Abstract
There is continuing belief that cardiac parasympathetic postganglionic fibres are sparse or absent from the ventricles. This review of the literature shows that the supposition is a myth. Early studies considered that fine silver-stained fibres coursing amongst ventricle myocardial cells were most likely cardiac parasympathetic postganglionic fibres. The conclusions were later supported by acetyl cholinesterase staining using a method that appeared not to be associated with noradrenaline nerve fibres. The conclusion is critically examined in the light of several recent histological studies using the acetyl cholinesterase method and also a more definitive technique (CHAT), that suggest a widespread location of parasympathetic ganglia and a relatively dense parasympathetic innervation of ventricular muscle in a range of mammals including man. The many studies demonstrating acetylcholine release in the ventricle on vagal nerve stimulation and a high density of acetylcholine M2 receptors is in accord with this as are tests of ventricular performance from many physiological studies. Selective control of cardiac functions by anatomically segregated parasympathetic ganglia is discussed. It is argued that the influence of vagal stimulation on ventricular myocardial action potential refractory period, duration, force and rhythm is evidence that vagal fibres have close apposition to myocardial fibres. This is supported by clear evidence of accentuated antagonism between sympathetic activity and vagal activity in the ventricle and also by direct effects of vagal activity independent of sympathetic activity. The idea of differential control of atrial and ventricular physiology by vagal C and vagal B preganglionic fibres is examined as well as differences in chemical phenotypes and their function. The latter is reflected in medullary and supramedullary control. Reference is made to the importance of this knowledge to understanding the normal physiology of cardiac autonomic control and significance to pathology.
 |
John H. Coote was formerly Bowman Chair of Physiology, Medical School University of Birmingham now Professor Emeritus. He is a specialist in Autonomic Neuroscience. After graduating from the Royal Free Hospital School of Medicine he continued studies of brain control of heart and circulation with Charles Downman, Sophia Jex-Blake Professor of Physiology. In Birmingham he established a group carrying out pioneering studies on cardiac innervation and renal nerves in exercise and blood volume control. He made important new observations on the role of nitric oxide in spinal genital reflexes and in cardiac vagal tone in humans and in the vagal innervation of the ventricle. He described central neuropeptide pathways controlling heart and Kidney.
Introduction
There is a consensus repeated in most medical text books that cardiac sympathetic nerves innervate the sinoatrial (SA) and atrioventricular (AV) nodes, the atria, the ventricles and conducting tissue, whereas the idea that parasympathetic nerve supply is mainly limited to the atria and nodal tissues is perpetuated. This has occurred in spite of many anatomical and physiological studies showing the presence of cardiac vagus nerves coursing throughout the ventricles as well as having a profound influence on ventricular rate and rhythm, and affecting contractility. Recent research accounts have drawn attention to the misconception (Crick et al. 1999; Dhein et al. 2001; Harvey & Belevych, 2003; Kawano et al. 2003; Hoover et al. 2004; Ulphani et al. 2010). Additionally, recent research of cellular and molecular events in ventricle muscle provide substantial knowledge of vagal cholinergic muscarinic regulation of cardiac ventricular function (Casadei, 2001) that further emphasises the importance of vagal innervation of the heart. Despite these contributions, the understanding of fundamental mechanisms of parasympathetic regulation of the heart lags behind our knowledge of sympathetic control. The present review attempts to refocus our sight onto vagal parasympathetic influence on the heart and particularly the ventricles.
Anatomical evidence
Do sympathetic efferents in the cervical vagus nerve project to the heart?
It is first important for me to deal with an oft-repeated supposition regarding the efferent fibre composition of the cervical vagus nerves. In contrast to common belief there are no cardiac sympathetic efferent nerves projecting to the heart via the nodose ganglion in these nerve bundles, at least in the rabbit, cat and dog (see Evans & Murray, 1954). This was clearly shown by Heinbecker & O’Leary (1933a,b) who stimulated the cervical vagus before and after allowing 10–20 days for vagal preganglionic efferent fibres to degenerate following extracranial supranodose ganglion vagotomy. This procedure left sympathetic postganglionic efferents undamaged. Significantly it abolished all cardiac effects on stimulating the cervical vagus. Early studies also showed that those sympathetic postganglionic fibres reported to pass from the cervical sympathetic nerve via the nodose ganglion were destined for the bronchial circulation and pulmonary blood vessels (McSwiney & Spurrel, 1933; Richardson & Hinsey, 1933). Confirmation came in two later studies using similar procedures (Daly & Hebb, 1952; Daly & Evans, 1953). The conjecture of a cardiac vago-sympathetic fibre composition in the cervical nerve trunk may have arisen because stimulation of the cervical vagus lower in the neck may excite cardiac sympathetic branches from the stellate ganglion (Randall & Armour, 1977). This may be the explanation for the recent report on two dogs that displayed a sympathetic tachycardia during stimulation of cervical vagus nerve via an implanted electrode (Onkka et al. 2013).
Therefore, providing stimulating electrodes are positioned on the rostral part of the vagus nerve nearer to the nodose ganglion one can be confident that cardiac sympathetic postganglionic nerves are not activated together with vagal preganglionic fibres.
Vagal nerve supply to the chambers of the heart
It is universally accepted that parasympathetic nerves densely innervate the atria, SA and AV nodes and conducting tissue. This has been extensively described in several reviews (Randall, 1984; Levy & Martin, 1996). Accordingly it is agreed that activity in vagal fibres to the heart can cause cardiac slowing via decreased excitability at the SA and AV nodes, and a decrease in force of atrial contraction and in rate of atrioventricular conduction. However, there is now substantial evidence for a vagal nerve influence on ventricular rhythm and contractility. These atrial and ventricular effects are dependent upon excitation of ganglion cells located in discrete clusters on the epicardium of the atria and in atrial and ventricular septum (Singh et al. 1996; Gatti et al. 1997; Dickerson et al. 1998). There is evidence that each cluster of postganglionic neurones targets different regions of the heart and selectively controls cardiac muscle action not only in adjacent regions but also in all four chambers of the heart (Yuan et al. 1994; Armour, 2008). This is described as a topographical functional organisation of the vagal cardiac ganglia (Randall et al. 1987; Billman et al. 1989; Sampaio et al. 2003). However, it had been generally thought that parasympathetic postganglionic fibres in the ventricles are sparse and effects on the ventricular muscle are minimal and highly dependent on interaction with sympathetic activity. This idea is critically examined in the following sections.
Evidence for vagal innervation of the cardiac ventricles
Historically, anatomical studies (Cullis & Tribe, 1913; Nonidez, 1939, 1943) have generally been considered to indicate an absence of direct parasympathetic innervation of the ventricles. A more careful examination shows that even Nonidez (1939), in his observations on autonomically decentralised hearts, interpreted the fine heavily silver-impregnated fibres coursing amongst ventricle muscle fibres to be postganglionic fibres of the vagus nerve. Using similar methods a more substantial parasympathetic innervation was demonstrated by Tcheng (1951) and Davis et al. (1952). Confirmation was forthcoming from subsequent studies in dog, cat and human hearts (Cooper, 1965; Jacobowitz et al. 1967) in which the thiocholine method developed by Karnovsky & Roots (1964) was used to identify acetylcholinesterase (AChE) the enzyme responsible for hydrolysing acetylcholine (ACh). These data were interpreted as showing a relatively rich parasympathetic postganglionic innervation distributed throughout the ventricles albeit less than in the atria. Not surprisingly, identification of cholinergic nerve fibres by AChE staining was questioned since other types of nerves have been observed to express the cholinergic enzyme. To get round this problem, Kent et al. (1974) compared the increase in ventricular fibrillation threshold caused by cervical vagus stimulation with effects of directly stimulating the cardiac ganglia. This was done before and after selectively destroying the postganglionic neurones in the heart ganglia with the neurotoxin vinblastine. Post-lesion there was an absence of AChE in the ventricles, and vagus nerve stimulation no longer increased fibrillation threshold. In a further experiment adrenergic denervation with 6-hydroxydopamine (6OHDA) did not affect the presence of AChE or the action of vagus nerve stimulation. Therefore, Kent et al. (1974) concluded that AChE was abundant in cholinergic neurones but sparse or absent in adrenergic or sensory neurones in the heart. A similar conclusion was reached by Bermani et al. (1982) who also used the thiocholine method to examine innervation of bronchi in rabbits. They showed that AChE-positive axons lacked catecholamine fluorescence. More recently several studies have refined the thiocholine method and interpreted the AChE staining as showing the cardiac cholinergic innervation to be widespread across all chambers of the heart. It is similar in mouse (Rysevaite et al. 2011), guinea pig (Batulevicius et al. 2005), rat (Zang et al. 2005), cat (Johnson et al. 2004), dog (Blomquist et al. 1987; Pauza et al. 2002), pig (Crick et al. 1999; Ulphani et al. 2010), sheep (Saburkina et al. 2010) and human (Kent et al. 1974; Pauza et al. 2000; Kawano et al. 2003) and in bats (O'Shea & Evans, 1985). These authors concluded that cholinergic nerves richly innervate the epicardial and endocardial surfaces of the ventricles as well as of the atria.
