Abstract
Cerebral amyloid angiopathy (CAA) is pathologically defined as the deposition of amyloid protein, most commonly the amyloid β peptide (Aβ), primarily within the media and adventitia of small and medium-sized arteries of the leptomeninges, cerebral and cerebellar cortex. This deposition likely reflects an imbalance between Aβ production and clearance within the brain and leads to weakening of the overall structure of brain small vessels, predisposing patients tolobar intracerebral haemorrhage (ICH), brain ischaemia and cognitive decline. CAA is associated with markers of small vessel disease, like lobar microbleeds and white matter hyperintensities on magnetic resonance imaging. Therefore, it can be now be diagnosed during life with reasonable accuracy by clinical and neuroimaging criteria. Despite the lack of a specific treatment for this condition, the detection of CAA may help in the management of patients, regarding the prevention of major haemorrhagic complications and genetic counselling. This review discusses recent advances in our understanding of the pathophysiology, detection and management of CAA.
Keywords: Amyloid angiopathy, intracerebral haemorrhage, cognitive decline, stroke, amyloid
Cerebral amyloid angiopathy (CAA) is a disorder of the central nervous system characterised by the deposition of amyloid proteins in the wall of small- to medium-sized vessels, most frequently arteries, within the leptomeninges and cortex of the brain.1 In vessels affected by CAA, local muscle and elastic elements of the arterial wall are lost and replaced by amyloid fibrils, primarily the amyloid-β (Aβ) peptide. Since the first description of neurovascular amyloid deposition in 1909 by Gustav Oppenheim, sound scientific evidence has supported the concept that the associated disruption of the overall structure of those small vessels predisposes to both ischaemic small vessel disease and cerebral haemorrhage.2–4
Sporadic CAA is a major cause of lobar intracerebral haemorrhage (ICH) and cognitive decline in the elderly, including the normotensive population.5,6 Hereditary forms of CAA are generally rare, usually more severe and earlier in onset. Rare non-Aβ familial CAAs can also present clinically with lobar ICH.7 Regarding sporadic CAA, two major challenges persist:
a definitive diagnosis requires a neuropathological exam; and8
no treatment or preventive strategy for CAA or CAA-ICH has been firmly established.
Nevertheless, in the last decades of research, there has been remarkable progress in our understanding of this condition. CAA pathology has been associated with markers of small vessel disease, including lobar cerebral microbleeds (CMB) and white matter hyperintensities on magnetic resonance imaging (MRI).9–11 The availability of MRI sequences that are particularly sensitive to susceptibility effects like the T2* gradient-recalled echo (GRE) and susceptibility weighted imaging (SWI) now allow reliable assessment of an individual’s haemorrhagic burden over time and reasonable accuracy by clinical and neuroimaging diagnostic criteria.10–13 As our understanding of CAA pathophysiology evolves, specific targets have been identified as candidates for the prevention and treatment of this condition.14 As new research tools such as the Pittsburgh Compound B (PiB) or other amyloid-imaging agents for positron emission tomography (PET) scan become incorporated into clinical practice, it may also be possible to detect vascular amyloid deposition in the brain noninvasively in living patients, perhaps before an ICH or significant cognitive decline.15
This article focuses on our current understanding of the pathophysiology of sporadic CAA, the new imaging modalities and laboratory biomarkers that may aid in its detection and the currently available evidence that could guide the management of patients with this condition.
Pathophysiology and Genetics of Sporadic Cerebral Amyloid Angiopathy
Sporadic CAA is the most frequent form of the disease, mostly occurring in the elderly and defined by the accumulation of Aβ in the vessel walls of capillaries, arterioles and small and medium-sized arteries of the cerebral cortex, leptomeninges, and cerebellum. Sporadic CAA is not associated with other systemic, primary or secondary, amyloidosis.
The Aβ peptide is the normal proteolytic product of the integral membrane protein Aβ precursor protein (APP), encoded by the APP gene on chromosome 21.16–19 Aβ occurs as 40 or 42 amino acid species (Aβ40, Aβ42) and its generation from APP requires two enzymatic events: a proteolytic cleavage at the amino terminus of the Aβ sequence by β-secretase; and a cleavage at the carboxyl terminus by γ-secretase.16–19 Via mechanisms that remain still unknown, soluble Aβ forms undergo a change in conformation, resulting in a predominantly β-sheet structure, highly prone to oligomerisation, fibrillisation and deposition. These deposits can trigger a secondary cascade of events that include release of inflammatory components, activation of the complement system, oxidative stress, alteration of the blood–brain barrier (BBB) permeability and cell toxicity.20–22
Aβ in CAA is structurally similar to the peptide that constitutes senile plaques in Alzheimer’s disease (AD). In contrast to Alzheimer plaques, however, a substantial proportion of vascular deposits in CAA is of the shorter Aβ40 species.20 Increases in the Aβ40/42 ratio appears to favour vascular over parenchymal amyloid deposition.22 The cascade of events that promotes cerebrovascular Aβ accumulation is still not fully understood but an imbalance between Aβ production and clearance is likely to play a key role.22 Deposited Aβ appears to derive from neurons although circulating Aβ may play a seeding function for brain deposition, since intraperitoneal inoculation with Aβ-rich extracts can induce CAA in transgenic mice over expressing APP.22–25 Recent data from animal models indicate that ischaemic strokes may trigger accelerated amyloid build-up, most likely through interference with clearance pathways.26
