Abstract
Introduction
Few randomized controlled trials (RCTs) have been conducted to establish evidence-based management protocols for provoked vestibulodynia (PVD), a chronic vulvar pain condition affecting approximately 14 million women in the U.S. We describe the rationale and design of an NIH funded multicenter clinical trial utilizing an extended release formulation of gabapentin (G-ER), an intervention that preliminary data suggest may be efficacious for this condition.
Objectives
1) to determine if pain from tampon insertion (primary outcome measure) is lower in PVD patients when treated with G-ER compared to when treated with placebo and 2) to determine if G-ER reduces vulvar mechanical hyperalgesia, vaginal muscle pain to palpation, the number and intensity of somatic tenderpoints, spontaneous and provoked pain to intradermal capsaicin with an accompanying increase in cardiac beat-to-beat variability and to identify mechanistically-based PVD subtypes. Additional outcomes include subject reported intercourse pain and summative 24-hour pain.
Methods
This 16-week, randomized, double-blind, placebo-controlled, crossover study will enroll 120 women 18 years and older who report tenderness localized to the vulvar vestibule, pain with tampon insertion, and, when sexually active, insertional dyspareunia. Electronically entered daily diaries will be used to determine if pain is lower in PVD subjects when treated with G-ER (up to 3000 mg/d) compared to when treated with placebo. Psychophysiological measures will be obtained at baseline and after 2 weeks at the maximum tolerated dose.
Conclusion
We will conduct the first multicenter RCT to confirm efficacy of an agent that is currently used in clinical practice for treating PVD.
Keywords: gabapentin, vulvodynia, provoked vestibulodynia, multicenter clinical trial, biological correlates
1. Introduction
Approximately 14 million U.S. women suffer from provoked vestibulodynia (PVD) [1], a type of localized vulvar pain triggered by pressure applied to the vestibule (outer vagina), including vaginal penetration and tampon insertion [2]. PVD causes major disruption in the personal lives of up to 60% of women and severely compromises sexual function in 45% [3,4]. These women report significantly worse quality of life than controls without reported vulvodynia [4] and women with other vulvar conditions [5]. The burden imposed on the health care system is also significant, as these women visit multiple clinicians and specialists [6], and try numerous, non-evidence based treatments [7].
In the few randomized clinical trials (RCTs) conducted to date, the efficacy of the tricyclic antidepressants (TCAs) (amitriptyline [8] and desipramine) [9], considered standard interventions [10], have shown lower efficacy than predicted from non-controlled studies [11–14]. Since PVD has features similar to chronic neuropathic pain [15–17], involving central nervous system pain regulatory pathways, gabapentin is a frequently used alternative [18]. Because pelvic floor hypertonicity and altered cognitive patterns have been shown in some women with PVD, the anxiolytic and antispasmodic effects of gabapentin may also contribute to its efficacy [19].
Numerous RCTs of immediate-release gabapentin (G-IR) (1800–3600 mg/d in three divided doses) [20–23] and recent RCTS with gabapentin extended release (G-ER) (1800–3000 mg/d in single or two divided doses) [24,25], have shown superiority over placebo in treating neuropathic pain. Gabapentin is listed as a first choice treatment in three evidence-based consensus guidelines on neuropathic pain, as well as being suggested as effective from data based on three retrospective trials in vulvodynia [26–31], buts its efficacy in this population has not been empirically tested [32].
Gabapentin efficacy may be dependent on clinical presentations or “subtypes” of PVD. Possible predictors of treatment response may include evidence of peripheral [33,34] or central sensitization [17,35], degree of pelvic floor dysfunction [36,37], presence of certain personality characteristics [38,39], onset of the disorder [16,40,41], and hormonal influence [42–45] Identifying predictors of treatment response in PVD appears to have clinical applicability to other chronic pain syndromes, especially in identifying common etiological pathways for developing therapeutic targets.
Based on these data, we are undertaking a multicenter RCT which will examine the efficacy of G-ER treatment in PVD patients. Since the original submission of our RCT, we have revised inclusion/exclusion criteria based on recently available data, an NIH consensus conference [46] and the input of authorities who study other types of chronic pain syndromes. [26,47–50].
We also hope to elucidate mechanisms by which treatment may improve clinical outcomes. We hypothesize that short-term treatment of G-ER vs. placebo will result in improvement in pain severity and quality of life in this population. We further hypothesize that short-term treatment of PVD with G-ER will identify and define psychophysiologic measures of treatment response and define mechanistically-based PVD subtypes (Table 1).
Table 1.
Goal, hypotheses and measurements.
| Goal 1. | ||
| Determine the efficacy of G-ERa for treatment of PVDb compared to placebo | 1a. Pain from tampon insertion will be lower in PVD patients when treated with G-ER compared to when treated with placebo (primary outcome variable. | Diary |
| 1b. Pain from intercourse will be lower in PVD patients when treated with G-ER compared to when treated with placebo. | Diary | |
| 1c. 24-hour vulvar pain will be lower in PVD patients when treated with G-ER compared to when treated with placebo. | Diary | |
| Goal 2. | ||
| Identify psychophysiological measures of treatment response and define mechanistically-based PVD subtypes including central vs. peripheral sensitization, pelvic hypertonicity, tender point tenderness and autonomic dysregulation | 2a. G-ER will reduce mechanical allodynia compared to placebo | Von frey hair, brush, Algesiometer |
| 2b. G-ER will reduce area and duration of hypersensitivity induced by intradermal capsaicin compared to placebo | Capsaicin skin sensitivity test | |
| 2c. G-ER will reduce vaginal muscle pain to palpation compared to placebo | Vaginal algometer | |
| 2d. G-ER will decrease the number and intensity of somatic tender points compared to placebo | Tender point tenderness test | |
| 2e. G-ER will increase cardiac beat-to-beat variability compared to placebo | Heart rate variability | |
G-ER: gabapentin extended release.
PVD: provoked vestibulodynia.
2. Methods
2.1. Study Sample
Inclusion Criteria
Women over 18 years of age with greater than 3 continuous months of insertional (entryway) dyspareunia, and/or pain to touch/tampon insertion (modified ‘Friedrich’s Criteria’ [51] and have an average pain level of “4” or greater on the 0–10 numeric rating scale (NRS) on tampon insertion during screening.
Our inclusion criteria differs from the original ‘Friedrich’s Criteria’ where women must report greater than 12 continuous months of vulvar symptoms [51]. A “modified” Friedrich’s Criteria was selected because pain that has persisted for at least 3 months has been considered chronic and this duration has served as an inclusion criterion in other clinical trials [9,26].
Exclusion criteria
Presence of other vulvar conditions, or vaginal infection, atrophic vaginitis, previous vestibulectomy, pregnancy or at risk for pregnancy, any unstable medical condition (e.g., renal impairment, significant hematological disease, cardiovascular disease, hepatic insufficiency, neurological disorder, autoimmune disease, or respiratory illness), gastric bypass surgery, multiple allergies, other severe pain disorder (pain more severe in an area outside of the vulvar vestibule), history of intolerance to gabapentin or pregabalin, or topical lidocaine. Lidocaine is excluded because its topical administration route, direct peripheral effects, and potential efficacy could contribute to a significant effect independent of gabapentin [32].
