Abstract
The primary objective of this trial was to establish the maximum tolerated dose (MTD) of oxaliplatin 130 mg/m2 preceded by escalating doses of docetaxel 60 mg/m2 (75, 90, 100 mg/m2) administered every 3 weeks. A total of 11 patients were entered; 10 evaluable for response: 4 stable disease (liver, ovary and esophagus) and 1 partial remission (esophagus). At dose level 1, there was 1 dose-limiting toxicity (DLT) (grade 3 allergic reaction). At dose level 2, there were 3 DLTs (3 grade 4 neutropenia, grade 3 gastritis, diarrhea, hypophosphatemia, neuro-mood). The MTD is docetaxel 60 mg/m2 with oxaliplatin 130 mg/m2.
Keywords: Phase I, Oxaliplatin, Docetaxel, Oxaliplatin and Docetaxel
Introduction
Oxaliplatin is a platinum analog of the 1,2-diaminocyclohexane (DACH) family [1]. Its cytotoxic effects are exerted through formation of DNA adducts that block both DNA replication and transcription, leading to cell death in actively dividing cells as well as the induction of apoptosis. Preclinical data reveal its non-overlapping spectrum of activity with cisplatin, as documented in cell lines including those with acquired and intrinsic platinum resistance (as KB-CP, A 2780, HT29, CaCo2 colon cancer) [2]. When combined with other cytotoxic agents (5-FU, SN38, cisplatin, carboplatin, gemcitabine, CPT-11, cyclophosphamide and taxanes), oxaliplatin has additive and/or synergistic antitumoral effects on various in vitro and in vivo models (colon, gastric, breast, ovarian and epidermoid tumors) [3].
Oxaliplatin has been approved in the United States for the treatment of metastatic colorectal cancer. Ongoing clinical trials have evaluated this drug in other malignancies, including esophageal, gastric, pancreas, lung and breast. The drug is often used as a standard option in gastrointestinal malignancies.
When evaluated in phase I studies, the dose-limiting side effects of oxaliplatin therapy were transient peripheral neuropathy manifested as paresthesia in the fingers, hands, toes and lips, and dysesthesias in the forearms, legs, mouth and throat triggered or enhanced by exposure to cold. The duration and intensity of the symptoms increased with the number of courses administered [4, 5].
Docetaxel is a cytotoxic drug that acts as a promoter of tubulin polymerization with a broad spectrum of antitumor activity in preclinical testing. Docetaxel has demonstrated activity in the treatment of breast, ovarian, lung, head and neck and gastric cancer. The main dose-limiting toxicity (DLT) of docetaxel reported in these trials is hematologic, specifically neutropenia.
Pre-clinical data have shown that the oxaliplatin when combined with taxanes may have additive and/or synergistic effects. When docetaxel and cisplatin or carboplatin have been combined, significant clinical activity has been demonstrated in head and neck, lung, breast, esophageal and gastric cancers.
These platinum agents differ in their spectrum of activity and toxicity. In trials of oxaliplatin alone or in combination with 5-FU, the dose of oxaliplatin has been 130 mg/m2 and is well tolerated. We designed a phase I trial with a fixed dose of oxaliplatin and escalating doses of docetaxel.
The primary objective of this study was to establish the maximum tolerated dose (MTD) of docetaxel given as a 1-h infusion every 3 weeks when followed by a 2-h infusion of oxaliplatin given at a dose of 130 mg/m2 and to describe the toxicities at each dose level studied. The secondary objectives of this study were to evaluate the pharmacokinetics and pharmacodynamics of oxaliplatin and docetaxel when a 2-h oxaliplatin infusion is preceded by a 1-h infusion of docetaxel.
Materials and methods
This was a multicenter, phase I, open-label study that evaluated oxaliplatin at a fixed dose and escalating doses of docetaxel in combination, to establish the MTD of docetaxel and to describe the toxicities of this combination. This study was conducted in the California Cancer Consortium (CCC) that includes 3 cancer centers at: City of Hope Medical Center (COH), University of Southern California (USC) and University of California Davis (UCD).