The mechanism of blood vessel rupture in CAA is still under debate. Amyloid deposition has been associated with reduced vascular compliance, cracking and weakening of the vessel wall that predispose to vascular rupture and subsequent extravasation of blood to brain parenchyma.27,28 The reason why some vessel ruptures lead to major lobar haemorrhage in CAA patients while others lead to microhaemorrhage may be related to differences in thickness of small vessel walls. Apparently, CAA patients with thicker vessel walls have more CMB and those with thinner walls are more prone to develop symptomatic lobar ICH.29
Pathology of Cerebral Amyloid Angiopathy
Pathological studies of CAA show that Aβ vascular deposits infiltrate the media and adventitia of the microvasculature, resulting in loss of smooth muscle cells with replacement of the vascular media and acellular thickening of the vessel wall.30,31 Advanced CAA is characterised by severe disruption of the vascular architecture, that includes the distinctive ‘vessel-within-vessel’ appearance, microaneurysm formation, fibrinoid necrosis, hyaline degeneration, obliterative intimal changes and perivascular leakage of blood products.30–32
CAA distribution is characteristically patchy and segmental, involving predominantly the lobar areas.8 For unknown reasons, CAA is most frequent in the occipital lobes. This contrasts with hypertensive arteriopathy, which is characterised by lipohyalinosis and fibrinoid necrosis of small deep perforating arteries and therefore typically affects deep brain structures including the basal ganglia, brain stem and thalamus.33 Nevertheless, since over 30 % of patients with CAA-related ICH have documented arterial hypertension (HTN), some patients exhibit both hypertensive and CAA microvascular alterations.3
CAA pathology is common in the elderly, with a prevalence from 10–50 % of the general elderly population, in autopsy series.34,35 Some CAA pathology is also present in nearly all brains with AD and advanced CAA is present in approximately 25 % of AD brains.35,36 However, fewer than 50 % of CAA cases meet the pathological criteria for AD.3
Genetics of Sporadic Cerebral Amyloid Angiopathy
The apolipoprotein E (APOE) ε4 and ε2 alleles are genetic risk factors associated with risk of developing sporadic CAA-related ICH.37,38 APOE ε2 exerts a protective effect on AD risk but increases risk of ICH in CAA patients.39,40 The ε2 allele is predominantly associated with vasculopathic changes that predispose to rupture of the diseased vessels, whereas ε4 is related to the severity of amyloid deposition within the vessel wall.41,42 Interestingly, recent studies have found that possession of APOE ε2 predisposes individuals with lobar ICH to haematoma expansion.43,44 This effect was more pronounced in patients with amyloid angiopathy-related ICH, consistent with the ε2 allele’s role in vascular amyloid deposition and vessel fragility. More recently, the CR1 gene has also been linked to risk of CAA-related ICH, recurrent CAA and CAA pathology burden.45
Detection of Cerebral Amyloid Angiopathy
Approximately 50 % of patients over 80 years of age display some pathological evidence of CAA, mostly without clinical symptoms. Among individuals with more advanced CAA, the clinical presentation can vary from incidental asymptomatic microbleeds on MRI to catastrophic lobar ICH. Dementia, cognitive impairment and transient neurological symptoms or signs are also increasingly recognised.46
Primary lobar ICH is the most common clinical presentation leading to the diagnosis of CAA. HTN and CAA are responsible for most primary ICHs in the elderly. The clinical manifestations of CAA-related ICH are similar to other types of ICH. The signs and symptoms depend on the size and location of the bleed and may include headache, focal neurological deficits, seizures and altered level of consciousness.45
In a retrospective analysis of consecutive patients with non-traumatic SAH, CAA was found to be a common cause of SAH in the elderly and clinically presents with single or recurrent focal transient neurological events of short duration.47 CAA has also been recognised as a probable cause of ischaemic small vessel disease.48,49 Indeed, amyloid deposits can narrow the vessel lumen, impair cerebral blood flow regulation, cause alterations in endothelial structure and function and influence vessel dilation in response to physiologic stimuli.50–52 Progression of CAA ischaemic burden may lead to cognitive decline and ultimately to vascular dementia.53
A subset of patients with CAA-related inflammation may present with subacute cognitive decline, seizures and diffuse radiographic white matter abnormalities attributed to vasogenic oedema.54,55 The APOE ε4/ε4 genotype has been associated with CAA-related inflammation and evidence of CAA-related vasculitis or perivascular inflammation on pathologic examination.56
Histological Diagnosis
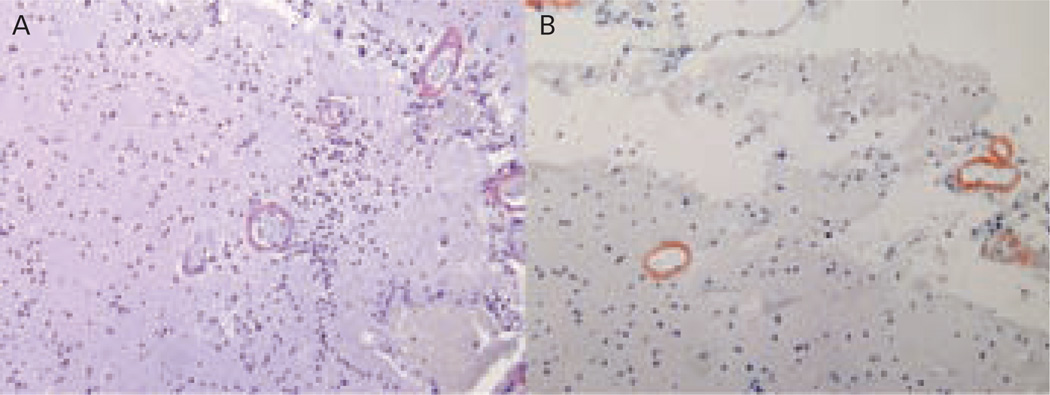
Definitive diagnosis of CAA requires histopathological exam. Congo red staining for amyloid under light microscopy (see Figure 1A) has been the classical method for amyloid staining, although its sensitivity is relatively low.57 Immunohistochemistry with fluorescent antibodies specific for Aβ (see Figure 1B) is increasingly used to identify amyloid accumulation in the brain.
