Abstract
(±)–Dragmacidin E was synthesized in 25 steps from commercially available 7-(benzyloxy)indole. Key transformations in this sponge metabolite’s preparation include (a) a Witkop cyclization to establish the bridging indole core, (b) cyclo-dehydrative pyrazinone formation to unite the two indole-bearing components, and (c) late-stage guanidine installation via chemoselective carbonyl activation.
Keywords: Organic synthesis, alkaloid, marine, Witkop cyclization, and pyrazinone
1. Introduction
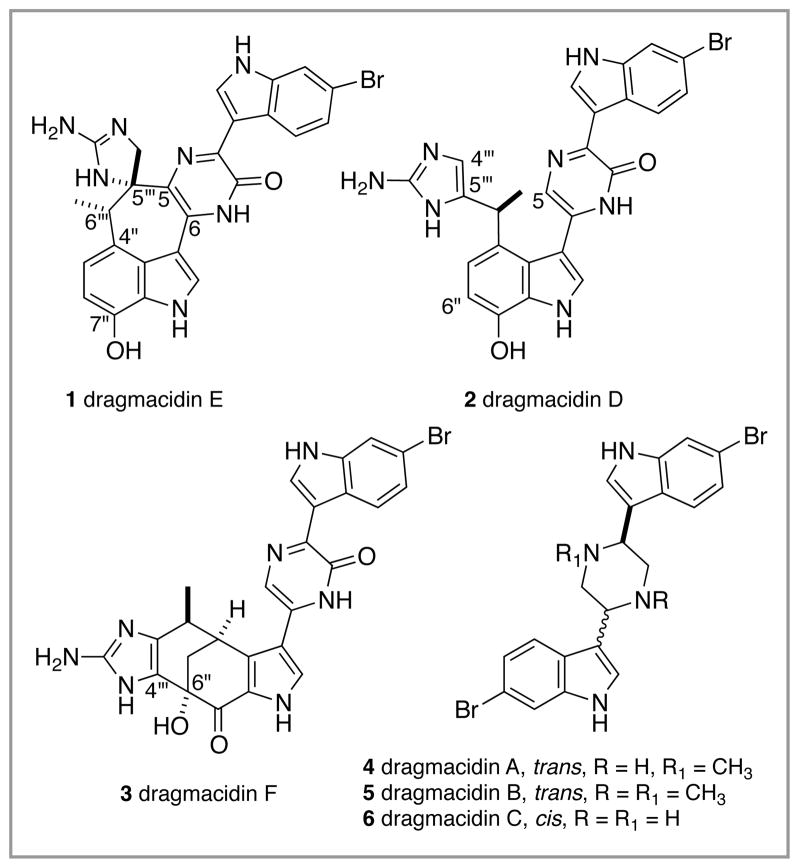
The dragmacidin family of sponge metabolites (Figure 1) is categorized by the formal union of two indole-3-(α-amino)ethylamine fragments via cyclocondensation to form either a central piperazine ring or a pyrazinone ring. Interest in this family of marine natural products has been sustained by a promising roster of biological activities, including enzyme inhibition, antiviral, cytotoxic, and anti-inflammatory capabilities, and by the unprecedented structures of the more complex members with their attendant challenges for chemical synthesis.1 The structurally simpler piperazine-containing dragmacidins A-C (and related compound dragmacidin itself, not shown) have been isolated from sponges identified as belonging to the Dragmacidon (148m depth, Bahamas) and Hexadella (40–200m, British Columbia) genera, and from a tunicate, D. candidum (1m, Mexico).2 Two of the more structurally complicated members of this grouping, the pyrazinone-containing dragmacidins DE, were recovered from a single Spongosorites sp. found in relatively deep water (90m) off of the coast of southern Australia, and the third complex dragmacidin, dragmacidin F (3), was found in a Halicortex sponge species at 45m off of the coast of Italy.3 Whereas it is not clear if the pyrazinone group shares any more than formal nomenclatural identity with the piperazine group, it seems more likely that the members of the pyrazinone dragmacidins are biosynthetically related to each other, as first proposed by Capon and Riccio and later discussed in detail by Garg and Stoltz.1 Thus, formal isomerization of dragmacidin D (2) with connection of C(5) to C(5‴) would deliver dragmacidin E (1), and oxidative cyclization within (2) with attachment of C(4‴) to C(6″) might serve as a blueprint for the biosynthesis of dragmacidin F (3). Neither of these putative biosynthesis transformations has been realized in vitro, although a large body of work on the total chemical synthesis of the dragmacidins, primarily from the Stoltz laboratory in the case of dragmacidins D and F, has emerged over the past two decades.4
Figure 1.
The dragmacidin family of marine metabolites.
Much of the synthesis work directed towards the pyrazinone-containing dragmacidins D-F extended from a perception that these targets are composed of modular aromatic units and consequentially are approachable by a succession of metal-mediated sp2-sp2 coupling transformations. The Stoltz group’s successful preparation of dragmacidins D and F,4g,i and the synthesis of dragmacidin D by Yamaguchi and Itami,4l all follow this paradigm. In addition, approaches to the construction of dragmacidin D by Jiang,5a and to dragmacidin E by Funk,5b continue this theme. All of these approaches introduce the pyrazinone unit as a preformed aromatic moiety, typically as a functionalized pyrazine ring ready for sp2-sp2 coupling.
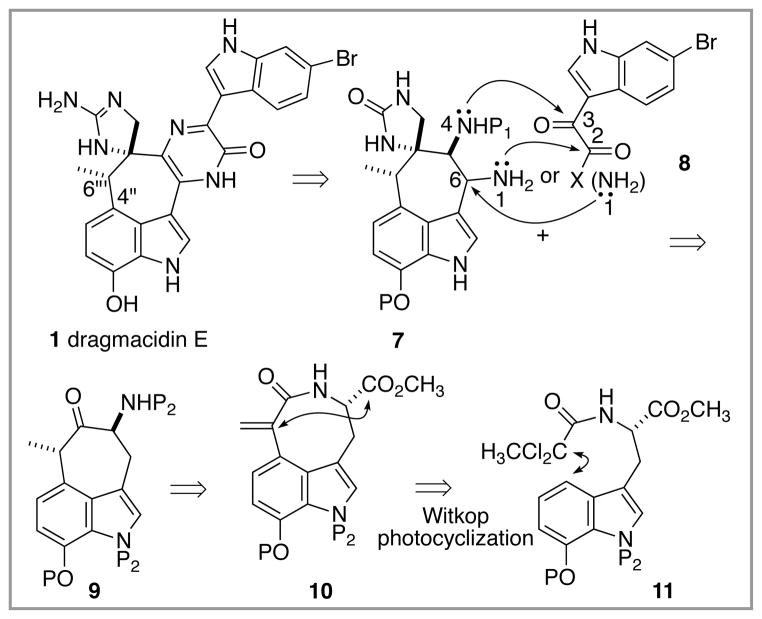
The dragmacidin E structure can also be viewed from a different angle,6 as illustrated in the retrosynthesis shown in Scheme 1. In particular, the C(6‴)–C(4″) bond must be forged in a rather sterically demanding environment, and we have an ongoing interest in the development of diradical cyclization strategies for the formation of such challenging bonds.7 From this perspective, a different conceptualization of the problem, which divides dragmacidin E into two indole-containing fragments 7 and 8, emerges. Thus, the relatively simply 6-bromoindole 3-acetic acid derived species 8, either as a bis electrophile or electrophile/nucleophile pair, can be cyclocondensed with the more complex bis nucleophilic partner 7 (or a nucleophile/electrophile equivalent) to form the central pyrazinone ring, a transformation hopefully driven by accrual of aromatic resonance energy. Several versions of indole 8 are known (X = NH2, Cl),8 and so preparation of tetracycle 7 with control of relative stereochemistry becomes the operational challenge of the first stage of this synthesis plan. We anticipated that 7 could be derived by manipulation of the peripheral functionality of tricycle 9, which in turn might result from a Dieckmann cyclization within the azocane sector of 10. The structural complexity inherent in 10 can be rapidly addressed by a diradical-based9 Witkop photocyclization10 of simple tryptophan derivative 11, and it is in this transformation that the focal point of the route, the C(6‴)–C(4″) bond, is secured. Thus, the valuable attributes of diradical cyclizations for C–C bond formation, such as low activation barriers even in sterically hindered systems and a relative insensitivity to solvent effects,11 can be exploited to deliver a complex tricyclic core upon which the remainder of the synthesis can be mounted.