Also excluded are those with a psychiatric disorder that could impact vaginal pain, risk patient safety, or may impact compliance at the discretion of the investigator (including major depressive disorder, current suicidal ideation with intent, manic or psychotic episode, severe anxiety, binge or anorexic behavior, or drug dependence or abuse). Women who have been prescribed a centrally-acting pharmacologic agent or started nonpharmacologic within the past month for vulvar pain are excluded. However, those who have been on stable concomitant therapy for at least 1 month without improvement in vulvar pain and remain on the same regimen throughout the study are eligible.
Women successfully treated for a vaginal infection as well as those with HPV or an abnormal Pap, and those with co-existing vaginismus (painful spasmodic contraction of the vagina) will be enrolled if a single index digit can be inserted into the introitus during the pelvic exam.
Women over 50 years of age without vaginal atrophy (<10% parabasal cells with maturation index) will be included. If atrophy is noted at baseline they will be treated with vaginal estrogen therapy for 6 weeks and rescreened. If they have a normal maturation index at rescreening and continue to have vulvar pain they will be enrolled and continued on estrogen therapy throughout the trial. A maturation index will be performed at each visit in subjects of all ages to provide an index of the degree of estrogenization (superficial/intermediate cell ratio) which varies according to the menstrual cycle phase, oral contraceptive use, and hormone therapy use [52]. Detailed inclusion and exclusion criteria are listed in Table 2.
Table 2.
Inclusion and exclusion criteria
| Inclusion Criteria |
| Women 18 years of age and older |
| Greater than 3 continuous months of insertional dyspareunia, pain to touch or tampon insertion |
| Average pain level of 4 or greater on 11-point tampon test during screening period |
| Score in vestibule greater than score in vulva or score n vagina on cotton swab test |
| Exclusion Criteria |
| Other vulvar conditions, including dermatoses (such as lichen sclerosis, lichen planus, desquamative inflammatory vaginitis), fissures, squamous cell carcinoma or other vaginal cancers, or vulvitis |
| Atrophic vaginitis. Atrophic vaginitis is indicated by a maturation index ≥ 10% parabasal cells. (Women with atrophic vaginitis may be treated with topical hormone replacement therapy for a minimum of 6 weeks and rescreened for eligibility). |
| Vestibulectomy |
| Active vaginal infection, including candida, bacterial vaginosis, trichomonas, chlamydia, gonorrhea, and herpes simplex virus. (Women who have active vaginal infections may be treated and retested prior to randomization). |
| Pregnancy or at risk for pregnancy and not using reliable birth control method for at least 3 months prior to study entry |
| Significant renal impairment (creatinine clearance of ≤ 60 mL/min, BUN > 30 mg/dL, serum creatinine > 2 mg/dL) |
| Significant hematological disease (leukopenia [WBC < 3.0 × 10−3 μl], leukocytosis [WBC > 20.0 × 10−3μl], neutropenia [ABC < 1.50 × 10−3 μl, < 20%], (thrombocytopenia [platelets < 100,000 μl], anemia [HCT < 27%, Hbg < 8 g/dL, RBC < 3 × 10−6]) |
| Noncontrolled cardiovascular disease (cardiac conduction disturbance, congestive heart failure, hypertension (≥ 140/90) |
| Hepatic insufficiency (serum AST, ALT or ALP ≥ 3 times upper limit of normal) |
| Neurological disorder, including seizures, syncopal episodes, peripheral neuropathy or other severe pain disorder. (“Other severe pain disorder” refers to a pain disorder where the pain is more severe in an area outside of the vulvar vestibule). |
| Coexisting vaginismus, fibromyalgia and/or interstitial cystitis where pain is greater than vulvar pain. (If vaginismus is present a single index digit must be able to be inserted into the introitus during the pelvic exam). |
| Any other unstable medical condition such as autoimmune disease or respiratory illness |
| Score of ≥ 12 on depression subscale of Hospital Anxiety and Depression Scale (HADS). (Mild depression is not an exclusion criteria). |
| Any psychiatric disorder that could impact vaginal pain, risk patient safety, or may impact compliance at the discretion of the investigator, including major depressive disorder, current suicidal ideation with intent, manic or psychotic episode, severe anxiety, binge or anorexic behavior, or drug dependence or abuse. |
| Recently prescribed a centrally-acting pharmacologic agent, including antidepressants, anticonvulsants, anxiolytics, muscle relaxants, and opiates or dosage adjustment. (Subjects are eligible for participation if they have been on stable concomitant therapy for at least 1 month without improvement in vulvar pain and remain on the same regimen throughout the study). |
| Recently began nonpharmacologic therapy, including physical therapy or individual/relationship and counseling or number of sessions has changed. (Subjects are eligible for participation if they have been on stable concomitant therapy for at least 1 month without improvement in vulvar pain and remain on the same regimen throughout the study). |
| Multiple allergies |
| Use of topical lidocaine within 2 weeks of randomization and during the study. |
| Previous use of gabapentin or pregabalin where side effects resulted in discontinuation |
| Gastric bypass surgery |
Subjects are permitted to take acetaminophen, aspirin, or a nonsteroidal anti-inflammatory drug as rescue medications. The medications allowed and prohibited were determined from a previous study of gabapentin in fibromyalgia [20]. Because naproxen may increase the amount of gabapentin absorbed by 12% to 15%, patients will be instructed to contact the study investigator prior to use [53–55].
2.2. Setting and design
This is a 16-week, randomized, double-blind, placebo-controlled, two-treatment, two-period crossover design study (Figure 1). Although a 2-week maintenance phase for each treatment is relatively short, it is consistent with most well-designed clinical trials in chronic pain [26] and with expert guidelines for gabapentin use in vulvodynia [10]. Women will be enrolled at any point in their menstrual cycle to maintain enrollment timelines, but the phase of their cycle will be documented and analyzed statistically for any confounding effects.
Fig. 1.
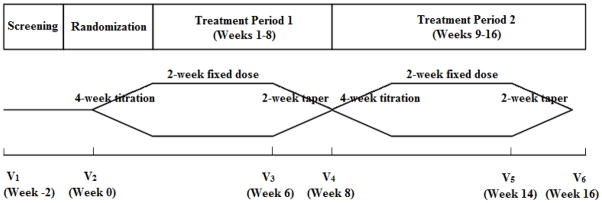
Schematic of 16-week, randomized, double-blind, placebo-controlled, two-treatment, two-period crossover design.
One-hundred twenty female subjects (40 per site) 18 years or older, who have been diagnosed with provoked vestibulodynia by one of the study clinicians will be recruited between July 1, 2012 and September 2013 from the University of Tennessee Health Science Center (UTHSC), the University Medical and Dental School of New Jersey (UMDNJ), and the University of Rochester Medical Center (URMC). The study design involves 8 phases: screening, randomization, dose titration, maintenance phase, dose-taper, dose titration, maintenance phase, and dose-taper phase. Eligible subjects will participate in the study for 18 weeks. There are a total of 6 clinic visits and 28 telephone contacts. Subjects will receive weekly telephone contacts by the nurse coordinator at each of the three sites, except during the two dose-titration phases when they will be contacted twice weekly. Study coordinators will follow a standardized script (see attached script, Appendix A). Primary recruitment will be referral-based from each of the collaborating institutions. As a secondary recruitment source, the National Vulvodynia Association (NVA) is displaying clinical trial information on their web site, and in their bimonthly electronic newsletter, where participants will be directed to a “vulvodynia website”, constructed by UTHSC Office of Biomedical Informatics (BMI), which contains information about the clinical trial.