Figure 1.
Histopathological Sections from a Patient with Definite Cerebral Amyloid Angiopathy Confirmed by Congo Red Stain (A) and Immunostaining (B), Demonstrating Vascular Amyloid Deposition
Neuroimaging
MRI sequences that are particularly sensitive to susceptibility effects like GRE and SWI can identify not only major bleeding in the brain but also CMBs, which may not be visible on other imaging modalities (see Figure 2).12,58 CMBs have hypointense signal on GRE sequences due to haemosiderin, a blood breakdown product that causes magnetic susceptibility-induced dephasing, leading to T2* signal loss. The appearance of microbleeds on GRE sequences is larger than the actual tissue lesions because of the so-called blooming effect of the magnetic resonance signal at the border of these lesions.12,57,58 Novel techniques such as SWI have considerably increased microbleed detection rates.59–61
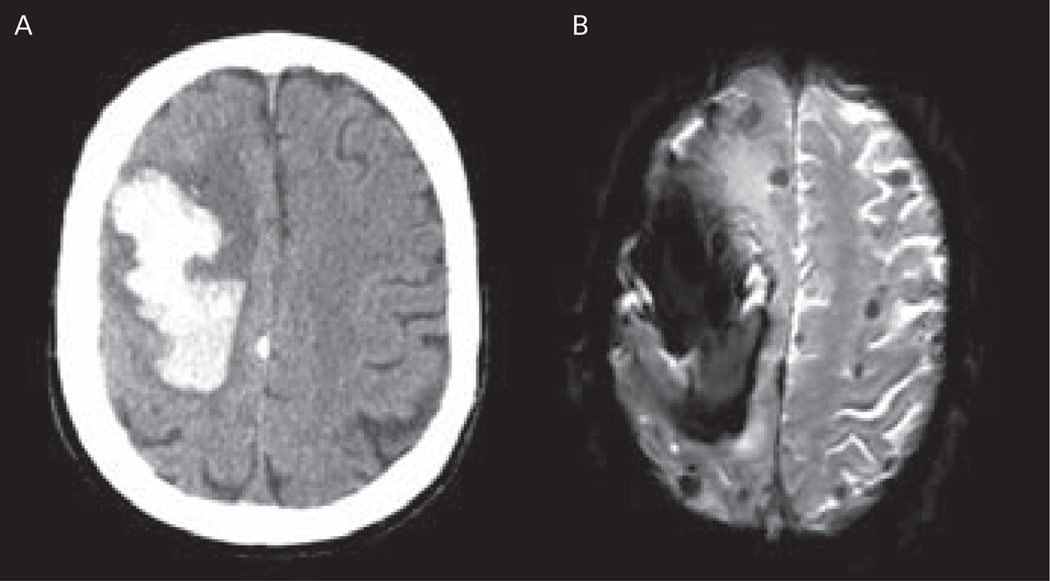
Figure 2.
Neuroimaging of a Patient with Probable Cerebral Amyloid Angiopathy-related Intracerebral Haemorrhage, According to the Boston Criteria
A: Computed tomography (CT) scan showing large right frontal intracerebral haemorrhage (ICH); B: magnetic resonance imaging (MRI) gradient recalled echo (GRE) sequence showing the large frontal lobar haematoma and several additional lobar microbleeds.