Eligibility
All patients participating in this study had histologically or cytologically confirmed metastatic or recurrent malignant solid tumors that did not respond to standard therapy and/or for which no known curative or standard palliative therapy was available. Patients were at least 18 years of age with a life expectancy of at least 12 weeks and a Southwest Oncology Group performance status of 0–2. In addition, patients were required to have adequate organ and marrow function determined by leukocytes ≥3,000/μl, absolute neutrophil count ≥1,500/μl, platelets ≥100,000/μl, total bilirubin within 1.5 times the normal institutional limits, AST (SGOT)/ALT (SGPT) within 2.5 times the normal institutional limits, creatinine within 1.25 times the normal institutional limits and baseline serum calcium ≤12 mg/dl. Patients must have recovered from previous therapy, and the last treatment must have been completed 30 days prior to entry into the study. Prior therapy with 5-FU and/or cisplatin was allowed. Patients who had received mitomycin C and nitrosourea must have completed therapy 6 weeks prior to entry. Patients with HIV or receiving anti-retroviral therapy (HAART) were excluded due to possible pharmacokinetic interactions. Patients who did not recover from surgery or previous therapy-related toxicity were excluded. Pregnant or nursing women were not eligible, and adequate contraception was required for men or women of childbearing age prior to study entry and for the duration of study participation. Additionally, patients with central nervous system metastases, history of allergy to platinum compounds or to antiemetics, uncontrolled intercurrent illness and neuropathy were also not eligible.
Patients were not required to have measurable or evaluable disease. Written informed consent was obtained from all patients before study entry. The study was approved by the Institutional Review Board at all 3 participating institutions.
Treatment plan and dose escalation
Registration and assignment to treatment schedule and dose were done centrally at the Data Coordinating Center of the CCC. Patients received docetaxel as a 1-h i.v. infusion followed by oxaliplatin given as a 2-h i.v. infusion on day 1, administered every 3 weeks. The dose of oxaliplatin was 130 mg/m2 and was maintained at this dose throughout. Oxaliplatin was an investigational drug supplied to investigators by the Division of Cancer Treatment and Diagnosis, NCI. The starting dose of docetaxel was 60 mg/m2 with increasing doses as follows: 75, 90 and 100 mg/m2. Docetaxel was commercially available and was supplied by Sanofi-Aventis (formerly, Rhone-Poulenc Rorer Pharmaceuticals, Inc.). No investigational or commercial drugs other than oxaliplatin and docetaxel were allowed to be administered with intent to treat the patient’s tumors. Supportive care including corticosteroids (i.e., dexamethasone) and anti-emetics was given to all patients before treatment and during treatment (if necessary).
Patients were evaluated for toxicity every 3 weeks with history and physical examination, and laboratory assessment. Patients were treated at the same dose level if no DLT was observed until disease progression, unacceptable adverse effects, intercurrent illness, withdrawal of consent by the patient or other conditions that may have prevented further administration of treatment.
Study design
Dose-limiting toxicity (DLT) was defined as any grade 3 non-hematologic toxicity not resolving to grade 2 or less within 96 h or any grade 4 toxicity. DLT was based on the first course of treatment. Patients who had received at least 1 complete course of treatment and had been observed for at least 3 weeks after completion of the first course or had experienced DLT were evaluable for toxicity. Toxicity was recorded using the descriptions and grading scales reported in the revised NCI common toxicity criteria version 2.0. Adverse events were reported using the NCI/NIH adverse drug experience reporting guidelines.
