Abstract
In this study, we screened field-caught mosquitoes for presence of Dirofilaria spp. by using a polymerase chain reaction (PCR) assay. Potential occurrence of Dirofilaria repens and Dirofilaria immitis microfilariae was examined in 3,600 mosquitoes of eight species (Aedes vexans, Aedes cinereus, Aedes rossicus, Culex pipiens, Culiseta annulata, Ochlerotatus sticticus, Ochlerotatus cantans and Ochlerotatus caspius) collected from five locations in two districts (Kosice and Trebisov) of Eastern Slovakia, endemic region of canine dirofilariasis. Collection of mosquitoes was performed between May and August 2012 in premises known to be inhabited by Dirofilaria-infected dogs. PCR assays were performed on 72 pools, each pool containing 50 mosquitoes of the same species, collected on the same location. Each pool was examined separately for the presence of D. immitis and D. repens, respectively. A positive finding of D. repens was recorded in one pool of A. vexans mosquitoes collected in Košické Olšany village. Minimum infection rate in A. vexans was 1:1,750, i.e. 0.57 per 1,000 mosquitoes. The identity of D. repens was confirmed by direct sequencing of PCR product which has shown 100 % homology with sequence attributed to D. repens (GenBank accession number AJ271614). This study represents the first molecular evidence of D. repens microfilariae in mosquitoes in Slovakia and highlights a need for better surveillance of zoonotic dirofilariasis in central Europe.
Introduction
Two filarial species represent causative agents of dirofilariasis of dogs and foxes in Slovakia: Dirofilaria repens (Railliet and Henry 1911) which is localised in the hypodermis of the host and causes the so-called subcutaneous form of dirofilariasis with the presence of nodular lesions and eczematous dermatitis (Rocconi et al. 2012) and Dirofilaria immitis (Leidy 1856), the agent of the cardiopulmonary form of the disease. Both filarial species have zoonotic potential and, despite the fact that a human represents only an occasional (dead-end) host and that microfilariae are not able to complete their development in a human, the number of infections in humans is rising, and D. repens is the most important causative agent of human dirofilariasis in Europe (McCall et al. 2008; Ondriska et al. 2010).
Mosquitoes represent an essential part of the dirofilarial life cycle and means of dispersion. They function not only as vectors but also as essential secondary hosts in which microfilariae develop to L3 larvae. Along with humidity, temperature is one of the most important environmental factors that regulate larval development of D. repens in mosquitoes. Temperature dictates the time requirements for the development of microfilariae to infective larvae (Sassnau and Genchi 2013). Their development in mosquitoes can last 8–10 days at 28–30 °C, 11–12 days at 24 °C and 16–20 days at 22 °C (Cancrini et al. 1988). L3 microfilariae then migrate to the mosquito’s proboscis, and from there, they are later inoculated to a new host during blood feeding. Transmission of dirofilariasis is dependent upon the presence of sufficient numbers of infected, microfilaraemic dogs, susceptible mosquitoes and a suitable climate to permit extrinsic incubation of parasite in the mosquito vector (Medlock et al. 2007; Genchi et al. 2009).
When assessing the role and importance of mosquitoes in the epidemiology of Dirofilaria spp., it is necessary to consider the bionomics as such and the vector’s capacity. Important attributes in the bionomics of mosquitoes in filarial transmission include the following: the vector’s behaviour on the basis of which it searches for competent hosts, its ability to disperse from the place of reproduction, the vector’s geographical distribution, the vector’s activity time horizon, the number of generations per year and the vector’s population size and seasonal occurrence. The interactions between animal/human, mosquito and nematode biology contribute to the clinical spectrum and geographical distribution of Dirofilaria (Genchi et al. 2009). A vector’s capacity relates to the potential for pathogen transmission via the insect population and includes the flying range of the insect, the host and the environmental variable parameters, including vector’s occurrence, vector’s survival, intensity of bite and transmission, preferences and occurrence of the host (Saegerman 2008).
To the best of the author’s knowledge, no studies have been carried out regarding mosquitoes as vectors of dirofilariasis in Slovakia. Entomological and molecular studies have been performed in this study to determine potential mosquito species involved in circulation of these zoonotic microfilariae in endemic region.
