clodrolip and the CT-lip groups. Data are normalized to the average value of the control mice group that did not receive any treatment or any surgery, and expressed as fold time increase (n = 6 per group and per timing, two-way ANOVA, P = 0.004 for the – treatment effect, P = 0.003 for the time effect, P = 0.19 for interaction). CT-lip = control-liposome; CCR2 = chemokine (C-C motif) receptor 2; CT-lip = control liposome; DAPI = 4',6'-diamidino-2-phenylindole; IP = intraperitoneal. Boxes and circles are means ± 95% confidence interval.
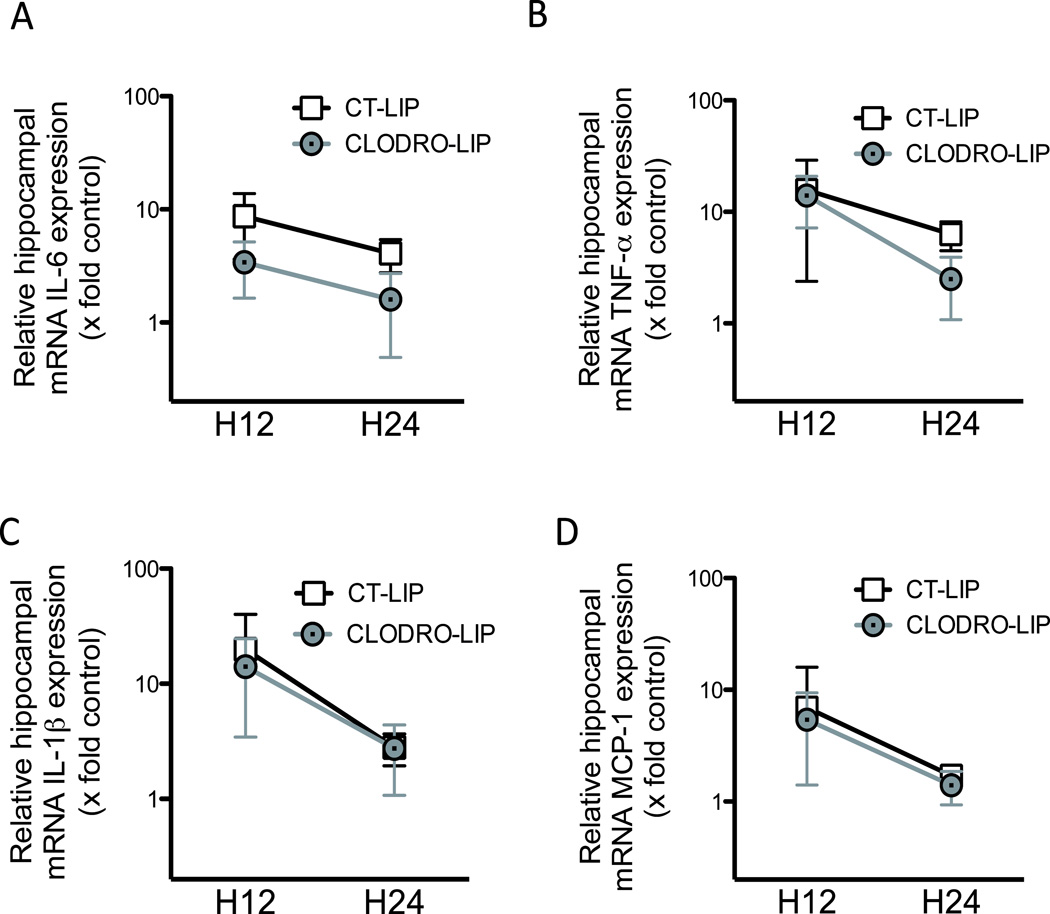
Between 12 and 24 h after surgery, the increase in mRNA hippocampal expression of IL-6, TNF-α and IL-1 induced by the surgery returned to almost baseline values at 24 h (fig. 5). Clodrolip exposure significantly inhibited the surgery-induced increased expression of mRNA IL-6 (two-way ANOVA, P < 0.001 for the treatment and P = 0.002 for the time effect, P = 0.51 for interaction), and interacted with the time-dependent decrease for TNF-α (two-way ANOVA, P = 0.03 for interaction). Clodrolip treatment did not change IL-1β mRNA expression (two-way ANOVA, P=0.42 for the treatment, P < 0.001 for the time effect, P = 0.66 for interaction, Figure 5).
Figure 5. Clodrolip effect and early kinetic of the mRNA hippocampal expression of IL-1β, TNF-α, IL-6 and MCP-1 after tibia fracture.