In this study, the standard 3 + 3 phase I trial design was used. Three patients were treated at each new dose level. If none of the three patients experienced DLT attributable to study drug, 3 new patients were treated at the next higher dose level. If one of the three patients experienced DLT attributable to study drugs, 3 more patients (for a total of 6) were treated at that dose level. If no additional DLT was observed at the expanded dose level, the dose was escalated to the next level. When 2 or more patients experience DLT at a given dose level, accrual to that dose level was stopped and escalation was terminated; the next lower dose was expanded, if 6 patients have not already been treated at that dose level. All patients who had not experienced any DLT were observed for a minimum 3 weeks after the first 1 course, before the dose level was escalated. No dose escalation was allowed within an individual patient. Those patients who were not evaluable for toxicity were replaced.
The MTD was defined as the highest dose tested in which none or at most one patient experienced DLT attributable to the study drugs, when at least 6 patients had been treated at that dose level and were evaluable for toxicity. The MTD is one dose level below the lowest dose tested in which 2 or more patients experienced DLT attributable to the study drugs. The phase I trial was closed once MTD had been identified.
Response evaluation
Measurable lesions were defined as bidimensionally measurable lesions by medical photograph, plain X-ray, CT, MRI or other imaging scans of at least 0.5 cm or greater (bone lesions excluded). Evaluable disease was defined as unidimensionally measurable lesions, masses with margins not clearly defined, lesions with both diameters <0.5 cm and palpable lesions with either diameter <2 cm. Patients with measurable disease who had received 2 complete courses of treatment, who had evident disease progression before completing the first two courses or who discontinued treatment because of toxicity prior to the complete 2 courses were evaluable for response. Response was defined as follows: complete response (CR) was complete disappearance of all measurable or evaluable disease for at least 6 weeks and no new lesions and partial response (PR) was defined as at least a 50 % decrease in measurable disease under baseline in the sum of products of perpendicular diameters of all measurable lesions for at least 6 weeks and no new lesions. Progressive disease (PD) was defined as progression of tumor by 25 %; stable disease was defined if CR, PR or PD was not achieved.
Pharmacokinetics
Peripheral blood samples were collected in heparinized vacuum tubes prior to treatment, just prior to the end of the oxaliplatin infusion and at 0.5, 1, 2, 4 and 24 h after the end of the infusion. Plasma was separated from whole blood, ultrafiltrates were prepared and free platinum levels in ultrafiltrates were determined using atomic absorption spectrometry as previously described [6]. Additional peripheral blood samples were collected during the first course of treatment in heparinized vacuum tubes just prior to the docetaxel infusion, prior to the end of the docetaxel infusion and at 0.5, 1, 2, 4 and 24 h after the end of the infusion. All blood sample tubes were placed on ice and promptly transferred into polypropylene microcentrifuge tubes for immediate centrifugation. Docetaxel levels in plasma were measured by HPLC as previously described [7].
Non-compartmental analyses were used to estimate the secondary pharmacokinetic parameters, maximum plasma concentration (Cmax), area-under-the-curve (AUC) and systemic clearance (CLsys). Secondary parameters for free platinum and docetaxel were summarized by dose level and compared using a two-tailed Student’s t test.
Results
Patient characteristics
A total of 11 patients with advanced malignant tumors were entered and treated in this study. The characteristics of the patients are listed in Table 1. The median age was 59 years (range 42–72). There were 8 male patients and 3 female patients. Three (27 %) patients had esophageal cancer, 2 (18 %) colon cancer, 2 (18 %) ovarian cancer and the remaining 4 (36 %) patients had a variety of solid tumors; including 1 hepatoma, 1 melanoma, 1 breast and 1 thyroid. Eight (73 %) patients had received prior chemotherapy. The dose escalation scheme and number of courses for docetaxel are also listed in Table 2.
Table 1.
