Abstract
Background
The success of antiretroviral therapy (ART) has led to dramatic changes in causes of morbidity and mortality in HIV-infected individuals. As chronic diseases rates have increased in HIV+ populations, modifiable risk factors such as obesity have increased in importance. Our objective was to evaluate factors associated with weight change among patients receiving ART.
Methods
ART-naïve patients initiating therapy at the University of Alabama - Birmingham 1917 HIV/AIDS Clinic from 2000– 2008 were included. Body Mass Index (BMI) was categorized as: underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9) and obese (≥30). Linear regression models were used to evaluate overall change in BMI and factors associated with increased BMI category 24 months following ART initiation.
Results
Among 681 patients, the mean baseline BMI was 25.4 ± 6.1; 44% of patients were overweight/obese. At 24 months, 20% of patients moved from normal to overweight/obese or overweight to obese BMI categories. Greater increases in BMI were observed in patients with baseline CD4 count < 50 cells/μl (3.4 ± 4.1, P<0.01) and boosted protease inhibitor use (2.5±4.1 P=0.01), but did not account for all of the variation observed in weight change.
Conclusions
The findings that almost half of patients were overweight or obese at ART initiation, and 1 in 5 patients moved to a deleterious BMI category within 2 years of ART initiation are alarming. ART therapy provides only a modest contribution to weight gain in patients. Obesity represents a highly prevalent condition in patients with HIV infection and an important target for intervention.
Keywords: obesity, HIV, body mass index
Introduction
Obesity, defined as a body mass index (BMI) at or above 30, has reached epidemic proportions in the United States.[1] Overweight (BMI 25–29.9) or obese status are risk factors for diabetes, hypertension, cardiovascular disease and malignancy in HIV-negative adults.[2–4] Since the advent of highly active anti-retroviral therapy (ART) the life expectancy of HIV-infected individuals in the developed world has steadily increased.[5–7] As patients live longer, the prevalence of several co-morbid conditions including obesity is increasing.[8] HIV infection and ART are also independent risk factors for diabetes, atherosclerosis, low bone density, and a number of other chronic diseases.[9–13] The convergence of HIV infection and obesity could compound the risk for non-AIDS related morbidity and mortality in the aging HIV-infected population. Indeed, recent studies highlight increased frequency of non-AIDS related events (e.g., malignancy and cardiovascular disease) as contributors to morbidity and mortality relative to AIDS-defining events.
Although overweight/obesity and HIV infection have each been studied in great detail individually, few studies have examined the prevalence of overweight/obesity among an HIV-infected population. The goals of this study were to identify both the frequency of and risk factors for excessive weight gain in treatment naïve HIV-infected patients in the first two years following ART initiation. Of note, few studies have investigated the concomitant rate of overweight/obesity and HIV infection in the United States (US) Deep South, a region disproportionately impacted by both epidemics. We hypothesized that prevalence of overweight/obesity in our HIV-infected cohort sample would mirror frequencies in non-HIV-infected individuals, and that a sizeable proportion of patients would experience significant weight gain within 2 years of ART initiation.
Methods
Established in 1992, The University of Alabama at Birmingham (UAB) 1917 HIV/AIDS Clinic Cohort Observational Database Project (UAB 1917 Clinic Cohort) is a prospective clinical cohort study that has collected detailed socio-demographic, psychosocial, and clinical information from over 6000 HIV-infected patients. There are currently more than 1800 active patients that receive primary and subspecialty care at the UAB 1917 HIV/AIDS Clinic who are enrolled in the Institutional Review Board (IRB) approved observational cohort project. The 1917 Clinic uses a locally programmed electronic medical record that imports all laboratory values from the central UAB laboratory, requires electronic prescription for all medications, and contains detailed encounter notes. As previously described, the electronic medical record and database are 100% quality controlled, with all provider notes reviewed within 72 hours of data entry to ensure appropriate data capture regarding diagnoses and medications, including start and stop dates for antiretroviral medications and all other prescribed drugs.[14] The UAB Institutional Review Board approved this retrospective study nested in the UAB 1917 Clinic Cohort.
Study sample
Treatment naïve patients who initiated ART between January 1, 2000 and December 31, 2008 who (1) maintained therapy for at least 2 years, and (2) had their weight recorded at least twice during the 2 year study period were included as study subjects. Only baseline ART regimen was included in this analysis. Patients were excluded if they did not remain on ART for at least 2 years, or if they were participants in a blinded ART-regimen investigation during the study period. Patients receiving an ART regimen that did not include a nucleoside reverse transcriptase inhibitor (NRTI) backbone with either a third NRTI, a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI) or ritonavir boosted PI as the third drug were also excluded from analyses (n=78). All data were acquired through queries of the 1917 Clinic electronic database.
