Abstract
George Ralph Mines studied the basic principles of reentry and published his data in The Journal of Physiology in 1913. Exactly 100 years later we discuss his first electrophysiological experiments and how his results lead to the insight that was the basis for the treatment of the clinical arrhythmias seen in Wolff–Parkinson–White syndrome.
 |
Bas J. Boukens was born in Hoorn on december 7, 1982. After studying Biomedical at the University of Utrecht, he started to work on his PhD-thesis at the Heart Failure Research Center, University of Amsterdam. He defended his thesis with honours in 2012. Then he worked as a postdoctoral researcher at the department of Anatomy, Embryology and Physiology, University of Amsterdam. He is currently a research scientist at the department of Biomedical Engineering, Washington University, St Louis. The goal of his research is to understand the molecular mechanism underlying electrophysiological remodeling during heart disease. Michiel J. Janse was born in Amsterdam on July 23, 1938. He Studied Medicine at the University of Amsterdam and is a staff member of the Department of Cardiology, University of Amsterdam since 1966. In 1985, he became professor of Experimental Cardiology. In 1993 he received the Distinguished Scientist Award of the North American Society of Pacing and Electrophysiology (NASPE; now Heart Rhythm Society). From 1995 to 2002 he was Editor-in-Chief of Cardiovascular Research. In 1997 he became an Honorary Fellow of the Royal College of Physicians. From 2004 on he is a visiting professor at Columbia University, New York. The main focus of his research is to understand mechanisms of arrhythmias.
Introduction
A hundred years ago, George Ralph Mines (Fig. 1) described in this journal the principles of reentrant excitation (Mines, 1913). He used a ring-like preparation of a tortoise heart (Figs 2 and 3).
Figure 1. George Ralph Mines.

A photograph probably taken by Mrs Dorothy Thacker (at that time Dorothy Dale) at the Marine Biological Laboratory Plymouth, in the summer of 1911. In the same issue of this journal in which Mines paper ‘On the Dynamic Equilibrium in the Heart’ was published, there is also a paper by Dale and Mines on the influence of novel stimulation on the electrocardiogram. The late Professor David A. Rytand, of Stanford University, made this picture available to one of us (M.J.J.).
Figure 2.

A schematic drawing of the tortoise preparation that Mines published in 1913 in his paper ‘On the Dynamic Equilibrium in the Heart’ (Mines, 1913).
Figure 3.

Mines's diagram to explain that sustained reentry will occur in the presence of unidirectional block if conduction is slowed and the refractory period duration is decreased. A stimulated impulse leaves in its wake absolutely refractory tissue (black area) and relative refractory tissue (dotted area). In both A and B, the impulse conducts in one direction only. In A, because of fast conduction and a long refractory, the tissue is still absolutely refractory when the impulse has returned to its site of origin. In B, because of fast conduction and a long refractory period the tissue has recovered excitability by the time the impulse has reached the site of origin, and the impulse continues to circulate in the ring.(from Mines, 1913).
‘On stimulating any part of the heart there was, after a light pause, contraction in each of the other parts. After stimulating several times, the following condition appeared. The four portions of the heart marked V1, V2, A1, A2, contracted in the order given, with distinct pauses between the successive portions. This cycle of events was repeated over and over again without any further external stimulation … I venture to suggest that a circulating excitation of this kind may be responsible for some cases of paroxysmal tachycardia as observed clinically.’
In 1914, he repeated this suggestion and described in detail the mechanism of a clinical syndrome that was not yet discovered (Mines, 1914).
Discovery of the mechanism underlying WPW
In 1913, Stanley Kent claimed to have described a human heart with an extra connection between right atrium and right ventricle (Fig. 4A; Kent, 1913). The claim of Kent was incorrect, as was shown before by William His Jr who found that only a single bundle connected the atria with the ventricular myocardium (His, 1893) and by Sumao Tawara who discovered that this bundle originated from a complicated network of small muscle fibres (Tawara, 1906). However, in 1914, Mines repeated his earlier suggestion (Mines, 1913) that circulating excitations might be responsible for clinical arrhythmias in the light of the new, albeit incorrect, histological demonstration of Stanley Kent: ‘that an extensive muscular connexion is to be found at the right hand margin of the heart. Supposing that for some reason an impulse from the auricle reached the main A–V bundle but failed to reach this “right lateral” connexion, it is possible then that the ventricle would excite the ventricular end of this right lateral connexion. The wave spreading then to the auricle might be expected to circulate around the path indicated’ (Mines, 1914). This was written 16 years before Wolff, Parkinson and White described the syndrome that now bears their name (Fig. 4B; Wolff et al. 1930), 18 years before Holzmann and Scherf ascribed the abnormal ECG in these patients to pre-excitation of the ventricles via an accessory atrioventricular bundle (Fig. 4C) (Holzmann & Scherf, 1932), and 19 years before Wolferth and Wood published diagrams showing the pathways of circulating excitation (Wolferth & Wood, 1933). Wood et al. also described the histology of a right-sided accessory pathway in 1943: ‘So far as we are aware, no one has yet presented histologic proof of the existence of accessory muscular connections between the auricles and ventricles of a patient with this type of electrocardiographic abnormality. The present paper furnishes this proof.’ (Wood et al. 1943). Öhnell was the first to describe in detail the histology of left-sided connections in 1944 (Fig. 4D; Öhnell, 1944).