Materials and methods
Study area
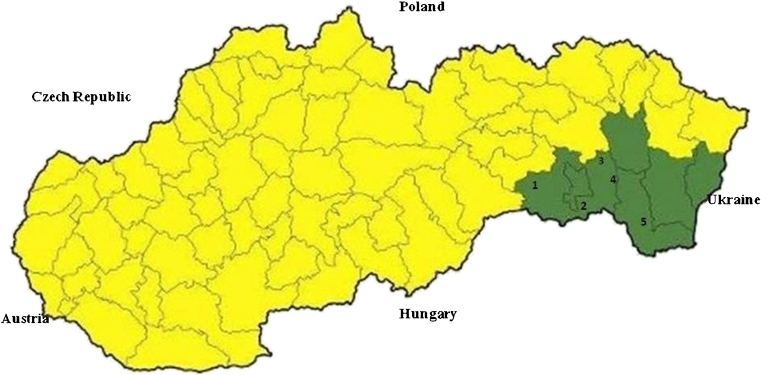
The research was concentrated on four locations situated in the area of the Košická Basin (Panovce, Gynov, Beniakovce, Košické Olšany) and one location in Michalany (District of Trebisov), located in the Eastern Slovak Lowland (Fig. 1). The Košická Basin lies in the south-eastern part of Slovakia. In the west, it borders with the Slovak Karst and the Slovak Ore Mountains, and in the north, with the Sarisska Highlands and Ondavska Highlands; in the east, it is connected with the Slanske Mountains, and in the south, it forms the border with Hungary. The total area of the basin is 1,153 km2; from the geomorphologic point of view, the territory is mostly of a plane type, and from the hydrological point of view, it contains the basins of Bodva, Hornad, Torysa and Ida Rivers. The basin has a warm and moderately dry climate. The average annual rainfall ranges between 600 and 850 mm; the air humidity is 60–70 %. Almost the entire area has an early onset of spring, summers are rather long (52–60 days) with high average daily temperatures (18–20 °C), and winters are short and mild with average daily temperatures between −3 and −6 °C (Slovak Hydrometeorological Institute 2011), with a low number of days with snow cover. The long-term average annual air temperature is 8.7 °C. The area of Eastern Slovak Lowland is situated near the borders with Ukraine and Hungary. In the north up to the north-east, it is surrounded by the Vihorlat Mountains; in the north, by the Beskidian Piedmont; in the north-west and west, by the Slanske Mountains; and in the south-west, by the Zemplín Mountains. The total area is 2,500 km2 with the altitude of 94–200 m above sea level. The area has a fan-pattern network of rivers comprising the Bodrog, Ondava, Latorica, Laborec, Uh and Topľa Rivers. The region has a mild and dry climate. The average annual rainfall is 600–750 mm. In winter months, the temperatures range between −2 and −4 °C. Summers are long (52–70 days) with average temperatures of 17–20 °C. The long-term average annual air temperature is 9–10 °C.
Fig. 1.
Territory of Slovakia, with administrative districts and study sites (1 Panovce, 2 Gynov, 3 Beniakovce, 4 Kosicke Olsany, 5 Michalany)
Mosquito trapping
Mosquitoes were sampled using CO2-baited CDC light traps which were exposed from 5–6 p.m. to 7–8 a.m. of the following day. Collections were done in each site every week during April to August. Mosquitoes were collected from traps each morning within 30 min of dawn. During transportation from the field, the collected individuals were kept on dry ice. After transport to the laboratory at the Department of Parasitology, mosquitoes were knocked down by placing trap containers in a −18 °C freezer for 15–30 min and subsequently separated by species and sex. Mosquitoes were identified using available identification keys (Kramar 1958; Becker et al. 2010).
Set of biological material for the PCR analysis
For the PCR analysis, we used 3,600 adult female mosquitoes which were divided, based on the species diagnostics, into 72 pools, each pool containing 50 individuals of the same species, collected on the same location. Each pool was examined separately for the presence of D. immitis and D. repens.
Homogenization of mosquitoes
The collected mosquitoes were mechanically disrupted using a ceramic blender in 500 μL of phosphate-buffered saline under sterile conditions.