Although the evidence strongly favoured the interpretation that histochemical identification of AChE in the heart is a marker of cholinergic fibres the interpretation needed to be confirmed with a more reliable marker. This has now been achieved in studies in rat and mouse that have identified cholinergic fibres with choline acetyltransferase (CHAT) immunohistochemistry (Yasahura et al. 2007; Rysevaite et al. 2011; Pauza et al. 2013). This method convincingly identifies only ACh fibres, since CHAT is the enzyme that catalyses the final step in the synthesis of ACh. In general the pattern of cholinergic parasympathetic postganglionic innervation of the heart conducting system and heart chambers, including the ventricles, was confirmed in these studies.
In summary, present knowledge is that the cardiac vagal preganglionic nerves pass close to the cardiopulmonary vessels and superior vena cava (Kaye et al. 1970) to synapse on ganglion cells located in several epicardial fat pads on the dorsum of the atria close to sites of entry of the major veins and surface of the ventricles as well as the interventricular groove and the interatrial and interventricular septa (Ardell & Randall, 1986; Randall et al. 1986a,b; Gatti et al. 1995, 1997). These are now considered the principal sites of preganglionic termination. The number of these principal parasympathetic ganglia varies across species, four identified in the rat, eight in the cat and ten in human hearts (Pardini et al. 1987; Singh et al. 1996). Parasympathetic postganglionic axons project from these ganglia to selective regions of the atria and cardiac conduction system and ventricles. With regard to both right and left ventricle innervation there are several supraventricular ganglia from which many parasympathetic postganglionic axons project across the atrioventricular groove (Priola et al. 1977; Blomquist et al. 1987). Presumably these are the AChE-stained fibres observed on the epicardium in recent studies (Ulphani et al. 2010; Taggart et al. 2011). There is also an important parasympathetic postganglionic innervation, shown in the dog, that projects from the interventricular septum to supply the right ventricle to decrease its contractility and another that projects from a ganglion near the cranial margin of the left ventricle that participates in the decrease in left ventricle contractility produced by cervical vagus nerve stimulation (Gatti et al. 1997; Dickerson et al. 1998). The anatomical data are summarised schematically in Fig. 1.
Figure 1. Summary of the parasympathetic innervation of the heart that is discussed in the text.
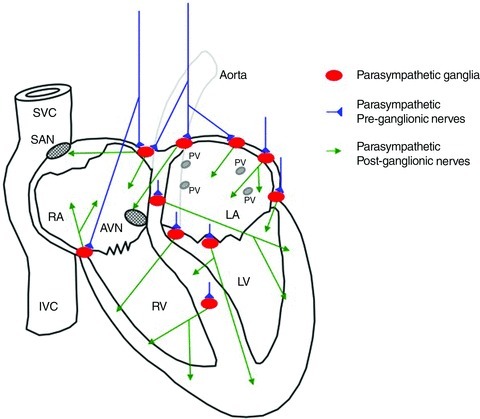
Schematic diagram of an anterior view of the human heart with the two vagal cardiac preganglionic nerves in blue that project to synapse in parasympathetic ganglia (red) that are distributed at several sites on the epicardium and within the atrial and ventricular septa. The diagram does not indicate the extent of the distribution of the right and left vagal nerves to either side of the heart. The parasympathetic postganglionic neurones send axons (green) to different regions of the heart including the sinoatrial node (SAN) and atrioventricular node (AVN) and all cardiac chambers including the ventricles and conducting tissue. The anatomical location is diagrammatic. The anterior portion of the heart has been excluded in the cut-away diagram. The major blood vessels, including the superior vena cava (SVC), inferior vena cava (IVC), the entry of the pulmonary veins (PV) and aorta are indicated (diagram by Kieran Brack based on original drawing by John Coote).
These data describing a significant parasympathetic postganglionic innervation of the ventricles are supported by numerous observations of the distribution of muscarinic receptors in both right and left ventricles (Fields et al. 1978; Yamada et al. 1980; Syrota et al. 1985; Hancock et al. 1987; Deighton et al. 1990; Yang et al. 1992; Dunlap et al. 2002; Mittmann et al. 2003) as well as a high density of chemically identified AChE (Crick et al. 1999; Dunlap et al. 2002; Gill et al. 2003; Zang et al. 2005).
There is also convincing evidence that ACh, detected by microdialysis, is released from various postganglionic sites in the left ventricular myocardium of mice and cats following vagal stimulation (Akiyama et al. 1994; Akiyama & Yamazaki, 2001; Shimizu et al. 2009). These studies showed that the concentration of released ACh was positively correlated with the frequency of stimulation of the cervical vagus nerve and with decreases in heart rate. Furthermore, ganglion blockade with hexamethonium prevented the effect of vagal stimulation on ACh release (Akiyama et al. 1994; Akiyama & Yamazaki, 2001; Shimizu et al. 2009). It is worth noting that the findings of a higher density of cholinergic fibres and of acetycholine in the atria may in part be due to the location of both pre- and postganglionic neurones in this region.
Functional evidence of vagal control of the cardiac ventricles
Supporting the anatomical and pharmacological data are several lines of physiological evidence showing that parasympathetic postganglionic nerve fibres must come into close apposition to ventricular myocardial fibres.
Eliakim et al. (1961) showed, in dogs with complete experimentally induced bundle branch block, that vagal stimulation caused a small but significant decrease in the rate of ventricular contraction that was blocked by atropine and mimicked by ACh. In subsequent studies by others, measurements of the monophasic action potential from surface electrodes on the left ventricle myocardium have shown that the duration and effective refractory period is lengthened during vagus nerve stimulation (Martins & Zipes, 1980a; Ng et al. 2001; Brack et al. 2007, 2011). A significant message that also follows from these and numerous other studies is that the parasympathetic postganglionic nerve fibres must innervate the cardiac ventricles (Hirsch, 1971; Martins & Zipes, 1980b, 1983; Itto & Zipes, 1994).
There are three ways in which the terminal fibres of the vagus can influence the myocardium: one is dependent on interaction with the sympathetic nerves, another is an independent direct action on the myocardium, and a third is via interaction with neurones of the intrinsic cardiac plexuses.
Dependent vagal effects
Sympathetic-dependent vagal effects on the myocardium
It is clear that in intact anaesthetised animals increased vagal activity has a significant negative inotropic effect on the ventricle of rat (Nalivaiko et al. 2009), rabbit (Brack et al. 2006), cat (Gatti et al. 1997), dog (De Geest et al. 1964), pig and human (Lewis et al. 2001). This is most marked in the presence of sympathetic tone where a muscarinic receptor activation by parasympathetic nerve-released ACh suppresses the action of sympathetic nerves on the myocardium (Henning & Levy, 1991; Levy & Schwartz, 2004; Brack et al. 2006). Much evidence suggests that this action is partly a pre-junctional effect whereby acetylcholine released from parasympathetic postganglionic terminal varicosities reduces the amount of catecholamine released from sympathetic nerves (Levy, 1977), an action which has been studied in detail in atria (Paterson, 2001; Dawson et al. 2008).
The sympatho-vagal interaction may also be post-junctional and due to the cholinergic intracellular elevation of cGMP that then inhibits cAMP so reducing the sympathetic adrenergic-activated hyperpolarization activated slow depolarizing (If current) and the L-type Ca2+ current in the myocyte. The interaction results in a decrease in contractility. The experimental observations of these phenomena, particularly by Levy & Zieske (1969) led to the formulation of the concept of accentuated antagonism (Levy, 1977; Levy & Schwartz, 2004). The postsynaptic mechanism for adrenergic–cholinergic interaction also has a firm molecular basis. Paterson's group using a guinea pig atrial preparation has shown that vago-sympathetic nerve interaction depends on the following events. Depolarisation at the parasympathetic nerve endings leads to the formation of nitric oxide (NO) derived from l-arginine by the activation of nitric oxide synthase. The NO facilitates parasympathetic nerve release of acetylcholine (Herring et al. 2000; Herring & Paterson, 2001; Paterson, 2001; Mohan et al. 2002) and inhibits sympathetic transmission (Paton et al. 2002; Li et al. 2007) via cGMP- and cAMP-dependent modulation of neuronal calcium levels, and this in turn regulates exocytosis (Wang et al. 2007).