2.3. Intervention
2.3.1. Drugs, dosages, and regimens
All patients will be scheduled to receive 2 weeks of stable-dose G-ER and 2 weeks of stable-dose placebo after a 4-week dose escalation period for each treatment arm. During the first four weeks of active treatment, the dose will be increased to a maximal tolerated dose or to the target ceiling dose of 3000 mg/d, whichever is reached first. This target dose was chosen on the basis of previous trials that suggested efficacy and tolerability of comparable doses of G-IR when used to treat symptomatic neuropathy [21–23], recent trials of G-ER in the treatment of diabetic peripheral neuropathy (PDN) [24], post herpetic neuralgia [25] and menopausal hot flashes [56,57], and from dosage range recommendations from a panel of international experts on vulvodynia [10]. Moreover, the dose-response curve observed in neuropathic pain trials (without disproportionate dropout rates), necessitates higher doses to establish efficacy [21]. Divided doses were selected based on lower fluctuation in plasma concentrations compared to single dosing [55] and in order to maximize dosage exposure during symptomatic periods. Asymmetric dosage was based on previous studies and with the intent of reducing adverse effects during the day [53–57]. Prior to study enrollment, the investigators received an Investigational New Drug Application (IND 108,795) to evaluate gabapentin ER for this unapproved indication.
2.3.2. Dosage titration
The number of tablets taken daily will be increased over 4 weeks in a step-up manner to a maximum total dose of 3000 mg/d, regardless of evidence of treatment efficacy at a lower dose. The titration schedule for G-ER asymmetric dosing is as follows: Week 0: 600 mg pm; Week 1: 600 mg am/600 mg pm; Week 2: 600 mg am/1200 mg pm; Week 3: 600 mg am/1800 mg pm; Week 4: 1200 mg am/1800 mg pm. Tablets will be taken with morning and evening meals. If side effects are intolerable or do not diminish within 3–4 days, the morning dose will be decreased by one level (600 mg), and an increase will be attempted one more time, at the next telephone call. If this next increase again results in intolerable side effects, the study drug will be decreased to the level of the previous dose, which will be defined as the maximal tolerated dose, and continued at that level for the remainder of the study (a minimal dose of 1200 mg/d will be permitted). Both tablet appearance and number of tablets taken will be the same during active and placebo phases of the trial to maintain blinding.
The slow titration period is necessary to reduce side effects and discontinuation rates. Each subject will receive her maximal tolerated dose during the 5th–6th week of each period. During the 7th and 8th week, subjects will undergo a 10-day dose tapering (of active drug; or sham decrease for placebo) and a 4-day complete washout. The 4-day washout is consistent with the 5–7 hour-half-life of gabapentin to prevent crossover to the next treatment period [53–57]. Similar washout periods have been used in clinical trials with both G-ER and G-IR because the elimination half-lives do not differ between the two dosage forms (53–57). Although it is conceivable that drug may remain in the tissue longer than its half-life, methods for serum and tissue assay of gabapentin are not well defined with respect to its anti-nociceptive effect. However, efficacy will be based on patient pain ratings during the final week of treatment (allowing additional 6 weeks for dissipation of drug effects after washout), and we will statistically test for carryover effects. (A previous gabapentin trial using a shorter crossover period [4-day taper and a 3-day washout] showed no carryover effects [23]. We will assess blinding through subjects’ guessing treatment at trial conclusion. (Our previous trial showed modest unblinding − 67% vs. chance [9]).
2.3.3. Baseline/screening
Informed consent will be obtained and eligibility determined at the patient’s first visit. Demographic data will be collected, including relationship status, length of time with current partner, number of marriages/.partnerships, age, education, race/ethnicity, employment status, income, and religious affiliation. A medical and gynecologic history and a general and genital-urinary exam will be performed, including a cotton swab test (CST) and assessment for vaginitis (Table 3). The pelvic exam will include insertion of a single index finger into the introitus to determine the presence of involuntary introital spasm (vaginismus), as well as palpation of the levator ani muscles, urethra, cervix, adnexa and uterus to determine tenderness. Moreover, muscle algometry with the load cell will be a precise measure of levator muscle tenderness/spasm and therefore vaginismus [58,59]. We will be able to distinguish normal iliococcygeus muscle tenderness from vaginismus by comparing our values to normal controls reported by Tu [59].
Table 3.
Schedule of Events.
| Measurements | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|
| Consent | X | |||||
| Demographics | X | |||||
| Medical and gynecologic history | X | |||||
| Vital signs | X | X | X | X | X | X |
| Physical Exam | X | |||||
| Pelvic Exam | X | X | X | X | X | X |
| Cotton swab test | X | X | X | X | X | X |
| VPIII microbial identification testa,b | X | |||||
| Urine pregnancy testb | X | |||||
| Laboratory testsc | X | |||||
| Diary training | X | |||||
| Dispense medication | X | X | ||||
| Medication compliance | X | X | X | X | ||
| Diary review | X | X | X | X | X | |
| Adverse events | X | X | X | X | X | X |
| Concomitant medications | X | X | X | X | X | X |
| Use of escape medications | X | X | X | X | X | X |
| Capsaicin test | X | X | X | |||
| Heart rate variability | X | X | X | |||
| Vaginal algometer | X | X | X | X | ||
| Algesiometer | X | X | X | X | ||
| Tender point tenderness | X | X | X | X | ||
| Psychometricsd | X | X | X |
VPIII microbial identification test (Affirm®) for vaginitis, including candida, trichomonas, and bacterial vaginosis.
If indicated.
Comprehensive metabolic panel, CBC + differential.
Brief Pain Interference (BPI) Scale, Patient Global Impression of Change (PGIC), Hospital Anxiety and Depression Scale (HADS), Locke-Wallace Scale, Female Sexual Function Index (FSFI), MOS 36-item short-form health survey (SF-36), and Leserman Sexual and Physical Abuse History Questionnaire.
Subjects will receive training on the use of an electronic diary and the tampon test [60] (Appendix B) to rate insertional pain. Biological measures, including the algesiometer, vaginal algometer and tender point tenderness will be conducted.
2.3.4. Randomization
Subjects will be randomized using a computer-generated random numbers technique, where a concealed allocation schedule will be prepared randomly assigning the two sequences, in blocks of four to each site, to a consecutive series of numbers. At this visit subjects will receive 8 weeks of either active drug or placebo, and at Visit 4 they will receive 8 weeks of the alternate treatment.
The Department of Pharmaceutical Sciences at the Core Center will be responsible for properly blinding the study drug and for dispensing and labeling for shipment to all clinical sites. Bottles will be labeled as “A” and “B”, and will contain either 600 mg of G-ER or matching placebo in randomized sequences. Subjects will receive detailed dosing instructions on how many tablets to take from each bottle during dosage titration, maintenance and tapering. They will take the same number of tablets when receiving G-ER and placebo. At this visit subjects will complete all psychometric measures. The capsaicin test and heart rate variability (HRV) will be performed in addition to the other biological measures obtained at baseline.
2.3.5. Treatment phases
Subjects will complete all psychometric and biological measures at Visits 3 and 5. They will receive study medication at Visit 4 for the second arm of the study and compliance will be checked at Visits 3–6. All visits include CST testing, pelvic exam, and use of concomitant and escape medications.
2.4. Primary outcome measures
The primary outcome measure is the Tampon Test, where subjects are instructed to report pain during insertion and removal of a tampon using a numeric rating scale (NRS) (0 = no pain to 10 = worse pain imaginable. Primary and secondary outcome measures are listed in Table 4.
Table 4.