Genomic DNA isolation
The total genomic DNA was extracted from 100 μL of the mosquito homogenate with QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions.
PCR procedure
Primers were designed to amplify approximately 200-bp region of mitochondrial cytochromoxidase subunit I gene of Dirofilaria spp. parasites (Rishniw et al. 2006). PCR amplification was performed with two sets of primers: DI COI-F1 (5′-AGT GTA GAG GGT CAG CCT GAG TTA -3′) and DI COI-R1 (5′- ACA GGC ACT GAC AAT ACC AAT-3′) for detection of D. immitis and DR COI-F1 (5′- AGT GTT GAT GGT CAA CCT GAA TTA-3′) and DR COI-R1 (5′- GCC AAA ACA GGA ACA GAT AAA ACT-3′) for detection of D. repens. Primers used in our study are routinely employed in molecular diagnostics and genotyping of D. immitis and D. repens in clinical samples (dog blood) as well as in mosquito vectors. Each reaction tube contained 75 mmol/L Tris–HCl (pH 8.8), 20 mmol/L (NH4)2SO4, 0.001 % Tween 20, 2.5 mmol/L MgCl2, 200 mmol/L mixture of dNTPs, 2.5 U Taq purple DNA polymerase (Top-Bio, Czech Republic) and 25 pmol of respective primer pair. PCR reaction was performed in PTC-200 Gradient Thermal Cycler (MJ Research, USA) under the following conditions: initial denaturating step at 94 °C for 2 min, followed by denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s consisting of 32 cycles and final extension at 72 °C for 7 min. The PCR products were then separated on 1.5 % agarose gel, stained with GelRed (Biotium, USA) and visualised by UV light. DNA extraction, PCR handling as well as post-PCR procedures were done in separate rooms to avoid possible cross-contamination of the samples. Specific PCR product was further characterised by sequence analysis.
Sequence analysis of PCR product
The PCR product was purified by means of precipitation in PEG/Mg/NaAc (26 % polyethylene glycol, 6.5 mM MgCl2·6H2O, 0.6 M NaAc·3H2O). Direct sequencing of purified PCR product was performed with the BigDye™ Terminator Cycle Sequencing Ready Reaction Kit version 1.1 (Applied Biosystems, USA) according to the manufacturer’s instructions and purified with EtOH/EDTA precipitation. The sequencing was performed on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems, USA). PCR amplicon was multiple sequenced from both directions to ensure high quality reads. The DNA sequences were edited and aligned using the Seqman module within Lasergene v. 6.0 (DNASTAR, Inc., USA) and also checked manually. The FASTA format and BLAST programme (http://www.ncbi.nlm.nih.gov/blast) of the National Center for Biotechnology Information (Bethesda, MD, USA) were used for database searches.
Results
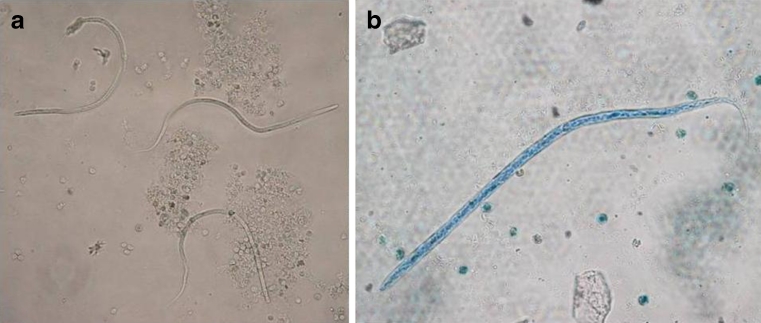
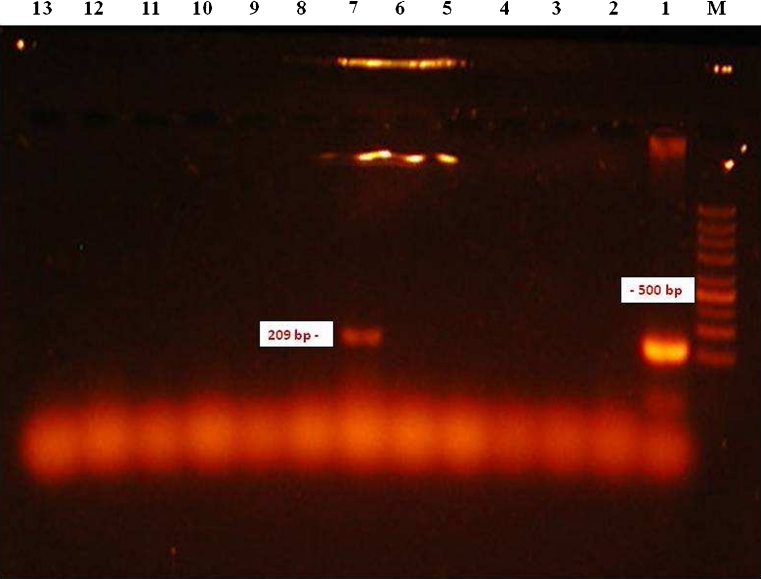
Molecular detection of microfilariae (Fig. 2) in vectors was carried out using 3,600 mosquitoes of eight species (Aedes vexans, Aedes cinereus, Aedes rossicus, Culex pipiens, Culiseta annulata, Ochlerotatus sticticus, Ochlerotatus cantans and Ochlerotatus caspius) (Table 1). The PCR analysis confirmed the presence of D. repens DNA in one pool (no. 10) of A. vexans mosquitoes (Fig. 3) collected on the location in Košické Olšany village (Fig. 1). The identity of positive specimen was confirmed by direct sequencing of PCR product which has shown 100 % homology with sequence attributed to D. repens (GenBank accession number AJ271614).