It is likely that similar molecular events are also involved in the vago-sympathetic interaction in the ventricles (Levy & Schwartz, 2004).
The description now needs to be further extended in the light of the discovery of numerous local factors such as peptides released from myocardial cells, vasculature and intrinsic neurones, that can alter the release of chemical transmitters of parasympathetic postganglionic or sympathetic nerves (Herring & Paterson, 2009).
In conclusion functional studies of cardiac sympatho-vagal balance provide clear robust and abundant evidence for a parasympathetic postganglionic nerve presence in the ventricles that interacts with sympathetic nerve terminals in all species so far studied.
Independent vagal effects
Sympathetic-independent vagal effects on the myocardium
Many studies have shown that stimulation of the vagus nerves to the heart can decrease heart rate and decrease atrio-ventricular conduction independent of sympathetic nerve activity (e.g. Conlon et al. 1996). This action is facilitated by neuronal nitric oxide (Conlon & Kidd, 1999).
There is also robust evidence that parasympathetic postganglionic nerve fibres have direct effects on the ventricular myocardial cells acting independently of sympathetic activity. Vagal stimulation is capable of significantly lengthening the effective refractory period and the duration of the monophasic action potential recorded from the surface of the ventricle, in the isolated innervated paced rabbit heart where there is an absence of sympathetic tone (Ng et al. 2001; Brack et al. 2007, 2011). Vagal stimulation has also been shown to directly depress the force of ventricular contraction in the absence of sympathetic tone. This is in contrast to several early studies that reported very small or no inotropic effects of vagus nerve stimulation on the ventricles (Sarnoff et al. 1960; Harman & Reeves, 1968; Higgins et al. 1973). On the contrary, Gatti et al. (1997) have demonstrated that cervical vagus nerve stimulation can reduce left ventricle contractility by around 20% in anaesthetised cats in which the heart is paced and the sympathetic influence prevented by β adrenoreceptor blockade. Similarly, Nakano et al. (1998) measured changes in the right heart and showed that cervical vagus stimulation decreased dP/dt of right atrium and right ventricle in autonomically decentralised paced hearts of anaesthetised dogs. This seemingly direct action was given a more convincing basis by a study also in dogs (Xenopoulos & Applegate, 1994). The authors measured the changes in the end-systolic pressure–volume relationship in the left ventricle obtained from pressure–volume loops to give a load-insensitive measure of ventricular contractile performance. Vagal stimulation caused a negative inotropic effect that was still present, although reduced, after sympathetic denervation and β adrenoreceptor blockade. Similar results have been described in pig and humans (Lewis et al. 2001) and in the working heart–brainstem preparation of rat (Nalivaiko et al. 2009). There are other studies reporting small but significant vagally induced negative inotropy of the ventricles in rabbit when sympathetic tone has been pharmacologically blocked (Garcia-Perez & Jordan, 2001). However, Brack et al. (2006) could not confirm this in their isolated innervated rabbit heart preparation. The different results from the two laboratories could be due to incomplete β adrenergic blockade in the study by Garcia-Perez & Jordan (2001). However, it may be worth noting that there is an interesting and possibly important difference in the two studies. In the Garcia-Perez & Jordan (2001) experiments the effect was a characteristic of unmyelinated vagal preganglionic efferents that were stimulated in the absence of larger vagal fibres, these larger fibres having been blocked by anodal current. Different actions of large and small vagal efferents have also been described in the rabbit (Ford & McWilliam, 1986; Woolley et al. 1987).
An action via intrinsic neural plexuses
Cardiac vagal preganglionic termination and location of ganglia containing parasympathetic postganglionic neurones is complicated by the presence of intrinsic neurones that form plexuses with numerous connections throughout all cardiac chambers (Randall et al. 1996; Pauza et al. 2002; Armour, 2008). These systems may interact within the network and also with the parasympathetic pre- and postganglionic nerves, but it is important to understand that the neurones forming the network of connections that are referred to as the intrinsic nerve plexuses are not the same as the parasympathetic postganglionic neurones (Randall et al. 2003) that I have depicted in Fig. 1.
How information is processed by cardiac parasympathetic postganglionic neurones is partly explained by data from intracellular studies of cardiac ganglion cells recorded in isolated atria in vitro (e.g. Edwards et al. 1995) and in a ground-breaking in vitro preparation in which the heart is functionally connected to the brainstem (McAllen et al. 2011) allowing definitive identification of postganglionic vagal neurones in the atrium. These show that the efficiency of synaptic transmission is high but not necessarily 1:1 and the cells are capable of gating high frequency inputs. Thus the cardiac vagal ganglia can determine the level of postganglionic parasympathetic activity transmitted to the heart and do not act as a simple relay. The question of how much influence the intrinsic nerve plexuses have on the efficacy of transmission at the parasympathetic ganglia and on extrinsic autonomic postganglionic terminals is still unclear. However, there is some evidence that the effect of parasympathetic action can be enhanced or depressed by intrinsic nerves (Armour, 2008). A more challenging concept is the extent to which the intrinsic plexuses act independently as a ‘Little Brain’ as proposed by Randall et al. (1996). This remains an important area for further investigation. This is particularly so in view of the scope for both physiological and pathophysiological factors to alter cardiac vagal parasympathetic transmission.
Vagal modulation of ventricular rhythm
Perhaps the most striking and well-documented action of the cardiac vagus nerves is their protective effect on the vulnerability for ventricular fibrillation (Verrier & Lown, 1984; Schwartz, 1996; Brack et al. 2012). This is in contrast to the effects of the parasympathetic nerves on the atrium where they reduce the action potential duration and effective refractory period of atrial muscle cells (Liu & Nattel, 1997) hence lowering the threshold for fibrillation. Part of the protective influence on the ventricle appears to be contingent on the level of cardiac sympathetic tone (Kolman et al. 1975). Thus in dogs, whilst the protective action was shown to be attenuated by atropine, it was prevented by β adrenergic blockade (Kolman et al. 1975; Rabinowitz et al. 1976; Verrier & Lown, 1984). Similarly in dogs and cats vagal stimulation prevented ventricular arrhythmias induced by raised cardiac sympathetic activity following coronary artery occlusion (Kent et al. 1973; Myers et al. 1974; Corr & Gillis, 1974; Zuanetti et al. 1987). These conclusions were then tested in a demanding series of studies on conscious animals (reviewed by Schwartz, 1996). In dogs with a healed myocardial infarction, cardiac vulnerability was tested by subjecting them to exhaustive exercise. It was shown that those animals with a higher resting vagal tone as measured by baroreflex heart rate sensitivity (BRS) were less likely to experience ventricular fibrillation and sudden death compared to dogs with low BRS. The data are consistent with the presence of parasympathetic postganglionic nerve fibres in the ventricles but having an indirect action. That is, where sympathetic tone is high the protective effect of vagal activity is due, at least in part, to accentuated antagonism involving cholinergic-induced formation of nitric oxide (Paterson, 2001).
There is also convincing evidence supporting a direct and non-cholinergic protective action of vagal nerve fibres on pathological alterations in ventricular rhythm (Brack et al. 2012). The most compelling evidence comes from a series of studies over the last 12 years by André Ng and colleagues. These experiments depended on the development of a novel isolated Langendorff rabbit heart preparation in which the parasympathetic and sympathetic nerves are patent but inactive and can be stimulated independently (Ng et al. 2001; Brack et al. 2004). The studies revealed that the parasympathetic nerves have two separate independent actions in the cardiac ventricle. One is a cholinergic–muscarinic effect on contraction rate and ventricular effective refractory period (Brack et al. 2009). The other concerns the protection from fibrillation by increased vagal activity. The latter turned out to be an independent effect of NO on action potential duration restitution. This action was selectively blocked by an neuronal nitric oxide synthase (nNOS) antagonist and unaffected by atropine. Thus it appeared to be mediated via NO release independently of ACh action at M2 post-junctional receptors (Brack et al. 2007, 2009, 2011). This highly novel discovery depended on a series of technically demanding studies, one of which used the selective NO fluorescent indicator technique developed by the group for use in the beating heart (Patel et al. 2008). In a parallel study it was confirmed that stimulation of the vagus nerve increased nNOS-dependent NO fluorescence (Brack et al. 2009). There are several ways in which this may occur. One is that NO released from cholinergic fibres acts at a different post-junctional site to ACh. Another suggested by the latter authors is that this anti-arrhythmic action of the vagus in the rabbit is exerted by a select population of parasympathetic postganglionic nitrergic nerves. There is anatomical data in support of this speculation (Klimaschewski et al. 1992; Tanaka et al. 1993; Hoover et al. 2009). The possibility of specific vagal nitrergic neuro-junctional transmission in the heart was also alluded to by Rubino et al. (1996). Otherwise it has always been assumed that the parasympathetic postganglionic nerve fibres are cholinergic and phenotypically homogeneous.
