Abstract
When two targets are shown in a rapid temporal stream of distractors, performance for the second target (T2) is typically reduced when presented between 200 and 500 ms after the first (T1). The present study used the steady-state visual evoked potential (ssVEP), a continuous index of electrocortical dynamics, to compare brain responses in trials with correct versus incorrect T2 responses. We found a reduction of the electrocortical response following T1, in trials with correct T2 identification. By contrast, incorrect T2 trials were characterized by enhanced electrocortical amplitude. Amplitude attenuation predictive of successful T2 report was sustained over time, suggesting a reduction of resources allocated to the distractor stream in correct trials. Across inter-target intervals, T2 performance was a linear function of the ssVEP amplitude reduction in correct trials, weighted by the SOA.
Descriptors: Attention, Dense-Array EEG, Rapid Serial Visual Presentation, Steady-State Potential
Humans process and perceive only a fraction of the visual array surrounding them at a given point in time, a phenomenon typically examined in studies of visual selective attention. Limitations of selection in the human visual system arise not only based on the spatial distribution of stimuli in the visual field, but also on the basis of their temporal proximity and density. Studies employing rapid visual serial presentation (RSVP) have focused on the ability of an observer to detect or identify relevant information in a rapid stream of distractor items. In RSVP experiments, stimuli are presented sequentially at a high rate, for instance 10 items per second (see Raymond, Shapiro, & Arnell, 1992). In a typical experimental design, participants search the stimulus stream for specified target items. At RSVP rates of 7 Hz and higher, attending to a first target (T1) embedded in the distractor stream often leads to a transient impairment in detecting or identifying a second target stimulus (T2). This so-called “attentional blink” (AB) effect (Raymond et al., 1992) has been demonstrated with a variety of stimuli such as symbols, letters, digits, and words (e.g., Raymond, 2003). Report rates for the second target are usually reduced for inter-target-intervals between 200 and 500 ms.
A number of theoretical views of the AB attribute the accuracy impairment for T2 stimuli to decreased availability of cognitive resources (e.g., Chun & Potter, 1995; Jolicoeur, Sessa, Dell’Acqua, & Robitaille, 2005) or attentional capacity (Vul, Nieuwenstein, & Kanwisher, 2008), which is assumed to incur as a consequence of encoding/selecting the T1 item. In this perspective, over-allocation of resources to T1 is associated with a lack of resources available for the second target (T2) in a trade-off fashion. Overspending resources to the T1 then prevents T2 from being transformed into a durable and reportable working memory representation.
A group of alternative theoretical notions of the AB have emphasized non-trade-off aspects during RSVP target identification (Di Lollo, Kawahara, Shahab Ghorashi, & Enns, 2005; Olivers, van der Stigchel, & Hulleman, 2007). For instance, work examining the so-called lag-1 sparing effect showed that two or more targets can be reported at high accuracy, if presented in a row, without intermittent distractor items present (Olivers et al., 2007). Furthermore, concurrent tasks added to RSVP protocols draw on resources, but have been reported to reduce, not to increase the AB impairment (Olivers & Nieuwenhuis, 2006). Thus, several authors have argued against limited capacity/resource sharing as a sole determinant of the AB effect. Among other perspectives, it has been suggested (Di Lollo et al., 2005) that processing of the T1 results in a transient loss of control over the perceptual “filter” used to select target items based on their features. The perceptual system then remains tuned to respond to the target features. Items that do not match the perceptual properties of the target will disrupt this metaphorical filter and will thus reduce sensitivity to subsequent targets. Another recent view of the AB (Olivers et al., 2007) is similar to the loss-of-control account, but has capitalized on “overzealously applied” filtering of the stream which is thought to induce strong enhancement of the perceptual set when a stimulus gets selected. In the case of a distractor following T1, selection of the T1 will lead to a period of attentional enhancement and may thus allow the T1+1 distractor to be erroneously selected. In response to this event, the metaphorical attentional gate will be closed and T2 (which closely follows the T1+1 distractor in time) cannot be adequately processed. In terms of physiological processes, it can be predicted from both limited capacity models and the alternative accounts presented above, that amplitude and latency of activity in the visual system in response to the first target are critical to understand the mechanisms mediating the AB effect.
Electrophysiological studies have generally reported evidence for a resource sharing account, showing trade-off between the two targets as indexed by evoked magneto- and electrocortical fields (Hommel et al., 2006). In the resource sharing account, resources over-allocated to T1 are at the expense of T2 processing, thus predicting greater neural responses evoked by T1 stimuli in those trials in which T2 is missed, compared to correct T2 trials. Later in time, the response to the second target is expected to mirror this pattern, with smaller neural activity following T2 in T2-incorrect trials and more activity evoked by correct T2s. Consistent with this prediction, relative increases in T1-related neural activity during RSVP as measured by means of magnetoencephalography have been related to impaired T2 report (Shapiro, Schmitz, Martens, Hommel, & Schnitzler, 2006). Likewise, Kranczioch and collaborators (Kranczioch, Debener, Maye, & Engel, 2007) studied the P3 component of the event-related potential and oscillatory activity during RSVP. They found similar evidence for resource sharing as indexed by smaller P3 for the T1 in correct T2 trials, compared to incorrect T2 trials. Work using steady-state visual evoked potentials (ssVEPs; see below) in a paradigm with T2s varying in emotional content also demonstrated augmented ssVEP amplitudes following T1 in trials with missed T2s (Keil, Ihssen, & Heim, 2006a).
