Abstract
In normal rats, central administration of orexin or exposure to certain forms of stress can induce significant increases in blood pressure and sympathetic nerve activity, which can be blocked by orexin receptor antagonists. The resting blood pressure is, however, unaffected by such antagonists, but is significantly lower in rodents with total loss of orexin, such as prepro-orexin knockout mice and orexin neuron-ablated orexin/ataxin-3 transgenic rats. We hypothesize that orexin is involved in the pathophysiology and maintenance of high blood pressure in the spontaneously hypertensive rat (SHR), a model of primary hypertension. To test this hypothesis, we measured orexin-A mRNA expression in the rostral ventrolateral medulla and antagonized both orexin receptors using an orally administered potent dual orexin receptor antagonist, almorexant, in SHRs and normotensive Wistar–Kyoto rats. In SHRs, there was a strong trend towards an increased orexin-A mRNA expression in the rostral ventrolateral medulla, and blocking orexin receptors markedly lowered blood pressure (from 182/152 ± 5/6 to 149/119 ± 9/8 mmHg; P < 0.001), heart rate (P < 0.001), sympathetic vasomotor tone (P < 0.001) and the noradrenaline levels in cerebrospinal fluid and plasma (P < 0.002). The significant antihypertensive effects of almorexant were observed in wakefulness and non-rapid eye movement sleep during both dark and light phases of the diurnal cycle only in SHRs. Blocking orexin receptors had no effect on blood pressure and sympathetic tone in normotensive Wistar–Kyoto rats. Our study links the orexin system to the pathogenesis of high blood pressure in SHRs and suggests that modulation of the orexin system could be a potential target in treating some forms of hypertension.
Key points
Orexin is involved in blood pressure regulation. Certain forms of stress or central administration of orexin increases blood pressure and sympathetic nerve activity in normal rats; orexin knockout mice and orexin neuron-ablated transgenic rats have lower basal blood pressure. The role of orexin in hypertension remains unknown.
RT-PCR shows a strong trend towards an increased orexin-A mRNA expression in the rostral ventrolateral medulla in adult spontaneously hypertensive rats.
In adult spontaneously hypertensive rats, blocking orexin receptors by oral administration of an antagonist, almorexant, significantly lowers blood pressure in wakefulness and non-rapid eye movement sleep during dark and light cycles, an effect accompanied by decreased sympathetic vasomotor tone and noradrenaline levels in cerebrospinal fluid and plasma.
Antagonizing orexin receptors had no effect on resting blood pressure in normal rats.
The orexin system participates in the pathogenesis of high blood pressure in spontaneously hypertensive rats. Modulation of the orexin system could be a potential target in treating some forms of hypertension.
Introduction
Essential hypertension is the most prevalent form of human high blood pressure (Chobanian et al. 2003). The spontaneously hypertensive rat (SHR) is the most commonly used animal model for human essential hypertension and, as in human hypertension, the blood pressure of the SHR rises with age, starting from about 5 weeks of age, accompanied by an overactive sympathetic nervous system and an impaired baroreflex (Simms et al. 2009; Tan et al. 2010).
Orexins (orexin-A and -B), the neuropeptides produced by neurons primarily located in the lateral hypothalamic area, are important in the regulation of food intake, arousal and autonomic functions (Antunes et al. 2001; Kayaba et al. 2003; Matsumura et al. 2003; Shirasaka et al. 2003; de Lecea, 2010; Nattie & Li, 2012; Shahid et al. 2012). Increasing evidence in the past decade shows that the orexin system is directly and/or indirectly involved in the regulation of blood pressure (Follwell & Ferguson, 2002; Kayaba et al. 2003; Matsumura et al. 2003; Shirasaka et al. 2003; Furlong et al. 2009; Huang et al. 2010; Nattie & Li, 2012; Shahid et al. 2012). Orexin projections and orexin receptors (OX1R and OX2R) are found in many brain sites that participate in the control of sympathetic nerve activity (SNA) and blood pressure, e.g., paraventricular nucleus (PVN), nucleus of the solitary tract, the rostral ventrolateral medulla (RVLM) and the spinal cord intermediolateral cell column and sympathetic preganglionic neurons (Swanson & Sawchenko, 1983; Peyron et al. 1998; Date et al. 1999; Shirasaka et al. 2003). In vitro, orexin depolarizes hypothalamic PVN neurons (Follwell & Ferguson, 2002; Shirasaka et al. 2003) and spinal cord sympathetic preganglionic neurons (Antunes et al. 2001). In vivo, central (focal or intracerebral) administration of orexin in conscious and anaesthetized animals increases arterial blood pressure, heart rate (HR), SNA and plasma catecholamine release, and these effects can be attenuated by orexin receptor antagonists (Matsumura et al. 2003; Shirasaka et al. 2003; Huang et al. 2010; Shahid et al. 2012). Given that administered orexins evoke high blood pressure and augmented SNA and that orexin is necessary for the increase in blood pressure and HR that occurs in response to certain stresses (Furlong et al. 2009; Johnson et al. 2010), we hypothesized that the orexin system is not only involved in the regulation of blood pressure in normal conditions but also may play an important role in the genesis and maintenance of the high blood pressure in the SHR. To test the hypothesis, we antagonized both OX1R and OX2R by using a potent dual receptor antagonist in SHRs and normotensive control Wistar–Kyoto (WKY) rats in wakefulness and sleep during both the dark and light periods of the diurnal cycle.