Study Variables
Independent variables
Patient-level information included age, sex, racial/ethnic group (White, Black/Other), risk transmission category [heterosexual, men who have sex with men (MSM)], health insurance status (public, private, uninsured), baseline (pre-ART) viral load, baseline (pre-ART) CD4 count, history of substance abuse, and affective mental health disorders (depression and anxiety) as recorded in patient problem lists. The variables risk transmission category and history of substance abuse were included based on the inverse associations of these factors with body weight.[15–17] Affective mental health disorders were included due to reports of both positive and negative associations with body weight.[18–20] ART regimen-level data included third drug by class (NRTI, NNRTI, PI, boosted PI) and composition of the NRTI backbone. NRTI backbones were assigned to three groups: didanosine (DDI) or stavudine (D4T)-containing regimens, zidovudine (ZDV)-containing regimens, and those containing abacavir (ABC) or tenofovir (TDF). These NRTIs were typically combined with either emtricitabine (FTC) or lamivudine (3TC) (98% of regimens). If a regimen contained NRTIs from more than one group (e.g. DDI and ZDV), the regimen was assigned to one group using a standardized hierarchy: DDI or D4T, then ZDV, and finally ABC or TDF.
Dependent variable
Body mass index (BMI) was calculated for each patient by obtaining height and weight from the clinic database (BMI=weight in kg/height in m2) at the time of ART initiation and subsequent measurements at 6 and 24 months within a window of 8 weeks. Individuals without recorded body weights for at least one time point at both 6 and 24 months were excluded from analyses. Patients were categorized into four different BMI groups at each time point according to the National Institutes of Health criteria: underweight (BMI<18.5), normal weight (BMI of 18.5–24.9, overweight (BMI of 25–29.9) and obese (BMI of ≥30).[21]
Statistical Analyses
Baseline descriptive statistics were evaluated by BMI category using chi-square/Fisher’s exact tests or analysis of variance (ANOVA) with Tukey’s post-hoc analysis. Paired t-tests were used to compare the percentage of participants in each BMI category at ART initiation versus 24 months on therapy. Pearson correlation testing revealed that baseline viral load (VL) and CD4 counts were highly correlated; hence only CD4 count was included in subsequent models. Stepwise linear regression was performed to analyze the relationships of patient characteristics with a change in absolute BMI, with model criteria set at entry as P < 0.15 and stay as P < 0.10. After inspection of residuals, the model for BMI change at 24 months was log-transformed to improve normality. The distributions of change in BMI at 6 months and 24 months were determined to be normally distributed, and values at either end of the distribution were double-checked and confirmed for accuracy. Change in BMI at 6 months and 24 months were then analyzed with general linear regression controlling for age, sex, racial/ethnic group, sexual risk factor, baseline CD4 count, history of substance abuse, and ART regimen composition (NRTI backbone and third drug). Sensitivity analyses were performed for all models in a subset 1) excluding patients who were underweight at baseline, and 2) excluding patients with a baseline CD4 count <50 cells/μL. All data was analyzed using SAS version 9.2 with a significance level of P<0.05.
Results
Among 681 patients, complete socio-demographic information was available for 603 individuals. The median age was 38 years, 22% were female, 54% were of minority race and 28% reported a history of illicit drug use. The initial log10 plasma HIV RNA was 4.4 ± 1.2 copies/ml and 27% of participants had baseline CD4 counts less than 50 cells/μL. The most commonly initiated NRTI backbone was ABC/TDF (57%), while D4T/DDI (7%) was least commonly used (Table 1). When stratified by BMI category, overweight and obese participants had higher rates of diagnosed diabetes, lower rates of tobacco use, and were less likely to report a history of homosexual activities (MSM). Tukey’s post-hoc analysis indicated that underweight participants had lower CD4 counts and higher log10 VL at ART initiation, with no significant differences in these factors among participants classified as normal weight, overweight, and obese (all at P<0.05).
Table 1.