In the present study, we examined the direction and duration of differences in the electrocortical response to first and second targets in a RSVP task using neutral German words. The ssVEP is an oscillatory response of visual cortex to flickering stimuli, in which the frequency of the brain response equals the flicker rate of the stimuli (Müller et al., 1998a; Regan, 1989). It has been used in studies of visual perception and cognition, because it is sensitive to fluctuations in selective attention (Müller, Malinowski, Gruber, & Hillyard, 2003), emotional content (Keil, Moratti, Sabatinelli, Bradley, & Lang, 2005), and low-level perceptual features such as brightness or contrast, among other variables of interest (Regan, 1989). As a major advantage, it reflects oscillatory brain activity that is tagged by the specific frequency used in the stimulation paradigm, and therefore can easily be separated from noise and quantified in the frequency domain (Wang, Clementz, & Keil, 2007). Time-frequency domain analyses as used in the present study are also possible, yielding time-varying ssVEP amplitude measures of the frequency of interest. As a sensory visual response, it has been suggested to reflect sensory gain enhancement for selectively attended stimuli (Müller et al., 2003), but also the re-entrant modulatory activity that is hypothesized to mediate changes in sensory gain (Keil et al., in press).
Based on previous ssVEP work with affective stimuli as targets in an attentional blink paradigm (Keil et al., 2006a), we expected that resource sharing should be present across all T1-T2 lags. In particular, we asked whether enhanced electrocortical responses to the T1 stimulus predicted low T2 response and low accuracy for T2 identification. We included lags 1, 2, 4, and 6, thus examining RSVP conditions in which there were 0, 1, 3, and 5 intervening distractors.
Methods
Participants
Twelve right-handed university students (nine females) whose age ranged from 20 to 26 years (mean age 23.9 years) gave informed consent to participate in the study. All participants were native speakers of German, were right-handed, and reported normal or corrected-to-normal vision. Because stimuli were presented very rapidly, only healthy students with a negative family history for seizure disorders were examined. They were given class credit or a small financial bonus of 10 Euros for participation.
Stimuli
Based on a rating study described elsewhere (Keil & Ihssen, 2004), we selected a total of 110 German verbs describing affectively non-engaging activities and procedures such as to accompany, to continue, and to install. Sixty verbs having a mean Lemma frequency of 195.9 (SEM = 51.7) per one million words in the CELEX database (Baayen, Piepenbrock, & Gulikers, 1995) served as target stimuli. The mean number of letters and syllables were 7.9 (SD = 2.8) and 2.5 (SD = 1.0), respectively. The remaining 50 verbs were used as distractor items and comprised on average of 8.2 (SD = 2.0) letters and 2.8 (SD = 0.7) syllables. Mean word frequency was 325.9 (SEM = 88.2).
Procedure
Participants entered a sound attenuated, dimly lit chamber and were seated comfortably, with their chin on a rest. Stimuli were presented on a computer screen with a retrace frequency of 60 Hz, located at 70 cm distance of the observer. Target words were shown in green, distractors in gray color on a black background, using Helvetica 26 fonts. The gray and green words appeared at a luminance of 24.9 cd/m2 and each word subtended a vertical visual angle of 0.82°. A script written using the Experimental Run Time System (ERTS) software controlled presentation and response registration.
The experimental session started with 4 practice trials to demonstrate the procedure and make sure that all individuals understood the task correctly. In total, there were 240 trials organized into two blocks. A schematic of an example trial is shown in Figure 1. Each trial contained the following series of events: A blank screen appeared for 1000 ms. Then, a stream of verbs at a frequency of 8.6 Hz was displayed at the center of the screen. The 8.6 Hz RSVP was effected by alternating the presentation of a word for 50 ms, followed by a black screen for 66 ms. Following the initial black screen, a baseline RSVP of neutral distractor words was displayed, with durations varying randomly between 8 and 25 items (i.e., about 928 to 2900 ms). This baseline RSVP was followed by the T1 (first target), a varying number of distractors, and the T2 (second target), again followed by a varying number of distractors. Inter-target intervals varied to contain none, one, three, or five intervening distractor verbs (i.e., lag1, lag 2, lag 4, lag 6). Accordingly, stimulus onset asynchronies (SOAs) were 116 ms (lag1), 232 ms (lag 2), 464 ms (lag 4), and 696 ms (lag 6). Each verb was shown four times during the experimental session, in different SOAs, respectively. Thus, there were 60 trials per SOA. The order of verbs and conditions was randomized, with the constraints that (i) immediate repetitions of trials belonging to the same lag condition could not occur, and (ii) that verbs were not repeated within blocks of 40 trials.
Figure 1.
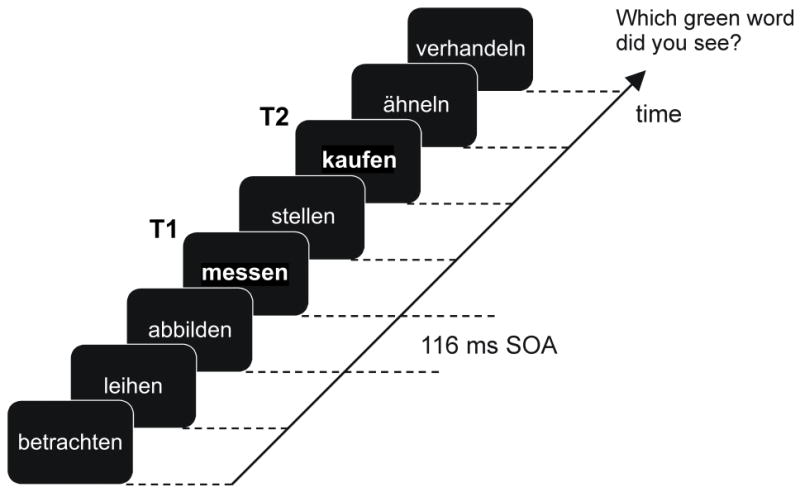
Example of one trial of the rapid serial visual presentation paradigm. Rapid stimulus presentation was effected by using on/off duty cycles of 50 ms (word) and 66 ms (blank screen), resulting in 116 ms per cycle and a stimulation frequency of 8.6 Hz. The two targets were verbs displayed in green font (T1 and T2; shown here in bold letters) interspersed in a sequence of distractor verbs shown in white font on the computer screen. The present example illustrates a trial with one intervening distractor between T1 and T2 (i.e., lag 2); T1 is “messen” (to measure) and T2 is “kaufen” (to buy).