Figure 4.
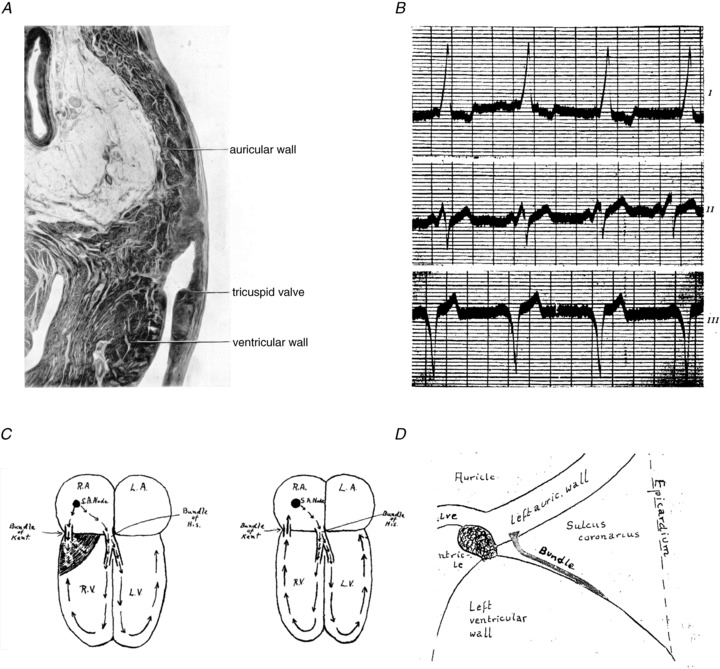
A, a section through the auriculo-ventricular junction at the right-hand margin of the heart that was published by Kent in 1913 (Kent, 1913). Note that this section does not show any structure crossing the plane of right-sided insulation. B, the electrocardiogram is shown of one of the patients from the study that Wolff, Parkinson and White published in 1930.(Wolff et al. 1930) C, left: a drawing that Wolferth made in 1933 in which he hypothesized that ‘premature transmission of the impulse through the bundle Kent to the right ventricle’ is the cause for the short PR interval. (Wolferth & Wood, 1933). C, right: the reentrant circuit during paroxysmal tachycardia is depicted, and is just as Mines described. D, a schematic picture giving the approximate position of the muscle bundle which was published in the thesis of Öhnell in 1944 ( Öhnell, 1944).
Ablating the accessory pathway
The essential feature of the findings presented in these studies is that the accessory connection skirts the annulus fibrosus and courses through the epicardial fat to join atrial and ventricular myocardium. This has important implications for the surgical interruption of the accessory connections. In the laboratory of Dirk Durrer a cartoon hang on the wall during the 1960s depicting Drs Howard B Burchell and Dirk Durrer, carrying long knives, standing along the bedside of a patient with WPW syndrome. The caption was: ‘Cut the Kent Bundle’. The idea was to make a long incision in the atrium, just above the annulus fibrosus, at the site where intra-operative mapping revealed earliest ventricular excitation just below the annulus. The early studies attempting to surgically abolish preexcitation often showed an initial disappearance of preexcitation, presumably due to the surgical trauma, but a recovery of preexcitation in the following weeks (Burchell et al. 1967; Cole et al. 1970; Lindsay et al. 1971; Wellens et al. 1974). In 1978, Becker et al. (1978) made a detailed histological study of seven hearts from patients with WPW syndrome who had died from other causes. They found that the accessory connection, similar to the connection that was found by Öhnell (1944), did not cross the annulus fibrosus but coursed in the epicardial fat. It was Sealy and colleagues (1974) who realized that in order to surgically remove the accessory connection it is necessary to scrape away the fat from the epicardial aspect of the annulus, and they developed a ‘fish hook’ for that purpose. In other words: do not cut the Kent bundle.