Methods
Ethical approval
All animal experiments and surgical protocols were within the guidelines of the National Institutes of Health for animal use and care and the American Physiological Society's Guiding Principles in the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at the Geisel School of Medicine at Dartmouth. The authors have read and complied with guidelines for research in rodents outlined for The Journal of Physiology and UK regulations (Drummond, 2009).
General methods
Spontaneously hypertensive rats (male, n= 7) and normotensive WKY control rats (male, n= 5; 240–320 g) used for the physiological experiments were individually housed in light- and temperature-controlled conditions subject to a 12 h light–12 h dark cycle. The light cycle was set as 07:00 h lights on and 19:00 h lights off. Food and water were provided ad libitum. The general methods are those in common use in our laboratory (Li et al. 2008; Li & Nattie, 2010). At the conclusion of the experiments, the rats were killed with an overdose of sodium pentobarbitone injected i.p. (>75 mg kg−1).
Surgery
Animals were anaesthetized with a ketamine (Putney, Inc. Portland, ME, USA) and xylazine (Lloyd Labs, Walnut, CA, USA) cocktail (90 and 15mg kg−1, respectively, i.m.) and implanted with a PhysioTel C50-PXT telemetric transmitter (DSI, St Paul, MN, USA) that allowed us to measure blood pressure, ECG and temperature simultaneously. The blood pressure catheter was secured in the descending aorta between the femoral bifurcation and renal artery. The positive ECG lead was secured to the xyphoid process, while the negative lead was sutured to the muscle tissue in the area of the right shoulder. The body of the transmitter was placed inside the abdominal cavity and secured to the abdominal wall. The rats were allowed to recover for 5–7 days before the EEG and EMG electrodes were implanted under anaesthesia as described earlier (Li et al. 2008; Li & Nattie, 2010). In brief, three EEG electrodes were screwed into the skull and two EMG electrodes were sutured onto the dorsal neck muscles. All the electrodes were connected to a six-prong plastic pedestal. The rats were then allowed to recover for at least 7 days before conducting any experiment. All the experiments were conducted in a Plexiglass chamber for at least 12 h with the light controlled at that appropriate for the phase of the rat's diurnal cycle under study. Food and water were continuously available.
Dual orexin receptor antagonist
Almorexant (Almxt) is a dual orexin receptor antagonist that can be administered orally, readily crosses the blood–brain barrier and reversibly blocks both OX1R and OX2R with high-affinity binding (Brisbare-Roch et al. 2007). The oral gavages (250–300 mg kg−1) were made using either dissolved Almxt in 0.25% methylcellulose solution or the 0.25% methylcellulose solution alone as the vehicle control (Veh), as previously described (Brisbare-Roch et al. 2007; Li & Nattie, 2010). Each rat had both Almxt and Veh treatment, each on separate days, and the order of drug administration was chosen randomly. The dosage was chosen based on the previous studies (Brisbare-Roch et al. 2007; Li & Nattie, 2010). Almorexant was generously supplied by Actelion Pharm, Ltd. (Gewerbestrasse, Allschwil, Switzerland).
Brain tissue for measuring orexin-A mRNA expression
Orexin-A mRNA expression in RVLM and PVN was measured by RT-PCR. After the rats were killed, the brains were quickly removed from SHRs (n= 5) and WKY rats (n= 5), immediately frozen on dry ice and subsequently stored in a freezer at −80°C until further processing. The frozen brains were sliced at 60 μm per block, and then the tissues from RVLM and PVN were punctured out using a 1 mm tissue punch (Interfocus). The RNA was TRIzol extracted using a Heavy Phase Lock Gel column (5 Prime), column purified (RNAeasy; Qiagen, Manchester, UK) and reverse transcribed (QuantiTect; Qiagen, Manchester, UK) ready for RT-PCR analysis. The RT-PCR was performed in triplicate for the orexin probes using an ABI 7500 Real-Time PCR system (The Applied Biosystems, Paisley, UK) with Roche Fast start universal SYBR green master mix (ROX). Ribosomal protein L19 (RPL19) & Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping primers were used for PVN and RVLM RT-PCR, respectively. The sequences of the orexin [Rattus norvegicus hypocretin (Hcrt)] primers are as follows: forward, CCACTGCACCGAAGATACCA and reverse, GCCCAGGGAACCTTTGTAGA. The relative quantification was performed using the comparative threshold cycle (Ct) with the ΔCt values determined by subtracting the values of the reference control gene from the target gene Ct values. The difference in the mRNA expression levels between the groups was expressed as 2(ΔΔCt), where ΔΔCt equals the difference in ΔCt between the SHRs and WKY rats. The level of orexin expression was calculated and presented as the fold change, shown in Fig. 1.
Figure 1. The levels of orexin-A mRNA expression in the rostral ventrolateral medulla (RVLM; A) and of adrenaline (Adr) and noradrenaline (NA) in cerebrospinal fluid (CSF; B) and plasma (C).