Patient characteristics and response to the therapy (n = 11)
| ID | Age | Sex | Ethnicity | Disease site | Performance status | No. of prior chemo | Cycles received | Response | Reason off study | DLT |
|---|---|---|---|---|---|---|---|---|---|---|
| Dose level 1 (oxaliplatin 130 mg/m2 ± docetaxel 60 mg/m2) | ||||||||||
| 1 | 57 | M | Black | Liver | 0 | 0 | 6 | SD | Progressive disease | Yes |
| 2 | 54 | F | Caucasian | Ovary | 1 | 9 | 5 | SD | Progressive disease | No |
| 3 | 62 | M | Caucasian | Colon | 2 | 4 | 1 | NE | Unacceptable toxicity | No |
| 4 | 66 | M | Asian | Esophagus | 1 | 0 | 6 | SD | Progressive disease | No |
| 5 | 42 | M | Caucasian | Esophagus | 0 | 6 | 5 | SD | Progressive disease | No |
| 6 | 65 | M | Caucasian | Esophagus | 1 | 0 | 8 | PR | Unacceptable toxicity | No |
| 11* | 59 | F | Caucasian | Ovary | 0 | 4 | 2 | PD | Progressive disease | No |
| Dose level 2 (oxaliplatin 130 mg/m2 ± docetaxel 75 mg/m2) | ||||||||||
| 7 | 72 | M | Caucasian | Thyroid | 1 | 2 | 2 | PD | Progressive disease | Yes |
| 8 | 62 | M | Caucasian | Colon | 1 | 6 | 2 | PD | Progressive disease | Yes |
| 9 | 52 | M | Caucasian | Melanoma | 1 | 6 | 2 | PD | Progressive disease | No |
| 10 | 59 | F | Caucasian | Breast | 2 | 10 | 2 | PD | Progressive disease | Yes |
Pt 11 was treated at dose level 1 since this patient had already been consented for dose level 2, when the 2nd DLT was observed at that level
Table 2.
Dose escalation schedule
| Dose level | Oxaliplatin (mg/m2) | Docetaxel (mg/m2) |
|---|---|---|
| Level 1 | 130 | 60 |
| Level 2 | 130 | 75 |
| Level 3 | 130 | 90 |
| Level 4 | 130 | 100 |
Treatment delivery
A total of 41 evaluable courses of docetaxel were administered (median: 2 courses; range 1–8 courses). All treated patient were assessable for toxicity, and there were no treatment-related deaths. At dose level 1 (60 mg/m2 of docetaxel), there was 1 DLT (grade 3 allergic reaction in the 1st patient), and this dose level was expanded (a total of 6 patients) with no additional DLT. At dose level 2 (75 mg/m2 of docetaxel), there were 3 DLTs among 4 patients treated. Three patients treated at dose level 2 experienced grade 4 neutropenia. Of these three, one patient also experienced grade 3 gastrointestinal toxicities (gastritis and diarrhea), grade 3 metabolic toxicity (hypophosphatemia) and grade 3 febrile neutropenia; another patient also experienced grade 3 neurologic toxicity (mood alteration-anxiety agitation). A 7th patient was treated at dose level 1 since this patient had already been consented for dose level 2, when the 2nd DLT was observed at dose level 2. This is summarized in Table 1.
Hematologic toxicities
Table 3 also lists the hematologic toxicities. At dose level 1, 1 patient had grade 3 neutropenia in the first and subsequent cycle of chemotherapy, and 2 patients had grade 4 neutropenia not in the first cycle, but in the subsequent cycles of chemotherapy. At dose level 2, 3 patients experienced grade 4 neutropenia in the first cycle of chemotherapy. There was no grade 3 or 4 anemia or thrombocytopenia beginning at cycle 1.
Table 3.