A further complexity is that diverse chemical phenotypes and receptors are expressed within the intrinsic cardiac plexuses and this expands the possible chemical messengers that might participate in cardiac performance if they interact with postganglionic fibres (Singh et al. 1999).
Conceptually, the idea of vagal pathways that are separate to the classical cholinergic ones is not new since it is established in parasympathetic control of the gastrointestinal tract. Medulla vagal preganglionic neurones innervating the gut have been shown to form two parallel projections: an excitatory one synapsing with cholinergic postganglionic neurones and another inhibitory one synapsing with non-cholinergic postganglionic neurones containing NO (Chang et al. 2003). A similar arrangement appears in the sacral parasympathetic innervation. Part of the parasympathetic innervation of the lower urinary tract and vas deferens in the rat has been identified as nitrergic (Persson et al. 1997; Ventura et al. 1998; Fry et al. 2010).
The central regulation of cardiac vagal neurones
The evidence reviewed above supports the notion of specialised functional organisation within the terminal parasympathetic innervation of the heart. This is reflected in the central nervous organisation of the vagal motor nuclei in the medulla and parallel functional pathways of the preganglionic neurones projecting to specific cardiac loci including the ventricles (Randall et al. 1986a,b; Pardini et al. 1987; Billman et al. 1989; Nakano et al. 1998; Dickerson et al. 1998; Sampaio et al. 2003; Cheng et al. 2004).
Preganglionic cardiac vagal neurones
Electrophysiological studies were the first to provide robust evidence for the location within the medulla of vagal cardiac preganglionic neurones (McAllen & Spyer, 1976; Jordan et al. 1982). Histological studies with tracer dyes injected either into the heart ganglia (retrograde; Izzo et al. 1993; Standish et al. 1995; Massari et al. 1995, 1998; Ter Horst et al. 1996; Hsieh et al. 1998; Corbett et al. 1999; Jones, 2001; Jordan, 2011) or into the medullary nuclei (anterograde; Cheng et al. 1999; Cheng & Powley, 2000; Cheng et al. 2004) show that preganglionic cardiac neurones mainly arise from two nuclei in the caudal medulla oblongata. A larger group of about 80% are located in the posterior ventrolateral nucleus ambiguus (NA). These have small myelinated axons (B fibres) that can powerfully slow heart rate, conduction and force of contraction. A second group comprising a significant population (20%) of cardiac vagal neurones originates from the dorsal motor nucleus (DMNV) and a scattering of neurones in an intermediate zone. These have more slowly conducting unmyelinated axons. Selective stimulation of the unmyelinated dorsal motor nucleus axons in the vagus nerve affects atrioventricular conduction and force of ventricular contraction and also reduces heart rate, but it has a slower onset and different pharmacology to that seen following stimulation of the B fibres in the vagus nerve (Jones et al. 1995; Garcia-Perez & Jordan, 2001; Jones, 2001). Apart from conduction speed the two groups of neurones have quite different discharge patterns. The NA cardiac neurones display a respiratory rhythm and receive baroreceptor input and chemoreceptor input. The DMNV neurones have a more irregular non-respiratory-dependent discharge (Jones et al. 1998) and appear to be unaffected by baroreceptors and chemoreceptors (Ford et al. 1990; Jones et al. 1998). Cardiac vagal neurones in the intermediate zone have similar characteristics to those in the DMNV (Kong et al. 2007). This arrangement has led to speculation that it reflects a differential function of the two groups (Jones et al. 1998; Wang et al. 2000). The idea is supported by a study showing that DMNV and NA terminals, each anterogradely labelled with different fluorescent tracers, target separate populations of principal neurones in an intrinsic cardiac ganglion, and furthermore DMNV neurones also innervated small intensely fluorescent (SIF) cells in ganglia (Cheng & Powley, 2000; Cheng et al. 2004). In contrast Jones (2001) has proposed that the rhythmic respiratory input conveyed by the faster vagal B fibres may interact at the same cardiac ganglia with the tonic input conveyed by the slow C fibres. This would suggest that B and C fibres converge on common postganglionic neurones but at present there is no direct evidence to support this. Another possibility is that some of the C fibres act independently and have a different action on the myocardium. In the rabbit the bradycardic effect of stimulating all the C fibres in the vagus nerve is not blocked by hexamethonium, the cholinergic nicotinic receptor antagonist, unlike its action on the effect of stimulating the B fibre component (Woolley et al. 1987; McWilliam & Woolley, 1988; Garcia-Perez & Jordan, 2001). This suggests that the action of C fibre stimulation on heart rate is mediated by a non-cholinergic mechanism. These studies on rabbits also showed that the effects of unmyelinated vagal preganglionic fibres on slowing atrioventicular conduction and decreasing ventricular contactility were also resistant to hexamethonium. The observation of differential actions of cardiac vagal fibres is supported by immunohistochemical characterisation showing that there are at least four chemically distinct types of cardiac vagal preganglionic neurones in the rat (Takanaga et al. 2003). Therefore, it appears that different cardiac functions may be regulated by different phenotypes of vagal preganglionic neurones. This has also been alluded to in studies in dog (Dickerson et al. 1998), cat (Wang et al. 2000) and rabbit (Woolley et al. 1987). Consistent with these data are results of experiments with microinjection of glutamate into different regions of the nucleus ambiguus in dogs that show a longitudinal cardiotopic organisation of negative chronotropic and dromotropic vagal preganglionic neurones (Gatti et al. 1996; Massari et al. 1998). In accord with this, Sampaio et al. (2003) showed different cardiac effects on the stimulation of two quite separate ganglionic plexuses in the heart: one at the junction of the superior vena cava and right atrium and the other at the junction of the pulmonary veins and left atrium. The first plexus elicits a bradycardia whilst stimulation of the second slows atrioventricular impulse conduction.
Adding further weight to this idea are experiments in both cat and dog showing two populations of cardiac vagal preganglionic neurones in the medulla, one that was inhibited by lung inflation and one that was unaffected (Daly & Kirkman, 1989).
Supramedullary control
Emotional events or clinically life-threatening brain lesions are associated with adverse electrocardiographic changes (Oppenheimer & Lima, 1998; Oppenheimer, 2006; Cheshire & Saper, 2006; Samuels, 2007). It is thus unsurprising that cardiac ventricular rhythm disturbances have long been known to accompany experimental manipulation of several brain regions above the level of the medulla. In many cases the effects are shown to be due to heightened sympathetic activity induced by regions such as the hypothalamic and midbrain ‘defence areas’ (Coote, 2004). Of relevance to the present discussion are those regions that have selective cardiac vagal effects.
For example, in the paraventricular nucleus of the hypothalamus oxytocin-type neurones have been shown to project to the dorsal motor nucleus of the vagus and the nucleus ambiguus of the medulla (Sawchenko & Swanson, 1982; Luiten et al. 1985) and some of their connections are on vagal cardiac preganglionic neurones. Rogers & Hermann (1986) showed that stimulation of these oxytocin neurones caused a bradycardia and later Darlington et al. (1989) demonstrated that this was a cholinergic vagal effect on heart rate, it being blocked by intravenous atropine and hexamethonium. The explicit functional role of this system is unclear.
Of more functionally explicit importance is an island of cerebral cortex lying deep within the fold of the lateral fissure (Sylvian fissure) on each side of the brain and known as the insula. Its posterior region has profound effects on autonomic control of the heart (Cechetto & Saper, 1987). In an ingenious series of experiments, it was shown that stimulation in human and monkey of this part of the right insula increases cardiac sympathetic tone whereas stimulation of the left insula increases cardiac vagal tone (in the rat it was opposite) (Oppenheimer & Cechetto, 1990; Oppenheimer et al. 1992; Zhang et al. 1998). The influence of the insula cortices is of considerable clinical importance since cerebrovascular events such as stroke and others like epilepsy are often accompanied by cardiac arrhythmias. The arrhythmias appear to occur more frequently after left insula infarction suggesting sympatho-vagal balance has been impaired. Also the long-term cardiac stability for stroke patients is improved if the vascular event mainly involves the right insula (Cheshire & Saper, 2006; Oppenheimer, 2006).