At the end of each trial, subjects were asked via a message on the computer screen to report aloud the green words using a microphone in the experimental chamber and type the first letter of the words on a computer keyboard. Participants started the subsequent trial after completing report, using the “space” key.
Analysis of behavioral data
Responses were labeled as being correct when they reflected the accurate temporal position (first and second target in correct order) of each target in the RSVP stream [Footnote 1]. Furthermore, only trials with correct T1 report were considered for determining T2 accuracy. Identification performance was then expressed as the percentage of correct responses for each of the four SOAs. Subsequently, separate F values for T1 and T2 responses were calculated using Analyses of Variance (ANOVAs) having the within-subject factor of lag (4; Lag1 = 116-ms SOA, Lag2 = 232-ms SOA, Lag4 = 464-ms SOA, Lag6 = 696-ms SOA). Significant effects were followed by means of planned comparisons reflecting the theoretical models described in the introduction (see Results).
EEG recording and data reduction
EEG was continuously recorded from 129 electrodes using an Electrical Geodesics™ (EGI) high-density EEG system and digitized at a rate of 250 Hz, using Cz as a recording reference. Impedances were kept below 50 kΩ, as recommended by the manufacturer. A subset of electrodes located at the outer canthi as well as above and below the right eye was used to determine the horizontal and vertical Electrooculogram (EOG). All channels were preprocessed on-line by means of 0.1 Hz high-pass and 100 Hz low-pass filtering. Epochs were extracted from the continuously recorded EEG relative to the onset of T1 for each stimulus, using 2800 ms pre-T1 and 2000 ms post-T1. The mean voltage of a 400-ms segment preceding T1 onset was subtracted as the baseline. In a next step, data were low-pass filtered at a frequency of 40 Hz (24 dB / octave) and then submitted to the procedure proposed by (Junghöfer, Elbert, Tucker, & Rockstroh, 2000), as implemented in the EMEGS software suite provided by Peter Peyk and Markus Junghöfer (see www.emegs.org). This procedure uses statistical parameters of the data to exclude channels and trials that are contaminated with artifacts. Recording artifacts are first detected using the recording reference (i.e., Cz), and then global artifacts are detected using the average reference. Subsequently, distinct sensors from particular trials are removed based on the distribution of their amplitude, standard deviation and gradient. Data at eliminated electrodes are replaced with a statistically weighted spherical spline interpolation from the full channel set (Junghöfer et al., 2000).
The mean number of approximated channels across conditions and participants was 21. It was ensured that the rejected sensors were not located within one region of the scalp, because this would make interpolation for this area invalid. In particular, we rejected epochs with bad channels accumulating in posterior regions, as these channels were the main focus of the present study. As in many other studies, channels with artifacts tended to cluster in anterior, rather than posterior locations. Exclusion of epochs with excluded channels, and the decision whether this epoch can be retained after interpolation was based on comparison of a forward model calculated using the actual remaining sensor set with a model that used the full set (see www.emegs.org, for a detailed description). Spherical spline interpolation was used throughout, both for approximation of sensors and illustration of ssVEP amplitude maps (Junghöfer, Elbert, Leiderer, Berg, & Rockstroh, 1997; Perrin, Pernier, Bertrand, Giard, & Echallier, 1987). Single epochs with excessive eye-movements and blinks or more than 21 channels containing artifacts were discarded. The resulting data were then visually inspected together with the vertical and horizontal EOG to exclude remaining artifacts. Subsequently, data were arithmetically transformed to the average reference, which was used for all analyses. After artifact correction, an average of 72% of the 240 trials was retained in the analyses, with the lag-6 condition showing a slightly higher attrition rate (64% of the trials retained) than the remaining lag conditions, t(11) = 2.2, p < .06. Epochs were averaged for each SOA and for correct versus incorrect T2 trials, yielding ssVEP time series for 8 conditions (lag x correct/incorrect) at 129 electrodes for each subject. The remaining epochs were then averaged according to the experimental condition and correct versus incorrect responses, as defined for the behavioral data (see above).
Steady-state VEP analyses
Time-varying amplitude at the stimulation frequency of 8.6 Hz was extracted by means of complex demodulation (Regan, 1989). To this end, the averaged data were multiplied with a sine and cosine function at the stimulation frequency. The resulting time series were then low-pass filtered at a cut-off of 0.75 Hz, leading to sensitivity of the resulting waveforms to amplitude changes between 7.85 Hz and 9.35 Hz, with a center frequency of 8.6 Hz. In a final step, sine and cosine time series were pooled as the Euclidian vector length for each time point, i.e., the square root of the sum of the squares, resulting in time-varying amplitude at each sensor. The mean voltage of each segment extending from −1000 to −160 ms relative to T1 onset was subtracted as the baseline and time-varying amplitude was thus expressed as change against this baseline. Duration of the baseline was maximized to ensure reliable estimation of the pre-T1 ssVEP amplitude, and was halted at −160 ms to avoid overlapping with post-T1 oscillatory activity, given the temporal smearing of complex demodulation. Subsequently, two aspects of the time-varying amplitude were examined statistically, for correct and incorrect T2 trials separately:
Amplitude following T1. In accordance with earlier work, we expected that effects of target processing on ssVEP amplitude recorded over posterior cortex would occur within 1–2 cycles after the target event (Belmonte, 1998; Müller, Teder-Salejarvi, & Hillyard, 1998b). We therefore formed temporal averages over time segments following T1 onset by 116 to 232 ms (corresponding to two cycles of the RSVP stream) for each lag condition. Note that the low-pass filter necessary for complex demodulation causes temporal smoothing, which renders the epoch selected sensitive to processes outside the indicated range.