Grade 3–4 toxicity
| ID | Cycle | Grade 3–4 hematologic toxicity | Grade 3–4 non-hematologic toxicity |
|---|---|---|---|
| Dose level 1 (oxaliplatin 130 mg/m2 ± docetaxel 60 mg/m2) | |||
| 1 | 1 | None | Grade 3 allergic reaction a |
| 2+b | Grade 3 leukocytes (total WBC) | Grade 3 infection without neutropenia | |
| 2 | 1 | None | None |
| 2+ | Grade 3 lymphopenia | Grade 3 nausea, vomiting, anorexia, other GI toxicity | |
| 3c | 1 | None | Grade 3 fatigue |
| 4 | 1 | Grade 3 neutropenia | None |
| 2+ | Grade 3 neutropenia | None | |
| 5 | 1 | None | Grade 3 diarrhea (without colostomy) |
| 2+ | Grade 3 leukocytes (total WBC) and grade 4 neutropenia | Grade 3 hyperglycemia | |
| 6 | 1 | None | None |
| 2+ | Grade 4 neutropenia | Grade 3 neuropathy-sensory | |
| 11 | 1 | None | Grade 3 hyperglycemia |
| 2d | None | Grade 3 hyperglycemia | |
| Dose level 2 (oxaliplatin 130 mg/m2 + docetaxel 75 mg/m2) | |||
| 7 | 1 | Grade 3 lymphopenia, grade 4 neutropenia and leukocytes (total WBC) |
Grade 3 gastritis, diarrhea, hypophosphatemia, depressed level of consciousness, tumor pain, febrile neutropenia and cardiac-ischemia/infarction |
| 2 | Grade 3 lymphopenia, neutropenia and leukocytes (total WBC) | None | |
| 8 | 1 | Grade 4 neutropenia and grade 3 leukocytes (total WBC) | Grade 3 neuro-mood |
| 2 | Grade 3 neutropenia | None | |
| 9 | 1 | None | Grade 3 headache |
| 2 | None | None | |
| 10 | 1 |
Grade 4 neutropenia, grade 3 lymphopenia and leukocytes (total WBC) |
Grade 3 pain-other |
| 2 | Grade 3 lymphopenia and leukocytes (total WBC) | ||
Toxicities in bold are DLTs
Pt received more than 2 cycles of treatment
Pt only received 1 cycle
Pt received 2 cycles of treatment
Non-hematologic toxicities
Table 3 lists the non-hematologic toxicities. Other than the aforementioned, non-hematologic toxicities were generally mild and limited to ≤NCI CTC grade 2. During the first cycle of chemotherapy at dose level 1, 1 patient had grade 3 fatigue, 1 patient had grade 3 diarrhea, 1 patient had grade 3 hyperglycemia and another 1 patient had grade 3 allergy—all of which resolved within 96 h and did not qualify as DLT. During subsequent cycles, 1 patient had grade 3 neurologic toxicity and 1 patient had grade 3 gastrointestinal toxicity. No patients had grade 4 toxicity. At dose level 2, 1 patient had grade 3 gastrointestinal, infection and metabolic toxicity, and another had grade 3 neurologic toxicity (see Table 1 for details).
Treatment efficacy
Of the 11 patients who participated in the study, 10 were evaluable for response to chemotherapy. The patient who was not evaluable only received 1 cycle of chemotherapy. Four (36 %) patients had stable disease (1 patient with hepatocellular carcinoma, 1 patient with ovarian carcinoma and 2 patients with esophageal carcinoma). There was 1 (9 %) partial response in a patient with esophageal carcinoma. The median overall survival (all of the 11 patients are dead) was 6.5 months (range 2.4–36.2 months). (Tables 1, 4)
Table 4 Responses
| Response | Frequency | % |
|---|---|---|
| Partial response | 1 | 9 |
| Stable disease | 4 | 36 |