A. Hippocampal mRNA expression of IL-6 relatively expressed as fold increase compared to control brain expression, 12 and 24 h after tibia fracture in the CT-lip and the clodrolip-treated mice (n = 6 per group and per timing, two-way ANOVA, P = 0.0003 for the treatment effect, P = 0.0023 for the time effect, P = 0.51 for interaction). B. Hippocampal mRNA expression of TNF-α relatively expressed as fold increase compared to control brain expression, 12 and 24 h after tibia fracture in the CT-lip and the clodrolip-treated mice (n = 6 per group and per timing, two-way ANOVA, P = 0.06 for the treatment, P < 0.0001 for the time effect, P = 0.035 for interaction). C. Hippocampal mRNA expression of IL-1β relatively expressed as fold increase compared to control brain expression, 12 and 24 h after tibia fracture in the CT-lip and the clodrolip-treated mice (n = 6 per group and per timing, two-way ANOVA, P = 0.42 for the treatment effect, P < 0.0001 for the time effect, P = 0.66 for interaction). D. Hippocampal mRNA expression of MCP-1 relatively expressed as fold increase compared to control brain expression, 12 and 24 h after tibia fracture in the CT-lip and the clodrolip-treated mice (two-way ANOVA, P = 0.64 for the treatment, P = 0.0005 for the time effect, P = 0.67 for interaction). CT-lip = control liposome; IL = Interleukin; MCP-1 = monocyte chemoattractant protein-1; mRNA = messenger RNA; TNF = tumor necrosis factor. Boxes are means ± 95% confidence interval and data are presented with a logarithmic scale.
Systemic macrophages are recruited into tissues by the chemoattractant MCP-1 that binds to CCR2, which is expressed on the surface of BMD macrophages.21,22 Following surgery, MCP-1 mRNA expression increases and is unaffected by prior exposure to clodrolip (two-way ANOVA, P = 0.64 for the treatment, P < 0.001 for the time effect, P = 0.67 for interaction, fig. 5D).
Clodrolip Prevents Surgery-induced Memory Impairment
During the preoperative training period, learning was similar in the clodrolip-exposed and the control (nonexposed) groups, with the percentage of freezing being highly associated with time (fig. 6).
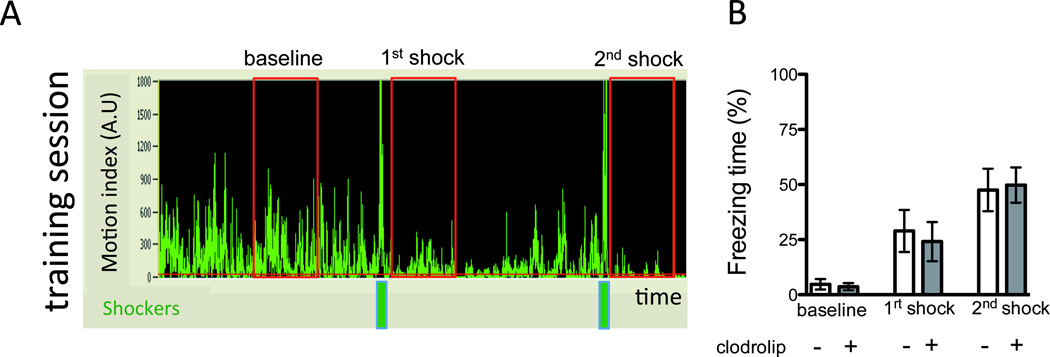
Figure 6. Clodrolip effect on the training session.
A. Representative record (A1) of a training session showing the motion (motion index expressed in arbitrary unit) of the mouse according to the time. The two green bars represent the two shocks while the three red rectangles represent the 40-s periods used to quantify the baseline, first and second shock freezing response. Bar graph (A2) quantifies the percentage of freezing time (n = 35, two-way ANOVA showing a significant effect of the time (P < 0.0001), no significant effect of the treatment (P = 0.68) and no interaction (P = 0.61).