Amplitude following T2. Paralleling the procedure for T1, temporal averages were calculated for time segments following T2 onset by 116 to 232 ms for each lag condition. All temporal averages were pooled for a sensor group containing Pz, Oz, and their nearest neighbors, respectively, where the ssVEP was most pronounced (see Figure 4). Mean ssVEP amplitudes were evaluated using ANOVA comprising within-subjects factors of target (2; post-T1, post-T2), lag (4; Lag1 = 116-ms SOA, Lag2 = 232-ms SOA, Lag4 = 464-ms SOA, Lag6 = 696-ms SOA), and accuracy (2; correct, incorrect). Whenever appropriate, significant effects were followed by contrast analyses.
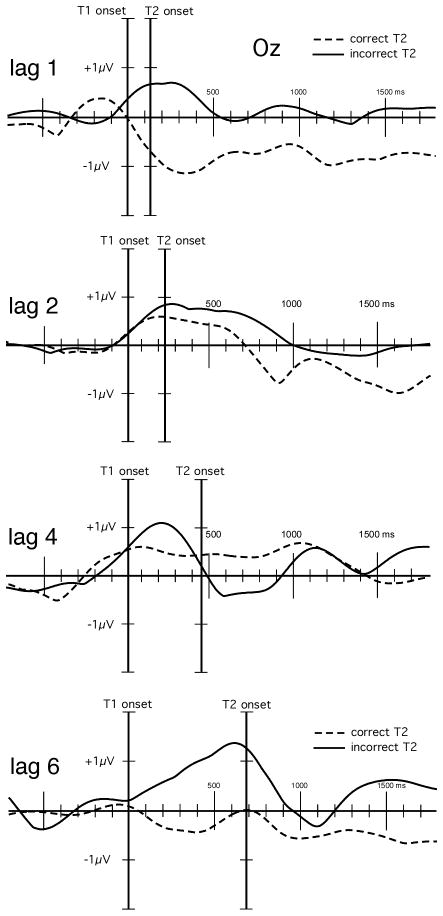
Figure 4.
Grand mean (n = 12) time-varying ssVEP amplitude changes (with respect to the pre-T1 baseline) for the four lag conditions. The time course of the amplitude was obtained by averaging across occipital electrode sites, separately for trials with correct (dashed lines) and incorrect T2 reports (solid lines). Successful T2 identification was generally associated with relative amplitude reduction following T1 onset compared to trials with missed second targets (T2s).
Results
Behavioral data
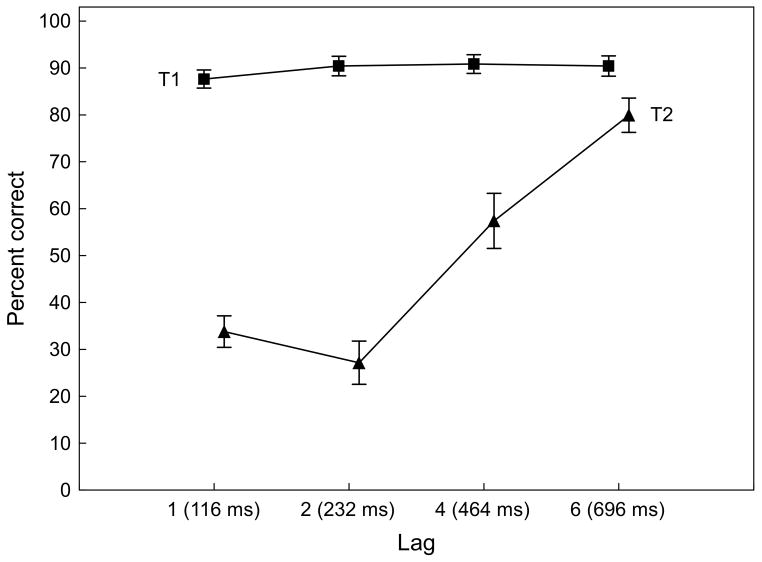
As expected, mean T2 accuracy varied systematically as a function of lag, F(3,33) = 54.4, p < .001, Partial Eta Squared = .83 (see Figure 2). Accuracy showed a linear increase from lag 2 to lag 6, F(1,11) = 116.3, p < .001, with T2 performance being significantly better for lag 1 than for lag 2, F(1,11) = 7.2, p <.05. No such differences were observed for T1 accuracy.
Figure 2.
Identification accuracy (percent correct) of first (T1) and second targets (T2) for four lags of the experimental task. Values represent a mean of 12 participants. Vertical bars indicate standard errors. T1 accuracy did not vary as a function of lag (squares), whereas T2 identification followed a typical hook-shaped performance profile (triangles).