Outcome measures.
| Primary Outcome Measures | |
| Tampon test | Measures pain with tampon insertion and removal on 0–10 NRSa |
| Secondary outcome measures | |
| Coital pain | Pain experienced with sexual intercourse in the last 24 hours on a 10-10 NRSa |
| 24-hours Pain | Average vulvodynia pain over last 24 hours on 0–10 NRSa |
| Psychometric measures | |
| BPI Interference Scaleb | Assesses degree to which pain interferes with physical and emotional functioning and sleep |
| HADSc | Measures dimensional and categorical aspects of anxiety and depression |
| PGICd | Measures improvement of symptoms with treatment |
| FSFIe | Measures sexual function including desire, arousal, lubrication, orgasm, satisfaction and pain subscales |
| Locke-Wallace Marital Adjustment Test | Measures degree of happiness in relationship, common interests and beliefs and conflict management |
| SF-36f | Measures quality of life |
| Leserman Sexual and Physical Abuse Questionnaire | Measures history of physical and sexual abuse |
| Biological measures | |
| Capsaicin skin sensitivity test | Measures central sensitization, a dimension of neuropathic pain |
| Heart rate variability (HRV) | Measures autonomic nervous system dysregulation |
| Vaginal algometer | Measures pressure pain thresholds in pelvic floor muscles |
| Tender point tenderness | Measures pain to pressure when applied to specific areas in the musculoskeletal system |
| Algesiometer | Measures vulvar mechanical hyperalgesia |
| Safety end points | |
| Medical history, physical exam, Pelvic examination | Assess for any findings that may increase risk for adverse events |
| Clinical laboratory testing | Complete blood cell count + differential, comprehensive metabolic panel, urine pregnancy test, urinalysis, Affirm Testg |
| Serum creatine phosphokinase (CK) | If signs or symptoms of rhabomyolysis occur |
| Serious adverse events (SAEs) | Death, life-threatening drug experience, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability/incapacity or congenital anomaly/birth defect |
NRS: Numeric Rating Scale
BPI: Brief Pain Inventory Interference Scale
HADS: Hamilton Anxiety and Depression Rating Scale
PGIC: Patient Global Impression Change Scale
FSFI: Female Sexual Function Index
SF-36: MOS 36-item short-form health survey
Affirm: VPIII microbial identification test for vaginal infection
Subjects will enter daily diaries electronically onto the web-based Scientific Laboratory & Patient-care Research Information Management System (Slim Prim) developed at the Core Center (61,62). They will be provided with standard cardboard applicators to provide uniform measurement (9) (See Appendix B for instructions). Excellent protocol adherence was demonstrated in our previous trials, with 96% compliance to tampon insertion (9).
Daily diaries will capture whether intercourse has occurred, the average pain in the past 24 hours, and whether a tampon has been inserted. Use of the tampon as the primary outcome will obviate any need to “encourage” women to have intercourse to collect sufficient data. The 24-hour pain will measure vulvar pain unrelated to intercourse which is either provoked or unprovoked in order to evaluate other sources of vulvar pain.
2.5. Secondary outcome measures
2.5.1. Coital pain
The severity of coital pain (when intercourse is attempted) and the average vulvodynia pain will be recorded using the 11-point (0 to 10) NRS in the electronic daily diary.
2.5.2. Psychometric measures
Psychometric measures will be used as secondary endpoints to predict treatment response, and include the 1) Brief Pain Inventory (BPI) Interference Scale [63], 2) Hospital Anxiety and Depression Rating Scale (HADS) [64,65], 3) Patient Global Impression Change Scale (PGIC) [66], 4) Female Sexual Function Index (FSFI) [67], 5) Locke-Wallace Marital Adjustment Test [68], 6) MOS 36-item short-form health survey (SF-36) [69], and the Leserman Sexual and Physical Abuse History Questionnaire [70].
The Brief Pain Inventory (BPI) Interference Scale is a 7-item self-report measure, designed to assess the extent to which pain interferes with various components of functioning, including physical and emotional functioning and sleep [63]. The items in this scale can be grouped into those that assess physical functioning (general activity; walking ability; normal work, including both work outside the home and housework), those that assess emotional functioning (mood; relations with people; enjoyment of life), and a single item that assesses the extent to which pain interferes with sleep. It has been used as an outcome measure in clinical trials of diverse treatments, including both pharmacological and psychological treatments.
The Hospital Anxiety and Depression Rating Scale (HADS) is widely used as a brief self-rating instrument for both dimensional and categorical aspects of anxiety and depression and has excellent psychometric properties [64,65]. It is a 14-item, 4-point questionnaire consisting of two subscales, HADS-A and HADS-D. Each subscale contains 7 items with score ranges from 0 to 21, indicating mild to severe impairment.
The Patient Global Impression Change Scale (PGIC) is a 7-point rating scale with the options “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” and “very much worse” [66]. There has been widespread use of the PGIC in recent chronic pain clinical trials and the measure provides a responsive and readily interpretable assessment of subjects’ evaluations of the importance of their improvement or worsening.
The Female Sexual Function Index (FSFI) is a 19-item, multidimensional, self-report measure comprised of a full scale and six subscales (i.e. Desire, Arousal, Lubrication, Orgasm, Satisfaction, and Pain) to measure sexual function [67]. The Desire, Arousal, Lubrication, Orgasm, and Pain subscales correspond to the five domains considered relevant for the sexual dysfunction disorders (hypoactive sexual desire disorder, sexual arousal disorder, sexual pain disorders, and orgasmic disorder). The sex subscale, Sexual Satisfaction, was included because it is considered one of the most important dimensions of sexual function. The measure was designed for use in clinical trials by the author and colleagues.
The Locke-Wallace Marital Adjustment Test. The Short form (15-item) was developed from the 35-item long form and has demonstrated excellent reliability and validity compared to the longer form [68]. It measures overall degree of happiness in the relationship, common interests and beliefs, and conflict management. A total score of ≤ 100 indicates a poor relationship.
The MOS 36-item short-form health survey (SF-36) produces eight scale scores for eight domains of health status: physical functioning, role functioning difficulties caused by physical problems, bodily pain, general health, vitality, social functioning, role functioning difficulties caused by emotional problems and mental health [69]. Scale scores range from 0 to 100, with higher scores indicating better functioning.
The Leserman Sexual and Physical Abuse History Questionnaire is an 8-item questionnaire that has been demonstrated to have acceptable levels of reliability and validity for sexual abuse, and to a lesser extent, physical abuse when compared to an interview [70]. Scale scores range from 0 to 18, with higher scores indicating a greater history of abuse.
The subject’s expectation of benefit from the study will be determined by the study coordinator asking the following question at baseline, “Now that you know about the study, how likely do you feel that you that you might get relief?,” using a 10-point VAS scale from 0 = no relief to 10 = complete relief. This question was modified from a question used by others and is designed to minimize placebo response due to a subject’s expected pain level when enrolled in a study [71].
2.5.3. Biological measures
2.5.3.1. Capsaicin skin sensitivity test
Enhanced cutaneous response to capsaicin has been used as a measure of central sensitization, a dimension of neuropathic pain [15,72–76]. We demonstrated that post-capsaicin injection (intradermal 10 μl of 0.1%, 10 μg) in both the foot and forearm increased punctuate hyperalgesia and dynamic allodynia in PVD cases compared to age-matched asymptomatic controls indicating the presence of central sensitization in PVD [15]. We will use our previous technique, using forearm injection only. Capsaicin will be manufactured by the Core Center.