Patient characteristics (mean ± SD or n(%)) stratified by body mass index (BMI) category among treatment-naïve HIV-infected patients at antiretroviral therapy initiation at the UAB 1917 HIV/AIDS Clinic, 2000–2008.
| Variable | Black Men n=655 | Black Women n=308 | White Men n=725 | White Women n=112 | P valuea |
|---|---|---|---|---|---|
| Age | 36.7 ± 9.6 | 37.6 ± 9.9 | 38.8 ± 9.7 | 38.4 ± 9.9 | 0.42 |
| Height (cm) | < 0.01 | ||||
| Weight (kg) | |||||
| Body mass index | 17.0 ± 1.2 | 21.9 ± 1.8 | 27.1 ± 1.4 | 35.0 ± 5.1 | < 0.01 |
| Health insurance | < 0.01 | ||||
| Uninsured | 16 (30.2%) | 91 (27.9%) | 49 (29.2%) | 41 (30.8%) | |
| Public | 28 (52.8%) | 99 (30.4%) | 40 (23.8%) | 38 (28.6%) | |
| Private | 9 (17.0%) | 136 (41.7%) | 79 (47.0%) | 54 (40.6%) | |
| Sexual risk factor | 0.02 | ||||
| Heterosexual | 26 (49.1%) | 128 (39.1%) | 76 (45.2%) | 75 (56.4%) | |
| MSM | 27 (50.9%) | 199 (60.9%) | 92 (54.8%) | 58 (43.6%) | |
| Baseline CD4 count | < 0.01 | ||||
| 0 – 49/μl | 28 (52.8%) | 93 (28.5%) | 34 (20.2%) | 26 (19.5%) | |
| 50 –199/μl | 14 (26.4%) | 86 (26.3%) | 45 (26.8%) | 25 (18.8%) | |
| 200 – 350/μl | 9 (17.0%) | 91 (27.8%) | 43 (25.6%) | 47 (35.3%) | |
| > 350/μl | 2 (3.8%) | 57 (17.4%) | 46 (27.4%) | 35 (26.3%) | |
| log10 plasma HIV | 0.03 | ||||
| RNA (copies/ml) | 4.8 ± 1.3 | 4.5 ± 1.2 | 4.3 ± 1.2 | 4.4 ± 1.3 | |
| History of diabetes | 1 (1.9%) | 11 (3.4%) | 15 (8.9%) | 19 (14.3%) | < 0.01 |
| ART Regimen | |||||
| NRTI Backbone | 0.22 | ||||
| TDF/ABC | 24 (50.0%) | 157 (53.6%) | 88 (59.5%) | 72 (63.2%) | |
| D4T/DDI | 5 (10.4%) | 18 (6.1%) | 13 (8.8%) | 4 (3.5%) | |
| AZT | 19 (39.6%) | 118 (40.3%) | 47 (31.7%) | 38 (33.3%) | |
| Third Drug | 0.93 | ||||
| NNRTI | 34 (70.8%) | 220 (75.1%) | 112 (75.7%) | 82 (7%) | |
| NRTI | 2 (4.2%) | 19 (6.5%) | 8 (5.4%) | 7 (6.1%) | |
| PI | 4 (8.3%) | 16 (5.5%) | 12 (8.1%) | 10 (8.8%) | |
| Boosted PI | 8 (16.7%) | 38 (12.9%) | 16 (10.8%) | 15 (13.2%) | |
P value based on analysis of variance (ANOVA) or chi-squared statistic
Race = Multiracial for one participant
Excludes alcohol and marijuana abuse
ABC, abacavir; AZT, zidovudine; ART, antiretroviral therapy; BMI, body mass index (weight [kg]/height [m2]); CI, confidence interval; D4T, stavudine; DDI, didanosine; MSM, men who have sex with men; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; TDF, tenofovir; PI, protease inhibitor.