Scheme 1.
Retrosynthetic analysis for dragmacidin E synthesis.
2. Results and Discussion
2.1 Model System Synthesis
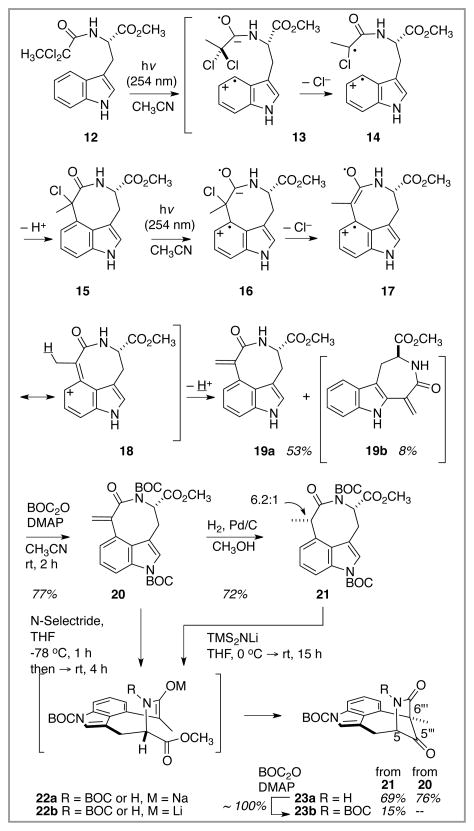
The starting point for the dragmacidin E synthesis effort was a model system designed to test the feasibility of the key Witkop cyclization, Scheme 2. In this vein, the simple and chiral tryptophan derivative 12, prepared by acylation of tryptophan methyl ester with 2,2-dichloropropionyl chloride, was subjected to irradiation through quartz at 254 nm in deoxygenated CH3CN solvent. The oxygen-free atmosphere was maintained by purging the reaction solution with an N2 stream throughout the irradiation. This simple protocol led to isolation of 53% of the azocane-containing bridged indole product 19a in accord with expectations.10 This product retained optical activity. The yield of this process was consistently in the 45 – 53% range over the scale of 150 mgs to 2.0 gms. In some runs, a small amount (< 10%) of the C(2) attached azacycloheptanoid product 19b could be isolated as well. Speculation about the mechanistic course of this transformation is based upon much prior art,9 and passage through the indicated radical intermediates is consistent with the conclusions drawn from that work. Diradical 14 is presumed to directly precede formation of the pivotal C(6‴)-C(4″) bond; the first-formed chloride product 15 appears to be susceptible to a second Witkop-like process,10j leading to formal loss of HCl to deliver the enone product 19a.
Scheme 2.
Exploratory Witkop cyclization of a model amide 12, and formation of the cycloheptane core.
The ready access to azocane 19a on a gram-scale prompted further work on this des-C(7″)-hydroxyl model system with the intent of pushing the chemistry as far as possible in preparation for work on the real system. The next transformation of merit involved forming the C(5‴)-C(6‴) bond to close the cycloheptanoid ring of the target structure. A Dieckmann cyclization within 23 between the amide enolate and the pendant ester was planned, but the method of formation and the stability of this reactive enolate was an issue. Initially, two approaches with the N-BOC protected imide derivative were envisioned: (a) hydrogenation to form the saturated azocane 21, from which enolate generation would follow standard deprotonation protocols, and (b) a more direct route utilizing conjugate addition of “H−” to the enone to generate the enolate directly. In advance of experimentation, concerns about steric hindrance to deprotonation clouded the prospects for route (a), whereas the issue of [H−] addition chemoselectivity made route (b) worrisome. In any event, both approaches proved serviceable, and both enolates 22a and 22b did participate in the Dieckmann cyclization to furnish the bridged system 23 as a mixture of primarily the N–H species 23a with varying amounts of the N-BOC derivative 23b. This crude mixture was subjected to BOC addition to formulate only the desired N-BOC product 23b. Thus, in just 4 steps from tryptophan methyl ester, a polycyclic species containing the cycloheptane-bridged indole core of dragmacidin E was in hand. The requisite C(5) nitrogen was in place as well, but there was an extraneous carbonyl as part of the imide bridge that had to be excised to further the route.
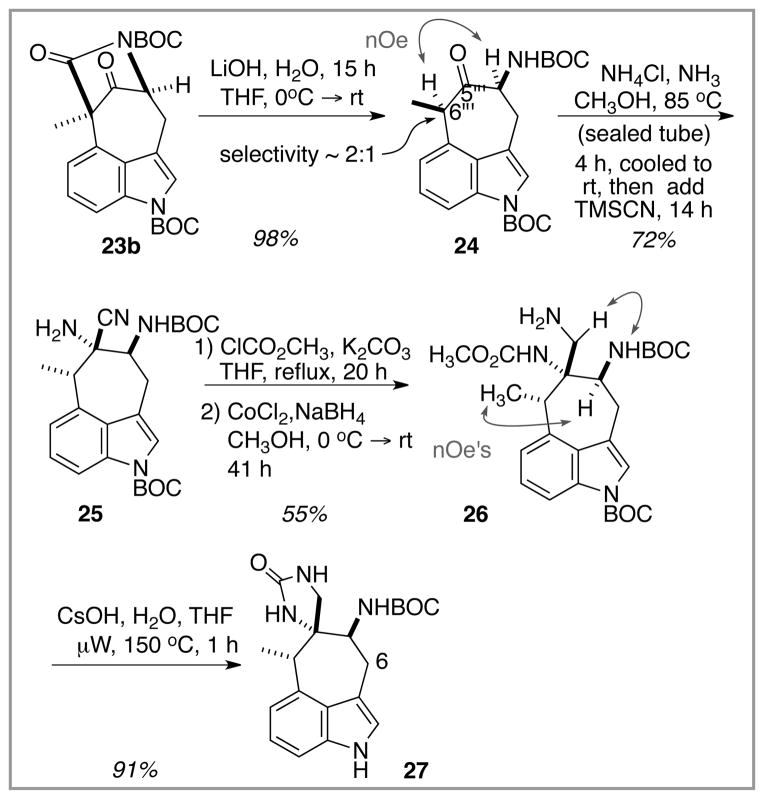
The model system work continued with the twin goals of first simplifying the bridged indole structure by cleaving the imide function and then elaborating the structure once again by installing the spiroimidazolone ring at C(5‴), Scheme 3. Imide hydrolysis with concomitant decarboxylation within 23b afforded the cycloheptanone product 24 as a separable diastereomer mixture, both of which displayed optical activity. Nuclear Overhauser enhancement measurements allowed assignment of relative stereochemistry (see 24), although the relative configuration at the methyl-bearing carbon C(6‴) proved to be irrelevant (vide infra). Thus, Strecker reaction with ketone 24 as the 2:1 mixture of diastereomers delivered the aminonitrile product 25 as a single diastereomer. Both the facial selectivity of cyanide addition to an intermediate iminium ion (not shown) and the configuration at C(6‴) emerged favorably disposed for dragmacidin E synthesis. Since the C(6‴)/C(5‴) relationship is the only stereochemical issue in the target structure, this transformation points the way to a complete solution for this part of the synthesis objective. Unfortunately, aminonitrile 25 was almost completely racemic. Thus, not only did C(6‴) epimerize under the iminium forming conditions (=> favorable), but the C(5) NHBOC-bearing carbon must have epimerized also (=> unfavorable!) Recovered starting ketone 24 from incomplete reaction was racemic as well. Much effort was expended in exploring the reaction conditions for this Strecker process in order to improve upon this disappointing turn, but in the final analysis, no experimental procedures were identified that overcame the epimerization problem. For example, when the Strecker reaction was carried out under milder conditions by treating ketone 24 with sat. NH3/MeOH in the presence of Ti(Oi-Pr)4 at room temperature prior to TMSCN addition, no reaction took place. In a different approach, addition of hydroxylamine to the 2:1 diastereomeric 24 mixture resulted in an ~ 2:1 mixture of diastereomeric oximes, suggesting that epimerization at both α-carbons did not occur in this instance. However, attempts to add cyanide to these diastereomeric oximes failed. Thus, it appeared that the synthesis of this dragmacidin E model system, and quite likely the natural product as well, was destined to produce a racemic target.