Bundle of Kent: a misnomer
The first article using the term ‘Kent bundle’ was that of Wolferth and Wood in 1933 (Wolferth & Wood, 1933). Subsequently, many papers, and even recent textbooks, continued the use of ‘Kent bundle’ (Hu et al. 2011; Aanhaanen et al. 2011a; Mills et al. 2013). Moreover, to date, lay sources found on the internet (http://www.uptodate.com; http://www.wikipedia.org) describe accessory connections that are present in WPW patients as ‘bundles of Kent’. It is ironical that Kent's mission was to show that multiple atrioventricular connections were responsible for atrioventricular conduction in the normal heart, (Kent, 1893), and also that what Kent described in 1913 is not at all the usual accessory pathway as found in the WPW syndrome (Kent, 1913). He did not describe a muscular connection but a node-like structure of an extensive atrioventricular ring of specialized tissue present during development (Becker & Anderson, 1981; Aanhaanen et al. 2011b). As stated by Anderson and Becker: ‘there are good scientific reasons for discontinuing the use of “Kent bundle”, the most important being that Kent did not describe connections in terms of morphology we know today. If an eponym is really necessary, then let us call them nodes of Kent’ (Anderson & Becker, 1981). Therefore, to avoid confusion, we suggest that the term ‘bundle of Kent’ be replaced by ‘accessory atrio-ventricular muscle bundles’ as suggested by Anderson et al. (1975).
The contributions of George Ralph Mines
In his 1913 and 1914 papers, written at the ages of 27 and 28 years, Mines formulated the essential characteristics of reentry:
Mines described an experiment on an isolated auricular preparation from a dog-fish, slit up in such a way as to form a ring. Normally, a stimulus provoked two contraction waves that ran in each direction and met on the opposite side of the ring where they died out. However: ‘Repeated the stimulus at diminishing intervals and after several attempts started a wave in one direction and not in the other. The wave ran all the way round the ring and continued to circulate going around about twice a second. After this had continued for two minutes extra stimuli were thrown in. After several attempts the wave was stopped.’(Mines, 1914). Thus, Mines also described the principle of anti-tachycardia pacing.
Mines described the relationship between conduction velocity and refractory period duration, as illustrated in Fig. 1, and thus, can be considered to be the first to formulate the ‘wavelength’ concept. In the 1913 paper he wrote: ‘With increasing frequency of stimulation, each wave of excitation in the heart muscle is propagated more slowly but lasts a shorter time at any point in the muscle. The wave excitation becomes slower and shorter.’.(Mines, 1913)
Mines realized that establishing the activation sequence during an arrhythmia is not sufficient to prove reentry: ‘The chief error to be guarded against is that of mistaking a series of automatic beats originating in one point of the ring and travelling round it in one direction only owing to complete close to the point of origin of the rhythm on one side of this point …. Severance of the ring will obviously prevent the possibility of circulating excitations but will not upset the course of a series of rhythmic spontaneous excitations unless by a rare chance the section should pass through the point actually initiating the spontaneous rhythm’.(Mines, 1914) Thus, Mines set the stage for catheter ablation.
These criteria for reentrant excitation are as valid now as they were 100 years ago.
Although it has often been suggested that Mines died during a self-experimentation, recent evidence casts doubt on this claim. We await with especial interest the outcomes of further ongoing investigations regarding the cause of his tragically early death.
Acknowledgments
We want to thank Dr. Ruben Coronel for critically reading the manuscript.
Additional information
Competing interests
None.
Funding
This work is supported by the Netherlands Heart Foundation (2008B062).
References
- Aanhaanen WT, Boukens BJ, Sizarov A, Wakker V, de Gier-de VC, van Ginneken AC, Moorman AF, Coronel R, Christoffels VM. Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice. J Clin Invest. 2011a;121:534–544. doi: 10.1172/JCI44350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanhaanen WT, Moorman AF, Christoffels VM. Origin and development of the atrioventricular myocardial lineage: insight into the development of accessory pathways. Birth Defects Res A Clin Mol Teratol. 2011b;91:565–577. doi: 10.1002/bdra.20826. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Becker AE. Stanley Kent and accessory atrioventricular connections. J Thorac Cardiovasc Surg. 1981;81:649–658. [PubMed] [Google Scholar]