Fig. 2.
D. repens microfilariae from a dog living in endemic area after concentration with the Knott’s test (a) (×200) and after Diff-Quick staining (b) (×400)
Table 1.
Locations, species and numbers of examined mosquitoes
| Location | Mosquito species | Number of examined mosquitoes/number of examined pools |
|---|---|---|
| Panovce | A. cinereus | 600/12 |
| A. rossicus | 50/1 | |
| A. vexans | 100/2 | |
| C. pipiens | 50/1 | |
| O. cantans | 250/5 | |
| O. sticticus | 150/3 | |
| Gynov | A. vexans | 150/3 |
| C. annulata | 50/1 | |
| C. pipiens | 50/1 | |
| O. sticticus | 50/1 | |
| Košické Olšany | A. vexans | 600/12 |
| C. pipiens | 150/3 | |
| Beniakovce | A. vexans | 300/6 |
| C. pipiens | 300/6 | |
| O. caspius | 100/2 | |
| Michalany | A. vexans | 600/12 |
| C. pipiens | 50/1 | |
| Total | 3,600/72 |
Fig. 3.
Gel electrophoresis of the amplified product of the COI gene of D. repens. Lane M 1,500–200 bp marker; 1 positive control; 2–6, 8–13 negative samples; 7 positive sample
Discussion
Previous research on dirofilariasis in carnivores in Slovakia has provided important information on causative agents of the disease and prevalence in dogs in different areas and districts (Miterpakova et al. 2008, 2009, 2010, 2012; Iglodyova et al. 2012a, b). Dirofilariasis has become a problem which occurs each year, and the number of canine cases is constantly growing. In spite of the fact that it is a zoonotic disease, in Slovakia, there is still lack of information on current and competent vectors. Available literature sources report more than 70 mosquito species of the Culicidae family (genera: Culex, Aedes, Ochlerotatus, Anopheles, Armigeres, Coquilettidia and Mansonia) that are able to transmit dirofilariae (Cancrini et al. 1995; Pampiglione and Rivasi 2000; Vezzani and Carbajo 2006; Vezzani et al. 2011). Many of these mosquito species occur in Slovakia as well (Orszagh et al. 2001; Jalili et al. 2000), and some of them are common. For the initial stage of the research, we have chosen the mosquitoes collected at five locations in Eastern Slovakia. Selection of individual locations was based on their geographical position (Košická Basin and Eastern Slovak Lowland), local and climatic conditions, habitats suitable for life of vectors and the information on infection-positive findings in definite hosts. The Kosice town district and the Kosice vicinity district lie in the Košická Basin and have very favourable climatic conditions for vector development. By our existing research on this territory, we have established the presence of 16 mosquito species (Bockova and Kocisova 2011). Results of the researches on canine dirofilariasis within the monitored territory indicate that the prevalence increased from 6.4 % in years 2007–2010 to 11.6 % in 2011 (Iglodyova et al. 2012b). At Košické Olšany, where we confirmed the presence of microfilariae in A. vexans, no research focused on detection of microfilaraemia in dogs has been carried until now. Michalany village is situated in one of the endemic areas of dirofilariasis in the district of Trebisov that belongs to the warmest areas in Slovakia. Prevalence of canine dirofilariasis in this district reaches 54.4 % (Iglodyova et al. 2012a).