Scheme 3.
Further model system work; installation of the imidazolone ring.
Despite this stereochemical setback, the model system synthesis continued with routine chemistry to convert the aminonitrile functionality to the desired spiroimidazolone. Earlier scouting experiments with a related aminonitrile (see compound 12 in ref. 6a) indicated that the free amine of 25 should be acylated to avoid returning either regenerated ketone 24 or forming the corresponding primary C(5‴) amine (via C(5‴) imine reduction) upon attempted nitrile reduction chemistry. Once the free amine was protected as a carbamate, the reduction of the nitrile function could be executed without event by using the cobalt-mediated procedure of Battersby.12 The aminocarbamate 26 did not spontaneously cyclize, perhaps due to strain accrual, but the desired closure could be forced by heating/microwave in the presence of cesium hydroxide. As an alternative, simply heating 26 in aqueous ethanol with LiOH present sufficed to form 27 in reproducibly good yields. The relative stereochemistry of the spiroimidazolone product 27 was established by nOe measurements on the precursor aminocarbamate 26, as illustrated in Scheme 3.
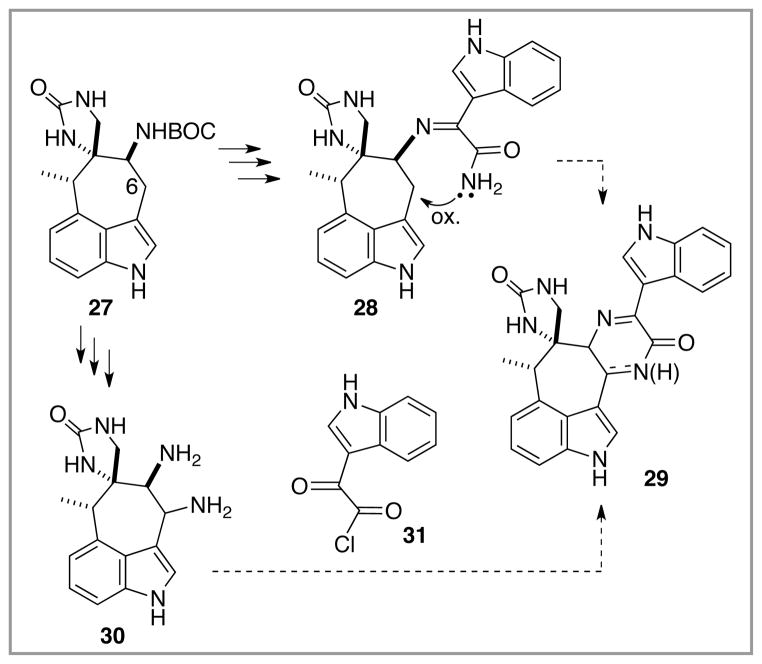
The next major task in probing the pathway for dragmacidin E synthesis with this model system involved establishing a procedure for preparing the central pyrazinone ring from 27 and a complimentary indole fragment related to 8 (Scheme 1). At this juncture, it was not clear which of several cyclocondensation-based approaches would emerge as the most productive. Stoltz et al. in their dragmacidin D synthesis work briefly explored one such strategy, utilizing a bis nucleophile version of 8 (i.e., α-amino-indoleacetamide) as a condensation partner with a bis electrophile version of the more structurally complex indole core (α-keto-indoleacetaldehyde).4g This cyclocondensation worked admirably in the simple model case, but failed with more functionalized versions of the bis electrophile partner featuring substitution at C(4″). With this limitation in mind, alternative cyclocondensation strategies to prepare a dragmacidin E-like heptacycle 29 were evaluated, Scheme 4. For example, since oxidation at C(6) in 27 was required no matter what approach was used, a strategy that first connected the two indole components via the nucleophilic C(5) nitrogen (after BOC removal) might have the advantage of relying on an intramolecular oxidative cyclization to close off the pyrazinone (or dihydropyrazinone) ring, 27 –> 28 –> 29. In this approach, each indolic component would offer complementary nucleophile/electrophile centers to ensure that the final pyrazinone ring would be fashioned with the desired regiochemistry. Alternatively, initial oxidative functionalization at C(6) in 27 might lead to a bis nucleophile version of the dragmacidin E core, 30, which could be joined with the bis electrophile 31 to deliver 29. In this “bis electrophile/bis nucleophile” scenario, the question of regiochemistry of addition (and hence connectivity of the eventual pyrazinone ring) arises.
Scheme 4.
Strategies for the convergent union of the two indole halves in a model system.
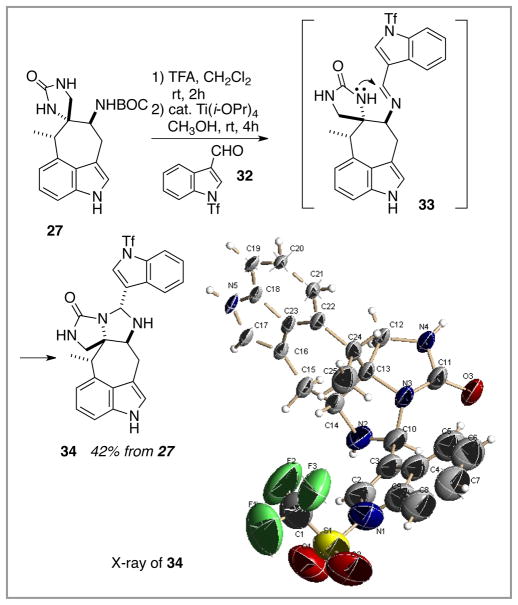
The intramolecular oxidative cyclization chemistry was pursued first in light of the potentially complicating regiochemistry issues of the alternative bis nucleophile/bis electrophile strategy, Scheme 5. The BOC group was cleaved from the C(5) carbamate within 27 to reveal the amine, which was condensed under mild Lewis acid catalysis with the test case indole-carboxaldehyde 32 as a model for a structure such as 8. Imine formation appeared to proceed without event, but an imine product was not isolated. Rather, a single, diastereomerically pure compound was formed in good yield. Preliminary mass spectrometry analysis indicated that a dehydrative condensation did occur (MS m/z = 530 (M + H) => 27 + 32 – H2O, CO2t-Bu). This isolate’s structure was secured by X-ray crystallography and is illustrated as 34 below.13a Apparently, intramolecular cyclization of one of the spiroimidazolone’s nitrogens is just too favorable and the simple imine does not survive. Attempts to cleave this aminal through reversible imine formation/cyanide addition failed. Although it might be possible to invest in a protection/deprotection strategy for the participating spiroimidazolone nitrogen in order to rescue this approach, this type of workaround would overly complicate the synthesis.