2.5.3.2. Autonomic dysregulation measurement
Blood Pressure (BP), pulse and heart rate variability (HRV) [77] will be performed prior to and during the intradermal capsaicin trial described above. Assessments will be performed 5 minutes prior to capsaicin injection and 1 minute, 5 minutes, 10 minutes, 20 minutes and 30 minutes after capsaicin injection, A Dynamapp physiologic monitor will measure BP and pulse rates and a pulse oximeter will read the “heart signal”, a more convenient measure than the ECG. Heart rate will be captured from the pulse oximeter signal and analyzed with a program developed by National Instruments using LabVIEW for Heart Rate Variability Analysis.
2.5.3.3. Muscular tenderness measurement
We will assess central muscular tenderness using the tender point (TP) examination as described by Okifuji et al [58]. Selective pelvic muscle tonometry (Vaginal Algometer) will be used to measure peripheral pain thresholds. We will apply standard digital pressure as evoked by the digital tonometer to the pelvic floor muscles using a modified technique of Tu et al [59]. The examiner’s gloved finger will have the calibrated digital load cell SLB-25 (Transducer Techniques, Inc.) affixed beneath the examining glove and will digitally press the three selected muscle groups and three pressures according to a randomization schedule.
2.5.3.4. Peripheral (mucocutaneous) pain measurement
We will use two techniques to measure mucocutaneous pain. The clinically used and standardized Cotton Swab Test (CST) will be performed to measure vestibular pain [78]. We will also use the Vulvar Algesiometer to measure vulvar mechanical hyperalgesia using our previous technique [9] and detailed by Eva et al. [79]. The Algesiometer consists of a mechanical pulse generator which drives a probe against the mucocutaneous surface of the vulva for a calibrated distance and force. We will use a “method of constant stimuli” with the pain threshold determined as the first of two consecutive reports of stimulus pain during the ascending scale of pulses.
2.5.4. Safety measures
Physical and pelvic exams and clinical lab testing will be performed at baseline and as indicated throughout the study. Blood pressure and pulse will be obtained at all visits. Gabapentin was recently placed on the U.S. Food and Drug Administration (FDA) Watch List for a possible association with rhabomyolysis [80]. Myotoxicity symptoms, as well as all other adverse events, will be assessed during weekly/bi-weekly telephone contacts and through monitoring electronic daily diaries,. Subjects with muscle pain will have a serum creatine phosphokinase (CK) level performed as soon as it is reported as recommended by guidelines [81]. Any FDA-defined Serious Adverse Event will be reported to the data safety monitoring board (DSMB), the NICHD, the IRB at each institution, the FDA, and the pharmaceutical sponsor.
2.6. Statistical analyses
2.6.1. Primary outcome
Study outcomes will be based on intent-to-treat (ITT), last observation carried forward (LOCF)[82]. SAS 9.3 (SAS Institute Inc., Cary, NC) will be used for analysis. For the primary outcome, the mean of the tampon test measured at baseline and during the placebo and the G-ER cycles will be compared by use of repeated measures analysis of variance, or more generally mixed models. A P value of less than 5% will be regarded as significant. To confirm that treatment response is not dependent on any of the demographic factors (including age, hormonal status [pre or post-menopausal, menstrual cycle phase, oral contraceptive or vaginal estrogen use, or changes in maturation index] or PVD subtype) or center effect, the change in the tampon test measure will be compared using ANOVA (or Kruskal-Wallis test if sample size is too small) or correlation as appropriate. This procedure will be repeated for the secondary outcome measures of intercourse pain and overall 24-hour pain. If the preliminary analyses demonstrate a significant effect on the test center or on any of the demographic variables, regression analyses will include these variables. Systematic error based on test center (center effect) will be examined by comparing the demographic factors and physiologic measures across the three centers: UTHSC, UMDNJ, and URMC.
2.6.2. Secondary outcome
For the secondary outcomes such as psychometric tests, physiologic tests and psychometric measures, G-ER response to treatment and placebo will be compared using two-sample t-test. When other variables are also included, multiple regression will be used to find their independent effect on G-ER response. All analyses will be done using two-sided tests.
2.6.3. Safety endpoints
The proportion of participants experiencing specific side effects will be statistically compared between the treatment arms. Serious adverse events (SAE)s will be subdivided and reported according to the following categories: “unrelated”, “unlikely related”, “possibly related”, “probably related” and “definitely related” to treatment.
2.7. Data management and master database
Data will be entered at each test center onto Slim-Prim, a web-accessible, modular database system mounted on an Oracle server located at the Data Coordinating Center at UTHSC [61,62]. By this means Slim-Prim will act as a central data repository, with PI controlled levels of permission to ensure secure, collaborative access to data. Electronic Protected Health Information (ePHI) within Slim-Prim are encrypted to federal standards as defined in the 2009 HITECH Act.
2.8. Data safety monitoring board
A data safety monitoring board (DSMB) was commenced to provide oversight and monitor the safety of participants and the validity and integrity of the trial and to meet the NIH requirements for conducting multi-site clinical trials. The DSMB will meet every 6 months for the duration of the trial. Safety monitoring reports will be generated periodically for the DSMB and any serious adverse events will be reported immediately to the DSMB and all institutional IRBs, the FDA, the NIH, and Depomed, Inc. for review.
No stopping rules are planned for efficacy or futility in order to reduce the possibility of alpha and beta errors and because there are escape pain medications in the protocol. No stopping rules are planned for safety because the safety profile of gabapentin is well-known. The DSMB will be blinded to treatment group, but the statistician will unmask data in a closed session upon the request of DSMB members.
2.9. Protection of human participants
The study was approved by the Institutional Review Boards (IRB) at all study sites. All Serious Adverse Events (SAEs) are reported to the IRBs, DSMB, FDA, and the pharmaceutical sponsor. Participation in the study will be terminated if the participant encounters any of the following stopping points: 1) an adverse event attributed to study drug that requires hospitalization, 2) intolerable side effects despite decreasing the dose to a minimum and the participant decides to withdraw, 3) development of severe myalgia with or without a CK evaluation and in whom other etiologies have been ruled out, 4) emergence or worsening of depression or development of suicidal intent, 5) pregnancy, 6) the participant is unwilling or unable to continue with study procedures, 7) hypersensitivity reaction to G-ER or to its inactive ingredients or 8) recommendation by the DSMB.
2.10. Estimation of power and sample size
We will not stratify the subjects according to ethnic, racial, and age groups prior to randomization but will conduct a post-hoc analysis to confirm that treatment response is not dependent on these variables. We will recruit sufficient subjects to detect a mean difference of at least 1 on the 11-point scale in the tampon test measure during G-ER and placebo cycles. In order to simplify the sample size calculation, we assume: (1) no carry-over effect, (2) no interactions between subjects, treatments, and periods, (3) no period effect, (4) no center effect, (5) standard deviation for our primary outcome measure during the gabapentin and placebo cycles is 2.2 (the SD was determined by a controlled study in neuropathic patients [23] since there are no data available in patients with vulvodynia), (6) the correlation between G-ER and placebo tampon test measure is 0.5, (7) significance level is 5%, and (8) power is 90%. Then the sample size required for the first aim is 53. Secondary measures (intercourse pain and 24-hour pain) are not considered for sample size requirement because they are not assessed at the same experimental significance level. Our preliminary data showed mean change in algesiometer ratings with and without G-ER was 9.8 ± 8.7 and 1.1 ± 11.1, respectively [9]. Since algesiometer ratings are one of our physiological measures; for the second aim, we estimate we will need 30 subjects per group. Hence we will recruit 120 patients to complete 60, assuming approximately 30% of subjects are ineligible and 30% dropout. Since the number needed to treat (NNT) in subjects with chronic neuropathic pain receiving gabapentin compared to placebo is 6.8 (5.6 to 8.7) for substantial benefit (50% score change)[83], the sample size of 60 is adequate to detect a clinically meaningful difference between the treatment arms.