Batches of mosquitoes for PCR analysis were chosen on the basis of theoretical information which suggests that A. vexans, O. caspius and C. pipiens (Latrofa et al. 2012; Yildirim et al. 2011) are the potential vectors of dirofilariae. Mosquitoes A. vexans, C. pipiens and Aedes (Stegomyia) albopictus are regarded to be the most important transmitting agents of D. repens and D. immitis in Europe. By the PCR analysis and subsequent sequencing, we have proved the presence of DNA of D. repens microfilariae in A. vexans mosquitoes. In similar trials carried out in north-east Italy (Latrofa et al. 2012), the authors report positive findings of D. repens microfilariae in C. pipiens and D. immitis in A. vexans, O. caspius and C. pipiens. Similar results were achieved in Turkey (Yildirim et al. 2011), where the authors state that the main vector of D. immitis is A. vexans and C. pipiens.
It is interesting to point out that in Slovakia, D. immitis in dogs has so far occurred only in co-infection with D. repens (seven cases, 2.1 % prevalence) (Miterpakova et al. 2008), while in Turkey or in Italy, it occurs alone in a prevalence between 2 and 30 % (Yildirim et al. 2011; Latrofa et al. 2012).
A. vexans and C. pipiens mosquitoes are among the most common mosquito species in Slovakia. A. vexans has several attributes of an ideal vector, especially its wide geographical distribution, short development cycle (in suitable conditions lasting 1–3 weeks), polycyclicity and ability to form multiple populations, especially after floods, ability of females to fly to distances more than 15 km away from the reproduction site and wide host preference.
C. pipiens is originally an ornitophilic mosquito (Kramar 1958; Becker et al. 2010) but has now adopted endophagic and anthropophagic behaviour in central and north Europe where it now also searches for human blood outdoors, as it happens in southern parts of the continent. This pattern also overlaps with the spread of canine D. immitis and D. repens infection in central and north-eastern countries (e.g. south Switzerland, Germany, Czechland, Hungary, Serbia and Slovakia) (Tasic et al. 2008; Genchi et al. 2009; Pantchev et al. 2009). In some parts of Eastern Slovakia (Kosice and Kosice vicinity), we very often encounter feeding on humans and domestic animals. Its role as a vector is primarily connected with the transmission of avian plasmodia, Sindbis alphavirus (Berezin et al. 1972) and West Nile Flavivirus (Anderson and Main 2006; Hubálek 2008).
Both these mosquito species are most abundant during the hottest months of the year, which increases the probability of spreading temperature-dependent pathogens, for example, dirofilariae. Our conclusion from this study corresponds to the finding by Iglodyova (personal information), which states that the largest number of microfilaraemic dogs occurs in the period between spring and summer and between summer and autumn, i.e. when the first generation or winter-surviving females emerge and are at maximum abundance.
Of the confirmed vectors of Dirofilaria spp. occurring in Slovakia, the most likely potential vectors include Anopheles maculipennis s.l., which occurs frequently in the Slovak lowlands, as well as O. caspius and A. cinereus. They could also include Anopheles hyrcanus, Ochlerotatus geniculatus and Coquilettidia richiardii, which, however, are only sporadically collected in the monitored areas.
Eastern Slovakia has often proved to have exceptionally favourable conditions for disease transmission via vectors. Until the 1950s, it was an endemic area for malaria; in the last 10 years, it has been shown to be a canine babesiosis focus, and dirofilariasis is now spreading in this area as well.