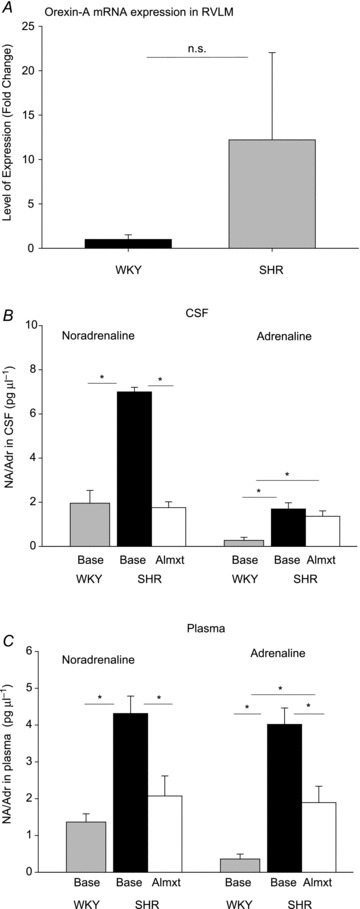
The level of orexin mRNA expression in the RVLM of Wistar–Kyoto (WKY) control rats (n= 5, black bar) and spontaneously hypertensive rats (SHRs; n= 5, grey bar) were measured by RT-PCT, and the levels of NA and Adr of WKY rats (n= 4, grey bars) and SHRs before (n= 3, black bars) and after almorexant (Almxt) treatment (open bars) were measured by HPLC. *Significantly different with Student–Newman–Keuls post hoc tests (P < 0.002, one-way ANOVA). The RT-PCR showed a 12-fold increase of orexin mRNA expression in the RVLM in SHRs compare with that of WKY rats, but it did not reach statistical significance (n.s., P= 0.28) due to the variability.
Collection of plasma and cerebrospinal fluid (CSF) for measuring monoamine
To assess monoamine content, HPLC analysis was performed on the plasma and CSF samples from SHRs (before and after Almxt treatment; n= 3) and WKY rats (n= 4). Under general anaesthesia (ketamine–xylazine cocktail), CSF (0.15–0.2 ml) was collected via a small puncture through the cistern using a 29 gauge needle insulin syringe, while a blood sample (0.5 ml) was withdrawn through a puncture in the jugular vein using a heparinized 29 gauge needle insulin syringe, with plasma collected following centrifugation (8,000 rmp, 1–2 min) of the blood. The baseline samples were collected during the surgery for EEG implantation at least 1 week before the experiment in both SHRs and WKY rats, and in SHRs post-Almxt samples were collected 3 h after Almxt treatment. All the samples were kept at −80°C until analysis.
Data collection and analysis
The rats were placed in a chamber with the light controlled on a 12 h light–12 h dark cycle and food and water provided ad libitum. The signals for blood pressure, ECG and body temperature from implanted telemetric probes were converted into an analog signal using a calibrated pressure output adapter (DSI, St Paul, MN, USA), and sampled at 1000 and 140 Hz, respectively. Raw EEG and EMG outputs from the skull and neck skeletal muscle electrodes were sampled at 140 Hz and filtered at 0.3–70 and 0.1–100 Hz, respectively. All analog signals were recorded continuously for at least 12 h using the DataPac 2K2 system (RUN Technologies, Laguna Hills, CA, USA), and then analysed using LabChart (ADInstruments, Colorado Springs, CO, USA). The systolic (SBP), diastolic (DBP) and mean arterial blood pressure (MAP) were averaged every 30 min based on the arousal and diurnal state in each SHR and WKY rat as shown in Fig. 2. The maximal decrease of MAP was defined as the difference between the mean baseline and the lowest mean 30 min data point of each rat. Heart rate was analysed using the R-wave traces of the ECG. The detailed methods for determination of vigilance states have been described in our earlier publications (Li et al. 2008; Li & Nattie, 2010). To evaluate the treatment effects on cardiac and vascular sympathetic tone, a fast Fourier transform function for power spectral analysis was performed on HR of ECG and on SBP using LabChart Pro (ADInstruments). The beat-to-beat heart rate derived from the ECG (R–R interval) and SBP from either wakefulness or non-rapid eye movement (NREM) sleep of every 1 h of the experiment were averaged and analysed in both light and dark periods of the diurnal cycles in SHRs and WKY rats. The maximal decrease of the low frequency (LF) and the low-frequency/high-frequency (LF/HF) ratio of SBP was the difference between the mean baselines and the lowest 1 h average data point of each rat. The frequency spectral bands of very low frequency (0.0–0.2 Hz), LF (0.2–0.7 5 Hz), high frequency (HF; 0.75–5 Hz) and LF/HF ratio of HR and SBP were assessed (Cerutti et al. 1991; Murasato et al. 1998; Waki et al. 2006; Abdala et al. 2012).
Figure 2. Effects of Almxt on blood pressure.
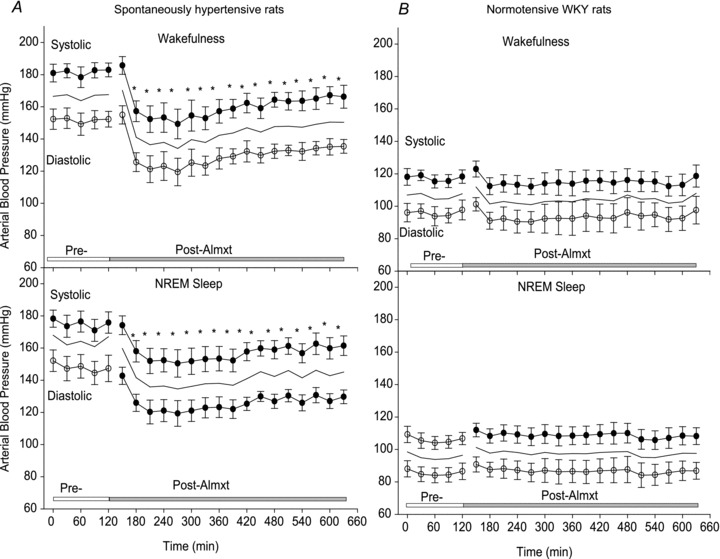
Systolic (filled circles; means ± SEM), diastolic (open circles; means ± SEM) and mean arterial blood pressure values (continuous lines) before and after Almxt treatment in wakefulness and non-rapid eye movement (NREM) sleep during the dark period in SHRs (A; n= 7) and WKY rats (B; n= 5) respectively. Each time point represents a 30 min segment of data. We first categorized the state as wakefulness or NREM sleep, and then applied one-way repeated measures (RM) ANOVA separately within the light and dark periods. *Significantly different from pretreatment with Student–Newman–Keuls post hoc tests (P < 0.002).