Scheme 5.
Attempt at an “imine-first” strategy for joining the two (model) indole fragments.
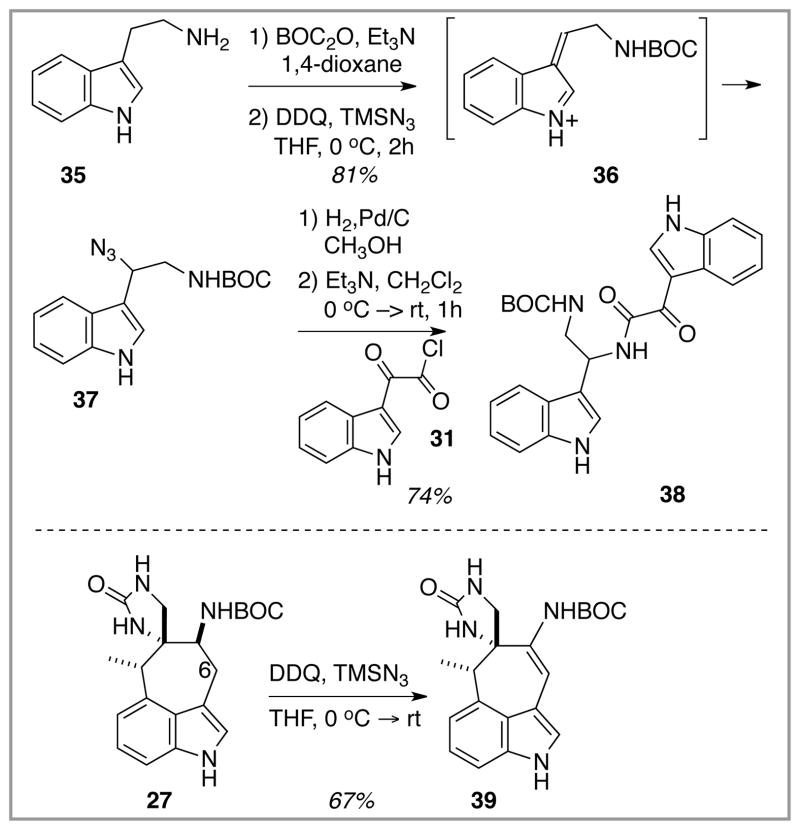
Recourse to the second pyrazinone assembly option was made in response to the failure of the initial strategy. Preliminary scouting experiments designed to test the possibility of direct oxidative azide introduction at C(6) commenced, Scheme 6. The simple tryptamine substrate 35 as its N-BOC derivative did perform as expected,14 and the indolic monoazide product 37 was formed in good yield. The feasibility of forming a pyrazinone-type ring from this species was demonstrated by its conversion through simple chemistry into the amide 38, a known species that had already been cyclized into a dihydropyrazinone product.15 Unfortunately, this model system success could not be exported to the dragmacidin core model system 27, which underwent simple dehydrogenation upon exposure to the oxidative azidation conditions. Presumably, a reactive intermediate unsaturated imine (or iminium ion) related to 36 simply deprotonated in preference to bimolecular azide trapping.
Scheme 6.
Preliminary attempts at C(6) nitrogen introduction (model system).
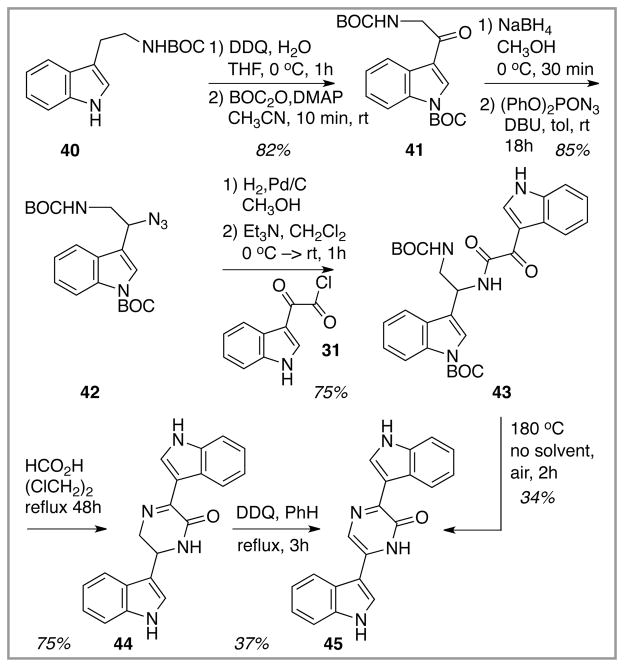
Option 3 in pyrazinone formation spun off from the above failed chemistry; the latter reactions of the 35 – > 38 sequence indicated that once the azide was in place at C(6) (dragmacidin numbering), the rest of the pyrazinone (or dihydropyrazinone) formation chemistry should follow without event. So, an alternative to direct C(6) oxidative azidation was required, and passage through an intermediate C(6) ketone was explored next, Scheme 7. Indolic oxidation of the indole-N-unprotected tryptamine derivative 40 with DDQ followed by N-protection afforded the indolic ketone 41 in good yield.16 Ketone reduction and subsequent azidation proceeded smoothly to give the indolic azide 42. Attempts at this chemistry without first deactivating the indole nitrogen via BOC protection did not lead to any chemical reaction. Unlike the indole N–H analogue 37 (Scheme 6), the N-BOC indole species 42 had not been processed on to a pyrazinone/dihydropyrazinone product, and so that chemistry was explored next. Azide reduction and acylation of the resulting amine with acid chloride 31 afforded the expected amide 43, now with a BOC group on the indole nitrogen (compare 38, Scheme 6). Two approaches for cyclization/oxidation were investigated to complete pyrazinone ring formation. Initially, global BOC removal with formic acid furnished the dihydropyrazinone 44 via dehydrative cyclization of the liberated amine and the proximate ketone. This light yellow species could be converted into the fully oxidized, bright yellow pyrazinone product 45 by exposure to DDQ. The low yield of this final step can, at least in part, be attributed to the limited solubility of 45 in all organic solvents tested for its chromatographic purification. The desired pyrazinone-containing product 45 also could be prepared in a more efficient manner by consolidating the deprotection and oxidation steps; simply heating 43 as a neat solid in air (180 °C) led to a 34% isolated yield of the pyrazinone product. Although the yield of pyrazinone formation was rather modest in this simple model system, the solubility problems with 45 left reason to hope that, perhaps in the more structurally complex dragmacidin species, this issue might not arise.
Scheme 7.
A second attempt at (indirect) C(6) oxidation/nitrogen introduction in a very simple model system.