Prevalence of Overweight/Obesity
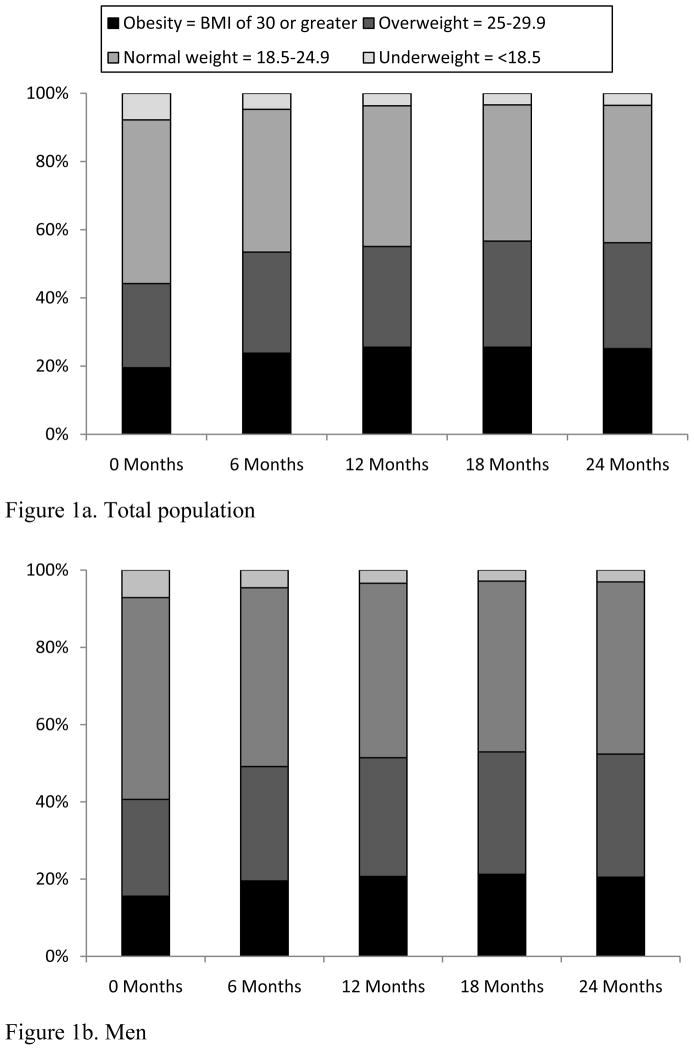
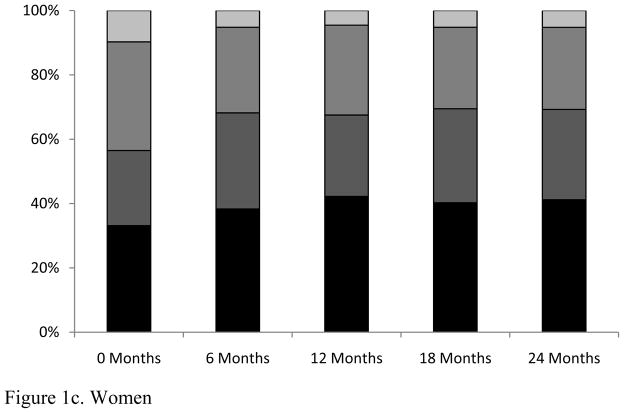
A total of 44% of patients were overweight (24%) or obese (20%) at the time of ART initiation, compared to only 8% of patients classified as underweight (Table 1). When change in BMI at 6-month increments was evaluated, an increase in the frequency of overweight (baseline: 24%; 24 months: 31%) and obese (baseline: 20%; 24 months: 25%) participants was observed (Figure 1). Notably, a greater proportion of females than males were overweight/obese at baseline (56% vs. 41%) and at 24 months (69% vs. 52%; Figure 1b and 1c). Repeated measures ANOVA revealed a significant increase in BMI over two years, with the majority of weight gain occurring during the first six months following initiation of ART (data not shown).
Figure 1.
Changes in body mass index (BMI) category by 6-month interval following antiretroviral therapy initiation among treatment-naïve HIV-infected patients at the UAB 1917 HIV/AIDS Clinic, 2000–2008.
Change in BMI at 6 months
Patients with lower CD4 counts (P<0.01) at initiation of therapy and those prescribed a protease inhibitor (PI) as third drug (P<0.05) had a significant increase in BMI at 6 months (r2 = 15.2) relative to other groups (Table 2). There was also a trend for greater change in BMI when D4T/DDI was the prescribed NRTI backbone (P<0.07). A history of substance abuse was associated with less weight gain at 6 months (P<0.01). No other patient or regimen level characteristics were associated with a 6-month change in BMI.
Table 2.
Multivariable linear regression models of characteristics associated with change in BMI (mean, SD) at 1) 6 and 2) 24 months following antiretroviral therapy initiation among treatment-naïve HIV-infected patients at the UAB 1917 HIV/AIDS Clinic, 2000–2008.