The ability of MRI to detect CMBs has greatly aided the non-invasive diagnosis of CAA during life. As haemosiderin remains in macrophages for many years after haemorrhage, MRI sequences that are particularly sensitive to susceptibility effects allow for reliable assessment of an individual’s haemorrhagic burden over time. Haemorrhage burden identified by MRI predicts clinically important events in survivors of lobar ICH.62 Detection of microhaemorrhages is therefore useful for assessing risk of functional deterioration in CAA patients as well as for risk of future ICH.62 It is noteworthy that CAA-related CMB, like CAA pathology and CAA-related-ICH, tend to cluster in posterior brain regions.60
Non-traumatic subarachnoid haemorrhage (SAH) and superficial siderosis are also common in patients with CAA.63,64 Superficial siderosis may be found at sites distant from ICH and in close vicinity to lobar CMBs, suggesting that SAH can also occur as a primary manifestation of CAA.65–67
Boston Criteria
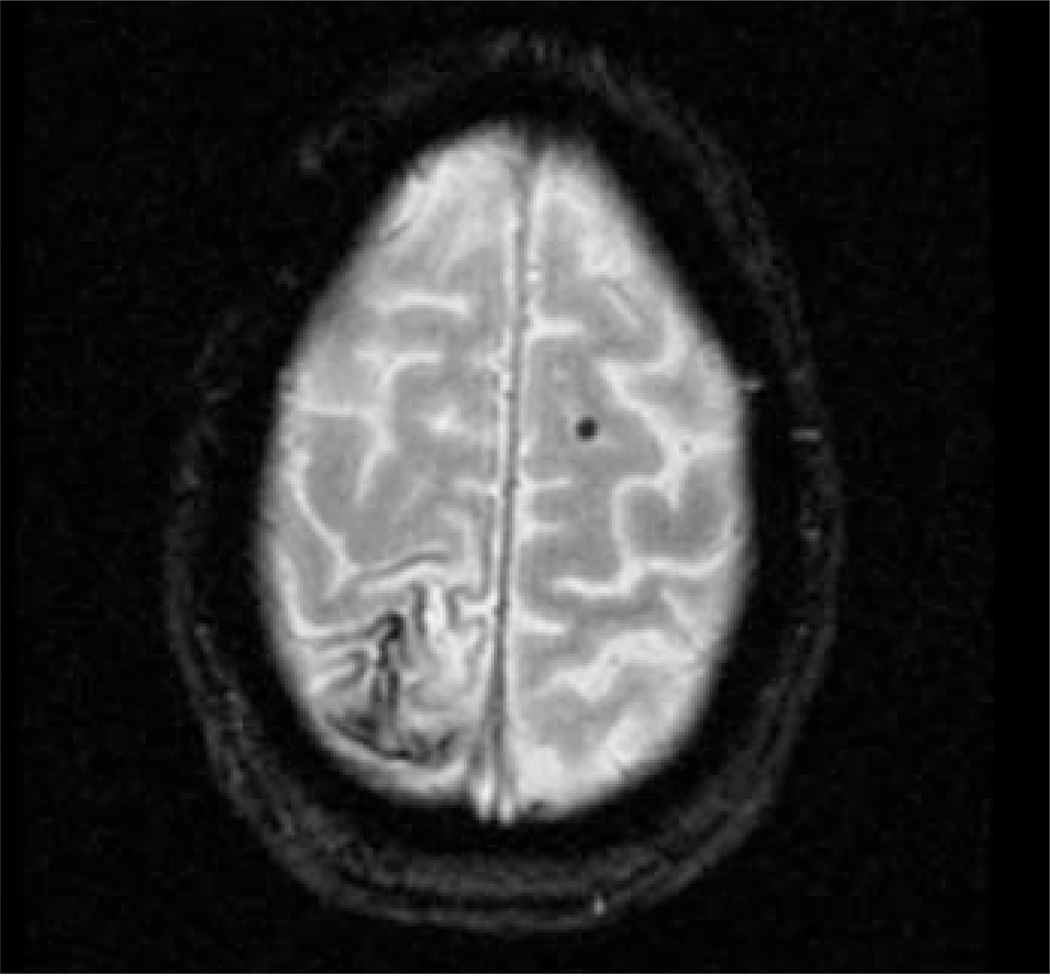
Using a combination of clinical, neuroimaging and pathological data, the Boston criteria establish three levels of certainty for the diagnosis of CAA: definite, probable and possible. These criteria were based on the tendency for CAA-related haemorrhages to occur in elderly patients, to be multiple and primarily located in lobar brain regions.13 As fully described in Table 1, the diagnosis of probable or possible CAA can be reached by clinical and neuroimaging findings alone without requiring pathological confirmation.13,68 The modified Boston criteria include superficial siderosis as one of the required lobar haemorrhagic lesion, which has been reported to improve the sensitivity of the diagnosis without lowering its specificity (see Figure 3).64
Table 1.
Boston Criteria for Cerebral Amyloid Angiopathy Diagnosis
| 1 Definite Cerebral Amyloid Angiopathy (CAA) |
Full post-mortem examination demonstrating:
|
| 2 Probable CAA with Supporting Pathology |
Clinical data and pathological tissue (evacuated haematoma or cortical biopsy) demonstrating:
|
| 3 Probable CAA |
Clinical data and MRI or CT demonstrating:
|
| 4 Possible CAA |
Clinical data and MRI or CT demonstrating:
|
Other causes of intracerebral haemorrhage include: excessive warfarin dosing (international normalised ratio [INR] >3.0), antecedent head trauma or ischaemic stroke, central nervous system (CNS) tumour, vascular malformation, CNS vasculitis, blood dyscrasia, coagulopathy.
CT = computed tomography; MRI = magnetic resonance imaging.
Figure 3.
Patient with Probable Cerebral Amyloid Angiopathy According to the Modified Boston Criteria
Gradient recalled echo (GRE) showing cortical siderosis on the right parietal lobe and a lobar microbleed on the left frontal lobe.
Positron Emission Tomography Imaging
PET imaging with Pittsburgh compound B (PiB) has been used in research to measure the burden and location of brain fibrillar Aβ deposits in animal models and in humans with AD or CAA.15,69–72 Global PiB retention is elevated in non-demented CAA subjects relative to healthy control subjects (see Figure 4), although lower in CAA than in AD subjects.3 Importantly, the occipital-to-global PiB ratio was found to be significantly greater in CAA than in patients with AD.15 Increased occipital PiB retention was further demonstrated in a young subject with early Iowa-type hereditary CAA, a rare hereditary form of CAA in which the fibrillar amyloid deposits appear to be entirely vascular.73 Taken together, these results suggest that PiB-PET can noninvasively detect CAA, possibly prior to overt signs of tissue damage such as haemorrhage or white matter lesions.