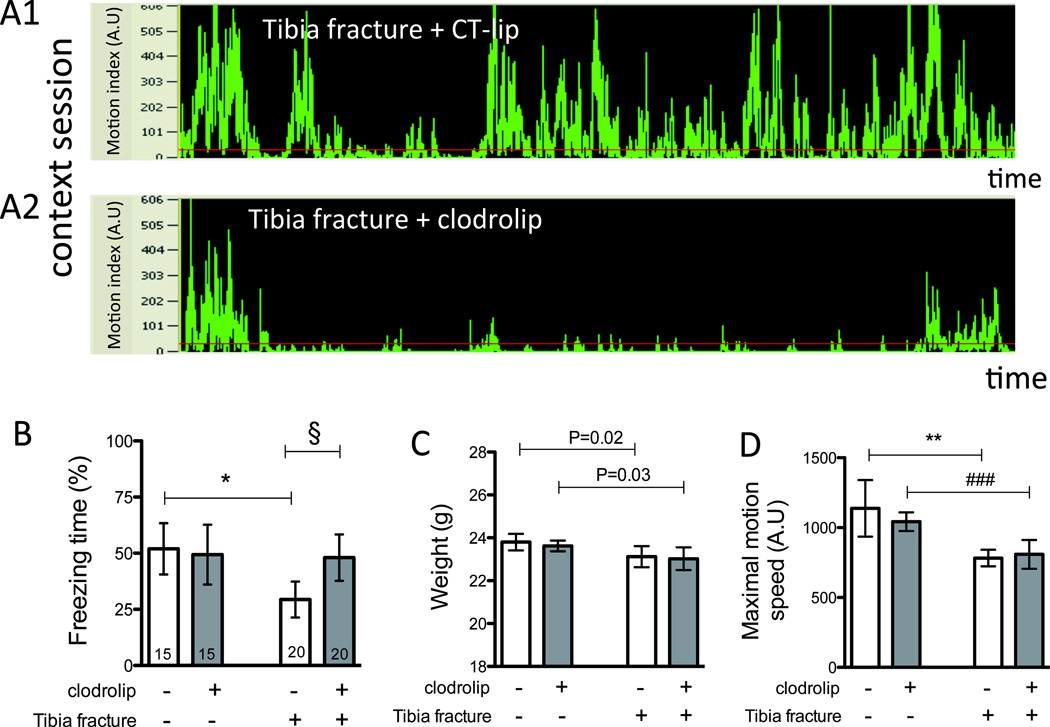
During the context session, surgery significantly decreased percentage of freezing time in comparison to the sham group (52%, 95% CI: 41 to 63% vs. 29%, 95% CI: 21 to 37%, P = 0.0012); pre-operative exposure to clodrolip resulted in significantly greater freezing time than in the non-exposed surgical cohort (29%, 95% CI: 21 to 38% vs. 48%, 95% CI: 38 to 58%, P = 0.004), reaching a similar level to that observed in the sham-operated clodrolip-exposed mice (49%, 95% CI: 36 to 63%, P = 0.86, fig. 7A–7B).
Figure 7. Depletion of systemic macrophage reduces the surgical-induced memory dysfunction.
A. Representative records of context sessions of tibia fracture mice treated with CT-lip (A1) and with clodrolip (A2) showing the motion (motion index expressed in arbitrary unit) of the mice according to the time. B. Quantification of the freezing time percentage according to the four groups (n = 15–20, * P = 0.0012 and §P = 0.004 respectively with one-way ANOVA and Bonferroni post-hoc analysis). C. Quantification of the body weight in the four groups three days after the surgery (n = 15–20, P = 0.02 and P = 0.03, respectively, not significant after adjustment for multiple comparisons with one-way ANOVA and Bonferroni post-hoc analysis and no significant effect of the clodrolip treatment. D. Quantification of the maximal motion speed in the four groups three days after the surgery (n = 15–20, ** P = 0.012 and ###P = 0.0003 respectively with one-way ANOVA and Bonferroni post-hoc analysis and no significant effect of the clodrolip treatment. Bars are means ± 95% confidence interval. ANOVA = analysis of variance; AU = arbitrary unit; CT-lip = control-liposome.
Clodrolip did not affect the body weight of the mice 3 days after the injection (fig. 7C). As for maximal motion speed, the clodrolip-treated groups were no different from the CT-lip groups, even though the maximal motion speed of the surgical groups was significantly slower than the sham groups (fig. 7D).
DISCUSSION
In this study, we report for the first time that BMD macrophages are required in the pathogenesis of the neuroinflammatory and memory dysfunction induced by surgery. Also, we report that a possible hippocampal signal through MCP-1 is involved in the recruitment of BMD macrophages to this brain region. Data from rodent surgical models have provided insight into the neuroinflammatory basis for postoperative cognitive decline. This usually transient process appears to be part of a motivational system that reorganizes the organism's priorities to facilitate recovery. To date, we have established a pivotal early role for the proinflammatory cytokine TNF-α,6 and our study demonstrated that hippocampal infiltration of BMD macrophages also plays a role in the initiation of neuroinflammation.