Statistical analysis
SigmaPlot statistics programs Systat Software Inc., San Jose, CA, USA were used for our statistical analysis. The data are presented as means ± SEM. To evaluate the treatment effect on MAP, SBP, DBP, HR, LF/HF ratio and body temperature, we first categorized the vigilance states into wakefulness and sleep and then applied repeated measures (RM) ANOVA separately for each vigilance state within both the light and dark periods, applying the Student–Newman–Keuls method post hoc test when appropriate. In addition, we compared Almxt with Veh by two-way RM ANOVA and found verification of the deductions from the one-way RM ANOVA. The methods for sleep analysis were as described previously (Li & Nattie, 2010). To evaluate whether the maximal effects of Almxt on blood pressure and HR are sleep–wake and diurnal cycle dependent, we compared the maximal effects in wakefulness and sleep in both light and dark phases of the diurnal cycle by applying a two-way RM ANOVA with sleep–wake and light–dark periods as factors and the treatments as RM. The data from the RT-PCR and neurochemistry were also compared between SHRs and WKY rats using an ANOVA.
Experimental protocol
All the animals were studied in both dark and light periods of the diurnal cycle with both control Veh and Almxt treatments, each administered on a separate day. To study each animal in all these conditions we set the diurnal light cycle as 07:00 h lights on and 19:00 h lights off. For study in the light period, the animals were administered one dose of control Veh or Almxt between 10:00 and 10:30 h. For study in the dark period, the animals were administered one dose of control Veh or Almxt between 22:00 and 22:30 h. On the day of the experiment, the rat was placed into the experimental chamber and allowed to acclimate for at least 1 h until the rat started to have normal wake–sleep cycles; then 2.5–3 h of pretreatment baseline data were collected while the rat was in the assigned light or dark period before an Almxt or control Veh gavage treatment was given. The animals were then returned to the recording chamber, and all the data were continuously recorded until the end of that assigned diurnal cycle. Rats were allowed at least 3 days of rest before another experiment.
Results
Orexin-A expression in the RVLM
The RT-PCR showed a 12-fold increase of orexin mRNA expression in the RVLM in SHRs compared with that of WKY rats (Fig. 1A), but due to the variability it did not reach statistical significance (P= 0.28, one-way ANOVA). In the PVN, a similar orexin mRNA expression was found in SHRs and WKY rats. The larger variability observed in the RVLM here might be due to variance in sampling sites within the RVLM and in the times of day when tissue was taken. Immunohistochemistry staining and Western blot for orexin receptors were not performed in the present study due to the lack of selective antibodies (Kukkonen, 2013).
Changes in adrenaline (Adr) and noradrenaline (NA) in CSF and plasma
The total adrenaline and noradrenaline levels in CSF and plasma in WKY rats and SHRs are shown in Fig. 1B and C. In CSF (Fig. 1B), the basal levels of NA and Adr are significantly higher in SHRs than those measured in WKY rats (P < 0.001, one-way ANOVA). In SHRs, Almxt significantly lowered NA to a level similar to that of WKY rats (P < 0.001, one-way RM ANOVA). The Almxt treatment did not significantly change the level of Adr in SHRs (P > 0.05, one-way ANOVA), and the levels of Adr in SHRs both pre- and post-Almxt are higher than those of WKY rats (P < 0.02, one-way ANOVA). In plasma (Fig. 2C), the basal levels of NA and Adr are significantly higher in SHRs than in WKY rats (P < 0.01, one-way ANOVA). In SHRs, Almxt significantly lowered both NA and Adr in plasma (P < 0.001, one-way RM ANOVA).
Arterial blood pressure and HR
In SHRs, one dose of Almxt produced a remarkable and long-lasting (∼8 h) decrease in MAP, SBP and DBP in both wakefulness and sleep during the dark and light periods of the diurnal cycle (Fig. 2A). In wakefulness during the dark period, values for SBP/DBP and HR were 182/152 ± 5/6 mmHg and 355 ± 8 beats min-1, respectively, before Almxt treatment and were lowered by 18–22% to 149/119 ± 9/8 mmHg and 302 ± 5 beats min-1, respectively, at the times of maximal effect (between 1 and 4 h) after Almxt treatment (Figs 2 and 3). The significant effects of Almxt were observed from 30 min post-Almxt treatment until 8 h, when the recording was terminated due to the change of light cycle (Fig. 2A; P < 0.002, one-way RM ANOVA), and SBP/DBP and HR were maintained below 167/136 ± 5/4 mmHg and 325 ± 11 beats min-1, respectively, during this 8 h period (Fig. 2A). Similar effects were observed in NREM sleep during the dark period (Fig. 2A) and in both wakefulness and sleep during the light period (data not shown). Mean arterial pressure and HR returned to the pretreatment level within 24 h (data not shown). In addition, we compared Almxt with Veh by two-way RM ANOVA and found verification of the deductions from the one-way RM ANOVA. As shown in Fig. 1B, Almxt had no effect on blood pressure in WKY control rats.
Figure 3. Changes in heart rate (HR) before and after vehicle (Veh) or Almxt treatment in wakefulness and sleep during the dark period in SHRs.