| Change in BMI after 6 months | P value | Change in BMI after 24 months | P value | |
|---|---|---|---|---|
| Gender | 0.10 | 0.50 | ||
| Male | 1.0 ± 3.3 | 1.5 ± 3.1 | ||
| Female | 1.5 ± 2.3 | 2.3 ± 4.3 | ||
| Age | 0.09 | 0.55 | ||
| ≤29 | 0.9 ± 2.4 | 1.5 ± 3.3 | ||
| 30–49 | 1.2 ± 2.6 | 1.7 ± 3.5 | ||
| ≥50 | 1.2 ± 2.7 | 1.6 ± 3.6 | ||
| Race | 0.77 | 0.44 | ||
| White | 0.9 ± 2.2 | 1.2 ± 2.8 | ||
| Minority | 1.3 ± 2.8 | 2.0 ± 3.9 | ||
| Risk Factor | 0.10 | 0.31 | ||
| Heterosexual | 1.5 ± 3.0 | 2.1 ± 4.0 | ||
| MSM | 0.8 ± 2.1 | 1.2 ± 2.8 | ||
| Baseline CD4 count (cells/μl) | < 0.01 | < 0.01 | ||
| <50 | 2.2 ± 3.1 | 3.4 ± 4.1 | ||
| 50 – 199 | 1.3 ± 2.5 | 1.8 ± 3.2 | ||
| 200 – 350 | 0.5 ± 1.8 | 0.7 ± 2.9 | ||
| > 350 | 0.2 ± 2.1 | 0.4 ± 2.4 | ||
| History of substance abuse | 0.01 | < 0.01 | ||
| Yes | 0.7 ± 2.5 | 0.8 ± 3.1 | ||
| No | 1.3 ± 2.6 | 2.0 ± 3.5 | ||
| NRTI Backbone | 0.07 | 0.71 | ||
| TDF/ABC | 1.1 ± 2.7 | 1.7 ± 3.7 | ||
| AZT | 1.2 ± 2.5 | 1.8 ± 3.3 | ||
| D4T/DDI | 2.1 ± 3.0 | 2.1 ± 3.1 | ||
| Third Drug | 0.02 | 0.01 | ||
| NNRTI | 1.2 ± 2.6 | 1.7 ± 3.5 | ||
| NRTI | 0.1 ± 2.2 | 0.6 ± 2.1 | ||
| PI | 1.7 ± 2.7 | 2.1 ± 3.1 | ||
| Boosted PI | 1.6 ± 3.0 | 2.5 ± 4.1 |
BMI, body mass index (weight kg/height m2); ABC, abacavir; AZT, zidovudine; CI, confidence interval; D4T, stavudine; DDI, didanosine; MSM, men who have sex with men; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; TDF, tenofovir; PI, protease inhibitor.
Change in BMI at 24 months
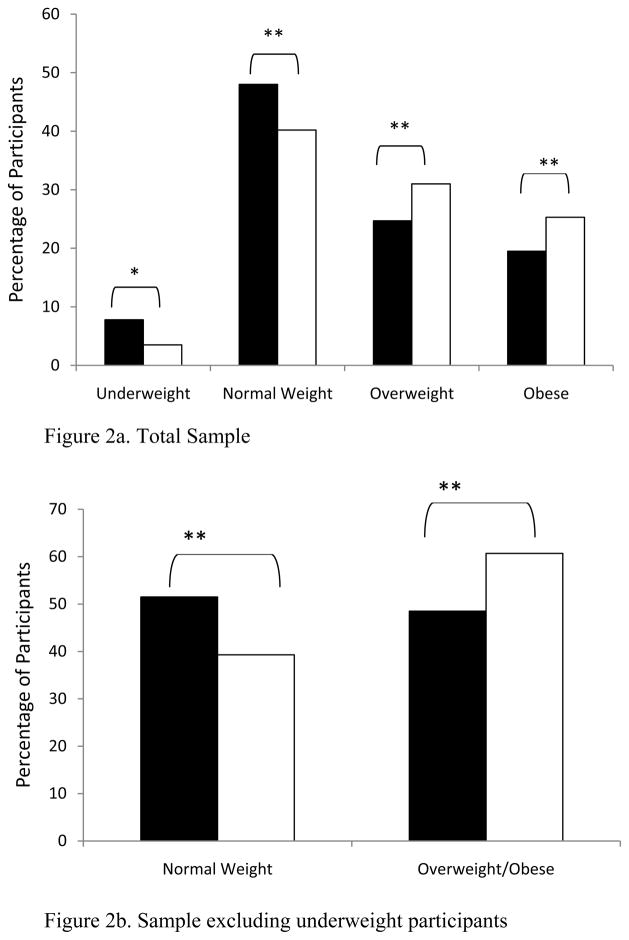
Linear regression analysis at 24 months revealed significant, positive associations of BMI change with lower baseline CD4 count, and use of a PI as a third drug (Table 2: r2= 16.4, all at P <0.01). When BMI classification of all participants at ART initiation was compared to BMI at 24 months using a paired t-test, we noted a significant increase in the percentage of patients transitioning to the overweight or obese categories (Figure 2a). Additionally, we observed that approximately 20% of patients initially classified as normal weight had become overweight or obese (Figure 2b), while the prevalence of underweight decreased to 4% of the population.