Acknowledgments
We thank Clive Boase (Pest Management Consultancy) for critically reviewing the manuscript and for his helpful suggestion and Juraj Pesko for the excellent technical assistance. This research was supported by grant VEGA no. 1/0236/12, basic research of the National Reference Laboratory for Pesticides of the University of Veterinary Medicine and execution of the Project “Centre of Excellence for Parasitology” (ITMS code: 26220120022) upon the support of the operation programme research and development, financed by the European Regional Development Fund (part 0.5). This study was partially funded by the EU grant FP7-261504 EDENext (http://www.edenext.eu); the publication is catalogued by the EDENext Steering Committee as EDENext147. We also thank the Operational Programme Education for Competiveness project CEB (CZ.1.07/2.3.00/20.0183).
Footnotes
The contents of this paper are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
References
- Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl CH, Kaiser A. Mosquitoes and their control. 2. Heidelberg: Springer; 2010. [Google Scholar]
- Berezin VV, Semenov BF, Reshetnikov IA, Bashkirtsev VN. Importance of birds in natural cycle of mosquito-borne viruses in the Volga delta. In: Cherepanov AI, editor. Transcontinental connections of migratory birds and their role in the distribution of arboviruses. Novosibirsk: Nauka; 1972. pp. 310–313. [Google Scholar]
- Bockova E, Kocisova A. Species composition of mosquitoes (Culicidae) in Bodrog and Hornád river-basins. Folia Veterinaria. 2011;55:45–46. [Google Scholar]
- Cancrini G, Yanchang S, Della Torre A, Coluzzi M. Influenza della temperatura sullo sviluppo larvale di Dirofilaria repens in diverse specie di zanzare. Parassitologia. 1988;30:38. [Google Scholar]
- Cancrini G, Pietrobelli M, Frangipane di Regalbono A, Ampieri MP, Della Torre A. Development of Dirofilaria and Setaria nematodes in Aedes albopictus. Parassitologia. 1995;37:141–145. [PubMed] [Google Scholar]
- Genchi C, Rinaldi L, Mortarino M, Genchi M, Cringoli G. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009;163:286–292. doi: 10.1016/j.vetpar.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Hubalek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103(1):29–43. doi: 10.1007/s00436-008-1064-7. [DOI] [PubMed] [Google Scholar]
- Iglodyova A, Miterpakova M, Hurnikova Z, Antolova D, Dubinsky P, Letkova V. Canine dirofilariosis under specific environmental conditions of the Eastern Slovak Lowland. Ann Agric Environ Med. 2012;19:57–60. [PubMed] [Google Scholar]
- Iglodyova A, Miterpakova M, Baksiova M (2012b) Dirofilariosis in Košice district (in Slovak). http://www.preveda.sk/conference/article/id=446/. 2012.06.12
- Jalili NA, Orszagh I, Halgos J, Labuda M. Mosquito (Diptera: Culicidae) of Slovakia. Europ Mosq Bull. 2000;6:20–26. [Google Scholar]
- Kramar J (1958) Biting mosquitoes—Culicinae (In Czech). Fauna ČSR Nakladatelství Československé akademie věd Praha
- Latrofa MS, Montarsi F, Ciocchetta S, Annoscia G, Dantas-Torres F, Ravagnan S, Capelli G, Otrando D. Molecular xenomonitoring of Dirofilaria immitis and Dirofilaria repens in mosquitoes from north-eastern Italy by real-time PCR coupled with melting analysis. Parasites & Vectors. 2012;5:76–82. doi: 10.1186/1756-3305-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy J (1856) A synopsis of Entozoa and some of their ecto‐congeners observed by the author. P Acad Nat Sci Phila 8:42–58
- McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans: a review. Adv Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- Medlock JM, Barras I, Kerrod E, Taylor MA, Leach S. Analysis of climatic predictions for extrinsic incubation of Dirofilaria in the United Kingdom. Vector Borne Zoonot Dis. 2007;7:4–14. doi: 10.1089/vbz.2006.0564. [DOI] [PubMed] [Google Scholar]