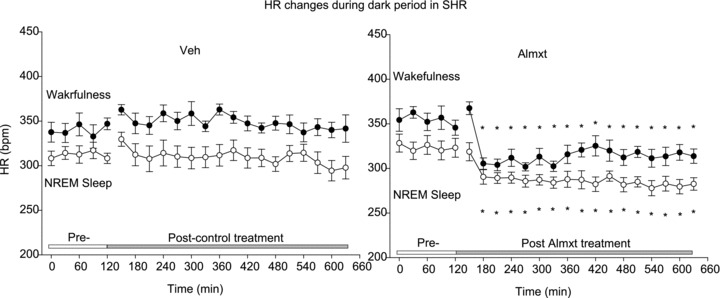
Each time point represents a 30 min segment of data. We first categorized the state as wakefulness or NREM sleep, and then applied one-way RM ANOVA separately within the light and dark periods. *Significantly different from pretreatment with Student–Newman–Keuls post hoc tests (P < 0.004).
With no treatment, the baseline HR was significantly higher in wakefulness compared with NREM sleep in both the light (340 ± 9 vs. 314 ± 7 beats min-1) and the dark period (354 ± 8 vs. 320 ± 10 beats min-1; Fig. 3; P < 0.05, one-way RM ANOVA). Mean arterial pressure was slightly but significantly higher in wakefulness compared with NREM sleep (∼4.9 mmHg, P= 0.021, two-way RM ANOVA) during the light period only. However, MAP and HR were not significant different in wakefulness or NREM sleep between the dark and the light period (P > 0.05, two-way RM ANOVA). In SHRs, comparing pre- with post-Almxt treatment, the maximal decreases of MAP and HR were not significantly different between wakefulness and NREM sleep within the dark period (∼37 mmHg and 64 beats min-1 in wakefulness and ∼34 mmHg and 54 beats min-1 in NREM sleep) or the light period (29 mmHg and 64 beats min-1 in wakefulness and 25 mmHg and 55 beats min-1 in NREM sleep, P > 0.05, two-way RM ANOVA; Fig. 4) or between dark and light cycles (P > 0.05, two-way ANOVA). The maximal decrease of MAP in wakefulness and NREM sleep during both light and dark periods after Almxt treatment was significantly larger than that observed with Veh treatment in the same SHRs (P < 0.001, one-way RM ANOVA; Fig. 4) and that observed with Almx or Veh treatment in WKY rats (P≤ 0.001, one-way ANOVA; Fig. 4). In wakefulness and sleep during the light period and in wakefulness in the dark period, the maximal decrease of HR after Almxt treatment was significantly larger than that after Veh treatment in the same SHRs (P < 0.001, one-way RM ANOVA; Figs 3 and 4). In the light period, the maximal decrease of HR after Almxt treatment was significantly larger than that observed in the WKY rats in the same conditions (P < 0.001, one-way ANOVA; Fig. 4).
Figure 4. The maximal decrease of mean arterial blood pressure (MAP) (A, B) and HR (C, D) after Almxt or Veh treatment in wakefulness and NREM sleep during dark (A, C) and light (B, D) periods in SHRs and WKY rats.
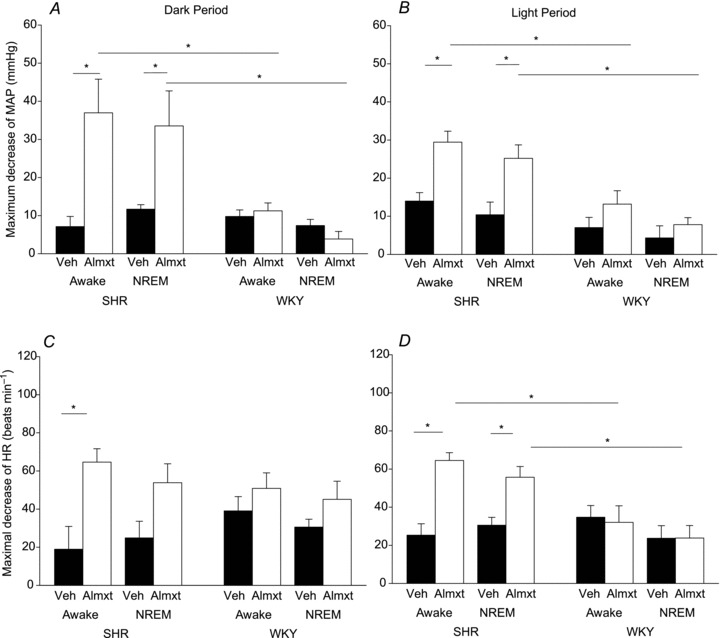
We first categorized the state as wakefulness or NREM sleep, and then applied a one-way ANOVA separately within the light and dark periods. *Significantly different with Student–Newman–Keuls post hoc tests (P < 0.002).
The HR changes before and after Veh or Almxt treatment in SHRs during the dark period are shown in Fig. 3. The Veh treatment had no significant effects on the HR in wakefulness and NREM sleep during either the light or the dark period in both SHRs and WKY rats (P≥ 0.05, one-way RM ANOVA).
In normotensive WKY rats, Almxt treatment had no significant effect on MAP or SBP/DBP comparing each post-treatment value with the pretreatment baseline (P≥ 0.05, one-way RM ANOVA) and with Veh treatment in the same rats (P≥ 0.05, two-way RM ANOVA) in wakefulness and sleep during either period of the diurnal cycle (Figs 2 and 4). The Almxt slightly lowered HR in wakefulness and NREM sleep in the dark and the light periods, but the changes were not significantly different compared with the pre-Almxt baseline (data not shown) and with that of Veh control conditions (Fig. 4C and D) in the same rats (P≥ 0.005, one-way RM ANOVA).