- Anderson RH, Becker AE, Brechenmacher C, Davies MJ, Rossi L. Ventricular preexcitation. A proposed nomenclature for its substrates. Eur J Cardiol. 1975;3:27–36. [PubMed] [Google Scholar]
- Becker AE, Anderson RH. The Wolff–Parkinson–White syndrome and its anatomical substrates. Anat Rec. 1981;201:169–177. doi: 10.1002/ar.1092010118. [DOI] [PubMed] [Google Scholar]
- Becker AE, Anderson RH, Durrer D, Wellens HJ. The anatomical substrates of Wolff–Parkinson–White syndrome. A clinicopathologic correlation in seven patients. Circulation. 1978;57:870–879. doi: 10.1161/01.cir.57.5.870. [DOI] [PubMed] [Google Scholar]
- Burchell HB, Frye RL, Anderson MW, McGoon DC. Atrioventricular and ventriculoatrial excitation in Wolff–Parkinson–White syndrome. (type B). Temporary ablation at surgery. Circulation. 1967;36:663–672. doi: 10.1161/01.cir.36.5.663. [DOI] [PubMed] [Google Scholar]
- Cole JS, Wills RE, Winterscheid LC, Reichenbach DD, Blackmon JR. The Wolff–Parkinson–White syndrome: problems in evaluation and surgical therapy. Circulation. 1970;42:111–121. doi: 10.1161/01.cir.42.1.111. [DOI] [PubMed] [Google Scholar]
- His W., Jr Die Thätigkeit des embryonalen Herzens und deren Bedeutung für die Lehre von der Herzbewegung beim Erwachsenen. (The function of the embryonic heart and its significance in the interpretation of the heart action in the adult.) Arbeiten aus der med Klin zu Leipzig. 1893;1:14–50. (Translation from: Willius FA & Keys TE (1941). Wilhelm His Jr. In Classics of Cardiology, vol. 2, pp. 695. Dover Publications, New York.) [Google Scholar]
- Holzmann M, Scherf D. Uber elektrokardiogramme mit verkurzter Vorhof-Kammer Distanz und positiven P. Zacken Z Klin Med. 1932;121:404–410. [Google Scholar]
- Hu R, Stevenson WG, Lilly LS. Mechanisms of Cardiac Arrhythmias. In: Lilly LS, editor. Pathophysiology of Heart Disease. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2011. pp. 261–278. [Google Scholar]
- Kent AF. Researches on the structure and function of the mammalian heart. J Physiol. 1893;14:i2–254. [PMC free article] [PubMed] [Google Scholar]
- Kent A. Observations on the auriculo-ventricular junction of the mammalian heart. Quart J Exp Physiol. 1913;7:193–195. [Google Scholar]
- Lindsay AE, Nelson RM, Abildskov JA, Wyatt R. Attempted surgical division of the preexcitation pathway in the Wolff–Parkinson–White syndrome. Am J Cardiol. 1971;28:581–585. doi: 10.1016/0002-9149(71)90101-9. [DOI] [PubMed] [Google Scholar]
- Mills KI, Anderson J, Levy PT, Cole FS, Silva JN, Kulkarni S, Shinawi M. Duplication of 20p12.3 associated with familial Wolff–Parkinson–White syndrome. Am J Med Genet A. 2013;161:137–144. doi: 10.1002/ajmg.a.35701. [DOI] [PubMed] [Google Scholar]
- Mines GR. On circulating excitations in heart muscle and their possible relation to tachycardia and fibrillation. Trans Roy Soc Can. 1914:43–52. [Google Scholar]
- Mines G. On dynamic equilibrium of the heart. J Physiol. 1913;46:349–382. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhnell RF. Pre-excitation, a cardiac abnormality, pathophysiological, patho-anatomical and clinical studies of excitatory spread phenomenon bearing upon the problem of the WPW. (Wolff–Parkinson–White) electrocardiogram and paroxysmal tachycardia. Acta Med Scand. 1944;152:74–167. [Google Scholar]
- Sealy WC, Wallace AJ, Ramming KP, Gallagher JJ, Svenson RH. An improved operation for the definitive treatment of the Wolff–Parkinson–White syndrome. Ann Thorac Surg. 1974;17:107–113. doi: 10.1016/s0003-4975(10)65617-2. [DOI] [PubMed] [Google Scholar]
- Tawara S. Das Reizleitungssystem Des Säugetierherzens. (The Conduction System of the Mammalian Heart.) London: Imperial College Press; 1906. (Originally published by Gustav Fischer, Jena, 1906. Translated into English by Kozo Suma and Munehiro Shimada.) [Google Scholar]
- Wellens HJ, Janse MJ, Van Dam RT, van Capelle FJ, Meijne NG, Mellink HM, Durrer D. Epicardial mapping and surgical treatment in Wolff–Parkinson–White syndrome Type A. Am Heart J. 1974;88:69–78. doi: 10.1016/0002-8703(74)90351-2. [DOI] [PubMed] [Google Scholar]
- Wolferth CC, Wood FC. This mechanism of production of short P-R intervals and prolonged QRS complexes in patients with pre-sumably undamaged hearts: Hypothesis of an accessory pathway of auriculo-ventricular conduction. (bundle of Kent) Am Heart J. 1933;8:297–311. [Google Scholar]
- Wolff L, Parkinson J, White PD. Bundle-branch block with short P–R interval in healthy young people prone to paroxysmal tachycardia. 1930. Am Heart J. 1930;5:685–704. doi: 10.1111/j.1542-474X.2006.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood FC, Wolferth CC, Geckeler GD. Histologic demonstration of accessory muscular connections between auricle and ventricle in a case of short P–R interval and prolonged QRS complex. Am Heart J. 1943;25:454–462. [Google Scholar]