Conclusion
In conclusion the myth that vagal fibres are sparse in the cardiac ventricles and only have weak actions on their physiology is incorrect. This account highlights the importance of cardiac vagal innervation to the normal functioning of the ventricles as well as to the atria (Fig. 1). There are many gaps still to be filled. There is no full description of the terminal innervation and neurochemistry of the parasympathetic postganglionic nerves in the atrial and ventricular myocardium. It is also unclear to what extent activity of the intrinsic plexuses influences the efficacy of extrinsic autonomic neurones. The normal functioning of a cardiotopic arrangement of parasympathetic pre- and postganglionic neurones is also a puzzle. Also much has still to be learned regarding the non-cholinergic mechanisms and molecular pathways targeted by multiple co-transmitters. Meanwhile present knowledge needs to be more explicitly presented in textbooks and lectures for the benefit of physicians and scientists alike. A sound and full knowledge of the anatomy and physiology of the parasympathetic nerves supplying the heart is essential if we are to fully understand heart dysfunction and in particular how brain damage or disease can have serious adverse cardiac effects (Taggart et al. 2011).
Additional information
Competing interests
None declared.
References
- Akiyama T, Yamazaki T. Effect of right and left vagal stimulation on left ventricular acetylcholine levels in cat. Acta Physiol Scand. 2001;172:11–16. doi: 10.1046/j.1365-201X.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Yamazaki T, Ninomiya I. In vivo detection of endogenous acetylcholine release in cat ventricles. Am J Physiol Heart Circ Physiol. 1994;266:H854–H860. doi: 10.1152/ajpheart.1994.266.3.H854. [DOI] [PubMed] [Google Scholar]
- Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in the canine heart. Am J Physiol Heart Circ Physiol. 1986;251:H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- Batulevicius D, Pauziene N, Pauza DH. Architecture and age-related analysis of the neuronal number of the guinea pig intrinsic cardiac nerve plexus. Ann Anat. 2005;187:225–243. doi: 10.1016/j.aanat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bermani A-W, Bloomquist EI, Montvilo JA. Distribution of pulmonary cholinergic nerves in the rabbit. Thorax. 1982;37:703–710. doi: 10.1136/thx.37.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Hoskins RS, Randall DC, Randall WC, Hamlin RL, Lin YC. Selective vagal postganglionic innervation of the sinoatrial and atrioventricular nodes in the non-human primate. J Auton Nerv Syst. 1989;26:27–36. doi: 10.1016/0165-1838(89)90104-5. [DOI] [PubMed] [Google Scholar]
- Blomquist TM, Priola DV, Romero AM. Source of intrinsic innervation of the canine ventricles: a functional study. Am J Physiol Heart Circ Physiol. 1987;252:H638–H644. doi: 10.1152/ajpheart.1987.252.3.H638. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Interaction between direct sympathetic and vagus nerve stimulation on heart rate in the isolated rabbit heart. Exp Physiol. 2004;89:128–139. doi: 10.1113/expphysiol.2003.002654. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci. 2006;124:69–80. doi: 10.1016/j.autneu.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brack KE, Coote JH, Ng GA. Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res. 2011;91:437–447. doi: 10.1093/cvr/cvr105. [DOI] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol. 2007;583:695–704. doi: 10.1113/jphysiol.2007.138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Patel VH, Mantravardi R, Coote JH, Ng GA. Direct evidence of nitric oxide release from neuronal nitric oxide synthase activation in the left ventricle as a result of cervical vagus nerve stimulation. J Physiol. 2009;587:3045–3054. doi: 10.1113/jphysiol.2009.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Winter J, Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation-tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmia in heart failure. Heart Fail Rev. 2012;18:389–408. doi: 10.1007/s10741-012-9314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei B. Vagal control of myocardial contractility in humans. Exp Physiol. 2001;86:817–823. doi: 10.1111/j.1469-445x.2001.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chang HY, Mashimo H, Goyal RK. Musings of the wanderer: What's new in our understanding of vagal-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 2000;424:588–606. [PubMed] [Google Scholar]
- Cheng Z, Powley TL, Schwaber JS, Doyle FJ., III Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 1999;410:320–341. [PubMed] [Google Scholar]
- Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over postganglionic neurones in rat cardiac ganglia by NA and DmnX neurons: anatomical evidence. Am J Physiol Regul Integr Comp Physiol. 2004;286:R625–R633. doi: 10.1152/ajpregu.00143.2003. [DOI] [PubMed] [Google Scholar]
- Cheshire WP, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66:1296–1297. doi: 10.1212/01.wnl.0000219563.87204.7d. [DOI] [PubMed] [Google Scholar]
- Conlon K, Collins T, Kidd C. Modulation of vagal actions on heart rate produced by inhibition of nitric oxide synthase in the anaesthetized ferret. Exp Physiol. 1996;81:547–550. doi: 10.1113/expphysiol.1996.sp003957. [DOI] [PubMed] [Google Scholar]
- Conlon K, Kidd C. Neuronal nitric oxide facilitates vagal chronotropic and dromotropic actions on the heart. J Auton Nerv Syst. 1999;75:136–146. doi: 10.1016/s0165-1838(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Cooper T. Terminal innervation of the heart. In: Randall WC, editor. Nervous Control of the Heart. Baltimore: Williams and Wilkins; 1965. pp. 130–153. [Google Scholar]
- Coote JH. The hypothalamus and cardiovascular regulation. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural Mechanisms of Cardiovascular Regulation. London: Kluver; 2004. pp. 117–146. [Google Scholar]
- Corbett EK, Batten TF, Kaye JC, Deuchars J, McWilliam PN. Labelling of rat vagal preganglionic neurones by carbocyanine dye DiI applied to the heart. Neuroreport. 1999;10:1177–1181. doi: 10.1097/00001756-199904260-00004. [DOI] [PubMed] [Google Scholar]
- Corr PB, Gillis RA. Role of vagus nerves in cardiovascular changes induced by coronary occlusion. Circulation. 1974;49:86–97. doi: 10.1161/01.cir.49.1.86. [DOI] [PubMed] [Google Scholar]
- Crick SJ, Anderson RH, Ho SY, Sheppard MN. Localisation and quantitation of autonomic innervation in the porcine heart. II: endocardium, myocardium and epicardium. J Anat. 1999;195:359–373. doi: 10.1046/j.1469-7580.1999.19530359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis W, Tribe EM. Distribution of nerves in the heart. J Physiol. 1913;46:141–150. doi: 10.1113/jphysiol.1913.sp001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly IdeBurgh, Hebb CO. Pulmonary vasomotor fibres in the cervical vago-sympathetic nerve of the dog. Quart J Exp Physiol. 1952;37:19–43. doi: 10.1113/expphysiol.1952.sp000978. [DOI] [PubMed] [Google Scholar]
- Daly MdeBurgh, Evans DLH. Functional and histological changes in the vagus nerve of the cat after degenerative section at various levels. J Physiol. 1953;120:579–595. doi: 10.1113/jphysiol.1953.sp004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeBurgh, Kirkman E. Differential modulation by pulmonary stretch afferents of some reflex cardioinhibitory responses in the cat. J Physiol. 1989;417:323–241. doi: 10.1113/jphysiol.1989.sp017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington DN, Miyamoto M, Keil LC, Dallman MF. Paraventricular stimulation with glutamate elicits bradycardia and pituitary responses. Am J Physiol Regul Integr Comp Physiol. 1989;256:R112–R119. doi: 10.1152/ajpregu.1989.256.1.R112. [DOI] [PubMed] [Google Scholar]
- Davis F, Francis ETB, King TS. Neurological studies of the cardiac ventricles of mammals. J Anat. 1952;86:130–143. [PMC free article] [PubMed] [Google Scholar]
- Dawson TA, Li D, Woodward TD, Barber ZE, Wang L, Paterson DJ. Cardiac cholinergic NO-cGMP signaling following acute myocardial infarction and nNOS gene transfer. Am J Physiol Heart Circ Physiol. 2008;295:H990–H998. doi: 10.1152/ajpheart.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest H, Levy MN, Zieske H. Negative inotropic effect of the vagus nerves upon the canine ventricle. Science. 1964;144:1223–1225. doi: 10.1126/science.144.3623.1223. [DOI] [PubMed] [Google Scholar]