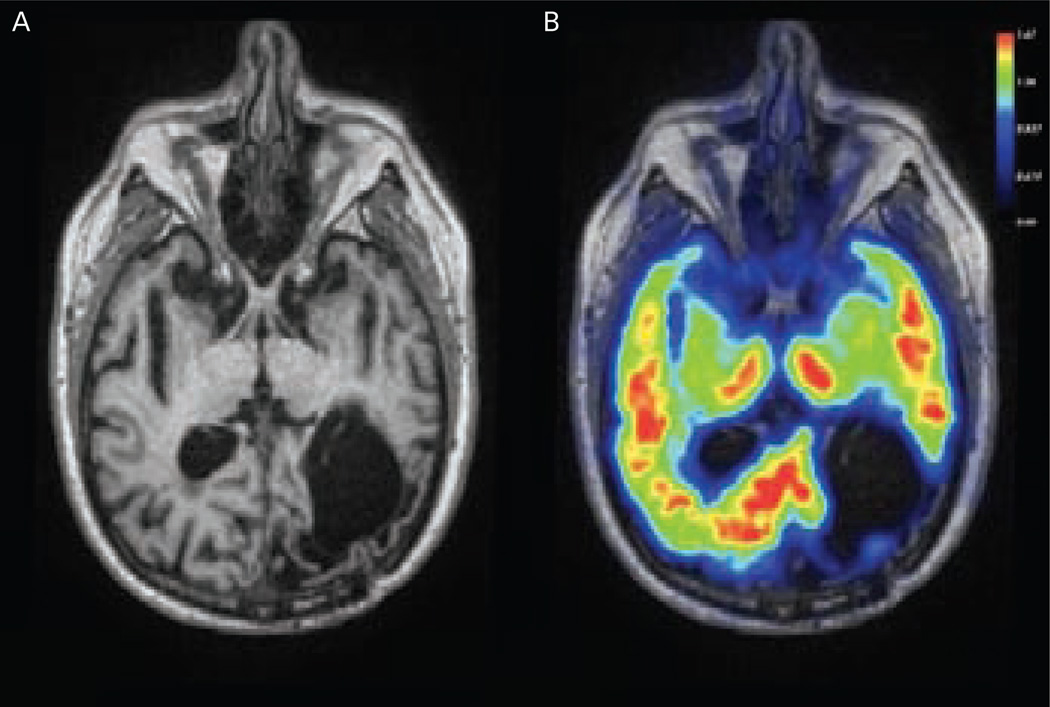
Figure 4.
Pittsburgh Compound B Positron Emission Tomography Scan of a Patient with Definitive Cerebral Amyloid Angiopathy Subsequently Confirmed at Autopsy
A: Magnetic resonance image (MRI) T1 showing encephalomalacia from a previous left occipital intracerebral haemorrhage (ICH). B: Pittsburg compound B (PiB) positron emission tomography (PET) scan co-registered to MR showing increased PiB retention in the posterior regions of the brain, including right occipital and bilateral temporal cortex.
At present, PiB-PET use is still considered investigational by the FDA for the diagnosis of AD or CAA. Other amyloid-binding compounds using the longer-lasting radionuclide fluorine-18 have more recently been tested to detect amyloid and may enter into clinical practice.74
Cerebrospinal Fluid Biomarkers
Patients with AD have decreased cerebrospinal fluid (CSF) concentrations of Aβ42 protein and increased tau protein, which allows reasonably accurate differentiation between AD patients and controls (sensitivity and specificity generally >80 %).75–83 More recently, CSF concentrations of both Aβ42 and Aβ40 were shown to be reduced in non-demented CAA subjects compared to both healthy controls and AD subjects.83 These results suggest that the large component of Aβ40 deposited in vessels in advanced CAA may deplete this peptide from CSF, similarly to AD-associated reductions of Aβ42. Total and phosphorylated tau protein levels were not significantly different in CAA from controls. The combination of Aβ42 and total tau strongly discriminated CAA from controls (area under the receiver operator curve, 0.98), although discrimination between CAA and AD was less distinct (area under the receiver operator curve, 0.82).83 Taken together, these data suggest that Aβ40, Aβ42, and tau protein levels in the CSF may be useful biomarkers of advanced CAA pathology.
Management of Patients with Cerebral Amyloid Angiopathy
There is no evidence-based treatment or preventive strategy specific for CAA or CAA-related ICH at this time. Given the increased risk of a first or recurrent ICH or progressive cognitive decline, the clinical diagnosis of CAA may nonetheless have impact on the management of some patients, especially regarding the use of anti-thrombotic medications, management of co-morbidities, long-term prognosis, cognitive follow-up and genetic counselling. Furthermore, patients who present with CAA-related inflammation have a potentially treatable form of the disease because of its responsiveness to immunosuppressive therapy.
Anti-thrombotic Medications
Anticoagulation with warfarin increases mortality after ICH and may not be safe following CAA-related ICH.84 While selected patients with deep hemispheric ICH at particularly high risk for thromboembolic stroke and low risk of ICH recurrence might benefit from long-term anticoagulation, patients with CAA-related ICH and atrial fibrillation should not be offered long-term anticoagulation.84 A small case-control study found an association between the presence of CMBs and warfarin-related ICH,85 but the relative risks and benefits of anticoagulation in patients with CMBs are less clear. At least two observational studies found that antiplatelet therapy increased the risk of recurrent CAA-related ICH, particularly for individuals with larger numbers of microbleeds.86,87 Larger, more definitive studies are required to settle these important questions, which could have considerable clinical implications.