Figure 2.
Proportion of the study sample by body mass index (BMI) category at antiretroviral therapy initiation vs. 2 years on therapy among treatment-naïve HIV-infected patients at the UAB 1917 HIV/AIDS Clinic, 2000–2008.
Black bars = therapy initiation; white bars = 24 months. * = P < 0.05; ** = P < 0.01
Sensitivity Analyses
As desirable weight gain among persons classified as underweight at ART initiation or with CD4 counts <50 c/μL could have affected study outcomes, we performed two separate sensitivity analyses: one excluding underweight study participants, and one excluding patients with a low baseline CD4 count (< 50 cells/μl). After excluding patients with low baseline CD4 count, choice of third drug was not significantly associated with BMI change at 24 months (P = 0.26). No other differences were found in these sensitivity models compared to models that included all participants (data not shown).
Discussion
Our findings suggest that while undesirable weight loss among HIV-infected patients in the Southern US is declining, these patients are now dealing with a prevalence of obesity in concordance to both regional and national trends for non-HIV-infected persons.[1] Prior to initiation of antiretroviral therapy, 45% of these patients were classified as overweight/obese; however, after two years the prevalence of overweight/obesity had increased significantly to 56%. Factors previously identified as contributing to weight gain among HIV-infected individuals, including ART regimen, sex, and baseline CD4 count,[22–24] contributed to but did not fully explain weight gain among this cohort. Comparable increases in obesity prevalence among non-HIV infected individuals are associated with increased chronic disease morbidity and mortality. Coupled with HIV-infection (e.g., inflammation and immune activation) and ART medication contributions to non-AIDS morbidity, this high prevalence of obesity places HIV+ patients at great risk.[9,25–28] The confluence of obesity and HIV infection likely exacerbates risk for co-morbidities such as diabetes, cardiovascular disease, and other conditions, highlighting the need to aggressively manage obesity and its related co-morbidities in the aging HIV-infected populace.
Investigators examining the trend toward obesity in HIV-infected patients have reported frequencies of overweight/obesity ranging from 45–61%.[22–24] The few studies that have evaluated change in weight status during the ART era have also noted a gradual trend for increasing BMI during the course of treatment. However, previous studies were predominately cross-sectional analyses that evaluated patients from regions with lower rates of obesity among the general population, and included patients who were not on an ART regimen. Among our cohort, after only 6 months of ART the percentage of overweight/obese patients had increased by 9% (Figure 1). This rapid increase in BMI and subsequent leveling off among patients recently starting therapy is possibly related to the “return to health” phenomenon observed in underweight patients.[29] However, rapid weight gain did not occur exclusively among underweight patients; 20% of patients starting therapy in the normal or overweight BMI category shifted to the deleterious overweight or obese categories by 24 months follow-up. These findings have not been well-documented in previous investigations and suggest additional factors not measured in this study could impact patient body weight during the initial stages of ART.
It is also unlikely that rapid weight gain in the first six months of treatment is due to the overall temporal trend for increasing overweight/obesity in the US alone. Interestingly, rates of overweight/obesity among the general US population have remained stable at approximately 65–68% (since 1999 among females and since 2003 among males) during the study period for the present investigation, suggesting that the continued weight gain among HIV-infected patients did not merely represent a reflection of general population trends.[1] At baseline, obesity prevalence was lower than that reported for Alabama at any time from 2000–2008.[30] While obesity prevalence at 24 months follow-up remained lower than Alabama obesity prevalence for men, there was no difference at 24 months in obesity prevalence of the women in our cohort compared to all women in Alabama. Hence, weight gain observed in our population could be partially attributable to the geographic location of the cohort, and additional studies that compare the cohort to a region-specific control population are warranted. Overall, our results suggest that rates of overweight/obesity among the Deep South HIV-infected population increase rapidly following initiation of ART. These changes warrant further investigation as they are likely to exert progressively more negative outcomes on patient health outcomes as the population ages.