Spectral analysis of SBP and HR
The maximal changes (decrease) of the LF and the LF/HF ratio of SBP before and after Almxt in wakefulness and NREM sleep during the dark and light periods of the diurnal cycle in both SHRs and WKY rats are shown in Fig. 5.
Figure 5. Effects of Almxt on maximal decrease of LF (A) and LF/HF (B) ratio in WKY rats (filled bars) and SHRs (open bars).
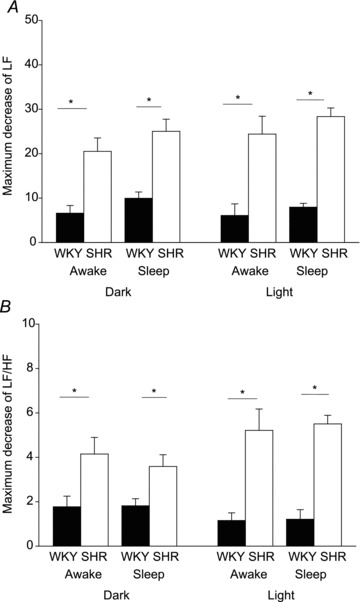
We first categorized the state as wakefulness or NREM sleep, and then applied a one-way ANOVA separately within the light and dark periods. *Significantly different with Student–Newman–Keuls post hoc tests (P < 0.001).
Frequency spectral analysis of SBP showed that in SHRs, Almxt treatment significantly lowered both the LF and the LF/HF ratio of SBP in wakefulness and NREM sleep in both the dark and the light period compared with the pretreatment baseline of the same SHRs (P < 0.002, one-way RM ANOVA) and with that observed in normotensive WKY rats (P < 0.001, one-way RM ANOVA; Fig. 5). The significantly decreased LF and LF/HF ratio was observed from 1 to 7 h after Almxt treatment (P < 0.003, one-way RM ANOVA). Almorexant also significantly decreased the very low frequency component of SBP in SHRs (P < 0.05, one-way RM ANOVA; data not shown). Similar effects were observed in NREM sleep in the dark period and in both wakefulness and sleep in the light period in SHRs (Fig. 5), and there was no significant difference in the responses between wakefulness and NREM sleep in both light and dark periods (P > 0.05, two-way RM ANOVA). The Veh control treatment yielded no significant changes of LF/HF of SBP in wakefulness and NREM sleep during either the light or the dark period in either SHRs or WKY rats (P≥ 0.05, one-way RM ANOVA). Almorexant treatment had no significant effect on LF, very low frequency and LF/HF ratio of SBP in wakefulness and NREM sleep in the light or the dark period in WKY rats (P≥ 0.05).
Spectral analysis of HR revealed that in SHRs following Almxt treatment there is a significant decrease of the LF/HF ratio of HR (P < 0.002, one-way RM ANOVA) similar to that of the LF/HF ratio of SBP and there are no significant changes in LF of HR (P≥ 0.05; data are not shown).
Discussion
The key novel findings of the study are as follows: (i) there is a strong trend towards an increased orexin mRNA expression in the RVLM in SHRs compare with WKY rats; and (ii) in conscious hypertensive rats, antagonism of the orexin receptors (OX1R and OX2R) by an orally administered dual orexin receptor antagonist, Almxt, markedly lowers blood pressure (by >30 mmHg) and HR, significantly reduces sympathetic vasomotor tone in wakefulness and sleep during both the dark and the light period of the diurnal cycle, as indirectly assessed by spectral analysis of SBP, and decreases levels of NA and Adr in CSF and plasma. Our data indicate that the orexin system is involved in the pathophysiology of hypertension in SHRs and that orexin receptor antagonism could constitute a potential new therapeutic strategy for some forms of hypertension.
Orexin and blood pressure regulation
Studies in normal animals in the past decade have shown that the orexin system participates in the regulation of blood pressure, HR and SNA (Chen et al. 2000; Antunes et al. 2001; Matsumura et al. 2001; Shirasaka et al. 2003; Shahid et al. 2012). For example: (i) orexinergic terminals and orexin receptors are expressed in many brain sites that are critical in the regulation of blood pressure and SNA, e.g. the PVN, nucleus tractus solitarii and RVLM (Swanson & Sawchenko, 1983; Peyron et al. 1998; Date et al. 1999; Shirasaka et al. 2003); (ii) in vitro, orexin depolarized neurons in the RVLM, nucleus tractus solitarii and hypothalamic PVN (Follwell & Ferguson, 2002; Shirasaka et al. 2003; Huang et al. 2010); (iii) in vivo, central, but not peripheral (Chen et al. 2000), administration of orexin intracerebrally or directly into the RVLM or nucleus tractus solitarii in conscious and anaesthetized normal rats or rabbits increased MAP, HR (Chen et al. 2000; Matsumura et al. 2001; Smith et al. 2002; Shirasaka et al. 2003; Shahid et al. 2012), SNA and plasma catecholamine levels (Matsumura et al. 2001; Shirasaka et al. 2003), and this excitatory response could be attenuated by an OX1R antagonist (Shahid et al. 2012); (iv) both prepro-orexin knockout mice and orexin neuron genetically ablated (orexin/ataxin-3) rats have lower resting arterial blood pressure and reduced autonomic responses to stress (Kayaba et al. 2003; Schwimmer et al. 2010); and (v) silencing prepro-orexin, the gene encoding orexin, in the dorsomedial-perifornical hypothalamus with small interfering RNA blocks the increases in blood pressure and HR that occur in response to panic in mice (Johnson et al. 2010).