Management of Co-morbidities
In the Perindopril protection against recurrent stroke study (PROGRESS), blood pressure lowering treatment using perindopril plus optional indapamide was found to provide protection against both ischaemic and haemorrhagic stroke.88 Further post hoc analysis indicated that randomisation to the blood pressure-lowering regimen reduced risk for CAA-related ICH in particular, suggesting that blood pressure reduction may have benefits in this population.89
Management of Cerebral Amyloid Angiopathy-related Intracerebral Haemorrhage
There is no evidence that the acute medical management of patients with CAA-associated ICH should differ from other causes of ICH.90 A subgroup analysis of the Surgical trial in intracerebral haemorrhage (STICH) II suggested better functional outcomes after surgery in patients with superficial as well as lobar ICH in the absence of IVH.91 The ongoing STICH II will specifically test the benefits of early surgical evacuation for lobar haematomas within 1 cm of the cortical surface without intraventricular extension.92 The results of this trial will have particular bearing on the surgical management of patients with CAA-related ICH, as lobar ICH is the hallmark of this condition.93
Management of Inflammatory Cerebral Amyloid Angiopathy
The inflammatory presentation of CAA represents a potentially treatable form of the disease. Corticosteroid treatment has been shown in some case reports and small series to ameliorate symptoms associated with CAA-related inflammation, possibly by reducing vasogenic oedema.94 Other immunosuppressant treatments have been reported to influence the course of inflammatory CAA, but available evidence is limited.95 DiFrancesco and colleagues reported a patient with CAA-related inflammation who was found to have elevated auto-antibodies to Aβ40 and Aβ42 in CSF with subsequent reductions following corticosteroid treatment, offering a potential biological mechanism for this syndrome.96 If this finding can be confirmed, titres of CSF anti-Aβ antibodies might serve as a biological marker for the diagnosis, monitoring and evaluation of treatment in CAA-related inflammation.
Genetic Testing and Counselling
The over-representation of the APOE ε2 allele in patients with warfarin-associated lobar ICH highlights a future potential for identifying high-risk patients for anticoagulation.97 The APOE ε2 allele has also been associated with larger baseline ICH volumes, haematoma expansion, presence of spot sign on initial CT angiography and poor outcome in lobar ICH.43,44 Nevertheless, there is currently not enough scientific evidence to support routine APOE genotyping of CAA patients for clinical purposes out of an investigational protocol.98,99
Mutations of APP as well as other genes unrelated to Aβ such as cystatin C, BRI and transthyretin have been associated to familial CAA.100–105 Interestingly, different carriers of the same mutation may have dramatically different clinical presentationss.105 Genetic testing and counselling may be appropriate to patients with a strong family history of lobar ICH or vascular dementia. When faced with those uncommon cases, it is reasonable to offer referral to an academic research centre for further genetic evaluation, differential diagnosis and follow-up.
Conclusions
Since the original description of neurovascular amyloid deposits in 1909, there has been remarkable progress in our understanding of CAA. A definitive diagnosis still relies on histopathological evaluation but MRI sequences now allow reliable assessment of an individual’s haemorrhagic burden over time and reasonable diagnostic accuracy via clinical neuroimaging. CAA has been associated with both ischaemic and haemorrhagic manifestations within the brain and is a major cause of lobar ICH and cognitive decline in the elderly. Despite the absence of specific treatment for this condition, the clinical diagnosis of CAA may have a significant impact on the management of some patients, especially regarding the use of antithrombotic medications, management of co-morbidities, long-term prognosis, cognitive follow-up and genetic counselling.
Acknowledgements
The authors would like to thank Matthew P Frosch, Andrew Dumas, Edip Gurol and Alison Ayres for their help with the pictures of this manuscript. Octavio M Pontes-Neto receives research support from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP).
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
References
- 1.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. [PubMed] [Google Scholar]
- 2.Oppenheim G. Über “drusige Nekrosen” in der Grosshirnrinde. Neurol Centralbl. 1909;28:410–413. [Google Scholar]
- 3.Vinters HV. Cerebral amyloid angiopathy, A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SM, Vonsattel JP, Stakes JW, et al. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology. 1993;43(10):2073–2079. doi: 10.1212/wnl.43.10.2073. [DOI] [PubMed] [Google Scholar]
- 5.Neuropathology Group, Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer LA, White LR, Ross GW, et al. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58(11):1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 7.Palsdottir A, Snorradottir AO, Thorsteinsson L. Hereditary cystatin C amyloid angiopathy: genetic, clinical, and pathological aspects. Brain Pathol. 2006;16(1):55–59. doi: 10.1111/j.1750-3639.2006.tb00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy, sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 9.Scharf J, Brauherr E, Forsting M, Sartor K. Significance of haemorrhagic lacunes on MRI in patients with hypertensive cerebrovascular disease and intracerebral haemorrhage. Neuroradiology. 1994;36:504–508. doi: 10.1007/BF00593508. [DOI] [PubMed] [Google Scholar]
- 10.Offenbacher H, Fazekas F, Schmidt R, et al. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. Am J Neuroradiol. 1996;17:573–578. [PMC free article] [PubMed] [Google Scholar]
- 11.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 14.Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14(4):343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62(3):229–334. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 16.Goldgaber D, Lerman MI, McBride OW, et al. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 17.Haass C, Schlossmacher MG, Hung AY, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 18.Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of beta- amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rostagno A, Lal R, Ghiso J. Protein misfolding, aggregation, and fibril formation: common features of cerebral and non-cerebral amyloid diseases. In: Dawbarn D, Allen S, editors. The Neurobiology of Alzheimer’s Disease. Oxford: Oxford University Press; 2007. pp. 133–160. [Google Scholar]