3. Discussion
Vulvodynia is a condition of vulvar discomfort that affects millions of women each year [1–4]. These women and their providers are challenged to find effective therapeutic interventions. Given the high placebo response rate, multicenter randomized placebo-controlled trials are needed using standardized outcomes. We will conduct the first multicenter RCT to determine the efficacy of gabapentin, an agent which is often used by clinicians without empiric evidence of its therapeutic benefit. We also hope to define which factors predict the level of treatment success and to explore their relative contribution to the prediction of pain reduction.
3.1. Issues that may constitute potential limitations
3.1.1. Issue 1: Placebo Effect
The placebo effect in neuropathic pain studies, described as an absolute effect of > 50% reduction in pain score compared to placebo, ranges from 15 – 43%, with a median of 22.5% [32] increases the likelihood of a negative trial. To minimize the placebo effect, we will use a script for follow-up calls to reduce the positive impact of patient interactions on therapeutic response (see Appendix A) and we will ask subjects a question about expected pain level, which may contribute to a placebo effect [71]. We will also conduct assessments at the conclusion of the trial and ask subjects and investigators to guess the subjects’ treatment group and the primary reason in an effort to reduce unblinding due to side effects. We elected not to include a wait list arm or a single blind run in prior to enrollment because the extended period may contribute to a placebo response due to natural history and spontaneous resolution, since annual remission rates have been reported to occur in 11% of women with vulvodynia [84]. We also elected not to use an active placebo because we believe it may increase the rate of adverse events and withdrawals and because we do not want to expose subjects to a medication that confers no benefit.
3.1.2. Issue #2: Number and Selection of Psychometric Instruments
There may be concern that the use of multiple pain measurements may result in false positives or that the frequent pain ratings in daily diaries may influence average pain scores. However, the number and selection of psychometric instruments are based on the recommendations of IMMPACT, an expert group which convened to establish guidelines in the conduction of chronic pain clinical trials. The expert panel suggested use of 6 core domains to fully assess pain and to use daily diaries as the primary efficacy endpoint for chronic pain clinical trials [87]. Recommendations did not include measurement of cognitive patterns of helplessness, rumination and magnification, coping mechanisms or ability to control symptoms which are captured in instruments such as the Pain Catastrophizing Scale [85], the Coping Strategy Questionnaire [86] or the Painful Intercourse self-efficacy scale [39]. Although these measures are important, have been used in PVD studies and changes in cognitive behaviors could contribute to a “placebo” response, we chose to prioritize measures of relationship and sexual function because of their significance in assessing treatment success in this population, yet at the same time minimizing subject burden. Completion of the currently used questionnaires take about 30 minutes for subjects to complete, and adding any more measures could conceivably affect dropout rate.
3.1.3. Issue 3: Limitations of Carryover Designs
Crossover designs have attractive features because subjects serve as their own control, variance is minimized and substantially fewer subjects are required to demonstrate a given treatment effect compared with parallel groups design [88]. In addition, patient recruitment can be easier for crossover trials since patients know that half of the time they will receive active drug. Moreover, crossover designs may be associated with reduced placebo group improvement [47,50]. However, carryover effects may also occur in crossover designs. These effects have been minimized in our trial by measuring efficacy during the final treatment week and statistically testing for carryover effects. It might be argued that it would be useful to evaluate gabapentin plasma concentrations to rule out carry over effects as well as to measure compliance. However, we elected not to measure levels as it would increase subject burden and study costs, and because a concentration-response effect has not been shown with seizure disorders [89] and the short half-life and marked interdosage fluctuations would make it difficult to use as a compliance measure.
Despite the use of a crossover design to reduce the necessary sample size, there have been no prospective studies evaluating gabapentin in women with PVD, so our sample size was determined from patients with neuropathic pain. Thus, it is possible that our estimation is inaccurate in this population; however, most clinical trials have shown similar variability regardless of the type of neuropathic pain or specific treatment [26,47].
Finally, crossover designs are typically longer in duration than parallel group designs. We decided to use a 4-week titration phase and a 2-week maintenance phase to minimize the dropout rate due to trial length and intolerable side effects. It is possible that the duration of the maintenance phase may be insufficient to adequately measure a treatment response. However, this length is consistent with most well-designed clinical trials in chronic pain [26,47]. We felt that 4-weeks of titration was important because we are escalating to the MTD (3000 mg/d) and current guidelines for G-ER in post herpetic neuralgia is 1800 mg/d over a 2-week period [53]. The long titration phase also is in line with expert guidelines for prescribing gabapentin in vulvodynia [10]. It has also been shown that longer clinical trials have been associated with a greater likelihood of a differential discontinuation rate occurring in active treatment and placebo groups, potentially complicating interpretation of results [47,49].
3.1.4. Issue 4: Validity and Reliability
External validity may be compromised because we will exclude women with mild vulvar pain and those with untreated medical or psychiatric conditions. However, internal validity may be affected because we have included women of all age groups receiving concomitant therapy. There are often challenging trade-offs between research design considerations and generalizability of results in clinical trials [90], but we believe that maintaining a balance between internal and external validity will maximize our ability to determine which particular populations benefit from gabapentin treatment and at the same time maintain adequate enrollment.
Nevertheless, we acknowledge that estrogen may have an effect on vestibular sensitivity [45], and it is possible that phase of the menstrual cycle [42,43,91], use of vaginal estrogen therapy [92], or oral contraceptives [44,91] could confound the results. We will control for all potential confounding factors, including hormonal and concomitant therapies by using a regression model if preliminary analyses demonstrate a hormonal effect.
There may be a concern with poor interrater reliability because multiple sites will be used. However, this possibility is minimized by use of a crossover design which allows for fewer sites to obtain an adequate sample size. In addition, all questionnaires are standardized and investigators were trained in performing physiological procedures during three site visits. We will also use a random effects model to determine inter-rater reliability.
3.2. Interpretation of findings
It is possible that we may not be able to reject the null hypotheses in our primary aims. If the main outcome (pain from tampon insertion) fails to demonstrate statistical significance, secondary outcome variables (intercourse pain and 24-hour pain) will be analyzed for significance with appropriate Bonferroni correction. If these variables fail to show statistical significance then additional exploratory subgroup analyses will be performed primarily as a guide for future research directions. Because gabapentin is the second most commonly used oral agent for vulvodynia on an empiric basis, the ultimate finding of a negative result for gabapentin will still provide an important clinical finding. Further, if the Null Hypothesis is not rejected, we will still be able to perform analyses of baseline data to try to identify clusters of patients based on profiles of signs, symptoms, demographics or other clinical features that might relate to pathophysiologic mechanisms and therefore could be predicted to respond differently to different treatments.
It is also possible gabapentin may fail to produce a beneficial effect on vulvodynia and that the selected physiologic measures may fail to reflect a beneficial effect if one indeed occurs. Further analysis of possible Type 2 error will be performed as well as additional exploratory analyses. Four possible outcomes may be found: 1) both physiologic measures may change and the clinical response may improve, 2) physiologic measures may change without improvement in clinical response, 3) clinical response may change without changes in physiologic measures, and 4) neither clinical response nor physiologic measures may improve or change.