- Deighton NM, Motomera S, Borquez D, Zerkowski HR, Doetsch N, Brodde OE. Muscarinic cholinoreceptors in the human heart: Demonstration, subclassification and distribution. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:14–21. doi: 10.1007/BF00195052. [DOI] [PubMed] [Google Scholar]
- Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res. 2001;44:161–182. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- Dickerson LW, Rodak DJ, Fleming TJ, Gatti PJ, Massari VJ, McKenzie JC, Gillis RA. Parasympathetic neurons in the cranial medial ventricular fat pad on the dog heart selectively decrease ventricular contractility. J Auton Nerv Syst. 1998;70:129–141. doi: 10.1016/s0165-1838(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Dunlap ME, Bibevsky S, Rosenberry TL, Ernsberger P. Mechanisms of altered vagal control in heart failure: influence of muscarinic receptors and acetylcholinesterase activity. Am J Physiol Heart Circ Physiol. 2002;285:H1362–H1640. doi: 10.1152/ajpheart.01051.2002. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol. 1995;486:453–471. doi: 10.1113/jphysiol.1995.sp020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliakim M, Bellet S, Tawil E, Muller O. Effect of vagal stimulation and acetylcholine on the ventricle. Circ Res. 1961;9:1372–1379. [PubMed] [Google Scholar]
- Evans DHL, Murray JG. Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat. 1954;88:320–337. [PMC free article] [PubMed] [Google Scholar]
- Fields JZ, Roeske WR, Morkin E, Yamamura HI. Cardiac muscarinic cholinergic receptors: biochemical identification and characterization. J Biol Chem. 1978;253:3251–3258. [PubMed] [Google Scholar]
- Ford TW, Bennett JA, Kidd C, McWilliam PN. Neurones in the dorsal vagal motor nucleus of the cat with non-myelinated axons projecting to the heart and lungs. Exp Physiol. 1990;75:459–473. doi: 10.1113/expphysiol.1990.sp003423. [DOI] [PubMed] [Google Scholar]
- Ford TW, McWilliam PN. The effects of electrical stimulation of myelinated and non-myelinated vagal fibres on heart rate in the rabbit. J Physiol. 1986;380:341–347. doi: 10.1113/jphysiol.1986.sp016289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci. 2010;154:3–13. doi: 10.1016/j.autneu.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez M, Jordan D. Effect of stimulating non-myelinated vagal axons on atrio-ventricular conduction and left ventricular function in anaesthetized rabbits. Auton Neurosci. 2001;86:183–191. doi: 10.1016/S1566-0702(00)00252-6. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Johnson TA, McKenzie JC, Lauenstein JM, Gray A, Massari VJ. Vagal control of left ventricular contractility is selectively mediated by a cranioventricular intracardiac ganglion in the cat. J Auton Nerv Syst. 1997;66:138–144. doi: 10.1016/s0165-1838(97)00071-4. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Johnson TA, Massari VJ. Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction. J Auton Nerv Syst. 1996;57:123–127. doi: 10.1016/0165-1838(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Johnson TA, Phan P, Jordan IK, Coleman W, Massari VJ. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline heart. J Auton Nerv Syst. 1995;51:255–259. doi: 10.1016/0165-1838(94)00139-b. [DOI] [PubMed] [Google Scholar]
- Gill DS, Lintern MC, Wetherell J, Coote JH, Smith ME. Guinea pig heart acetylcholinesterase after continuous physostigmine administration. Hum Exp Toxicol. 2003;22:373–381. doi: 10.1191/0960327103ht363oa. [DOI] [PubMed] [Google Scholar]
- Hancock SC, Hoover DB, Houghland MW. Distribution of muscarinic receptors and acetylcholinesterase in rat heart. J Auton Nerv Syst. 1987;19:59–66. doi: 10.1016/0165-1838(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Harman MA, Reeves TJ. Effects of efferent vagal stimulation on atrial and ventricular function. Am J Physiol. 1968;215:1210–1217. doi: 10.1152/ajplegacy.1968.215.5.1210. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol. 2003;139:1074–1084. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinbecker P, O’Leary J. Nature and function of certain fibres of the vagus–a new concept in peripheral nerve organization. Proc Soc Exp Biol Med. 1933a;30:506–508. [Google Scholar]
- Heinbecker P, O’Leary J. The mammalian vagus nerve–a functional and histological study. Am J Physiol. 1933b;106:623–646. [Google Scholar]
- Henning RJ, Levy MN. Effects of autonomic nerve stimulation, asynchrony, and load on dP/dtmax and on dP/dtmin. Am J Physiol Heart Circ Physiol. 1991;260:H1290–H1298. doi: 10.1152/ajpheart.1991.260.4.H1290. [DOI] [PubMed] [Google Scholar]
- Herring N, Golding S, Paterson DJ. Pre-synaptic NO-cGMP pathway modulates vagal control in isolated adult guinea pig atria. J Mol Cell Cardiol. 2000;32:1795–1804. doi: 10.1006/jmcc.2000.1214. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide–cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Higgins CB, Vatner SF, Braunwald E. Parasympathetic control of the heart. Pharmacol Rev. 1973;25:119–155. [PubMed] [Google Scholar]
- Hirsch EF( The Innervation of the Vertebrate Heart. Springfield, IL, USA: Charles C Thomas Publisher; 1971. pp. 80–101. [Google Scholar]
- Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea-pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res. 2004;62:112–121. doi: 10.1016/j.cardiores.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009;164:1170–1179. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hsieh JH, Chen RF, Wu JJ, Yen CT, Chai CY. Vagal innervation of the gastrointestinal tract arises from dorsal motor nucleus while that of the heart largely from nucleus ambiguus. J Auton Nerv Syst. 1998;70:38–50. doi: 10.1016/s0165-1838(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Itto M, Zipes DP. Efferent sympathetic and vagal innervation of the canine right ventricle. Circulation. 1994;90:1459–1468. doi: 10.1161/01.cir.90.3.1459. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Cooper T, Barner HB. Histochemical and chemical studies of the localization of adrenergic and cholinergic nerves in the normal and denervated cat hearts. Circ Res. 1967;20:289–298. doi: 10.1161/01.res.20.3.289. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Gray AL, Lauestein JM, Newton SS, Massari VJ. Parasympathetic control of the heart 1. An interventriculo-septal ganglion is the major source of the vagal intracardiac innervation of the ventricles. J Appl Physiol. 2004;96:2265–2272. doi: 10.1152/japplphysiol.00620.2003. [DOI] [PubMed] [Google Scholar]
- Jones JFX. Vagal control of the heart. Exp Physiol. 2001;86:791–801. doi: 10.1111/j.1469-445x.2001.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C-fibres in anaesthetized cats and rabbits. J Physiol. 1995;489:203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Activity of C-fibre cardiac vagal efferents in anaesthetized cats and rats. J Physiol. 1998;507:869–880. doi: 10.1111/j.1469-7793.1998.869bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Parasympathetic preganglionic neurones. In: Llewellyn-Smith IJ, Verberne AJM, editors. Central Regulation of Autonomic Functions. New York: OUP; 2011. pp. 120–139. [Google Scholar]
- Jordan D, Khalid MEM, Schneiderman N, Spyer KM. The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Pflugers Arch. 1982;395:244–250. doi: 10.1007/BF00584817. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A “direct colouring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Kaye MP, Geesbreght JM, Randall WC. Distribution of autonomic nerves to the canine heart. Am J Physiol. 1970;218:1025–1029. doi: 10.1152/ajplegacy.1970.218.4.1025. [DOI] [PubMed] [Google Scholar]
- Kent KM, Epstein SE, Cooper T, Jacobowitz DM. Cholinergic innervation of the canine and human ventricular conducting system: Anatomic and electrophysiological correlations. Circulation. 1974;50:948–955. doi: 10.1161/01.cir.50.5.948. [DOI] [PubMed] [Google Scholar]
- Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation. 1973;47:291–298. doi: 10.1161/01.cir.47.2.291. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Kummer W, Mayer B, Courand JY, Preissler U, Phillipin B, Heyin C. Nitric oxide synthase in cardiac nerve fibers of rat and guinea-pig heart. Circ Res. 1992;71:1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Kolman BS, Verrier RL, Lown B. The effect of vagus nerve stimulation upon vulnerability of the canine ventricle: Role of the sympathetic-parasympathetic interactions. Circulation. 1975;52:578–585. doi: 10.1161/01.cir.52.4.578. [DOI] [PubMed] [Google Scholar]