Orexin and hypertension
The input of the orexin system into blood pressure regulation is probably not functionally active in normal resting conditions. The evidence for this is as follows: (i) in vitro, the OX1R antagonist SB334867 and OX2R antagonist TCS-OX2-29 had no effect on spontaneous neuronal activity in the RVLM, but when applied together they completely abolished orexin-induced depolarization of the RVLM neurons (Huang et al. 2010); (ii) in vivo, in normal animals, the OX1R antagonist SB334867 and the dual orexin receptor antagonist Almxt have no effect on resting blood pressure, but can block orexin- or stress-evoked increases in BP and SNA, respectively (Furlong et al. 2009; Huang et al. 2010; Johnson et al. 2010; Shahid et al. 2012); and (iii) in our study, Almxt significantly decreased the existing high blood pressure in SHRs but had no effect on the normal blood pressure in WKY rats.
Increased sympathetic tone has been linked to the pathogenesis of essential hypertension in humans and SHRs (Guyenet, 2006; Simms et al. 2009; Tan et al. 2010), but the mechanisms that lead to increased sympathetic tone and why antagonism of orexin receptors has such an antihypertensive effect remain unclear at present. The LF and LF/HF ratio of HR and SBP have been used as indirect indices to assess cardiac and vasomotor sympathetic tone in conscious rats and humans (Cerutti et al. 1991; Murasato et al. 1998; Waki et al. 2006; Abdala et al. 2012). Here, we showed that the significantly reduced hypertension in Almxt-treated SHRs was accompanied by a significant decrease in the LF and LF/HF ratio of SBP and a decrease of noradrenaline in CSF and plasma, suggesting that Almxt may lower existing high blood pressure by reducing an overactive sympathetic vasomotor tone in SHRs. The observed strong trend towards an increased orexin-A mRNA expression in the RVLM suggests that one source of the enhanced sympathetic tone may be these orexin-producing neurons.
We conclude that in SHRs there is an overactive orexin system that pathologically enhances sympathetic vasomotor tone and plays an important role in the pathogenesis and maintenance of high blood pressure, and reducing the effects of an overactive orexin system by blocking orexin receptors can significantly lower the existing high sympathetic vasomotor tone and blood pressure in SHRs.
It is also worthwhile noting that Almxt treatment had an impressively long-lasting effect (∼8 h) on lowering both blood pressure and sympathetic tone in this study. The long-lasting effect may reflect the fact that orexin receptors are G-protein-coupled receptors that exert their effects via second messenger signalling and possibly subsequent epigenetic and protein changes. A binding study also showed that Almxt exerted a non-competitive and long-lasting antagonism as a result of its very slow rate of dissociation from OX2R (Malherbe et al. 2009).
Orexin and activity, sleep or stress
Orexin promotes wakefulness and is important in the maintenance of arousal. Given that stress responses are associated with arousal, it has been proposed that orexin is also involved in stress responses and defense mechanisms (Kayaba et al. 2003; Furlong et al. 2009; Iigaya et al. 2012; Johnson et al. 2010).
The effects of antagonizing orexin receptors by Almxt on sleep have previously been reported in great detail by the original study describing the properties of Almxt and by researchers in our laboratory (Brisbare-Roch et al. 2007; Li & Nattie, 2010). Brisbare-Roch et al. (2007) showed that Almxt reduced wakefulness and locomotor activity in rats, dogs and humans when administered systemically. We further reported that in the dark period of the diurnal cycle Amxt (300 mg kg−1) decreased wakefulness (−56%) and increased NREM (+39%) and rapid eye movement (+146%) sleep compared with pre-Almxt treatment in normal adult Sprague–Dawley rats, while not significantly promoting sleep in the light period of the diurnal cycle (Li & Nattie, 2010). Orexin has also been proved to be involved selectively in some forms of stress, e.g. blocking orexin receptors: (i) significantly reduced the pressor, tachycardic and renal sympathoexcitatory responses to stress evoked by injection of bicuculline in the perifornical hypothalamus (Iigaya et al. 2012); (ii) decreased the pressor, tachycardic and locomotor responses induced by fear, but had no effect on the pressor and tachycardic responses induced by restraint or cold exposure (Furlong et al. 2009); and (iii) decreased the hypercapnia-induced increase in ventilation without affecting breathing at rest (Li & Nattie, 2010). We cannot exclude the potential contributions of better sleep and/or a lower level of stress to the antihypertensive effects of Almxt reported in this study. However, the facts that the sleep-promoting effect was observed only in the dark period of the diurnal cycle, while antihypertensive effects were observed in both the light and the dark periods in wakefulness and sleep, suggest that the antihypertensive effect of Almxt cannot be achieved through promoting sleep alone. Moreover, in the normotensive WKY rats, Almxt generated the same sleep effect but did not significantly affect blood pressure and SNA. Our data also suggest that the modulation of BP and SNA by orexin in SHRs is not dependent on the state of arousal or diurnal cycle, as was the effect of orexin on central ventilatory chemoreception (Li & Nattie, 2010). Circadian changes of BP and HR are clearly observed in normal humans and in the early stages in patients with uncomplicated hypertension, with a typical 10–20% fall at night during sleep (nocturnal dip) compared with the values measured during the daytime (Peixoto & White, 2007). In rats, the circadian changes of BP and HR are complicated because, unlike humans, rats do sleep a significant amount in both the dark and the light periods. To detect the diurnal effects of orexin receptor antagonism on MAP and HR, if they are present, may require greater time periods of continuous recording.