4. Conclusion
Gabapentin is used in clinical practice to treat women with PVD. This double-blind, placebo-controlled, randomized controlled trial will provide clinicians with scientific evidence of gabapentin efficacy. It will also provide insight into the possible subtypes of PVD by assessing response to various psychophysiological measures.
Supplementary Material
Acknowledgments
Support: This work is supported by grant number 1R01HD065740-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Drs. Brown, Bachmann and Foster. Support was also provided by the University of Tennessee General Clinical Research Center (GCRC) and by Depomed, Inc. who provided gabapentin extended release and matching placebo for the study. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NICHD, the National Institutes of Health, GCRC or Depomed, Inc.
Footnotes
Financial disclosures: There are no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164:1609–14. doi: 10.1016/0002-9378(91)91444-2. [DOI] [PubMed] [Google Scholar]
- 2.Moyal-Barraco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: A historical perspective. J Reprod Med. 2004;49:772–777. [PubMed] [Google Scholar]
- 3.Arnold LD, Bachmann GA, Rosen R, Rhoads GG. Assessment of vulvodynia symptoms in a sample of US women: A prevalence survey with a nested case control study. Am J Obstet Gynecol. 2007;196:128, e1–128.e6. doi: 10.1016/j.ajog.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidity and quality of life. Obstet Gynecol. 2006;107:617–24. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponte M, Klemperer E, Sahay A, Chren MM. Effects of vulvodynia on quality of life. J Am Acad Dermatol. 2009;60:70–6. doi: 10.1016/j.jaad.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: Have we underestimated the prevalence of vulvodynia? J Amer Med Wom Assoc. 2003;58:82–88. [PubMed] [Google Scholar]
- 7.Sadownik LA. Clinical profile of vulvodynia patients: a prospective study of 300 patients. J Reprod Med. 2000;45:679–84. [PubMed] [Google Scholar]
- 8.Brown CS, Wan J, Bachmann G, Rosen R. Self-management, amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women’s Health. 2009;18:1–7. doi: 10.1089/jwh.2007.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster DC, Kotok MB, Huang L-S, Watts A, Oakes A, Howard FM, et al. Oral desipramine and Topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116(3):583–93. doi: 10.1097/AOG.0b013e3181e9e0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann E, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51. doi: 10.1097/00128360-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 11.McKay M. Dysesthetic “essential” vulvodynia: Treatment with amitriptyline. J Reprod Med. 1993;38:9–12. [PubMed] [Google Scholar]
- 12.Munday PE. Response to treatment in dysaesthetic vulvodynia. J Obstet Gynaecol. 2001;6:610–3. doi: 10.1080/01443610120085591. [DOI] [PubMed] [Google Scholar]
- 13.Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynecol. 1999;39(1):79–83. doi: 10.1111/j.1479-828x.1999.tb03450.x. [DOI] [PubMed] [Google Scholar]
- 14.Reed BD, Caron AM, Gorenflo DW, Gorenflo DW, Haefner HK. Treatment of vulvodynia with tricyclic antidepressants: Efficacy and associated factors. J Lower Genital Tract Dis. 2006;10:245–251. doi: 10.1097/01.lgt.0000225899.75207.0a. [DOI] [PubMed] [Google Scholar]
- 15.Foster DC, Dworkin RH, Wood RW. Effects of intradermal foot and forearm capsaicin injections in normal and vulvodynia-afflicted women. Pain. 2005;117:128–136. doi: 10.1016/j.pain.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Granot M, Friedman M, Yaranitsky D, Tamir A, Zimmer EZ. Primary and secondary vulvar vestibulitis syndrome: Systemic pain perception and psychophysical characteristics. Am J Obstet Gynecol. 2004;191:138–42. doi: 10.1016/j.ajog.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 17.Granot M, Zimmer EZ, Friedman M, Lowenstein L, Yarnitsky D. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J Pain. 2004;5:226–232. doi: 10.1016/j.jpain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CP, Gee NS, Su TZ, Kocsiis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 19.Zolnoun D, Hartman K, Lamvu L, As-Sante S, Maixner W, Steege J. A conceptual model for the pathophysiology of vulvar vestibulitis syndrome. Obstet Gynecol Survey. 2006;61:395–401. doi: 10.1097/01.ogx.0000219814.40759.38. [DOI] [PubMed] [Google Scholar]
- 20.Arnold LM, Goldenberg DL, Standford SB, Lalonde JK, Sandhu HS, Keck PE, et al. Gabapentin in the treatment of fibromyalgia: A randomized, double-blind, placebo-controlled, multicenter trial. Arthritis & Rheumatism. 2007;56:1336–1344. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 21.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of post herpetic neuralgia: A randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 22.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 23.Gilron I, Bailey JM, Tu D, Dongsheng T, Holden RR, Weaver DF, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Eng J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 24.Sandercock D, Cramer M, Biton V, Cowles VE. A gastroretentive gabapentin formulation for the treatment of painful diabetic peripheral neuropathy: Efficacy, tolerability in a double-blind, randomized, controlled clinical trial. Diabetes Res Clin Pract. 2012;97:438–45. doi: 10.1016/j.diabres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Wallace MS, Irving G, Cowles VE. Gabapentin extended-release tablets for the treatment of patients with post herpetic neuralgia: a randomized, double-blind, placebo-controlled, multicenter study. Clin Drug Investig. 2010;30(11):765–76. doi: 10.2165/11539520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. For the EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 28.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. For the Canadian Pain Society. Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg. 1999;89:1459–60. doi: 10.1097/00000539-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia, unprovoked. Reprod Med. 2007;52(2):103–6. [PubMed] [Google Scholar]
- 31.Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet Gynecol. 2008;112(3):579–85. doi: 10.1097/AOG.0b013e3181827c77. [DOI] [PubMed] [Google Scholar]
- 32.Andrews JC. Vulvodynia interventions: Systematic review and evidence grading. Obstet Gynecol Surv. 2011;66:299–315. doi: 10.1097/OGX.0b013e3182277fb7. [DOI] [PubMed] [Google Scholar]
- 33.Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80:638–44. [PubMed] [Google Scholar]
- 34.Pukall CF, Binik YM, Kalif S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96:263–75. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 35.Pukall CF, Strigo IA, Binik YM, Amsel R, Khalif S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118–27. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Genitilcore-Saulnoier E, McLean L, Goldfinger C, Pukall CF, Chamberlain S. Pelvic floor muscle assessment outcomes in women with and with provoked vestibulodynia and the impact of physical therapy. J Sex Med. 2010;7:1003–1022. doi: 10.1111/j.1743-6109.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 37.White G, Jantos M, Glazer H. Establishing the diagnosis of vulvar vestibulitis. J Reprod Med. 1997;42:157–60. [PubMed] [Google Scholar]
- 38.Granot M, Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther. 2005;31:285–302. doi: 10.1080/00926230590950208. [DOI] [PubMed] [Google Scholar]
- 39.Deroschers G, Bergeron S, Khalife S, Dupuis MJ, Jodoin M. Provoked vestibulodynia: Psychological predictors of topical and cognitive-behavioral treatment outcome. Behav Res Ther. 2010;48:106–115. doi: 10.1016/j.brat.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstet Gynecol. 2011;117:1307–13. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: a prospective study. Am J Obstet Gynecol. 2010;202:614–21. doi: 10.1016/j.ajog.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain. 2013;154:548–59. doi: 10.1016/j.pain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley JL, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–35. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 44.Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156:254–61. doi: 10.1093/aje/kwf037. [DOI] [PubMed] [Google Scholar]