Electrocortical data
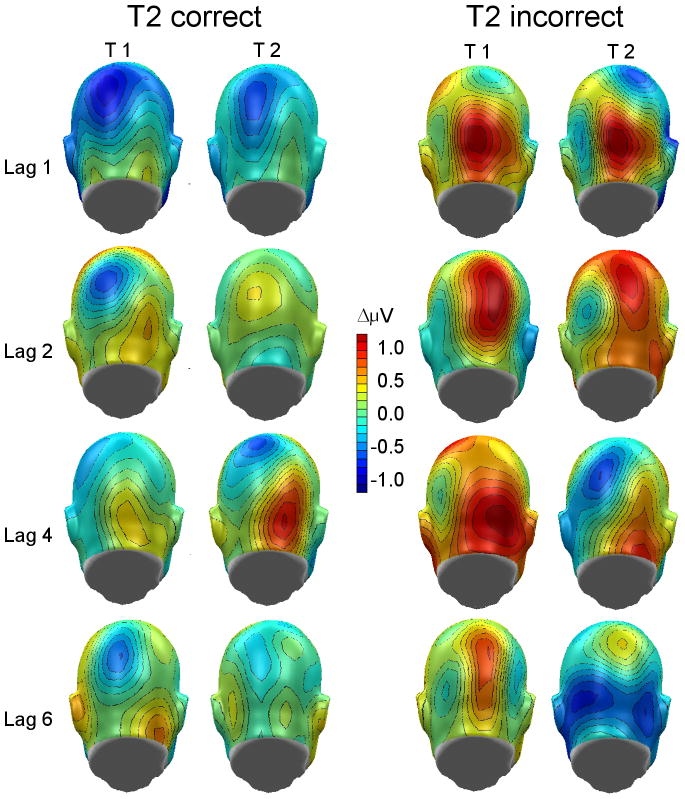
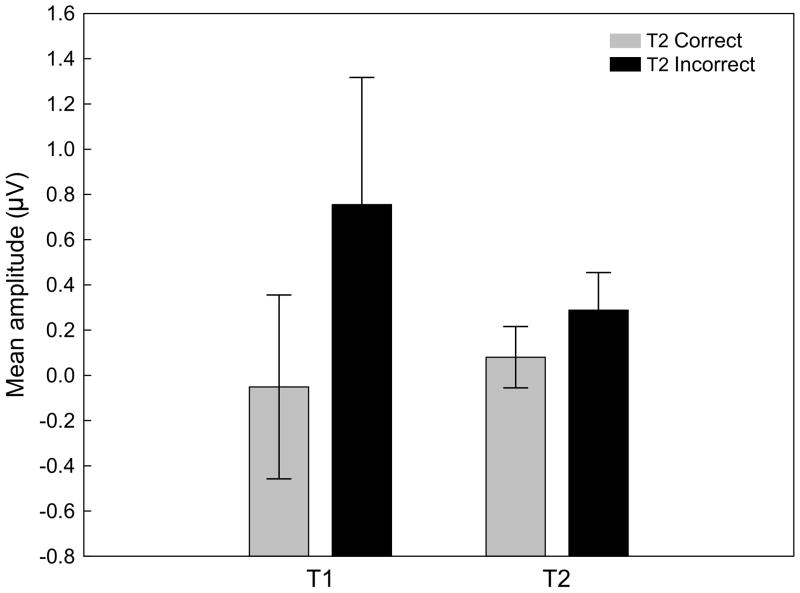
Grand mean time-locked averages of the voltages recorded in three example conditions are shown in Figure 3. As can be seen form this figure, the RSVP stream evoked a reliable and pronounced 8.6 Hz oscillation that was clearly time locked across conditions and separable from noise. Thus this signal was submitted to complex demodulation and the time varying amplitude on 8.6 Hz (i.e. the stimulation frequency) was extracted. The time-varying ssVEP amplitude for the four lag conditions is shown in Figure 4 separately for RSVP trials with correct and incorrect T2 responses. Mean time varying amplitudes in two time windows (post-T1 and post-T2) were averaged across an electrode cluster comprising Pz and Oz as well as 16 neighboring sensors (see Figure 5 for the topographical distribution of the mean ssVEP amplitude in the post-T1 [left] and post-T2 [right] time windows). Repeated measures ANOVA having target, lag, and accuracy as within-subject factors showed that incorrect responses were generally associated with higher mean ssVEP amplitudes than correct responses, F(1,11) = 37.3, p < .001, Partial Eta Squared = .77. As illustrated in Figure 6, this difference was more pronounced for the post-T1 than the post-T2 segment, F(1,11) = 6.9, p < .05, Partial Eta Squared = .38.
Figure 3.
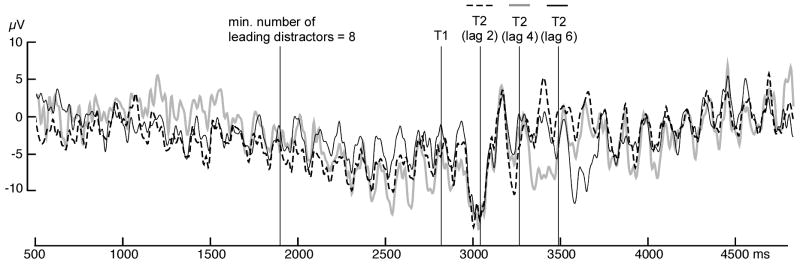
Grand mean time-domain representation of the ssVEP signal, after averaging across artifact-free epochs, at electrode site Poz (central parieto-occipital). Time series are shown for trials with correct responses in the lag 2, lag 4, and lag 6 conditions.
Figure 5.
Topographical distribution of time varying ssVEP amplitudes following onset of the first (T1) and second target (T2) for the four lag conditions. Left and right panels show the grand mean topography (n = 12; back view) in two time windows (see Methods) for trials with correct and incorrect T2 reports, respectively. The topographical map illustrates that failure of T2 identification is related to elevated posterior cortical activity following T1 onset.
Figure 6.
Interaction plot for the mean ssVEP amplitude between factors of target type (T1 versus T2) and accuracy (correct versus incorrect T2 reports). Mean values of 12 participants are depicted. Vertical bars indicate standard errors. Amplitude enhancement in incorrect (black) compared to correct trials (gray) is significantly more pronounced for T1-related brain activity.
Prediction of behavioral performance by electrocortical activity post-T1
As an explorative analysis, the behavioral accuracy for T2 stimuli was predicted as a linear function of relative amplitude reduction and SOA. In that simple linear model, each participant’s behavioral accuracy was modeled as the inverse of the z-transformed amplitude change following T1 plus the z-transformed SOA in ms, i.e.
Thus, performance was assumed to linearly increase as a function of amplitude reduction after T1 and with increasing SOA. The latter factor could be regarded as time to recover resources, or to re-establish control over the attentional set that is applied to the visual stream. The predicted accuracy (see Figure 6) was calculated for each participant, and an overall R2 was computed based on the overall sums of squares. This procedure resulted in a model fit with R2 = .76, p<.01.