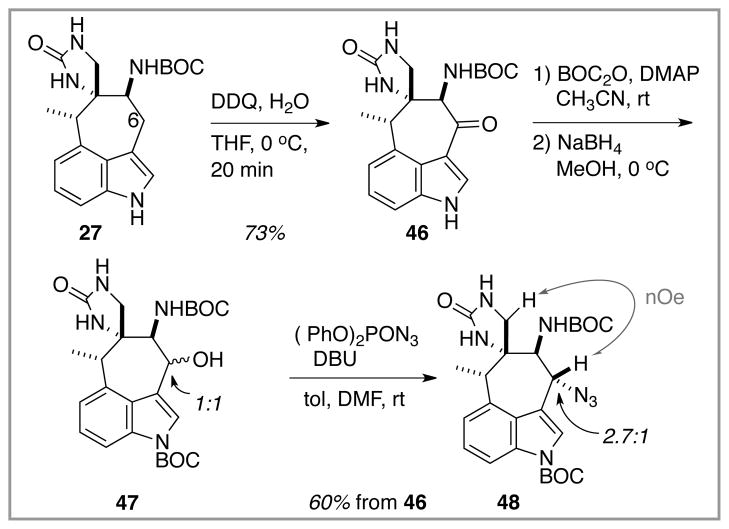
The application of this successful C(6) oxidation/amination approach to the more relevant model system 27 is described in Scheme 8. DDQ-mediated oxidation of the indolic position of the free NH indole unit proceeded as per the simpler model substrate 40 to furnish the C(6) ketone-containing tetracyclic species 46. Following the experimental protocol established with 41, indole nitrogen protection and then sequential ketone reduction and N-for-O exchange at C(6) delivered the requisite azide 48 as a 2.7:1 mixture of inconsequential diastereomers. The stereochemistry of the major isomer was established by the nOe correlation shown, and that of the minor isomer by default. The mechanistic course of the alcohol azidation is not clear. Simple SN2 displacement on both α- and β-isomers of 47 is possible, with a bias for more efficient replacement of the β-OPPh3+ intermediate and some yield loss attributed to less efficient reaction of the α-isomer. No alkene (N-BOC version of 39) was detected. Alternatively, this substitution could operate through an SN1 process via an unobserved alkenyl iminium ion. In this speculative scenario, the lack of alkene formation from 47, in contrast to exclusive alkene formation from 27 (Scheme 6), might be attributed to the intermediacy of a more electrophilic alkenyl iminium ion derived from the N-BOC species 47 when compared to a less electrophilic alkenyl imine (from deprotonation of a first-formed iminium ion) that might precede 39 in the N–H series.
Scheme 8.
Application of the C(6) oxidation chemistry to an advanced model system; introduction of a C(6) azide.
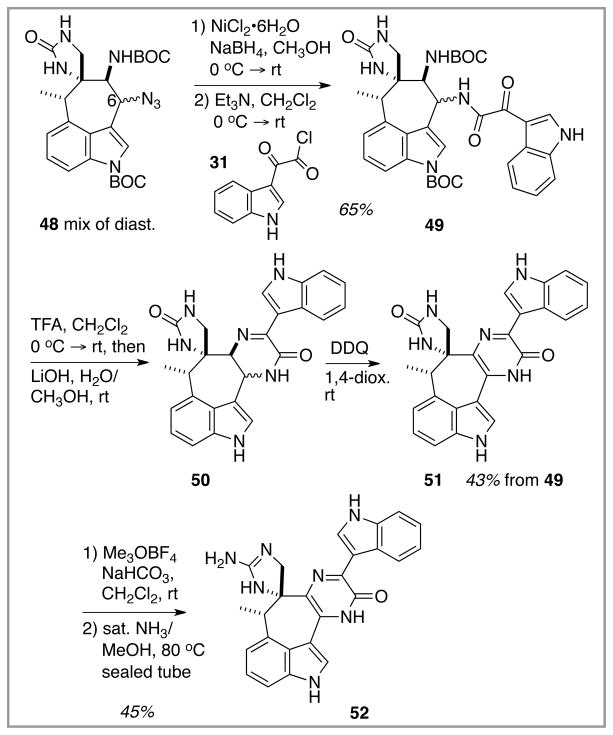
Continuation of this advanced model system exercise in order to reach a fully functionalized dragmacidin E analogue is illustrated in Scheme 9. As per the chemistry with 42 (Scheme 7), the azide mixture 48 was reduced to the primary amines and immediately acylated with 31 to give the amides 49 as a mixture of diastereomers. Unlike the simpler model systems (Schemes 6 and 7) where hydrogenolysis sufficed to convert azide to amine, the more complex azides 48 did not respond well to this chemistry. Fortunately, an alternative procedure based upon low-valent nickel16 did perform satisfactorily, and so the route remained on track. N-BOC removal with TFA and conversion of the first-formed primary ammonium salt to the free amine with base set up the cyclocondensation to generate the dihydropyrazine-containing heptacyclic product 50 containing all of the rings of dragmacidin E. In some trials, 50 air-oxidized in part to the desired pyrazinone 51, as evidenced by the development of the characteristic bright yellow color. However, adventitious air oxidation never led to more than trace amounts of 51, and so deliberate DDQ-mediated oxidation was employed to drive all of 50 into the pyrazinone 51.
Scheme 9.
Completion of an advanced model system synthesis; pyrazinone and guanidine assembly.
One final operation was required to complete this model system for dragmacidin E synthesis; the conversion of the urea function of the spiroimidazolone ring into a guanidine moiety. This procedure was not without its concerns, primarily because chemoselective functionalization of the urea carbonyl in the presence of the pyrazinone carbonyl was required. A two-stage procedure introduced by Kishi18 and utilized in a recent dibromophakellin synthesis19 was adapted for this system. Treatment of 51 with the very “hard” methylating agent Me3OBF4 began the sequence. Exclusive but incomplete methylation of the urea carbonyl was observed, leading to an intermediate methoxyamidine function. That this reaction proceeded without any evidence for pyrazinone O-methylation was a bit of a surprise,20 given the increase in aromatic character that might accompany O-methylation at the pyrazinone ring. In any event, after chromatographic removal of recovered starting urea 51, the final NH2 introduction could be accomplished by exposure of the methoxyamidine intermediate to ammonia under forcing conditions. Thus, the “almost dragmacidin E” model system 52 could be accessed from the simple tryptophan derivative 12 in 19 chemical steps. These efforts lend support to the notion that the real system, dragmacidin E, might be assembled by application of this chemistry to a more suitably functionalized starting tryptophan derivative.
2.2 (±)–Dragmacidin E Synthesis
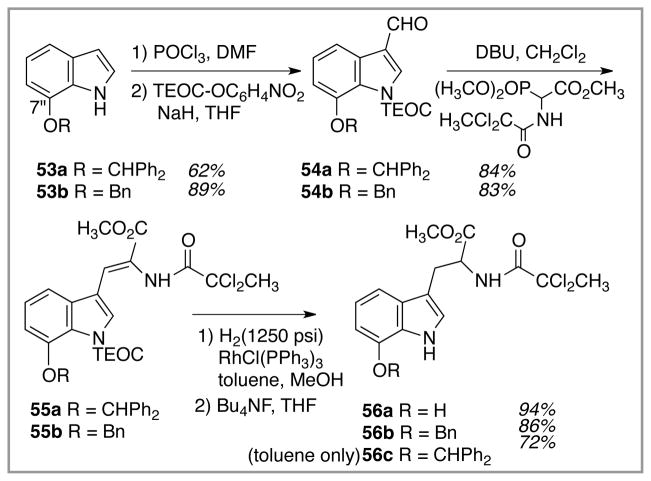
Synthesis of dragmacidin E commenced with the preparation of suitable Witkop photocyclization precursors, Scheme 10. At the outset, it was not clear if the C(7″)-oxygen moiety would benefit the Witkop reaction more as a free hydroxyl or in protected form. Therefore, both options were examined. The free C(7″)-OH version of the dichloroamide, 56a, was prepared via initial Bartoli indole synthesis to give the benzhydryl protected starting material 53a.21 Vilsmeier formylation of 53a installed the C(3) aldehyde, and Emmons-Horner extension of this aldehyde provided the unsaturated amido ester tryptophan derivative 55a. Hydrogenation of 55a at high pressure in a methanol/toluene solvent mixture served the dual purposes of alkene reduction and benzhydryl removal, leading to the first Witkop photocyclization precursor, the free phenol 56a, following deprotection of the indole’s nitrogen. The O-benzyl protected version of 56a, 56b, could in principle be formed by benzylation of 56a, but in practice a shorter route presented itself. The 7″-O-benzyl indole starting material 53b is commercially available, thus eliminating the 2-step Bartoli procedure for indole preparation. Similar formylation of 53b followed by Emmons-Horner chemistry furnished the alkene, benzyl ether 55b, which, upon exposure to identical hydrogenation conditions as described for 55a, did not undergo benzyl group hydrogenolysis. Thus, the benzyl ether-containing Witkop photocyclization precursor 56b was in hand also. Finally, conducting the rhodium-mediated hydrogenation of 55a in toluene only left the benzhydryl protecting group intact, and so the benzhydryl-protected version 56c was available as well following TEOC removal.
