Introduction
Epigenetic modifications are a group of chemical changes to DNA and histones, such as DNA methylation and histone methylation, phosphorylation, and acetylation, which are capable of regulating gene expression through chromatin remolding without altering DNA sequence [1]. These alterations could be stable through mitotic or meiotic cell division thus facilitating heritable changes in gene expression and cellular phenotypes, potentially across generational boundaries. Recently, Greer et al. [2] reported an amazing example of this phenotype: the transgenerational inheritance of lifespan and age-related phenotypes. To our knowledge, this is the first study reporting a transgenerational phenotype mediated by histone methylation.
Epigenetic transgenerational phenotypes
The global information of epigenetic modifications in a cell is called the epigenome. The epigenome is reprogrammed, or essentially erased, in the germ line through generations to ensure the totipotency of germ cells. Primordial germ cells for example undergo a rapid genome-wide erasure of the majority of DNA methylation [3]. However, in specific regions of genome, some epigenetic modifications can escape the reprogramming events and be passed down to the next generation. Therefore, changes in heritable epigenetic modifications could affect organismal phenotypes over several generations without alteration of DNA sequences. This phenomenon has been coined transgenerational inheritance [4]. For example, when rats are treated with an environmental endocrine disruptor vinclozolin during the period of gonadal sex determination, the adult disease phenotype of decreased spermatogenic capacity and increased male infertility occurs over several generations, even though the following generations are not exposed to the compound. The inherited changes of DNA methylation in male germ cells are believed to cause such phenotype [5]. Several studies also report the transmission of parental metabolic phenotypes to their offspring [6–8]. When rats are fed a low protein diet during pregnancy and lactation, the progeny of their female offspring develop insulin resistance and compromised glucose homeostasis [6]. Similarly, when male rodents are fed a high-fat or low-protein diet, their offspring exhibit severe metabolic defects, which are possibly caused by altered DNA methylation of specific metabolic genes [7, 8]. In addition, several other transgenerational phenomena have been reported in various species, such as paramutation in plants and tumor suscepibility in drosophila [9, 10]. In their recent publication, Greer et al. [2] extend the transgenerational phenotype to lifespan. This study provides experimental evidence that animal lifespan is also heritable over several generations, possibly through epigenetic mechanisms.
Aging and epigenetics
Aging is thought to be the result of the accumulation of wear and tear, leading to the ultimate decline of cellular and organismal function. Over the last decade, we have come to realize that the rate of aging is in part regulated by evolutionarily conserved signaling pathways. Remarkably, single gene mutations have been shown to influence the rate of aging and lifespan across multiple species including yeast, worms, flies, mice, and potentially humans. These “aging genes” function in essential cell biological pathways including, metabolism, nutrient signaling, mitochondrial function, reproduction, and protein synthesis [11]. Chromatin status has also been demonstrated to play significant roles in lifespan determination. Two RNAi screens in C. elegans identified several chromatin factors as a group of potential lifespan regulators [12, 13]. Reduced expression of proteins regulating histone H3 lysine 4 trimethylation (H3K4me3), such as WDR-5, SET-2 or ASH-2, results in an increased lifespan phenotype in C. elegans [14]. In addition, the histone demethylase UTX-1 regulates C. elegans lifespan by modifying H3K27me3 on insulin/IGF-1 signaling components [15].
Transgenerational inheritance of lifespan: histones surviving epigenome reset?
Since some of the epigenetic modifications can be passed down to subsequent generations, Greer et al. questioned if epigenetic change-mediated lifespan is transgenerationally heritable. To answer this question, they mated long-lived wdr-5 or set-2 mutant animals with genetically wild-type males to generate heterozygous F1 generation progeny. These F1 animals were allowed to self-fertilize to generate both homozygous wild-type and homozygous mutant F2 generation progeny, and similarly F3, F4, and F5 generation offspring were generated. Surprisingly, the genetically wild-type F2, F3, and F4 generation progeny displayed a similar long-lived lifespan as the homozygous mutant control animals, indicative of a transgenerational mechanism of inheritance of the longevity phenotype.