| Progressive disease | 5 | 46 |
| Inevaluable | 1 | 9 |
Pharmacokinetics
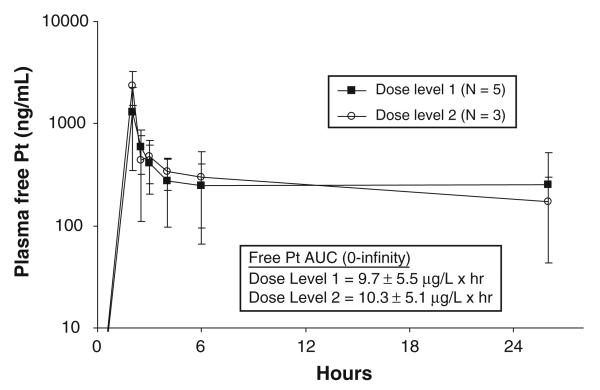
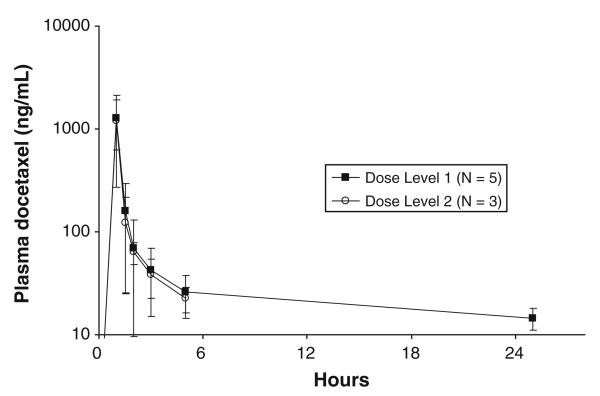
Free platinum and docetaxel pharmacokinetic data were available in 8 patients. Figure 1 shows the average plasma free platinum concentration versus time curves for the 5 subjects treated on dose level 1 and the 3 subjects on level 2. There were no significant differences in Cmax, AUC or CLsys between the two groups. Average plasma docetaxel concentration versus time data for the 2 different dose levels is depicted in Fig. 2. Because none of the 3 patients studied at the 75 mg/m2 dose level had 24 h post-infusion blood samples drawn, a more detailed comparison of the docetaxel pharmacokinetics was not possible. However, despite the difference in docetaxel doses (60 vs. 75 mg/m2), the 2 curves were very similar during the first 6 h following the infusion.
Fig. 1.
Average plasma free platinum concentration versus time curves for the 5 subjects treated on dose level 1 and the 3 subjects on level 2
Fig. 2.
Average plasma docetaxel concentration versus time data for the 2 different dose levels
Conclusion
Based on these data, the MTD of docetaxel is 60 mg/m2 given in combination with oxaliplatin 130 mg/m2 every 3 weeks. The results of the pharmacokinetic studies indicate that docetaxel co-administration does not affect the disposition or elimination of free platinum.
Discussion
This joint phase I study was conducted to determine the MTD of docetaxel given as a 1-h infusion when followed by a 2-h infusion of oxaliplatin at 130 mg/m2 every 3 weeks and define the toxicity of a combination of docetaxel with oxaliplatin. Eleven patients were treated and evaluated for toxicity. The MTD of docetaxel is 60 mg/m2 when given in combination with oxaliplatin 130 mg/m2 every 3 weeks. The dose-limiting toxicities were largely hematologic, specifically neutropenia, although at 75 mg/m2, 2 of 4 patients experienced significant grade 3 non-hematologic toxicity. The combination showed activity in patients with esophageal and ovarian cancer. One patient with hepatocellular carcinoma had stable disease. Interestingly, patients who had received platinum agents previously experienced a response or stable disease (Tables 3, 4).
A previous phase I reporting this combination of agents in patients with advanced breast cancer and non-small cell lung cancer reported a dose of docetaxel 75 mg/m2 on day 1 and 70 m/m2 for oxaliplatin on day 2 without rh G-CSF support and 85 and 130 mg/m2, respectively, with rh G-CSF support. Consistent with our study, the main dose-limiting toxicities were neutropenia and febrile neutropenia (diarrhea and fatigue were also reported) [8].
The combination has now been evaluated in patients with metastatic gastric cancer, a phase I/II study achieving a dose of docetaxel 75 mg/m2 on day 1 and oxaliplatin 130 mg/m2 as 2-h infusion every 3 weeks. The study reported a response rate of 55 % and a median overall survival of 12.7 months. The main toxicities were hematologic, gastrointestinal and neurotoxicity [9].