Scheme 10.
Synthesis of two Witkop cyclization precursors containing C(7″) oxygenation.
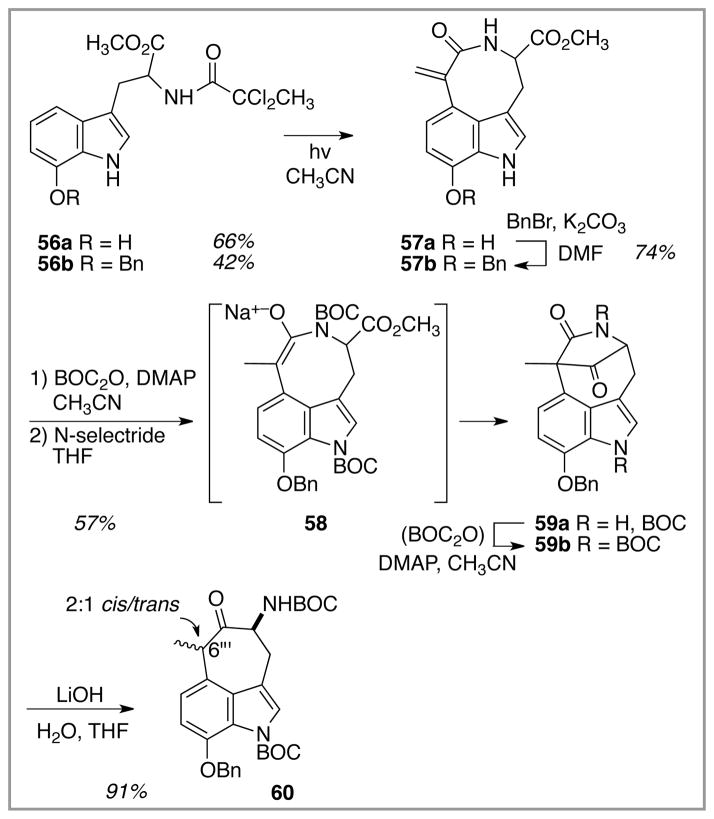
Witkop photocyclizations of all three tryptophan derivatives 56a-56c were run under the conditions optimized for the model system 12, Scheme 11. The benzhydryl-protected version 56c produced a complex mixture of compounds from which no azocane-containing material could be identified. Fortunately, both the free hydroxyl substrate 56a and the benzyl ether variant 56b did participate in the Witkop reaction in the anticipated manner. The free phenol 56a gave consistently higher yields of the Witkop product 57a. The chemical yield of the des-oxy model system 12 (53%) fell in between the yields of the two oxygenated substrates, and so it would be premature to draw any conclusions about the effect of C(7″) oxygenation on the overall efficiency of this Witkop process. The benzyl ether 57b actually was the desired species for continuing the dragmacidin E synthesis, and so the first transformation with the phenolic azocane product 57a was O-benzylation to produce 57b. Thus, as a practical matter, gram-scale photolyses were typically conducted with the more readily available benzyl ether substrate 56b to give benzyl ether 57b directly. Benzyl ether 56b also had the advantage of being a shelf-stable light yellow solid whereas the free phenol 56a was isolated as a foam that decomposed after a few days upon storage even at −20 °C. The alkene reduction/Dieckmann cyclization sequence on 57b followed the pathway precedented by the des-7″-benzyloxy model system (Scheme 2), leading to moderate yields of the bridged bicyclic system 59a/59b. As with the model system, cleavage of the lactam bridge within 59b proceeded without event upon treatment with aqueous base to yield the O-benzyloxy version of the cycloheptanone-spanning indole core of dragmacidin E, 60, as an inconsequential mixture of diastereomers at C(6‴).
Scheme 11.
Witkop cyclization of the hydroxy and benzyloxy substrates 56a and 56b, respectively, and conversion of the lactam product to the cycloheptanone core of dragmacidin E.
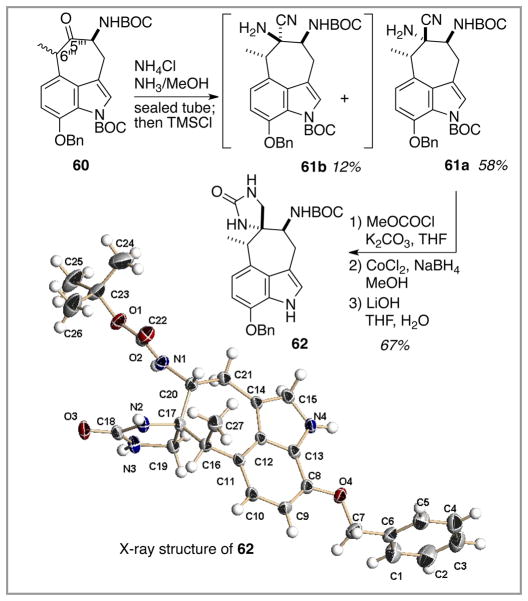
The chemistry of the C(7″)-benzyloxy indole series seemed to track the des OBn model system quite closely up to this juncture. The next operation, installation of the spiroimidazolone ring (Scheme 12), was not expected to deviate from this correspondence, given the structural disconnect between C(7″) and the cycloheptanone carbonyl. That expectation was not met. In fact, initial attempts to perform a Strecker reaction on cycloheptanone 60 under the conditions used successfully with 24 furnished the desired product aminonitrile 61a in only 38% yield along with 19% of its diastereomer 61b. This surprising result prompted a new round of optimization studies, leading to the observation that yield maximization required (a) freshly prepared NH3 in methanol, and (b) strict temperature control (T < 80 °C) to avoid product decomposition. Under this new experimental protocol, as much as 58% of the desired isomer 61a could be isolated along with only 12 % of the unwanted diastereomer 61b. The underlying basis for the disparate reactivity between ketone 60 and ketone 24 remains a matter of speculation. One scouting experiment to assess the effect of an alternative C(7″) oxygen protecting group was explored; replacement of the benzyl ether with the more electron withdrawing BOC group led to a C(7″)-OBOC analogue of 60 (free phenol 57a was treated in sequence with (i) BOC2O/DMAP, (ii) N-selectride/BOC2O, and (iii) LiOH/H2O/THF). Reaction of this substrate under the optimized Strecker conditions led to only an 18% yield of the C(7″)-OBOC analogue of 61a. Given the insulating C(6‴) methylene, it is not clear how the C(7″) oxygen protecting group could be exerting an electronic influence on the C(5‴) carbonyl. However, it may be possible that steric interactions between the C(7″) OR group and the indole’s N-BOC group (worse with OBOC than OBn) might contribute to subtle conformational effects that influence both the yield and the facial selectivity of this Strecker reaction. With 61a in hand as a single diastereomer following chromatographic purification, spiroimidazolone construction could continue. Fortunately, the remainder of the chemistry (amine carbamolyation, nitrile reduction, cyclization) did follow the earlier path set out in the model system, and spiroimidazolone 62 was formed in good yield and without complications. The relative stereochemistry of this species was firmly established by X-ray crystallographic analysis.13b
Scheme 12.