One explanation for the inherited longevity phenomenon is that heritable epigenetic modifications either directly or indirectly regulate the expression pattern of longevity-regulating genes through several generations. As mentioned above, each generation of the germ line undergoes a reset event on its epigenome; therefore, any inherited epigenetic modification status must be capable of surviving or escaping these erasing effects. A well-known example is genomic imprinting, by which one allele (imprinted allele) is silenced such that the gene is only expressed by the non-imprinted allele. For example, it has been demonstrated that the igf-2 gene is only expressed by the paternally inherited allele [16]. Furthermore, genomic imprinting is controlled by DNA methylation and these specific methylations are resistant to the genome-wide erase event of DNA methylation. Therefore, it is conceivable that mechanisms similar to genomic imprinting may contribute to trangenerationally heritable phenotypes mediated by DNA methylation.
Interestingly, canonical DNA methylation is not present in the C. elegans genome. Although there is a methyl-DNA binding domain protein mbd-2, there is no reported DNA methylation or conventional DNA methylase in C.elegans. Nevertheless, this species is capable of imprinting individual loci or chromosomes [17]. For instance, a sperm-specific chromatin-based X chromosome imprinting that is restricted to histone H3 modifications is reported in C.elegans [18], suggesting a histone mark may be resistant to epigenome reprogramming. This is consistent with the discovery of transgenerational inheritance of longevity in worms as the gene responsible for the phenotype are all components of H3K4me3 complex, indicating that inherited histone methylation status other than DNA methylation is crucial for the phenomenon. In contrast to DNA demethylation, histone methylation marks are not reset directly in the parental genome. Instead, protamines are exchanged for histones during gametogenesis [19]. Interestingly, a small portion of retained histones is found significantly enriched at specific loci during protamine replacement [20], providing a mechanism for inherited histone modifications over several generations. These findings are reported in mammals, and thus should be applied to worms with caution, despite the presence of conserved players and mechanisms across evolutionary boundaries.
It is not surprising that Greer et al. found no obvious changes in the global levels of H3K4me3 in long-lived wild-type animals, because it is unlikely that all histones lacking H3K4me3 survive the genome-wide protamine replacement through generations. Perhaps the histones with changed H3K4me3 in P0 mutant animals in some specific loci survive the resetting of histone modifications over several generations. In addition, these affected histones must be capable of resisting enzyme modification from F2 through F4 generations, since H3K4 methylase is functional in these generations. Therefore, although yet-to-be determined, there must be a biochemical event that endows the histones with the ability to resist methylase modification. Based on genome-wide transcriptional profile analyses, the authors found that the long-lived wild-type F4 but not the F5 animals with normal lifespans shared a significant subset of differentially regulated genes with long-lived mutant animals. Moreover, some of these shared genes are enriched for longevity regulating genes, suggesting that the genes with transgenerational inheritance of expression potentiate the increased lifespan of wild-type descents of mutant animals. Therefore, we propose that the loss of H3K4me3 may be inherited at some central loci, such as miRNA, transcription factors or signaling regulators, which are capable of affecting expression of groups of genes, leading to a longevity expression pattern. The analysis of these loci linked to the transgenerational inheritance of longevity will be paramount to the understanding of how this phenotype is propagated.
Of particular interest is the finding that the F5 generation re-shortens its lifespan to a normal level, with no obvious intermediated phenotype in the previous generations. The divergence between genotype and phenotype is reminiscent of a maternal effect mechanism where the phenotype of an organism is determined by genotype of its mother, irrespective of its own genotype. This effect often results from maternally-derived proteins or RNA supplied to the offspring. It is possible that P0 generation-derived demethylated histones are supplied to following generations, which disappear gradually. While this is plausible for the F1 generation, it would be remarkable for this to persist through four generations. However, post-fertilization, the C. elegans zygote begins preparations for assymetric cell division to specify the germ cell lineage. Maternally-derived epigenetic modifications could be maintained in this cell lineage until F5 generation, when the H3K4me3 levels in specific region reaches some yet-to-be-determined threshold. Therefore, although lacking an obvious intermediate phenotype, an intermediate H3K4me3 signature and resulting altered gene expression profile may exist through generations. From generations F2 through F4, the intermediate gene expression profile may still be above this threshold, which is sufficient to result in the observed longevity phenotype. By the F5 generation, the levels of the H3Kme3 modification and the change of gene expression might be too “wild-type” to induce an increased lifespan. Global transcripts analysis on additional F2 and F3 generations will help to answer this interesting question. Lastly, while no obvious difference in the mean and maximal lifespans were documented in the F2 through F4 generation, it is possible that subtle difference in mortality rates might be present. As such, a closer look at the mortality probability might be informative and revealing of differences across generations.