Discussion
This study aimed to explore the temporal fluctuations of visual resource allocation during RSVP, focusing on changes of electrocortical oscillatory activity in response to the first target, comparing trials in which T2 was missed against those with correctly identified T2s. As a main result, we found strong evidence for greater allocation of resources to T1 in trials in which T2 was missed, as indexed by greater electrocortical amplitude in these trials. This pattern for missed-T2 trials was very similar across the lag conditions, with an enhancement compared to baseline levels, immediately following presentation of the T1. By contrast, T2-correct trials showed marked amplitude reductions compared to the missed trials in the same time range, suggesting that less resource allocation to T1 is predictive of successful T2 processing. The inverse linear relationship between T1-linked brain activity and behavioral performance was confirmed by predicting behavioral performance (i.e., accuracy) on the basis of electrocortical amplitude and SOA, using a simple linear-additive model. Interestingly, relative amplitude reductions in correct trials were protracted in time, extending beyond T2 presentation across all lag conditions. Providing evidence for resource sharing across multiple targets in a temporal stream, the present results replicate and extend earlier electrophysiological work (Kranczioch et al., 2007; Shapiro et al., 2006), which has suggested that T1 and T2 compete for resources.
Notably, although we found evidence for a benefit of reduced electrocortical activity following T1, we did not observe the opposite pattern of greater amplitude increase following T2 in T2 correct trials. This may suggest that the resources that are “saved” during the T1 stage are not expended to facilitate successful T2 identification later on. Our analysis focused on posterior ssVEP amplitude however, that has been argued to be a primary visual signal biased by top-down modulatory feedback (Müller et al., 1998a). Clearly, additional neurophysiological processes beyond sensory gain may be involved in resource sharing during feature-based identification tasks as in the present study (Hamker, 2005). Previous work examining large-scale phase relationships between structures in cortical networks (Gross et al., 2004; Kessler, Gross, Schmitz, & Schnitzler, 2006) has emphasized the important role of coherent coupling in these structures for successful rapid temporal stimulus processing. Furthermore, increasing the perceptual load of T1 has been reported to diminish residual semantic processing of T2 (Giesbrecht, Sy, & Elliott, 2007), which highlights the fact that lower-order and higher-order cognitive processes are both affected by competition for resources in sensory processing. Together with research into spatial attention (Belmonte, 1998) and into the perception of emotionally salient stimuli (Keil et al., 2005), this latter finding supports the use of a sensory measure such as the ssVEP to study stimulus processing during RSVP.
As another methodological issue that might have occluded potential compensatory T2 enhancement in T2-correct trials, we used a narrow-band filter for the complex demodulation to ensure a high degree of frequency specificity, but we did so at the expense of time resolution (cf., Müller et al., 1998b). This choice was made to avoid alpha-band changes and other lower band activity to leak into the ssVEP band. We also focused on occipital and parieto-occipital sites of the electrode array, to increase the specificity of the dependent variable to activity in the visual brain and, at the same time, decrease the impact of potential alpha fluctuations around parietal recording sites (Keil, Mussweiler, & Epstude, 2006b) on the dependent variable. Taken together, these points suggest that resource tradeoff in the sense of compensatory T2 enhancement, when T1-evoked activity was small in amplitude, awaits further investigation. Research is underway that capitalizes on improved temporal resolution of ssVEPs with greater stimulation rate.
One limitation of the present study arises from our efforts to keep the duration of the overall session short and participants’ vigilance and motivation high: because we did not add a condition in which the T1 was to be ignored, it is unclear whether salience of the color change associated with T1, or the task has contributed to the T1-related changes in ssVEP amplitude. Work from our and other laboratories however suggests that salient but unattended T1 stimuli and distractor items do not affect the subsequent target processing (see e.g., Ihssen & Keil, in press). Furthermore, a purely sensory effect of T1 might not interact with SOA and T2 correctness in the way observed here. Taken together, it seems more likely that the T1-related changes in ssVEP amplitude are task-related and attentional in nature, rather than purely sensory responses to color change.
In showing relatively greater activation in response to T1 during incorrect trials over occipital sensors, the previous study is in line with two recent hemodynamic brain imaging studies (Kranczioch, Debener, Schwarzbach, Goebel, & Engel, 2005; Shapiro, Johnston, Vogels, Zaman, & Roberts, 2007). Both studies reported metabolic enhancement for missed-T2 relative to baseline, correct-T2, or no-T2 conditions, in brain regions typically associated with sensory processing, object representation, and attention modulation. The present study adds to this body of research that the reduction of sensory gain associated with successful T1 and T2 processing is prolonged in time, starting immediately after T1 onset, with no evidence for reversal after T2 across all lag conditions.
In terms of the competing views of the attentional blink effect presented in the introduction, the present pattern of results suggests that electrocortical dynamics partly support resource sharing accounts (Shapiro et al., 2006) without ruling out “loss of control” accounts and related views that emphasize the role of the distractor items following T1 (e.g., Di Lollo et al., 2005; Olivers et al., 2007). In these views, the ability to configure the perceptual filters according to the task demands is disrupted by the T1 + 1 item, but control is re-gained over time. The present analysis did not point to an interaction effect of lag and accuracy (i.e., correct – incorrect), and did not suggest a special role of the lag-1 condition vis-à-vis the other conditions. Given that the ssVEP is a measure of neural mass activity, it is also difficult to test predictions regarding changes of the configuration of the “input filter” as mentioned by notions emphasizing overinvestment (Olivers et al., 2007). The limitations of our dependent variable regarding spatial and temporal resolution were discussed above and caution is therefore warranted when linking metaphorical concepts on the cognitive level to physiological data sensitive to a subset, but not all of the relevant electrocortical dynamics. On the level of neural mass activity, however, findings are supportive of theoretical views predicting T2 errors when T1 processing is enhanced. The linear model applied to our electrophysiological data predicted 76 % of the variance in the behavioral accuracy, using equal weighting for the SOA and for the electrocortical activity evoked by T1, which was considered an index of resource deployment, linked to T1. This seems to suggest that both initial resource deployment and time to re-set the optimum state of the visual system after processing the first target contribute to the behavioral response pattern.