Additional patient and regimen-level characteristics were associated with cross-sectional overweight/obesity category at baseline prior to ART initiation. We observed that a baseline CD4 count lower than 200 cells/μl was associated with a greater increase in BMI at 6 and 24 months. These results are in agreement with other studies showing a correlation between successful immune recovery and increased weight.[29,31–33] Nevertheless, these analyses remained significant even after excluding underweight patients, suggesting that some immune-compromised patients at a healthy weight are at risk for excessive weight gain. When evaluating factors associated with weight gain after ART initiation, use of protease inhibitors was associated with a significant increase in BMI, while patients on an NRTI-containing regimen gained significantly less weight over time. Previous literature has demonstrated that PI use may promote weight gain consisting primarily of increased fat mass with little change in lean body mass.[34] Although we were unable to evaluate fat and lean mass separately, the greater increase in body weight among our patients on a PI-based regimen could be associated with a proportionately greater increase in fat mass. Additionally, while affective mental health disorder was not associated with change in body weight in this sample in preliminary analyses, it remains possible that specific medications, such as antidepressants, used by these patients could impact weight gain. Dietary intake, physical activity patterns, and diagnoses/medications for conditions including sleep disorders, type 1 or 2 diabetes, and hypertension could also impact obesity risk; however, these data are not routinely captured on all patients in our routine care setting. We were also Future investigations that include these variables will be invaluable to further elucidate specific factors that play a role in weight gain among patients with HIV, and how this change in weight may influence chronic disease risk.
Gender and racial/ethnic patient characteristics could also influence BMI among patients living with HIV/AIDS. Women typically present with higher rates of obesity compared to men, [1] and Boodram et al. noted higher rates of overweight and obesity among an all-female cohort living with HIV/AIDS compared to studies that include men only or both sexes.[24] Consistent with these findings, a higher percentage of women were overweight/obese throughout the study period, suggesting that women may be at greater risk for concomitant disease from HIV infection and obesity and could particularly benefit from weight maintenance interventions. However, in contrast to previous research, we found no difference in overweight/obesity rates between racial/ethnic groups. This could be partially attributable to geographic location, with a greater percentage of all racial/ethnic groups classified as overweight/obese in the Deep South region (http://www.cdc.gov/obesity/data/trends.html#State). While a race*sex interaction was not significant in statistical analyses, a high percentage of African-American women (44%) shifted from normal weight to overweight/obese categories at 24 months follow-up (data not shown). As the incidence of HIV infection among African-American women is rapidly increasing, our results indicate interventions targeted at weight maintenance for this group are greatly needed.[35]
The results of this study should also be interpreted within the context of study limitations. Our study population was limited to one academic HIV treatment center in a state with high rates of obesity, thus these results may not be applicable to other regions of the country. However, US population trends for overweight/obesity have generally followed those of the Southern US, a phenomenon which may also be observed within patients living with HIV/AIDS. BMI is an imprecise predictor of deleterious weight gain, and may not actually measure total fat mass or fat distribution, although monitoring BMI over time in a large sample size does provide insight into population weight gain.[36,37] This study only includes baseline ART regimen data, and we are unable to comment on the association of frequent regimen adjustments with changes in body weight over time. We were unable to assess the contribution of other influential factors such as diet and physical activity to change in BMI as these data were not captured routinely on all patients in our regular HIV care setting. A strength of the current investigation is the evaluation of longitudinal cross-sections after ART initiation which allowed us to follow participants through the course of therapy. Future studies would benefit from inclusion of these measures to evaluate their association with weight change among HIV-infected individuals.
In summary, we confirm a high prevalence of overweight/obesity among Southern HIV-infected individuals. These rates continue to increase for up to two years following ART initiation, but use of protease inhibitors and other ART regimens appears to only moderately contribute to weight gain. Treatment decisions for these patients must consider the paradigm shift from HIV as a disease of wasting to one with an increasing prevalence of obesity. Additional studies are required to determine the impact of this increase in weight on treatment outcomes, determining which factors promote undesirable weight changes, and whether nutrition/physical activity interventions can prevent undesirable weight gain among this population. Such interventions will be necessary to maximize individual health outcomes and to stem the associated increase in health care costs for overweight/obese patients that results from preventable chronic co-morbid diseases.
Acknowledgments
The investigators would like to thank the University of Alabama at Birmingham 1917 Clinic HIV/AIDS Clinic Cohort management team for their assistance with study data collection. This work was supported by the University of Alabama at Birmingham Center for AIDS Research (grant P30 AI27767), the Mary Fisher CARE Fund and the CFAR Network of Integrated Clinical Sites (CNICS; grant 5R24 AI067039). A.L.W. received financial support from a National Institutes of Health Ruth L. Kirschstein National Research Service Award (grant 5T32AI52069-08SI) and the UAB-VA Health Services Research/Comparative Effectiveness Research Training Program. The funders had no input into the design and conduct of the study, or analysis and reporting of the data.
Footnotes
Disclosure Statement
The authors declare no competing interests.