Hippocampal Infiltration of BMD Macrophages After Surgery
Monocyte infiltration into the brain is mainly described in acute brain injuries such as stroke23 and traumatic brain injuries,24 as well as chronic inflammatory brain injuries such as multiple sclerosis.13 Using long-bone fracture as a surrogate for a peripheral orthopedic surgical insult, we previously reported that CCR2+ cells were present in the hippocampus.6 Because microglia can also express CCR2 under certain conditions,25,26 we could not ascertain whether these CCR2-expressing cells arose from the resident macrophage population (microglia) or through an infiltration from outside of the central nervous system. Using clodrolip to specifically deplete the systemic pool of phagocytes, including BMD macrophages, we were able to demonstrate that the CCR2+ cells in the hippocampus are a result of the recruitment of BMD macrophages into the brain.
For passage into the brain, monocytes are required to overcome the blood-brain and/or blood-cerebral spinal fluid barriers;27,28 these barriers can be disrupted by direct acute brain injury.29,30 Interestingly, after peripheral surgery, the blood-brain barrier is disrupted although there is no discernible brain lesion.6 Now we show that after surgery, the hippocampus expresses MCP-1, which is capable of attracting CCR2+ expressing cells migrating through the disrupted blood-brain barrier. This increased expression of MCP-1 is unaffected by clodrolip treatment, indicating that BMD macrophages are not a self-perpetuating source of this chemoattractant for its own recruitment. Future understanding of the source and the triggers for hippocampal MCP-1 following peripheral surgery may result in interventional strategies designed to prevent recruitment of BMD macrophages into the brain.
Hippocampal BMD Macrophage Infiltration and Memory Dysfunction
Our recent data suggest that transient hippocampal inflammation is the key element in postoperative memory dysfunction because: 1) hippocampal areas are known to be involved in memory tasks;7 2) hippocampal neuroinflammation profile correlates with the level of memory dysfunction;4,5 and 3) hippocampal neuroinflammation leads to long-term potentiation disruption.31,32 Now we report here that the absence of BMD macrophage infiltration, produced by systemic depletion by clodrolip, decreases surgery-induced hippocampal inflammation and memory dysfunction . Therefore, postoperative BMD macrophage recruitment into the hippocampus plays a key role in the initiation of postoperative memory dysfunction.
In the context of postoperative cognitive decline, determining which cells are involved in the initiation of the inflammation response is important because, when exaggerated, this could overwhelm resolving responses and produce persistent postoperative cognitive decline. Earlier, we described that surgical trauma induces systemic release of alarmins (i.e., high-mobility group protein 1) and proinflammatory cytokines (i.e., TNF-α and IL-6).4,5 Improving our knowledge of cellular and molecular initiation mechanisms will allow insight into an ex-vivo bioassay to prospectively determine if patients are at risk.
Limitations of the Study
We used experimental tibial fracture to generate animal postoperative memory acute dysfunction. With this model, we traumatized the bone marrow directly, which could play a key role. However, other models that did not damage bone marrow with a splenectomy33 also showed that surgery generated postoperative cognitive dysfunction.
Fibrin is deposited in the hippocampus after tibia fracture,6 suggesting that the blood-brain barrier becomes disrupted and may allow the passage of clodrolip to act directly on the microglia population.34 Yet, we found that the systemic administration of clodrolip acts only on the number of CCR2+ cells without significantly affecting the number of CX3CR1+ cells (fig. 3); if there is a clodrolip effect on microglia, it may be to functionally modify them. For this reason, we cannot exclude the possibility that clodrolip does not affect microglia’s function, and that microglia do not play a key role in post-operative cognitive dysfunctions.
For this study, we used a pharmacological strategy in order to quickly deplete the pool of systemic macrophages. However, because clodrolip is highly toxic for the monocytes and macrophages,35 it can increase the risk of post-surgery infections, generating a phenotype of its own. With a single dose, we did not observe loss of weight or other signs of sickness within the 3 days. We performed a very short-term study, focusing on the acute exaggeration phase of the neuroinflammation, and did not perform any long-term study with clodrolip. Clodrolip should be considered as a tool for mechanistic studies but cannot be proposed for clinical therapy.
To distinguish if these recruited cells were resident or recruited systemic macrophages, we used CCR2RFP/+CX3CR1GFP/+ mice. CCR2 is receptor for MCP-1 and is mainly expressed in BMD monocytes/macrophages. We previously showed that CD11b+ macrophages/microglia cells were recruited in the hippocampus after tibial fracture4. In this study, however, we did not determine that CCR2+ cells were only BMD macrophages. Further study on the role of other systemic phagocytes, including neutrophils, in our phenotype will be of most interest. .