- 21.Hartz AM, Bauer B, Soldner EL, et al. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–523. doi: 10.1161/STROKEAHA.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16(1):40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuchi K, Ho L, Younkin SG, et al. High levels of circulating beta-amyloid peptide do not cause cerebral beta-amyloidosis in transgenic mice. Am J Pathol. 1996;149(1):219–227. [PMC free article] [PubMed] [Google Scholar]
- 24.Burgermeister P, Calhoun ME, Winkler DT, Jucker M. Mechanisms of cerebrovascular amyloid deposition. Lessons from mouse models. Ann N Y Acad Sci. 2000;903:307–316. doi: 10.1111/j.1749-6632.2000.tb06381.x. [DOI] [PubMed] [Google Scholar]
- 25.Eisele YS, Obermüller U, Heilbronner G, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Alloza M, Gregory J, Kuchibhotla KV, et al. Cerebrovascular lesions induce transient β-amyloid deposition. Brain. 2011;134:3697–3707. doi: 10.1093/brain/awr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinters HV, Wang ZZ, Secor DL. Brain parenchymal and microvascular amyloid in Alzheimer’s disease. Brain Pathol. 1996;6:179–195. doi: 10.1111/j.1750-3639.1996.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 28.Winkler DT, Bondolfi L, Herzig MC, et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40(7):2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45(1):79–90. [PubMed] [Google Scholar]
- 31.Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 32.Revesz T, Ghiso J, Lashley T, et al. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62:885–898. doi: 10.1093/jnen/62.9.885. [DOI] [PubMed] [Google Scholar]
- 33.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study (MRC CFAS), Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 35.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 36.Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 37.Sudlow C, Martínez González NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and metaanalyses of 31 studies among 5961 cases and 17,965 controls. Stroke. 2006;37:364–370. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicoll JA, Burnett C, Love S, et al. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1997;41:716–721. doi: 10.1002/ana.410410607. [DOI] [PubMed] [Google Scholar]
- 39.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis, APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 40.Holtzman DM. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer’s disease and cerebral amyloid angiopathy. J Mol Neurosci. 2001;17:147–155. doi: 10.1385/JMN:17:2:147. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- 42.Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol. 1998;57:353–359. doi: 10.1097/00005072-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Brouwers HB, Biffi A, Ayres AM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43(6):1490–1495. doi: 10.1161/STROKEAHA.111.643262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouwers HB, Biffi A, McNamara KA, et al. Apolipoprotein E genotype is associated with CT angiography spot sign in lobar intracerebral hemorrhage. Stroke. 2012 doi: 10.1161/STROKEAHA.112.659094. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biffi A, Shulman JM, Jagiella JM, et al. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology. 2012;78:334–341. doi: 10.1212/WNL.0b013e3182452b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan A, Greenberg SM. Chapter 38: Intracerebral hemorrhage. Handb Clin Neurol. 2008;93:767–790. doi: 10.1016/S0072-9752(08)93038-4. [DOI] [PubMed] [Google Scholar]
- 47.Raposo N, Viguier A, Cuvinciuc V, et al. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol. 2011;18:597–603. doi: 10.1111/j.1468-1331.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- 48.Thomas T, Thomas G, McLendon C, et al. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 49.Christie R, Yamada M, Moskowitz M, Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–1071. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008;71:1424–1430. doi: 10.1212/01.wnl.0000327887.64299.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 52.Dumas A, Dierksen GA, Gurol ME, et al. Functional MRI detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012 doi: 10.1002/ana.23566. (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fountain NB, Eberhard DA. Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy: report of two cases and review of the literature. Neurology. 1996;46:190–197. doi: 10.1212/wnl.46.1.190. [DOI] [PubMed] [Google Scholar]
- 55.Eng JA, Frosch MP, Choi K, et al. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol. 2004;55:250–256. doi: 10.1002/ana.10810. [DOI] [PubMed] [Google Scholar]
- 56.Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68:1411–1416. doi: 10.1212/01.wnl.0000260066.98681.2e. [DOI] [PubMed] [Google Scholar]
- 57.Puchtler H, Waldrop FS, Meloan SN. A review of light, polarization and fluorescence microscopic methods for amyloid. Appl Pathol. 1985;3:5–17. [PubMed] [Google Scholar]
- 58.Ripoll MA, Siosteen B, Hartman M, Raininko R. MR detectability and appearance of small experimental intracranial hematomas at 1.5 T and 0.5 T. A 6–7-month follow-up study. Acta Radiol. 2003;44:199–205. doi: 10.1080/j.1600-0455.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 59.Alemany Ripoll M, Stenborg A, Sonninen P, et al. Detection and appearance of intraparenchymal haematomas of the brain at 1.5 T with spin-echo, FLAIR and GE sequences: poor relationship to the age of the haematoma. Neuroradiology. 2004;46:435–443. doi: 10.1007/s00234-004-1191-5. [DOI] [PubMed] [Google Scholar]
- 60.Haacke EM, DelProposto ZS, Chaturvedi S, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. Am J Neuroradiol. 2007;28:316–317. [PMC free article] [PubMed] [Google Scholar]
- 61.Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol. 2009;30(2):338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenberg SM, Eng JA, Ning M, et al. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35(6):1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 63.Feldman HH, Maia LF, Mackenzie IR, et al. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke. 2008;39(10):2894–2897. doi: 10.1161/STROKEAHA.107.510826. [DOI] [PubMed] [Google Scholar]