Reference List
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S. 2000–2002. Diabetes Care. 2005;28:1599–603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–8. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Smit E, Skolasky RL, Dobs AS, et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol. 2002;156:211–8. doi: 10.1093/aje/kwf039. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Sabin CA, Youle M, et al. Changes in AIDS-defining illnesses in a London Clinic, 1987–1998. J Acquir Immune Defic Syndr. 1999;21:401–7. [PubMed] [Google Scholar]
- 8.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22:17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 11.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–70. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 13.Masia M, Padilla S, Garcia N, et al. Endothelial function is impaired in HIV-infected patients with lipodystrophy. Antivir Ther. 2010;15:101–10. doi: 10.3851/IMP1491. [DOI] [PubMed] [Google Scholar]
- 14.Willig JH, Abroms S, Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–60. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deputy NP, Boehmer U. Determinants of body weight among men of different sexual orientation. Prev Med. 2010;51:129–31. doi: 10.1016/j.ypmed.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Campa A, Yang Z, Lai S, et al. HIV-related wasting in HIV-infected drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:1179–85. doi: 10.1086/444499. [DOI] [PubMed] [Google Scholar]
- 17.Tang AM, Forrester JE, Spiegelman D, et al. Heavy injection drug use is associated with lower percent body fat in a multi-ethnic cohort of HIV-positive and HIV-negative drug users from three U.S. cities. Am J Drug Alcohol Abuse. 2010;36:78–86. doi: 10.3109/00952990903544851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lykouras L, Michopoulos J. Anxiety disorders and obesity. Psychiatrike. 2011;22:307–13. [PubMed] [Google Scholar]
- 19.Scott D, Happell B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment Health Nurs. 2011;32:589–97. doi: 10.3109/01612840.2011.569846. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: National Health and Nutrition Examination Survey 2005–2006. BMC Psychiatry. 2011;11:130. doi: 10.1186/1471-244X-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutues of Health, National Heart LaBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Institutes of Health; 1998. Ref Type: Report. [Google Scholar]
- 22.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–61. [PubMed] [Google Scholar]
- 23.Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS. 2008;22:925–30. doi: 10.1089/apc.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boodram B, Plankey MW, Cox C, et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women’s Interagency HIV Study. AIDS Patient Care STDS. 2009;23:1009–16. doi: 10.1089/apc.2009.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen NT, Nguyen XM, Wooldridge JB, Slone JA, Lane JS. Association of obesity with risk of coronary heart disease: findings from the National Health and Nutrition Examination Survey, 1999–2006. Surg Obes Relat Dis. 2010;6:465–9. doi: 10.1016/j.soard.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Zannou DM, Denoeud L, Lacombe K, et al. Incidence of lipodystrophy and metabolic disorders in patients starting non-nucleoside reverse transcriptase inhibitors in Benin. Antivir Ther. 2009;14:371–80. doi: 10.1177/135965350901400307. [DOI] [PubMed] [Google Scholar]
- 28.Polsky S, Floris-Moore M, Schoenbaum EE, Klein RS, Arnsten JH, Howard AA. Incident hyperglycaemia among older adults with or at-risk for HIV infection. Antivir Ther. 2011;16:181–8. doi: 10.3851/IMP1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones CY, Hogan JW, Snyder B, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis. 2003;37 (Suppl 2):S69–S80. doi: 10.1086/375889. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. BRFSS Prevalence and Trends Data. 2010 2-17-2012. Ref Type: Report. [Google Scholar]
- 31.Shor-Posner G, Campa A, Zhang G, et al. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000;23:81–8. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 32.Malvy D, Thiebaut R, Marimoutou C, Dabis F. Weight loss and body mass index as predictors of HIV disease progression to AIDS in adults. Aquitaine Cohort, France, 1985–1997. J Am Coll Nutr. 2010;20:609–15. doi: 10.1080/07315724.2001.10719065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palenicek JP, Graham NM, He YD, et al. Weight loss prior to clinical AIDS as a predictor of survival. Multicenter AIDS Cohort Study Investigators. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:366–73. [PubMed] [Google Scholar]
- 34.Silva M, Skolnik PR, Gorbach SL, et al. The effect of protease inhibitors on weight and body composition in HIV-infected patients. AIDS. 1998;12:1645–51. doi: 10.1097/00002030-199813000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. 2005;16 Ref Type: Report. [Google Scholar]
- 36.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol. 2010;115:982–8. doi: 10.1097/AOG.0b013e3181da9423. [DOI] [PMC free article] [PubMed] [Google Scholar]