This study is consistent with the doses we were able to achieve, although in comparison with other phase I, the doses of oxaliplatin was much higher. The DLT of oxaliplatin is generally reported as neuropathy; we did not find the combination with docetaxel to exacerbate this toxicity. In fact, neuropathy was not a significant toxicity or DLT. This may be due to the fact that neuropathy associated with oxaliplatin is cumulative and the median number of cycles administered per patient was 2.
The combination of oxaliplatin and docetaxel was combined to find the dosing of these drugs when used in combination. This regimen was well tolerated and requires further exploration in phase II trials, specifically head and neck, esophageal and gastric cancer.
Acknowledgments
Supported in part by Grants No. U01CA062505, P30CA033572 and P30CA14089 from the National Institutes of Health.
Footnotes
Conflict of interest None.
Contributor Information
Syma Iqbal, USC/Norris Comprehensive Cancer Center, Oncology, Los Angeles, CA, USA.
Heinz-Josef Lenz, USC/Norris Comprehensive Cancer Center, Oncology, Los Angeles, CA, USA.
David R. Gandara, University of California, Davis, Sacramento, CA, USA
Stephen I. Shibata, City of Hope, Oncology, Los Angeles, CA, USA
Susan Groshen, USC/Norris Comprehensive Cancer Center, Oncology, Los Angeles, CA, USA.
Timothy W. Synold, City of Hope, Oncology, Los Angeles, CA, USA
Edward M. Newman, City of Hope, Oncology, Los Angeles, CA, USA
References
- 1.Cvitkovic E. Ongoing and unsaid on oxaliplatin: the hope. Br J Cancer. 1998;77(Suppl 4):8–11. doi: 10.1038/bjc.1998.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvestro L, Anal H, Sommer F. Comparative effects of a new platinum analog (trans-1-diamine-cyclohexane oxalato-platinum: L-OHP) with CDDP on various cells: correlation with intracellular accumulation. Proc Anticancer Res. 1990 pp Abstract # 115. [Google Scholar]
- 3.Pendyala L, Kidani Y, Perez R, et al. Cytotoxicity, cellular accumulation and DNA binding of oxaliplatin isomers. Cancer Lett. 1995;97:177–184. doi: 10.1016/0304-3835(95)03974-2. [DOI] [PubMed] [Google Scholar]
- 4.Extra JM, Espie M, Calvo F, et al. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol. 1990;25:299–303. doi: 10.1007/BF00684890. [DOI] [PubMed] [Google Scholar]
- 5.Extra JM, Marty M, Brienza S, et al. Pharmacokinetics and safety profile of oxaliplatin. Semin Oncol. 1998;25:13–22. [PubMed] [Google Scholar]
- 6.Synold TW, Takimoto CH, Doroshow JH, et al. Dose-escalating and pharmacologic study of oxaliplatin in adult cancerpatients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res. 2007;13:3660–3666. doi: 10.1158/1078-0432.CCR-06-2385. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RJ, Jr, Doroshow JH, Synold T, et al. Phase I trial of intraperitoneal docetaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity: dose-limiting toxicity and pharmacokinetics. Clin Cancer Res. 2003;9:5896–5901. [PubMed] [Google Scholar]
- 8.Kouroussis C, Agelaki S, Mavroudis D, et al. A dose escalation study of docetaxel and oxaliplatin combination in patients with metastatic breast and non-small cell lung cancer. Anticancer Res. 2003;23:785–791. [PubMed] [Google Scholar]
- 9.Kim KH, Park YS, Chang MH, Kim HS, Jun HJ, Uhm J, Yi SY, Lim do H, Ji SH, Park MJ, Lee J, Park SH, Park JO, Lim HY, Kang WK. A phase I/II trial of docetaxel and oxaliplatin in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2009;64(2):347–353. doi: 10.1007/s00280-008-0878-4. doi:10.1007/s00280-008-0878-4. [DOI] [PubMed] [Google Scholar]