- 64.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74(17):1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar S, Goddeau RP, Jr, Selim MH, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010;74(11):893–899. doi: 10.1212/WNL.0b013e3181d55efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linn J, Brückmann H. Superficial siderosis in cerebral amyloid angiopathy. Am J Neuroradiol. 2010;31(2):E29. doi: 10.3174/ajnr.A1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vernooij MW, Ikram MA, Hofman A, et al. Superficial siderosis in the general population. Neurology. 2009;73(3):202–205. doi: 10.1212/WNL.0b013e3181ae7c5e. [DOI] [PubMed] [Google Scholar]
- 68.Von sattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 69.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 70.Bacskai BJ, Hickey GA, Skoch J, et al. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci. 2003;100:12462–12467. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–444. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 72.Ly JV, Donnan GA, Villemagne VL, et al. 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010;74:487–493. doi: 10.1212/WNL.0b013e3181cef7e3. [DOI] [PubMed] [Google Scholar]
- 73.Greenberg SM, Grabowski T, Gurol ME, et al. Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol. 2008;64(5):587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fleisher AS, Chen K, Liu X, Roontiva A, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68(11):1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 75.De Meyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vemuri P, Wiste HJ, Weigand SD, et al. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75:143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 79.de Jong D, Kremer BP, Olde Rikkert MG, Verbeek MM. Current state and future directions of neurochemical biomarkers for Alzheimer’s disease. Clin Chem Lab Med. 2007;45:1421–1434. doi: 10.1515/CCLM.2007.320. [DOI] [PubMed] [Google Scholar]
- 80.Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 81.Verbeek MM, De Jong D, Kremer HP. Brain-specific proteins in cerebrospinal fluid for the diagnosis of neurodegenerative diseases. Ann Clin Biochem. 2003;40:25–40. doi: 10.1258/000456303321016141. [DOI] [PubMed] [Google Scholar]
- 82.Sunderland T, Linker G, Mirza N, et al. Decreased betaamyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 83.Verbeek MM, Kremer BP, Rikkert MO, et al. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–249. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eckman MH, Rosand J, Knudsen KA, et al. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 85.Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72:171–176. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 86.Soo YO, Yang SR, Lam WW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255:1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 87.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arima H, Tzourio C, Anderson C, et al. PROGRESS Collaborative Group. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41:394–396. doi: 10.1161/STROKEAHA.109.563932. [DOI] [PubMed] [Google Scholar]
- 89.Chapman N, Huxley R, Anderson C, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke. 2004;35:116–121. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- 90.Morgenstern LB, Hemphill JC, III, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomized trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 92.Mendelow AD, Gregson BA, Mitchell PM, et al. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials. 2011;12:124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mehndiratta P, Manjila S, Ostergard T, et al. Cerebral amyloid angiopathy-associated intracerebral hemorrhage: pathology and management. Neurosurg Focus. 2012;32(4):E7. doi: 10.3171/2012.1.FOCUS11370. [DOI] [PubMed] [Google Scholar]
- 94.Kloppenborg RP, Richard E, Sprengers ME, et al. Steroid responsive encephalopathy in cerebral amyloid angiopathy: a case report and review of evidence for immunosuppressive treatment. J Neuroinflammation. 2010;7:18. doi: 10.1186/1742-2094-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luppe S, Betmouni S, Scolding N, Wilkins A. Cerebral amyloid angiopathy related vasculitis: successful treatment with azathioprine. J Neurol. 2010;257:2103–2105. doi: 10.1007/s00415-010-5665-6. [DOI] [PubMed] [Google Scholar]
- 96.DiFrancesco JC, Brioschi M, Brighina L, et al. Anti-Aβ autoantibodies in the CSF of a patient with CAA-related inflammation: a case report. Neurology. 2011;76:842–844. doi: 10.1212/WNL.0b013e31820e773c. [DOI] [PubMed] [Google Scholar]
- 97.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 98.Maia LF, Mackenzie IR, Feldman HH. Clinical phenotypes of cerebral amyloid angiopathy. J Neurol Sci. 2007;257:23–30. doi: 10.1016/j.jns.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 99.Eckman MH, Wong LK, Soo YO, et al. Patient-specific decisionmaking for warfarin therapy in nonvalvular atrial fibrillation: how will screening with genetics and imaging help? Stroke. 2008;39:3308–3315. doi: 10.1161/STROKEAHA.108.523159. [DOI] [PubMed] [Google Scholar]
- 100.Palsdottir A, Snorradottir AO, Thorsteinsson L. Hereditary cystatin C amyloid angiopathy: genetic, clinical, and pathological aspects. Brain Pathol. 2006;16:55–59. doi: 10.1111/j.1750-3639.2006.tb00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roks G, Van Harskamp F, De Koning I, et al. Presentation of amyloidosis in carriers of the codon 692 mutation in the amyloid precursor protein gene (APP692) Brain. 2000;123:2130–2140. doi: 10.1093/brain/123.10.2130. [DOI] [PubMed] [Google Scholar]
- 102.Vidal R, Frangione B, Rostagno A, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 103.Vidal R, Garzuly F, Budka H, et al. Meningocerebrovascular amyloidosis associated with a novel transthyretin mis-sense mutation at codon 18 (TTRD 18G) Am J Pathol. 1996;148:361–366. [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, et al. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greenberg SM, Shin Y, Grabowski TJ, et al. Hemorrhagic stroke associated with the Iowa amyloid precursor protein mutation. Neurology. 2003;60:1020–1022. doi: 10.1212/01.wnl.0000050140.10044.a8. [DOI] [PubMed] [Google Scholar]