The question arises as to the neurophysiological mechanism mediating the prolonged reduction of electrocortical processing following T1 processing in trials with successful identification of T2. In line with previous work on ssVEPs, we interpreted the time-varying amplitude as a continuous measure of selective attention to the letter stream (Müller, Andersen, & Keil, 2008) that reflects top-down modulation of visual cortical activity (Keil et al., in press). In the lag-1 condition, such an interpretation of the time-varying ssVEP amplitude is intuitively plausible: With correct identification of T1 and T2, the letter stream following T2 will contain only task-irrelevant distractor items that can be ignored (see Figure 3, top). This was mirrored in the ssVEP amplitude, which remained below the level of the pre-target baseline, during which participants were awaiting the onset of the target exemplars. No such reduction was observed following T2 in the lag-2 and lag-4 conditions. Here, correct T2 identification was associated with relative reduction compared to trials with incorrect responses, but the overall level of the ssVEP was above the baseline, suggesting heightened, sustained attention to the letter stream despite the fact that both targets were successfully processed. Finally, the lag-6 condition was characterized by a prolonged near-baseline level of the ssVEP in the T2-correct trials that suggested absence of attentional modulation whereas missed trials were related to slow amplitude enhancement until T2 was presented, again suggesting that over-expending resources in the anticipation of T2 is a dysfunctional strategy.
As mentioned above, a useful framework to account for detrimental effects of enhanced T1 processing is the concept of coherent large-scale cortical networks mediating selective attention to events in the temporal stream (Hommel et al., 2006). According to this perspective, bias signals are generated in parietal and frontal cortical areas and change thresholds in visual areas such as temporal-occipital cortex. Generally, high coupling/strong biasing of the T1 features may create difficulty to disengage attention after T1 has been encoded, and to re-engage the system to process T2. Together with previous work on oscillatory activity during RSVP (Kessler et al., 2006), the present results are supportive for such a biased competition account of the attentional blink effect, a hypothesis that has been proven successful in other areas of selective processing such as spatial (Müller & Hübner, 2002) and feature-based attention (Wang et al., 2007), among others. The specific predictions of this account regarding time-varying connectivity during rapid visual processing can be examined using directional measures of functional connectivity together with the ssVEP technique (Keil et al., in press). Future work will therefore employ such measures in combination with hemodynamic imaging, to increase the sensitivity to spatial and temporal dynamics underlying attention shifts during rapid visual processing.
Figure 7.
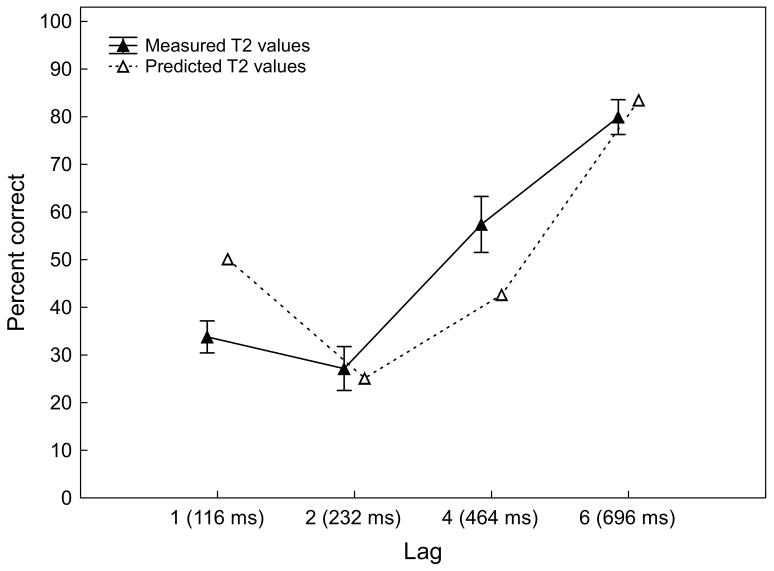
Identification accuracy (percent correct) for the second target (T2) at the different lags as measured in the experimental task (filled triangles) and predicted as a linear function of relative amplitude reduction and lag interval (open triangles). For illustration purposes, predicted values were linearly transformed from z-scores to a percent scale. Values reflect a mean of 12 participants. Vertical bars indicate standard errors.
Acknowledgments
Research was supported by grants from the Deutsche Forschungsgemeinschaft and from the NIMH. The authors would like to thank Alexander Dienst and Niklas Ihssen for help in data acquisition and data reduction.
Footnotes
Footnote 1: Previous AB work with word stimuli (Potter et al., 2005; Potter, Staub, & O’Connor, 2002) has suggested that at short SOAs, the report of the order of T1 and T2 is often reversed, as a consequence of T2 being processed first. We have therefore repeated our analyses, allowing reversal of target report as a correct response. Because there were only 9 reversal errors (defined as correct identification of the two targets, but in reversed order) at lag1 in the entire sample of participants, the results of this analysis were close to identical to the results reported below, and are not included in this manuscript. The absence of reversal errors in our design may well be attributable to the fact that we used longer SOAs and slower rates as the studies mentioned above, which may reduce the likelihood of T2 being processed prior to T1.