- Miterpakova M, Antolova D, Hurnikova Z, Dubinsky P, Pavlacka A, Nemeth J. Dirofilaria infections in working dogs in Slovakia. Helminthologia. 2010;84:173–176. doi: 10.1017/S0022149X09990496. [DOI] [PubMed] [Google Scholar]
- Miterpakova M, Antolova D, Hurnikova Z, Dubinsky P. Dirofilariosis in Slovakia—a new endemic area in Central Europe. Helminthologia. 2008;45:20–23. doi: 10.2478/s11687-008-0003-6. [DOI] [Google Scholar]
- Miterpakova M, Hurnikova Z, Antolova D, Dubinsky P (2009) Climate changes implicated for Dirofilaria dissemination in Slovakia. Wiad Parazytol 55:429–431 [PubMed]
- Miterpakova M, Iglodyova A, Hurnikova Z. Canine subcutaneous dirofilariosis—noteless and neglected (difficulties in disease monitoring) Helminthologia. 2012;49:225–228. doi: 10.2478/s11687-012-0042-x. [DOI] [Google Scholar]
- Ondriska F, Lengyel D, Miterpakova M, Lengyelova B, Streharova A, Dubinsky P. Human dirofilariosis in the Slovak Republic—a case report. Ann Agric Environ Med. 2010;17:169–171. [PubMed] [Google Scholar]
- Orszagh I, Halgos J, Jalili N, Labuda M. Mosquito (Diptera, Culicidae) of Slovakia II. Europ Mosq Bull. 2001;11:1–26. [Google Scholar]
- Pampiglione S, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Parassitologia. 2000;42:231–154. [PubMed] [Google Scholar]
- Pantchev N, Norden N, Lorentzen L, Rossi M, Rossi U, Brand B, Dyachenko V. Current surveys on the prevalence and distribution of Dirofilaria spp. in dogs in Germany. Parasitol Res. 2009;105:S63–S74. doi: 10.1007/s00436-009-1497-7. [DOI] [PubMed] [Google Scholar]
- Railliet A, Henry A (1911) Researches sur les ascarides des carnivores. Completed Rendus des Séances de Société Biologique de Paris 70:12–16
- Rishniw M, Barr SC, Simpson KW, Frongillo M, Franz M, Dominquez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006;135:303–314. doi: 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Rocconi F, Di Tommaso M, Traversa D, Palmier C, Pampurini F, Boari A. Allergic dermatitis by Dirofilaria repens in a dog: clinical picture and treatment. Parasitol Res. 2012;111:493–496. doi: 10.1007/s00436-012-2833-x. [DOI] [PubMed] [Google Scholar]
- Saegerman C. Epizootology of Bluetongin European Union (in Slovak) Slov Vet Cas. 2008;33:217–220. [Google Scholar]
- Sassnau R, Genchi C. Qualitative risk assessment for the endemisation of Dirofilaria repens in the state of Brandenburg (Germany) based on temperature-development vector competence. Parasitol Res. 2013 doi: 10.1007/s00436-013-3431-2. [DOI] [PubMed] [Google Scholar]
- Slovak Hydrometeorological Institute (2011) Flood situation in eastern Slovakia in winter 2010/2011. http://www.shmu.sk/File/HIPS/Povodn_situacia_toky_vychod_Slov_v_zime_2010-2011.pdf. pp 7–13. 2012.04.10
- Tasic A, Rossi L, Tasic S, Miladinovic-Tasic N, Ilic T, Dimitrijevic S. Survey of canine dirofilariasis in Vojvodina, Serbia. Parasitol Res. 2008;103:1297–1302. doi: 10.1007/s00436-008-1132-z. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Carbajo AE. Spatial and temporal transmission risk of Dirofilaria immitis in Argentina. Int J Parasitol. 2006;36:1463–1472. doi: 10.1016/j.ijpara.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Mesplet M, Eiras DF, Fontanarrosa MF, Schnittger L. PCR detection of Dirofilaria immitis in Aedes aegypti and Culex pipiens from urban temperate Argentina. Parasitol Res. 2011;108:985–989. doi: 10.1007/s00436-010-2142-1. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Inci A, Duzlu O, Biskin Z, Ica A, Sahin I. Aedes vexans and Culex pipiens as the potential vectors of Dirofilaria immitis in Central Turkey. Vet Parasitol. 2011;178:143–147. doi: 10.1016/j.vetpar.2010.12.023. [DOI] [PubMed] [Google Scholar]