- Kong S, Liu J-H, Ramage AG, Wang Y. Cardiac vagal preganglionic neurones in the intermediate zone of the brainstem in anaesthetized cats. Exp Physiol. 2007;92:1023–1028. doi: 10.1113/expphysiol.2007.039230. [DOI] [PubMed] [Google Scholar]
- Levy MN. Parasympathetric control of the heart. In: Randall WC, editor. Neural Regulation of the Heart. New York: OUP; 1977. pp. 97–129. [Google Scholar]
- Levy MN, Martin PJ. Autonomic control of cardiac conduction and automaticity. In: Shepherd JT, Vatner SF, editors. Nervous Control of the Heart. Amsterdam: HAP; 1996. pp. 201–225. [Google Scholar]
- Levy MN, Schwartz PJ. Vagal Control of the Heart: Experimental Basis and Clinical Implications. New York: Futura; 2004. [Google Scholar]
- Levy MN, Zieske H. Effect of enhanced contractility on the left ventricular response to vagus nerve stimulation in dogs. Circ Res. 1969;24:303–311. doi: 10.1161/01.res.24.3.303. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Al-Khalidi AH, Bonser RS, Clutton-Brock T, Morton D, Paterson DJ, Coote JH. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J Physiol. 2001;534:547–552. doi: 10.1111/j.1469-7793.2001.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang L, Chee-Wan L, Dawson TA, Paterson DJ. Noradrenergic cell specific gene transfer with neuronal nitric oxide synthase reduces cardiac sympathetic neurotransmission in hypertensive rats. Hypertension. 2007;50:69–74. doi: 10.1161/HYPERTENSIONAHA.107.088591. [DOI] [PubMed] [Google Scholar]
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol Heart Circ Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, Ter Horst GJ, Karst H, Steffins AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Salo LM, Paton JFR, Pickering AE. Processing of central and reflex drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol. 2011;589:5801–5818. doi: 10.1113/jphysiol.2011.214320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol. 1976;258:187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiney BA, Spurrel WR. The gastric fibres of the vagus nerve. J Physiol. 1933;77:447–458. doi: 10.1113/jphysiol.1933.sp002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam PN, Woolley DC. The actions of myelinated and non-myelinated vagal fibres on atrial contraction in the rabbit. J Auton Nerv Syst. 1988;22:67–73. doi: 10.1016/0165-1838(88)90155-5. [DOI] [PubMed] [Google Scholar]
- Martins JB, Zipes DP. Epicardial phenol interrupts refractory period responses to sympathetic but not vagal stimulation in canine left ventricular epicardium and endocardium. Circ Res. 1980a;47:33–40. doi: 10.1161/01.res.47.1.33. [DOI] [PubMed] [Google Scholar]
- Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res. 1980b;46:100–110. doi: 10.1161/01.res.46.1.100. [DOI] [PubMed] [Google Scholar]
- Martins JB, Zipes JB. Distribution of local repolarization changes produced by efferent vagal stimulation in the canine ventricles. J Am Coll Cardiol. 1983;2:1191–1199. doi: 10.1016/s0735-1097(83)80350-7. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Dickerson LW, Gray AL, Lauenstein J-M, Blinder KJ, Newsome JT, Rodak DJ, Fleming TJ, Gatti PJ, Gillis RA. Neural control of left ventricular contractility in the dog heart: synaptic interactions of negative inotropic vagal preganglionic neurons in the nucleus ambiguus with tyrosine hydroxylase immunoreactive terminals. Brain Res. 1998;802:205–220. doi: 10.1016/s0006-8993(98)00613-1. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Johnson TA, Gatti PJ. Cardiotopic organization of the nucleus ambiguus? An anatomical and physiological analysis of neurons regulating atrioventricular conduction. Brain Res. 1995;679:227–240. doi: 10.1016/0006-8993(95)00227-h. [DOI] [PubMed] [Google Scholar]
- Mittmann C, Pinkepank G, Stamatelopoulou S, Wieland T, Nürnberg B, Hirt S, Eschenhagen T. Differential coupling of m-cholinoceptors to Gi/Go-proteins in failing human myocardium. J Mol Cell Cardiol. 2003;35:1241–1249. doi: 10.1016/s0022-2828(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Heaton DA, Danson EJF, Krishnan SPR, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- Myers RW, Pearlman AS, Hyman RM, Goldstein RA, Kent KM, Goldstein RE, Epstein SE. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974;49:943–947. doi: 10.1161/01.cir.49.5.943. [DOI] [PubMed] [Google Scholar]
- Nakano H, Furukawa Y, Inoue Y, Sawaki S, Oguchi T, Chiba S. Right ventricular responses to vagal stimulation of fibers to discrete cardiac regions in dog hearts. J Auton Nerv Syst. 1998;74:179–188. doi: 10.1016/s0165-1838(98)00156-8. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Antunes VR, Paton JFR. Control of cardiac contractility in rat working heart–brain preparation. Exp Physiol. 2009;95:107–119. doi: 10.1113/expphysiol.2009.048710. [DOI] [PubMed] [Google Scholar]
- Ng GA, Brack KE, Coote JH. Effect of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart – a novel Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol. 2001;86:319–329. doi: 10.1113/eph8602146. [DOI] [PubMed] [Google Scholar]
- Nonidez JF. Studies on the innervation of the heart 1. Distribution of the cardiac nerves with special reference to the identification of sympathetic and parasympathetic postganglionics. Am J Anat. 1939;65:361–413. [Google Scholar]
- Nonidez JF. The structure and innervation of the conductive system of the heart of the dog and Rhesus monkey, as seen with a silver impregnation technique. Am Heart J. 1943;26:577–597. [Google Scholar]
- Onkka P, Maskoun W, Rhee K-S, Hellyer J, Patel J, Tan J, Chen LS, Vinters HV, Fishbein MC, Chen P-S. Sympathetic nerve fibers and ganglia in canine vagus nerves: Localization and quantitation. Heart Rhythm. 2013;10:585–591. doi: 10.1016/j.hrthm.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM( Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance. Clin Auton Res. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gebb AW, Girvan JP, Hachinski VC. Cardiovascular effects of human insular stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Lima J. Neurology and the heart. J Neurol Neurosurg Psychiatry. 1998;64:289–297. doi: 10.1136/jnnp.64.3.289. [DOI] [PubMed] [Google Scholar]
- O'Shea JE, Evans BK. Innervation of bat heart: cholinergic and adrenergic nerves innervate all chambers. Am J Physiol Heart Circ Physiol. 1985;249:H876–H882. doi: 10.1152/ajpheart.1985.249.4.H876. [DOI] [PubMed] [Google Scholar]
- Pardini BJ, Patel KP, Schmid PG, Lund DD. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst. 1987;20:91–101. doi: 10.1016/0165-1838(87)90106-8. [DOI] [PubMed] [Google Scholar]
- Patel VH, Brack KE, Coote JH, Ng GA. A novel method of measuring the nitric oxide-dependent fluorescence using 4,5-diaminofluorescein (DAF2) in the isolated Langendorff-perfused rabbit heart. Pflugers Arch. 2008;456:635–645. doi: 10.1007/s00424-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Paterson DJ( Nitric oxide and the autonomic regulation of cardiac excitability. Exp Physiol. 2001;86:1–12. doi: 10.1113/eph8602169. [DOI] [PubMed] [Google Scholar]
- Paton JRF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Saburkina I, Rysevaite K, Inokaitis H, Jokubauskas M, Jalife J, Pauziene N. Neuroanatomy of the murine cardiac conduction system. A combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci. 2013;176:32–47. doi: 10.1016/j.autneu.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs. 2002;172:297–320. doi: 10.1159/000067198. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution and variability of the epicardial neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Persson K, Johansson K, Alm P, Larsson B, Andersson KE. Morphological and functional evidence against a sensory and sympathetic origin of nitric oxide synthase-containing nerves in the rat lower urinary tract. Neuroscience. 1997;77:271–281. doi: 10.1016/s0306-4522(96)00443-5. [DOI] [PubMed] [Google Scholar]
- Priola DV, Spurgeon WA, Geis WP. The intrinsic innervation of the canine heart. A functional study. Circ Res. 1977;40:50–56. doi: 10.1161/01.res.40.1.50. [DOI] [PubMed] [Google Scholar]
- Rabinowitz SH, Verrier RL, Lown B. Muscarinic effects of vagosympathetic trunk stimulation on the repetitive extrasystole (RE) threshold. Circulation. 1976;53:622–627. doi: 10.1161/01.cir.53.4.622. [DOI] [PubMed] [Google Scholar]
- Randall WC. Nervous Control of Cardiovascular Function. New York: OUP; 1984. [Google Scholar]