Central or peripheral effect of Almxt
Almorexant is an orally active dual orexin receptor antagonist that can effectively cross the blood–brain barrier and reversibly block both OX1R and OX2R (Brisbare-Roch et al. 2007), and we anticipate that the main effects observed in this study are through effects in the CNS. However, our present study cannot exclude potential peripheral influences.
Orexin neurons are exclusively located in the lateral hypothalamus and send projections to many brain locations. Orexins and their functions have mainly been described in the CNS, but some recent studies suggest that orexin may also be present in some peripheral tissues, e.g. mRNAs of orexins and their receptors are detected in the gastrointestinal tract, pancreas and adipose tissue (Heinonen et al. 2008). Some have reported that OX1R mRNA is expressed in the adrenal gland, but this finding is controversial (Heinonen et al. 2008; Kukkonen, 2013). The potential peripheral effects of orexins on blood pressure and SNA have been investigated (Chen et al. 2000; Matsumura et al. 2001) and, in vivo, peripheral administration of orexin had no effect on SNA and blood pressure. Little evidence suggests any role of peripheral orexins in the regulation of cardiovascular function at the present time (Heinonen et al. 2008; Kukkonen, 2013).
In conclusion, our findings link the orexin system to the pathogenesis and maintenance of high blood pressure in spontaneously hypertensive rats and suggest that modulation or rebalance of the orexin system could be a potential target in treating hypertension.
Acknowledgments
The authors thank Actelion Pharmaceuticals Ltd for almorexant; Dr Andy Daubenspeck for helping with understanding the bases of power spectral analysis; and Dr Jay Leiter for helping with the statistics.
Glossary
- Almxt
almorexant
- BP
blood pressure
- CSF
cerebrospinal fluid
- DBP
diastolic blood pressure
- Adr
adrenaline
- HR
heart rate
- HRV
heart rate variability
- i.c.v.
intracerebroventricular
- LF
low frequency
- LF/HF ratio
low/high-frequency ratio
- MAP
mean arterial blood pressure
- NA
noradrenaline
- NREM
non-rapid eye movement
- OX1R
orexin receptor-1
- OX2R
orexin receptor-2
- PVN
hypothalamic paraventricular nucleus
- RM
repeated measures
- RVLM
rostral ventrolateral medulla
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
- SNA
sympathetic nerve activity
- SBP
systolic blood pressure
- Veh
vehicle control
- WKY rat
Wistar–Kyoto rat
Additional information
Competing interests
None declared.
Author contributions
The RT-PCR experiment was conducted at the University of Bristol, and all other experiments were conducted at Geisel School of Medicine at Dartmouth. A.L., E.E.N. and J.F.R.P. all contributed equally to conceive the hypothesis, interpret the data, conceptualize the findings, edit and finalize the manuscript. A.L. designed and performed all physiological experiments, analysed data and composed the manuscript. C.C.T.H. performed the RT-PCR experiment. All authors approved the final version of the manuscript.
Funding
The study was supported by the National Heart, Lung and Blood Institute (NHLBI), R37 HL 28066. J.F.R.P. was supported by a Royal Society Wolfson Research Merit Award. C.C.T.H. was supported by MOHE High Impact Research Grant (H-20001-00-E000055).
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman Z, Fudim M, Sobotka PA, Gourine A, Paton J. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–R1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol Heart Circ Physiol. 1991;261:H1292–H1299. doi: 10.1152/ajpheart.1991.261.4.H1292. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–R697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2010;198:203–208. doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Heinonen MV, Purhonen AK, Makela KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol (Oxf) 2008;192:471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Iigaya K, Horiuchi J, McDowall LM, Lam AC, Sediqi Y, Polson JW, Carrive P, Dampney RA. Blockade of orexin receptors with Almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro- or chemoreceptor reflex responses. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1011–R1022. doi: 10.1152/ajpregu.00263.2012. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol. 2013;304:C2–C32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol. 2008;605:371–376. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76:618–631. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37:1382–1387. doi: 10.1161/01.hyp.37.6.1382. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Fujii K, Iida M. Neural regulation of blood pressure by leptin and the related peptides. Regul Pept. 2003;114:79–86. doi: 10.1016/s0167-0115(03)00116-2. [DOI] [PubMed] [Google Scholar]
- Murasato Y, Hirakawa H, Harada Y, Nakamura T, Hayashida Y. Effects of systemic hypoxia on R–R interval and blood pressure variabilities in conscious rats. Am J Physiol Heart Circ Physiol. 1998;275:H797–H804. doi: 10.1152/ajpheart.1998.275.3.H797. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto AJ, White WB. Circadian blood pressure: clinical implications based on the pathophysiology of its variability. Kidney Int. 2007;71:855–860. doi: 10.1038/sj.ki.5002130. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer H, Stauss HM, Abboud F, Nishino S, Mignot E, Zeitzer JM. Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J Appl Physiol. 2010;109:1053–1063. doi: 10.1152/japplphysiol.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165:2292–2303. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Takasaki M, Kannan H. Cardiovascular effects of leptin and orexins. Am J Physiol Regul Integr Comp Physiol. 2003;284:R639–R651. doi: 10.1152/ajpregu.00359.2002. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory–sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension. J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Katahira K, Polson JW, Kasparov S, Murphy D, Paton JF. Automation of analysis of cardiovascular autonomic function from chronic measurements of arterial pressure in conscious rats. Exp Physiol. 2006;91:201–213. doi: 10.1113/expphysiol.2005.031716. [DOI] [PubMed] [Google Scholar]