Formation of the spiroimidazolone moiety; X-ray confirmation of relative stereochemistry.
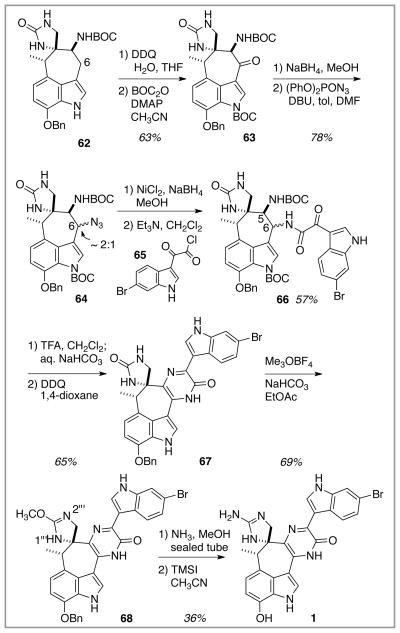
Completion of the dragmacidin E synthesis effort relied heavily on the model system chemistry. Once again, the C(7″) benzyloxy series performed faithfully and little deviation from the chemistry established for the conversion of 27 into 52 (Schemes 8 and 9) was required (Scheme 13). Indolic oxidation at C(6) in 62 followed by indole nitrogen protection produced the ketone substrate 63 that fed into the pyrazinone construction sequence detailed earlier with the model system. Ketone reduction, azide substitution, azide reduction, and amine acylation all proceeded in good yield. In this instance, of course, the 6-bromoindole acid chloride 65 was used rather than the simple indole acid chloride 31 employed in the model system chemistry. In a minor twist relative to the model system, basification of the ammonium salt produced from TFA-mediated deprotection of the C(5) NBOC moiety of 66 did not proceed cleanly to dihydropyrazinone product with LiOH as the base (model system conditions). The milder base NaHCO3 was required in this instance to maximize the yield of 67. With this intermediate dihydropyrazinone in hand, the next three steps (DDQ oxidation to give the pyrazinone unit, urea O-methylation, and ammonia displacement of OCH3 to form the guanidine unit) mirrored the chemistry established in the model system, and the (C7″) benzyl ether of the target dragmacidin E was in hand. A key ingredient to the success of the urea O-methylation step within 67 was substitution of EtOAc as solvent compared to CH2Cl2 in the model system series. In this instance, EtOAc helped solubilize the urea substrate 67, which promoted rapid reaction and minimized product overmethylation as observed with CH2Cl2 solvent. Furthermore, it is possible that the carbonyl of EtOAc played a relay role in methyl electrophile transfer. The ultimate step of the synthesis, debenzylation, was not part of the model system chemistry, and so it became the focus of some concern. Could the benzyl ether be removed under conditions that did not compromise the rest of the molecule? Hydrogenolysis was out, given the C(6′) bromide. Fortunately, Stoltz had described a similar but low-yielding debenzylation with TMSI in the presence of both guanidine and pyrazinone groups in his dragmacidin D synthesis.4g The similarities of his substrate to the guanidine-containing species derived from 68 boded well, and it was gratifying to observe that TMSI-mediated debenzylation did proceed smoothly and without much decomposition to deliver racemic dragmacidin E (1) as a bright yellow solid.
Scheme 13.
Completion of the synthesis of (±)–dragmacidin (1).
3. Conclusions
The deepwater sponge metabolite dragmacidin E was prepared in racemic form over 25 chemical steps (0.25% overall yield, ~ 79%/step average) from commercially available 7-benzyloxyindole. The synthesis route benefited greatly from preliminary model system work that provided guidance in all of the key transformations; (a) Witkop photocyclization to prepare the polycyclic indole core, (b) spiroimidazolone installation, (c) pyrazinone assembly by convergent cyclocondensation, and (d) late-stage guanidine formation. Unfortunately, unavoidable epimerization of a middle-stage intermediate derailed any plans for an asymmetric synthesis. Nevertheless, this work highlights the value of diradical-based C–C bond formation strategies for the construction of pivotal core bonds in sterically crowded environments.
Acknowledgments
We thank the National Institutes of Health, General Medical Sciences (GM 72572) for financial support of this work; the X-Ray facility is supported by NSF CHE 0131112.
Biographies

Dr. Ken Feldman
Ken Feldman received his B.S. degree from Harvey Mudd College in 1978, his Ph.D. from Stanford University in 1983, and, following a one-year post-doctoral stay at E.I. DuPont de Nemours & Company, he joined the faculty at the Pennsylvania State University as an Assistant Professor. He was promoted to Professor in 1996. His research interests include synthesis methodology development and natural products total synthesis. In addition to these studies, he is an avid amateur aquarist who conducts water chemistry experiments on his 175-gallon tropical reef tank.

Dr. Paiboon Ngernmeesri
Paiboon Ngernmeesri was born in 1977 and raised in Roi Et, Thailand. He received his B.S. degree in chemistry in 2000 from Indiana University, where he conducted undergraduate research on physical organic chemistry under the supervision of Professor Joseph Gajewski. He subsequently pursued his interest in organic chemistry at Pennsylvania State University, where he conducted graduate research on the synthesis of a natural product, dragmacidin E, under the mentorship of Professor Ken Feldman. After receiving his Ph.D. in 2008, he joined the Chemistry Department at Kasetsart University, Thailand, where he is now a lecturer. His primary research interests focus on the total synthesis of biologically active natural products.
References
- 1.Garg NK, Stoltz BM. J Chem Soc, Chem Commun. 2006:3769–3779. doi: 10.1039/b605929e. [DOI] [PubMed] [Google Scholar]
- 2.(dragmacidin) Kohmoto S, Kashman Y, McConnell OJ, Rinehart KL, Jr, Wright A, Koehn F. J Org Chem. 1988;53:3116–3118.(dragmacidins A and B) Morris SA, Andersen RJ. Tetrahedron. 1990;46:715–720.(dragmacidin C) Fahy E, Potts BCM, Faulkner DJ. J Nat Prod. 1991;54:564–569.
- 3.(dragmacidin D) Wright AE, Pomponi SA, Cross SS, McCarthy P. J Org Chem. 1992;57:4772–4775.(dragmacidins D and E) Capon RJ, Rooney F, Murray LM, Collins E, Sim ATR, Rostas JAP, Butler MS, Carrol AR. J Nat Prod. 1998;61:660–662. doi: 10.1021/np970483t.(dragmacidin F) Cutignano A, Bifulco G, Bruno I, Casapullo A, Gomez-Paloma L, Riccio R. Tetrahedron. 2000;56:3743–3748.
- 4.(dragmacidin) Jiang B, Smallheer JM, Amaral-Ly C, Wuonola MA. J Org Chem. 1994;59:6823–6827.(dragmacidin B) Whitlock CR, Cava MP. Tetrahedron Lett. 1994;35:371–374.(dragmacidin B) Miyake FY, Yakushijin K, Horne DA. Org Lett. 2000;2:3185–3187. doi: 10.1021/ol0001970.(dragmacidin A) Kawasaki T, Enoki H, Matsumura K, Ohyama M, Inagawa M, Sakamoto M. Org Lett. 2000;2:3027–3029. doi: 10.1021/ol006394g.(dragmacidin A) Yang CG, Wang J, Tang XX, Jiang B. Tetrahedron: Asymm. 2002;13:383–394.(dragmacidins B and C) Kawasaki T, Ohno K, Enoki H, Umemoto Y, Sakamoto M. Tetrahedron Lett. 2002;43:4245–4248.(dragmacidin D) Garg NK, Sarpong R, Stoltz BM. J Am Chem Soc. 2002;124:13179–13184. doi: 10.1021/ja027822b.(dragmacidin B) Garg NK, Stoltz BM. Tetrahedron Lett. 2005;46:2423–2426.(dragmacidin F) Garg NK, Caspi DD, Stoltz BM. J Am Chem Soc. 2005;127:5970–5978. doi: 10.1021/ja050586v.(dragmacidins A, B, and C) Tonsiengsom F, Miyake F, Yakushijin K, Horne DA. Synthesis. 2006:49–54.(dragmacidin A) Anstiss M, Nelson A. Org Biomol Chem. 2006;4:4135–4143. doi: 10.1039/b608910k.(dragmacidin D) Mandal D, Yamaguchi A, Yamaguchi J, Itami K. J Am Chem Soc. 2011;133:19660–19663. doi: 10.1021/ja209945x.