Hippocampal cytokine expression was performed with mRNA and not with protein. This is a limitation, but we considered that the potential extravasation from blood may affect protein levels. Indeed, in this model we found blood-brain barrier leakage after the tibia fracture,6 and the increase of circulating IL-6 protein in the serum could contaminate the hippocampal samples with passive movement in the parenchyma (this phenomenon could be amplified by the perfusion itself). By analyzing mRNA expression in the brain collected after purging blood from the vessels, we ensured that hippocampal cells were the source of pro-inflammatory cytokines.
In conclusion, we showed in this study that BMD macrophage activation after experimental tibial fracture is directly involved in the tibia fracture-induced hippocampal BMD macrophage infiltration and animal memory dysfunction. Understanding the cellular and biological pathways involved in postoperative cognitive decline is a key element in designing interventions to prevent this disease. Reducing activation and/or migration of innate immune cells, such as systemic macrophages, into the brain represents a viable preemptive strategy.
Final Boxed Summary Statement.
What we already know about this topic
Animal models of postoperative cognitive dysfunction implicate an innate immune response, with increased circulating inflammatory mediators and migration of bone marrow derived cells into the brain
Inhibitors of inflammatory mediators reduce postoperative memory deficits in mice, but also reduce wound healing
What this article tells us that is new
In mice, treatment with a drug which depletes bone marrow derived macrophages reduced circulating inflammatory mediators after surgical orthopedic injury, reduced migration of immune cells into the brain, and reduced postoperative memory deficits
Acknowledgments
Supported by grant nos. R01NS027713, P01NS044155 and R21NS070153 from the National Institutes of Health, Bethesda, Maryland; AHA10GRNT3130004 from the American Heart Association, Dallas, Texas. Additional support from the University of California, San Francisco, San Francisco, California; INSERM, Paris, France; Société Française d’Anesthésie Réanimation, Paris, France; Fondation des Gueules Cassées, Paris, France; and Institut Servier, Paris, France. Dr. van Rooijen provided complimentary clodronate and control liposomes.
The authors thank members of the University of California, San Francisco Center for Cerebrovascular Research and the Maze laboratory for their support, Niccolo Terrando Ph.D. (Assistant Professor, Karolinska Institute, Stockholm, Sweden) for the setting of the memory test, as well as Israel F. Charo, M.D. (Associate Director, University of California, San Francisco, Gladstone Institute, San Francisco, California), for providing the CCR2RFP/+CX3CR1GFP/+ mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work is presented by the Department of Anesthesiology and Perioperative Care of the University of California, San Francisco, California.
Summary Statement: Hippocampal recruitment of bone marrow-derived macrophages plays a causative role in postoperative cognitive decline. Interventions designed to prevent its activation and/or migration into the brain may represent a feasible preemptive strategy.
REFERENCES
- 1.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Mucke L, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie Z, Maze M. Perioperative cognitive decline with the aging population. Mayo Clin Proc. 2011;86:885–893. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 8.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1β inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 β inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res Brain Res Protoc. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ. Interleukin-1 beta (IL-1 β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro . Neurosci Let. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 11.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 12.Ruyssen-Witrand A, Gossec L, Salliot C, Luc M, Duclos M, Guignard S, Dougados M. Complication rates of 127 surgical procedures performed in rheumatic patients receiving tumor necrosis factor alpha blockers. Clin Exp Rheumatol. 2007;25:430–436. [PubMed] [Google Scholar]
- 13.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 16.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 17.Gaca JG, Palestrant D, Lukes DJ, Olausson M, Parker W, Davis RD., Jr Prevention of acute lung injury in swine: Depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112:19–25. doi: 10.1016/s0022-4804(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18:911–918. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- 24.Szmydynger-Chodobska J, Strazielle N, Gandy JR, Keefe TH, Zink BJ, Ghersi-Egea JF, Chodobski A. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab. 2012;32:93–104. doi: 10.1038/jcbfm.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Salafranca MN, Adhikari S, Xia Y, Feng L, Sonntag MK, deFiebre CM, Pennell NA, Streit WJ, Harrison JK. Chemokine receptor expression in cultured glia and rat experimental allergic encephalomyelitis. J Neuroimmunol. 1998;86:1–12. doi: 10.1016/s0165-5728(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 26.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: Role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, Stocklein V, Bazarian JJ. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: A possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Dehghani F, Conrad A, Kohl A, Korf HW, Hailer NP. Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp Neurol. 2004;189:241–251. doi: 10.1016/j.expneurol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 35.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]