- 45.Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold on the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49:888–92. [PubMed] [Google Scholar]
- 46.Eunice Kennedy Shriver National Insti6tue of Child Health and Human Development (NICHD) Facilitating Scientific Advancement on Vulvodynia through the Development of Research Diagnostic Criteria; May 15–16, 2013; Bethesda, MD. [Google Scholar]
- 47.Dworkin RH, Turk DC, Peirce-Sandner S, Baron F, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. and IMMPACT Consensus Group. Interpreting the clinical importance of treatment outcomes for chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, et al. Considerations for improving assay sensitivity in chronic pain trials: IMMPACT recommendations. Pain. 2012;153:1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Katz J, Finnerup NB, Dworkin RH. Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurol. 2008;70:263–72. doi: 10.1212/01.WNL.0000275528.01263.6c. [DOI] [PubMed] [Google Scholar]
- 51.Friedrich EG., Jr Vulvar vestibulitis syndrome. J Reprod Med. 1987;32:110–4. [PubMed] [Google Scholar]
- 52.Foster DC. Vulvar disease. Obstet Gynecol. 2002;100:145–163. doi: 10.1016/s0029-7844(02)02080-x. [DOI] [PubMed] [Google Scholar]
- 53.Depomed, Inc. Menlo Park, CA 94025. Prescribing Information. Aug, 2012. Gralise® (gabapentin) tablet. [Google Scholar]
- 54.Chen C, Cowles VE, Hou E. Pharmacokinetics of gabapentin in a novel gastric retentive extended-release formulation: Comparison with an immediate-release formulation and effect of dose escalation and food. J Clin Pharmacol. 2011;51:346–358. doi: 10.1177/0091270010368411. [DOI] [PubMed] [Google Scholar]
- 55.Gordi T, Hou E, Screeneeranj K, Berner B. Pharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive extended-release and immediate-release tablets: A randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjects. Clin Therapeutics. 2008;30 (5):909–916. doi: 10.1016/j.clinthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Sweeney M, Sathyanarayana R, Gordi T, Cowles VE, Heritier M. Positive efficacy data from a Phase 2 trial of gabapentin extended-release in the treatment of menopausal hot flashes. 19th Annual Meeting of the North American Menopause Society; Orlando, FL. Sep 2008. [Google Scholar]
- 57.Cowles VE, Gordi T, Hou SY. Steady-state pharmacokinetics of gabapentin after administration of a novel gastroretentive extended-release formulation in postmenopausal women with vasomotor symptoms. Clin Drug Investig. 2012;32(9):593–601. doi: 10.1007/BF03261914. [DOI] [PubMed] [Google Scholar]
- 58.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24(2):377–83. [PubMed] [Google Scholar]
- 59.Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Harden RN. Vaginal pressure-pain thresholds: Initial validation and reliability assessment in healthy women. Clin J Pain. 2008;24:45–50. doi: 10.1097/AJP.0b013e318156db13. [DOI] [PubMed] [Google Scholar]
- 60.Foster DC, Kotok MB, Huang LS, Watts BS, Oakes D, Howard FM, et al. The Tampon Test for vulvodynia research: reliability, construct validity, responsiveness. Obstet Gynecol. 2009;113:825–32. doi: 10.1097/AOG.0b013e31819bda7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viangteeravat T, Brooks IM, Ketcherside WJ, Houmayouni R, Furlotte N, Vuthipadadon S, et al. Biomedical informatics unit (BMIU): Slim-prim system bridges the gap between laboratory discovery and practice. Clin Transl Sci. 2009;2(3):238–41. doi: 10.1111/j.1752-8062.2009.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viangteeravat T, Brooks IM, Vuthipadadon S, Kuscu E, Nagisetty N, Smith E, et al. Slim-Prim: an integrated data system for clinical and translational research. BMC Bioinformatics. 2010;11(Suppl 4):5. [Google Scholar]
- 63.Cleeland C, Mendoza T. The Brief Pain Inventory: meaningful changes in pain interference. Presented at the fourth meeting of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials IMPACT-IV); June 2004; ( www.immpact.org/immpact4/Cleeland.pdf) [Google Scholar]
- 64.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 65.Olsson I, Mykletun A, Dahl AA. The hospital anxiety and depression rating scale: A cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatr. 2005;5:46–52. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guy W. DHEW Publication No ADM 76–338. Washington DC: US. Government Printing Office; 1976. ECDEU assessment manual for psychopharmacology. [Google Scholar]
- 67.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): A Multidimensional Self-Report Instrument for the assessment of female sexual function. J Sex Mar Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 68.Locke HJ, Wallace KM. Short Marital-Adjustment and Prediction Tests: Their reliability and validity. Mar Fam Living. 1959 Aug;:251–255. [Google Scholar]
- 69.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 70.Leserman J, Drossman DA, Zhiming L. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behavioral Med. 1995;21:141–150. doi: 10.1080/08964289.1995.9933752. [DOI] [PubMed] [Google Scholar]
- 71.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 73.Culp WJ, Ochoa J, Cline M, et al. Heat and mechanical hyperalgesia induced by capsaicin. Cross modality threshold modulation in human C nociceptors. Brain. 1989;112:1317–31. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- 74.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–64. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen KL, Fields HL, Brennum J, et al. Capsaicin evoked pain and allodynia in post-herpetic neurolgia. Pain. 2000;88:125–33. doi: 10.1016/S0304-3959(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 76.Sikand P, Shimada SG, Green BG, et al. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–94. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Appelhaus BM, Leucken LJ. Heart rate variability and pain: association of two interrelated homeostatic processes. Biol Psych. 2008;77:174–82. doi: 10.1016/j.biopsycho.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Bergeron S, Binik YM, Khalife S, et al. Vulvar vestibulitis syndrome: Reliability of diagnosis and evaluation of current diagnostic criteria. Obstet Gynecol. 2001;98:45–51. doi: 10.1016/s0029-7844(01)01389-8. [DOI] [PubMed] [Google Scholar]
- 79.Eva LJ, Reid WM, MacLean AB, Morrison GD. Assessment of response to treatment in vulvar vestibulitis syndrome by means of the vulvar algesiometer. American Journal of Obstetrics & Gynecology. 1999;181:99–102. doi: 10.1016/s0002-9378(99)70442-4. [DOI] [PubMed] [Google Scholar]
- 80.Medscape. FDA Puts 16 drugs on Watch List. Apr 17, 2012. [Google Scholar]
- 81.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(suppl):89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 82.Ware JH. Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348:2136–7. doi: 10.1056/NEJMe030054. [DOI] [PubMed] [Google Scholar]
- 83.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011 Mar;16(3) doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: A 2-year follow-up study. Obstet Gynecol. 2008;112:231–7. doi: 10.1097/AOG.0b013e318180965b. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 86.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 87.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 89.Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T. Serum concentrations and effects of gabapentin and vigabatrin: Observations from a dose titration study. Ther Drug Monitoring. 2003;25:457–462. doi: 10.1097/00007691-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 90.Rothwell PM. External validity of randomized controlled trials: “to whom do the results of this trial apply? Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 91.Johannesson U, Sahlm L, Masironi B, Rylander E, Bohm-Starke N. Steroid receptor expression in the vulvar vestibular mucosa – effects of oral contraceptives and menstrual cycle. Contraception. 2007;76:319–25. doi: 10.1016/j.contraception.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 92.Bachmann G, Rosen R. Vulvodynia and menopause. Menopause Management. 2006:14–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