- Randall WC, Ardell JL, Calderwood D, Milosavljevic M, Goyal SC. Parasympathetic ganglia innervating the canine atrioventricular nodal region. J Auton Nerv Syst. 1986a;16:311–323. doi: 10.1016/0165-1838(86)90036-6. [DOI] [PubMed] [Google Scholar]
- Randall WC, Ardell JL, Wurster RD, Milosavljevic M. Vagal postganglionic innervation of the canine sinoatrial node. J Auton Nerv Syst. 1987;20:13–23. doi: 10.1016/0165-1838(87)90077-4. [DOI] [PubMed] [Google Scholar]
- Randall WC, Armour JA. Gross and microscopic anatomy of the cardiac innervation. In: Randall WC, editor. Neural Regulation of the Heart. New York: OUP; 1977. pp. 15–41. [Google Scholar]
- Randall WC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1066–R1075. doi: 10.1152/ajpregu.00167.2003. [DOI] [PubMed] [Google Scholar]
- Randall WC, Milosavljevic M, Wurster RD, Geis GS, Ardell Selective vagal innervation of the heart. Ann Clin Lab Sci. 1986b;16:198–208. [PubMed] [Google Scholar]
- Randall WC, Wurster RD, Randall DC, Xi-Moy SX. From cardioaccelerator and inhibitory nerves to a “heart brain”: an evolution of concepts. In: Shepherd JT, Vatner SF, editors. Nervous Control of the Heart. Amsterdam: HAP; 1996. pp. 173–199. [Google Scholar]
- Richardson AP, Hinsey JC. A functional study of the nodose ganglion of the vagus with various degeneration methods. Proc Soc Exp Biol Med. 1933;30:1141–1143. [Google Scholar]
- Rogers RC, Hermann GE. Hypothalamic paraventricular nucleus stimulation-induced gastric acid secretion and bradycardia suppressed by oxytocin antagonist. Peptides. 1986;7:695–700. doi: 10.1016/0196-9781(86)90046-x. [DOI] [PubMed] [Google Scholar]
- Rubino A, Hasall JS, Burnstock G. Autonomic control of the myocardium: Non-adrenergic non-cholinergic (NANC) mechanisms. In: Shepherd JT, Vatner SF, editors. Nervous Control of the Heart. Amsterdam: HAP; 1996. pp. 139–171. [Google Scholar]
- Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, Pauza DH. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011;8:732–739. doi: 10.1016/j.hrthm.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburkina I, Rysevaite K, Pauziene N, Mischke K, Schauerte P, Jalife J, Pauza DH. Epicardial neural ganglionated plexus of ovine heart: anatomical basis for experimental cardiac electrophysiology and nerve protective cardiac surgery. Heart Rhythm. 2010;7:942–950. doi: 10.1016/j.hrthm.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio KN, Mauad H, Spyer KM, Ford TW. Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp Physiol. 2003;88:315–327. doi: 10.1113/eph8802525. [DOI] [PubMed] [Google Scholar]
- Samuels MA. The brain–heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- Sarnoff SJ, Brockman SK, Gilmore JP, Linden RJ, Mitchell JH. Regulation of ventricular contraction: Influence of cardiac sympathetic and vagal nerve stimulation on atrial and ventricular dynamics. Circ Res. 1960;8:1108–1122. doi: 10.1161/01.res.8.5.1108. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ( The autonomic nervous system and life-threatening arrhythmias. In: Shepherd JT, Vatner SF, editors. Nervous Control of the Heart. Amsterdam: HAP; 1996. pp. 329–356. [Google Scholar]
- Shimizu S, Akiyama T, Kawada T, Shishido T, Yamazaki T, Kamiya A, Mizuno M, Sano S, Sugimachi M. In vivo direct monitoring of vagal acetylcholine release to the sinoatrial node. Auton Neurosci. 2009;148:44–49. doi: 10.1016/j.autneu.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monoamine and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation. 1999;99:411–419. doi: 10.1161/01.cir.99.3.411. [DOI] [PubMed] [Google Scholar]
- Singh S, Johnson PI, Lee RE, Orfei E, Lonchyna VA, Sullivan HJ, Montoya A, Tran H, Wehrmacher WH, Wurster RD. Topography of cardiac ganglia in the adult human heart. J Thorac Cardiovasc Surg. 1996;112:943–953. doi: 10.1016/S0022-5223(96)70094-6. [DOI] [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neural circuit innervating the rat heart defined by neuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrota A, Comar D, Paillotin G, Davy J-M, Ammont M-C, Stulzaft O, Maziere B. Muscarinic cholinergic receptor in the human heart evidenced under physiological conditions by positron emission tomography. Proc Natl Acad Sci U S A. 1985;82:584–588. doi: 10.1073/pnas.82.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart P, Critchley H, Lambiase PD. Heart–brain interactions in cardiac arrhythmia. Heart. 2011;97:698–708. doi: 10.1136/hrt.2010.209304. [DOI] [PubMed] [Google Scholar]
- Takanaga A, Hayakawa T, Tanaka K, Kawabata K, Maeda S, Seki M. Immunohistochemical characterization of cardiac vagal preganglionic neurons in the rat. Auton Neurosci. 2003;106:132–137. doi: 10.1016/S1566-0702(03)00127-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hassal CJS, Burnstock G. The distribution of intracardiac neurones and nerve terminals that contain a marker for nitric oxide, NADPH-diaphorase, in the guinea-pig heart. Cell Tissue Res. 1993;73:293–300. doi: 10.1007/BF00312831. [DOI] [PubMed] [Google Scholar]
- Tcheng KT. Innervation of the dog's heart. Am Heart J. 1951;41:512–524. doi: 10.1016/0002-8703(51)90019-1. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RW, De Jongile MJ, Korf J. Neuroanatomy of cardiac activity regulating circuitry: a transneuronal retrograde viral labeling study in the rat. Eur J Neurosci. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Ulphani JS, Cain JH, Inderyas F, Gordan D, Gikas PV, Shade G, Mayor D, Arora R, Kadish AH, Goldberger JJ. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm. 2010;7:1113–1119. doi: 10.1016/j.hrthm.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Ventura S, Bavetta S, Milner P, Ralevic V, Burnstock G. Nitric oxide synthase is co-located with vasoactive intestinal polypeptide in postganglionic parasympathetic nerves innervating the rat vas deferens. Neuroscience. 1998;83:607–616. doi: 10.1016/s0306-4522(97)00416-8. [DOI] [PubMed] [Google Scholar]
- Verrier RL, Lown B. Behavioural stress and cardiac arrhythmias. Ann Rev Physiol. 1984;46:155–176. doi: 10.1146/annurev.ph.46.030184.001103. [DOI] [PubMed] [Google Scholar]
- Wang L, Henrich M, Buckler KJ, McMenamin M, Mee CJ, Sattelle DB, Paterson DJ. Neuronal nitric oxide synthase gene transfer decreases [Ca2+]i in cardiac sympathetic neurons. J Mol Cell Cardiol. 2007;43:717–725. doi: 10.1016/j.yjmcc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones JFX, Jeggo RD, Daly MB, Jordan D, Ramage AG. Effect of pulmonary C-fibre activation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats. J Physiol. 2000;526:157–165. doi: 10.1111/j.1469-7793.2000.t01-1-00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DC, McWilliam PN, Ford TW, Clarke RW. The effect of selective electrical stimulation of non-myelinated vagal fibres on heart rate in the rabbit. J Auton Nerv Syst. 1987;21:215–221. doi: 10.1016/0165-1838(87)90024-5. [DOI] [PubMed] [Google Scholar]
- Xenopoulos NP, Applegate RJ. The effect of vagal stimulation on left ventricular systolic and diastolic performance. Am J Physiol Heart Circ Physiol. 1994;266:H2167–H2173. doi: 10.1152/ajpheart.1994.266.6.H2167. [DOI] [PubMed] [Google Scholar]
- Yamada S, Yamamura HI, Roeske WR. Alterations in cardiac autonomic receptors following 6-hydroxydopamine treatment in rats. Mol Pharmacol. 1980;18:185–192. [PubMed] [Google Scholar]
- Yang CM, Yeh HM, Sung TC, Chen FF, Wang YY. Characterisation of muscarinic receptor subtypes in canine left ventricular membranes. J Recept Res. 1992;12:427–449. doi: 10.3109/10799899209074805. [DOI] [PubMed] [Google Scholar]
- Yasahura O, Matsuo A, Bellier JP, Aimi Y. Demonstration of choline acetyltransferase of a peripheral type in the rat heart. J Histochem Cytochem. 2007;55:287–299. doi: 10.1369/jhc.6A7092.2006. [DOI] [PubMed] [Google Scholar]
- Yuan B-X, Ardell JL, Hopkins DA, Armour JA. Gross and microscopic anatomy of canine intrinsic cardiac neurons. Anat Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- Zang W-J, Chen L-N, Yu X-J. Progress in the study of vagal control of cardiac ventricles. Acta Physiol Sinica. 2005;57:659–672. [PubMed] [Google Scholar]
- Zhang ZH, Rashba S, Oppenheimer SM. Insular cortex lesions alter baroreceptor sensitivity in the urethane anaesthetized rat. Brain Res. 1998;813:73–81. doi: 10.1016/s0006-8993(98)00996-2. [DOI] [PubMed] [Google Scholar]
- Zuanetti G, De Ferrari GM, Priori SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61:429–435. doi: 10.1161/01.res.61.3.429. [DOI] [PubMed] [Google Scholar]