References
- Belmonte M. Shifts of visual spatial attention modulate a steady-state visual evoked potential. Brain Research: Cognitive Brain Research. 1998;6(4):295–307. doi: 10.1016/s0926-6410(98)00007-x. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(1):109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J, Shahab Ghorashi SM, Enns JT. The attentional blink: Resource depletion or temporary loss of control? Psychological Research. 2005;69(3):191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Sy JL, Elliott JC. Electrophysiological evidence for both perceptual and postperceptual selection during the attentional blink. Journal of Cognitive Neuroscience. 2007;19:2005–2018. doi: 10.1162/jocn.2007.19.12.2005. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, et al. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proceedings of the National Academy of Sciences of the USA. 2004;101(35):13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamker FH. The reentry hypothesis: The putative interaction of the frontal eye field, ventrolateral prefrontal cortex, and areas v4, it for attention and eye movement. Cerebral Cortex. 2005;15(4):431–447. doi: 10.1093/cercor/bhh146. [DOI] [PubMed] [Google Scholar]
- Hommel B, Kessler K, Schmitz F, Gross J, Akyurek E, Shapiro K, et al. How the brain blinks: Towards a neurocognitive model of the attentional blink. Psychological Research. 2006;70(6):425–435. doi: 10.1007/s00426-005-0009-3. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Keil A. The costs and benefis of processing emotional stimuli during rapid serial visual presentation. Cognition and Emotion in press. [Google Scholar]
- Jolicoeur P, Sessa P, Dell’acqua R, Robitaille N. On the control of visual spatial attention: Evidence from human electrophysiology. Psychological Research. 2005;70:414–424. doi: 10.1007/s00426-005-0008-4. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Leiderer P, Berg P, Rockstroh B. Mapping eeg-potentials on the surface of the brain: A strategy for uncovering cortical sources. Brain Topography. 1997;9(3):203–217. doi: 10.1007/BF01190389. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array eeg/meg studies. Psychophysiology. 2000;37(4):523–532. [PubMed] [Google Scholar]
- Keil A, Ihssen N, Heim S. Early cortical facilitation for emotionally arousing targets during the attentional blink. BMC Biology. 2006a;4:23. doi: 10.1186/1741-7007-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Moratti S, Sabatinelli D, Bradley MM, Lang PJ. Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cerebral Cortex. 2005;15(8):1187–1197. doi: 10.1093/cercor/bhi001. [DOI] [PubMed] [Google Scholar]
- Keil A, Mussweiler T, Epstude K. Alpha-band activity reflects reduction of mental effort in a comparison task: A source space analysis. Brain Research. 2006b;1121:117–127. doi: 10.1016/j.brainres.2006.08.118. [DOI] [PubMed] [Google Scholar]
- Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Re-entrant projections modulate visual cortex in affective perception: Directional evidence from granger causality analysis. doi: 10.1002/hbm.20521. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler K, Gross J, Schmitz F, Schnitzler A. Cortical dynamics and synchronization related to multiple target consolidation under rapid-serial-visual-presentation conditions. Journal of Physiology Paris. 2006;99(1):21–28. doi: 10.1016/j.jphysparis.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Maye A, Engel AK. Temporal dynamics of access to consciousness in the attentional blink. Neuroimage. 2007;37(3):947–955. doi: 10.1016/j.neuroimage.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Schwarzbach J, Goebel R, Engel AK. Neural correlates of conscious perception in the attentional blink. Neuroimage. 2005;24(3):704–714. doi: 10.1016/j.neuroimage.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Müller MM, Andersen S, Keil A. Time course of competition for visual processing resources between emotional pictures and a foreground task. Cerebral Cortex. 2008;18:1892–1899. doi: 10.1093/cercor/bhm215. [DOI] [PubMed] [Google Scholar]
- Müller MM, Hübner R. Can the spotlight of attention be shaped like a doughnut? Evidence from steady-state visual evoked potentials. Psychological Science. 2002;13(2):119–124. doi: 10.1111/1467-9280.00422. [DOI] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424(6946):309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Müller MM, Picton TW, Valdes-Sosa P, Riera J, Teder-Salejarvi WA, Hillyard SA. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 hz range. Brain Research: Cognitive Brain Research. 1998a;6(4):249–261. doi: 10.1016/s0926-6410(97)00036-0. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Salejarvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nature Neuroscience. 1998b;1(7):631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Nieuwenhuis S. The beneficial effects of additional task load, positive affect, and instruction on the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(2):364–379. doi: 10.1037/0096-1523.32.2.364. [DOI] [PubMed] [Google Scholar]
- Olivers CN, van der Stigchel S, Hulleman J. Spreading the sparing: Against a limited-capacity account of the attentional blink. Psychological Research. 2007;71:126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology. 1987;66(1):75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Potter MC, Dell’Acqua R, Pesciarelli F, Job R, Peressotti F, O’Connor DH. Bidirectional semantic priming in the attentional blink. Psychonomic Bulletin and Review. 2005;12(3):460–465. doi: 10.3758/bf03193788. [DOI] [PubMed] [Google Scholar]
- Potter MC, Staub A, O’Connor DH. The time course of competition for attention: Attention is initially labile. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(5):1149–1162. doi: 10.1037//0096-1523.28.5.1149. [DOI] [PubMed] [Google Scholar]
- Raymond JE. New objects, not new features, trigger the attentional blink. Psychological Science. 2003;14(1):54–59. doi: 10.1111/1467-9280.01418. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an rsvp task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Regan D. Human brain electrophysiology: Evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier; 1989. [Google Scholar]
- Shapiro K, Schmitz F, Martens S, Hommel B, Schnitzler A. Resource sharing in the attentional blink. Neuroreport. 2006;17(2):163–166. doi: 10.1097/01.wnr.0000195670.37892.1a. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Johnston SJ, Vogels W, Zaman A, Roberts N. Increased functional magnetic resonance imaging activity during nonconscious perception in the attentional blink. Neuroreport. 2007;18(4):341–345. doi: 10.1097/WNR.0b013e32801299e2. [DOI] [PubMed] [Google Scholar]
- Vul E, Nieuwenstein M, Kanwisher N. Temporal selection is suppressed, delayed, and diffused during the attentional blink. Psychological Science. 2008;19(1):55–61. doi: 10.1111/j.1467-9280.2008.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Clementz B, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45(7):1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]