Concluding remarks
This study for the first time reports that lifespan, one of the most complicated organismal phenotypes, could be transgenerationally inherited. It raises the possibility that for several longevity-regulating genes, epigenetic marks could modify their activity and be inherited transgenerationally. Although in the current study the epigenetic modification in P0 animals is established by genetic mutation or RNAi, it is conceivable that some environmental factors, such as chemicals or diet, may affect lifespan transgenerationally through epigenetic inheritance as shown in transgenerational diseases [4]. In this regard, this study opens a new avenue for future aging studies. We anticipate that these epigenetic markers might become valuable for predictions or diagnosis of aging and aging-related diseases.
Figure 1.
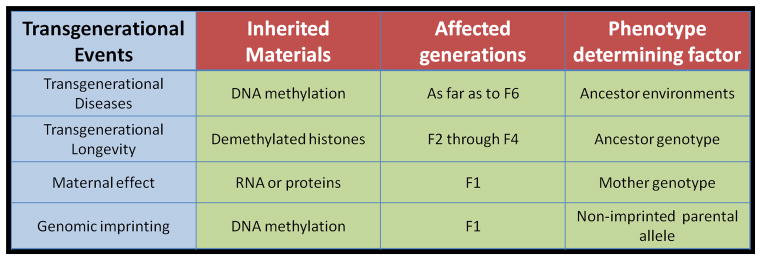
Comparison of several transgenerational events: transgenerational diseases, transgenerational longevity, maternal effect inheritance and genomic imprinting.
Acknowledgments
Special thanks to Tammy Nguyen, Jacqueline Lo and Percy Genyk for critical reading of the manuscript. SPC is an Ellison Medical Foundation Young Investigator.
References
- 1.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 2.Greer EL, Maures TJ, Ucar D, Hauswirth AG, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–71. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan HD, Santos F, Green K, Dean W, et al. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(Spec No 1):R47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 4.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–22. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–69. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–36. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng SF, Lin RC, Laybutt DR, Barres R, et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–6. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 8.Carone BR, Fauquier L, Habib N, Shea JM, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–96. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzin F, Grandjean V, Rassoulzadegan M. Inherited variation at the epigenetic level: paramutation from the plant to the mouse. Curr Opin Genet Dev. 2008;18:193–6. doi: 10.1016/j.gde.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Xing Y, Shi S, Le L, Lee CA, et al. Evidence for transgenerational transmission of epigenetic tumor susceptibility in Drosophila. PLoS Genet. 2007;3:1598–606. doi: 10.1371/journal.pgen.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon CJ. The genetics of aging. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton B, Dong Y, Shindo M, Liu W, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–55. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greer EL, Maures TJ, Hauswirth AG, Green EM, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–7. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin C, Li J, Green CD, Yu X, et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 2011;14:161–72. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Efstratiadis A. Parental imprinting of autosomal mammalian genes. Curr Opin Genet Dev. 1994;4:265–80. doi: 10.1016/s0959-437x(05)80054-1. [DOI] [PubMed] [Google Scholar]
- 17.Sha K. A mechanistic view of genomic imprinting. Annu Rev Genomics Hum Genet. 2008;9:197–216. doi: 10.1146/annurev.genom.122007.110031. [DOI] [PubMed] [Google Scholar]
- 18.Bean CJ, Schaner CE, Kelly WG. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat Genet. 2004;36:100–5. doi: 10.1038/ng1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–74. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- 20.Hammoud SS, Nix DA, Zhang H, Purwar J, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]