- 5.(a) Yang CG, Liu G, Jiang B. J Org Chem. 2002;67:9392–9396. doi: 10.1021/jo026450m. [DOI] [PubMed] [Google Scholar]; (b) Huntley RJ, Funk RL. Org Lett. 2006;21:4775–4778. doi: 10.1021/ol0617547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Feldman KS, Ngernmeesri P. Org Lett. 2005;7:5449–5452. doi: 10.1021/ol0522081. [DOI] [PubMed] [Google Scholar]; (b) Feldman KS, Ngernmeesri P. Org Lett. 2010;12:4502–4505. doi: 10.1021/ol1018008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Feldman KS, Ngernmeesri P. Org Lett. 2011;13:5704–5707. doi: 10.1021/ol202535f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Feldman KS, Iyer MR, López CS, Faza ON. J Org Chem. 2008;73:5090–5099. doi: 10.1021/jo8008066. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Feldman KS, Selfridge BR. Heterocycles. 2010;81:117–143. doi: 10.3987/COM-09-11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(Chloride) Guinchard X, Vallee Y, Denis JN. J Org Chem. 2007;72:3972–3975. doi: 10.1021/jo070286r.(amide) Santos LS, Pilli RA, Rawal VH. J Org Chem. 2004;69:1283–1289. doi: 10.1021/jo035165f.
- 9.(a) McCall MT, Hammond GS, Yonemitsu O, Witkop B. J Am Chem Soc. 1970;92:6991–6993. [Google Scholar]; (b) Naruto S, Yonemitsu O. Tetrahedron Lett. 1975;16:3399–3402. [Google Scholar]; (c) Hamada T, Ohmori M, Yonemitsu O. Tetrahedron Lett. 1977;18:1519–1522. [Google Scholar]; (d) Naruto S, Yonemitsu O. Chem Pharm Bull. 1980;28:900–909. [Google Scholar]
- 10.(a) Kobayashi T, Spande TF, Aoyagi H, Witkop B. J Org Chem. 1969;12:636–638. doi: 10.1021/jm00304a017. [DOI] [PubMed] [Google Scholar]; (b) Anderson NG, Lawton RG. Tetrahedron Lett. 1977:1843–1846. [Google Scholar]; (c) Bosch J, Amat M, Sanfeliu E, Miranda MA. Tetrahedron. 1985;41:2557–2566. [Google Scholar]; (d) Klohr SE, Cassady JM. Synth Commun. 1988;18:671–674. [Google Scholar]; (e) Beck AL, Mascal M, Moody CJ, Slawin AMZ, Williams DJ, Coates WJ. J Chem Soc Perkin Trans 1. 1992:797–811. [Google Scholar]; (f) Beck AL, Mascal M, Moody CJ, Coates WJ. J Chem Soc Perkin Trans 1. 1992:813–822. [Google Scholar]; (g) Mascal M, Moody CJ, Slawin AMZ, Williams DJ. J Chem Soc Perkin Trans 1. 1992:823–830. [Google Scholar]; (h) Nagata R, Endo Y, Shudo K. Chem Pharm Bull. 1993;41:369–372. [Google Scholar]; (i) Mascal M, Moody CJ, Morrell AI, Slawin AMZ, Williams DJ. J Am Chem Soc. 1993;115:813–814. [Google Scholar]; (j) Mascal M, Wood IG, Begley MJ, Batsanov AS, Walsgrove T, Slawin AMZ, Williams DJ, Drake AF, Siligardi G. J Chem Soc Perkin Trans 1. 1996:2427–2433. [Google Scholar]; (k) Bennasar ML, Zulaica E, Ramirez A, Bosch J. Heterocycles. 1996;43:1959–1966. [Google Scholar]; (l) Ruchkina EL, Blake AJ, Mascal M. Tetrahedron Lett. 1999;40:8443–8445. [Google Scholar]; (m) Bremner JB, Russell HF, Skelton BW, White AH. Heterocycles. 2000;53:277–296. [Google Scholar]; (n) Mascal M, Modes KV, Durmus A. Angew Chem Int Ed. 2011;50:4445–4446. doi: 10.1002/anie.201006423. [DOI] [PubMed] [Google Scholar]; (o) Qin H, Xu Z, Cui Y, Jia Y. Angew Chem Int Ed. 2011;50:4447–4449. doi: 10.1002/anie.201100495. [DOI] [PubMed] [Google Scholar]
- 11.Tsang W, Hampson RF. J Phys Chem Ref Data. 1986;15:1087–1222.(see pg. 1095) Parkes DA, Quinn CP. J Chem Soc Farad Soc I. 1976;72:1952–1970.Tsang W. J Am Chem Soc. 1985;107:2872–2880.Choo KY, Beadle PC, Piszkiewicz W, Golden DM. Int J Chem Kinet. 1976;8:45–58.
- 12.Aucken CJ, Leeper FJ, Battersby AR. J Chem Soc, Perkin Trans 1. 1997:2099–2109. [Google Scholar]
- 13.(a) Cambridge Crystallographic Data Centre deposition number for 34: CCDC 865924 (b) Cambridge Crystallographic Data Centre deposition number for 62: CCDC 826169. The data can be obtained free from Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif
- 14.(a) Guy A, Lemor A, Doussot J, Lemaire M. Synthesis. 1988:900–902. [Google Scholar]; (b) Magnus P, Lacour J, Weber W. J Am Chem Soc. 1993;115:9347–9348. [Google Scholar]
- 15.Guinchard X, Vallée Y, Denis JN. Org Lett. 2007;9:3761–3764. doi: 10.1021/ol701626m. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaou KC, Chen DYK, Huang X, Ling T, Bella M, Snyder SA. J Am Chem Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i. [DOI] [PubMed] [Google Scholar]
- 17.(a) Giuliano RM, Deisenroth TW. J Carb Chem. 1987;6:295–299. [Google Scholar]; (b) Wood JL, White RD. Org Lett. 2001;3:1825–1827. doi: 10.1021/ol015828k. [DOI] [PubMed] [Google Scholar]
- 18.(a) Tanino H, Nakata T, Kaneka T, Kishi Y. J Am Chem Soc. 1977;99:2818–2819. doi: 10.1021/ja00450a079. [DOI] [PubMed] [Google Scholar]; (b) Jacobi PA, Martinelli MJ, Slovenko P. J Am Chem Soc. 1984;106:5594–5595. [Google Scholar]
- 19.Feldman KS, Skoumbourdis AP, Fodor MD. J Org Chem. 2007;72:8076–8086. doi: 10.1021/jo701487j. [DOI] [PubMed] [Google Scholar]
- 20.Gerritsma DA, Brindle ID, Jones TRB, Capretta A. J Labeled Cmpds Radiopharm. 2003;46:243–253. [Google Scholar]
- 21.Dobson D, Todd A, Gilmore J. Synth Commun. 1991;21:611–617. [Google Scholar]