Abstract
Objective
Neuronal damage is the morphological substrate of persisting neurological disability. Neurofilaments (Nf) are cytoskeletal proteins of neurons and their release into cerebrospinal fluid has shown encouraging results as a biomarker for neurodegeneration. This study aimed to validate the quantification of the Nf light chain (NfL) in blood samples, as a biofluid source easily accessible for longitudinal studies.
Methods
We developed and applied a highly sensitive electrochemiluminescence (ECL) based immunoassay for quantification of NfL in blood and CSF.
Results
Patients with Alzheimer’s disease (AD) (30.8 pg/ml, n=20), Guillain-Barré-syndrome (GBS) (79.4 pg/ml, n=19) or amyotrophic lateral sclerosis (ALS) (95.4 pg/ml, n=46) had higher serum NfL values than a control group of neurological patients without evidence of structural CNS damage (control patients, CP) (4.4 pg/ml, n=68, p<0.0001 for each comparison, p=0.002 for AD patients) and healthy controls (HC) (3.3 pg/ml, n=67, p<0.0001). Similar differences were seen in corresponding CSF samples. CSF and serum levels correlated in AD (r=0.48, p=0.033), GBS (r=0.79, p<0.0001) and ALS (r=0.70, p<0.0001), but not in CP (r=0.11, p=0.3739). The sensitivity and specificity of serum NfL for separating ALS from healthy controls was 91.3% and 91.0%.
Conclusions
We developed and validated a novel ECL based sandwich immunoassay for the NfL protein in serum (NfLUmea47:3); levels in ALS were more than 20-fold higher than in controls. Our data supports further longitudinal studies of serum NfL in neurodegenerative diseases as a potential biomarker of on-going disease progression, and as a potential surrogate to quantify effects of neuroprotective drugs in clinical trials.
Introduction
Neurofilaments (Nf) are highly specific major structural proteins of neurons, consisting predominantly of four subunits: Nf light (NfL), Nf medium (NfM) and Nf heavy (NfH) chain and alpha-internexin [1]. Nf are released in significant quantity following axonal damage or neuronal degeneration. Disruption to the axonal membrane releases Nf into the interstitial fluid and eventually into cerebrospinal fluid (CSF) and blood. Therefore, blood Nf levels could be useful for both predicting and monitoring disease progression and for assessing the efficacy and/or toxicity of future neuroprotective treatment strategies.
Several previous studies have demonstrated the presence of NfH and NfL in CSF, which has been assumed to reflect brain pathology more accurately than the peripheral blood compartment [2–12]. However, obtaining longitudinal CSF samples is considered too invasive outside the clinical trial arena, precluding the broader clinical use of Nf. In contrast to CSF, serial blood samples can readily be collected, hence reliable quantification of NfL in blood would be a major stride towards a biomarker of the course of neurodegeneration. Several reports have suggested peripheral blood levels of NfH as a potential marker of neurodegeneration [13–22]. In contrast to this, there is only one recent study investigating serum NfL; this paper examined the relationship between serum NfL and neurological outcome following cardiac arrest [23].
A commercially available ELISA (UmanDiagnostics NF-light® assay) uses two highly specific, non-competing monoclonal antibodies (47:3 and 2:1) to quantify soluble NfL in CSF samples but it cannot in its present form be used for analysis of blood samples [24].
We recently compared our highly sensitive electrochemiluminescence (ECL)-based solid-phase sandwich immunoassay for NfHSMI35 (adhering to a previously proposed nomenclature, the soluble fraction of NfH measured is indicated with the capture antibody in the superscript [8]) with NfL, determined by the NF-light® assay in CSF samples [6]. Importantly, the conventional ELISA showed higher sensitivity compared with the ECL-NfHSMI35 immunoassay [25].
The aim of this study was to develop and validate (both analytically and clinically) a sensitive ECL-based NfL assay suitable for the quantification of NfL in serum at concentrations relevant to clinical settings.
Materials and Methods
Antibodies and chemicals
The following mouse antibodies were used: Capture monoclonal antibody (mAB) 47:3, and the biotinylated detector mAB 2:1 [10,24]. MSD SULFO-TAGTM labelled streptavidin was used as detection reagent to generate electrochemiluminescence (MSD, Gaithersburg, MD). Bovine serum albumin (BSA), ethylenediaminetetraacetic disodium salt (EDTA), NaCl, phosphate buffered saline, pH 7.5 (PBS), tris base and Tween 20 were of analytical grade (Sigma-Aldrich, Saint Louis, MO).
Standards
Bovine lyophilized NfL was obtained from UmanDiagnostics (N Norgren). Standards were diluted in tris buffered saline (TBS) containing 1% BSA, 0.1% Tween 20, pH 7.5 and ranged from 0 to 10,000 pg/ml. Batch prepared standards were stored at -80°C.
Patients and Control persons and ethics statement
Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the study was approved by the Common Institutional Review Board of the Cantons of Basel. Paired CSF and serum samples were collected during routine diagnostic investigations as indicated by the treating physicians.
Samples were collected and processed at room temperature within two hours. Serum samples were spun at 2,000 g, CSF samples at 400 g at room temperature for 10 minutes, aliquoted in polypropylene tubes and stored at -80°C.
Serum samples from 67 healthy control subjects (HC) were included in the study. For ethical reasons CSF samples were not available from these subjects. The group of control patients (CP) (n=68) consisted of patients who, based on extensive diagnostic evaluation had no objective clinical, structural (cranial magnetic resonance imaging, MRI), laboratory (CSF analysis) or functional (electroencephalography, EEG) deficit. These patients suffered from tension type headache (n=21), lower back pain (n=7), psychiatric disorders (n=26) or miscellaneous non-specific symptoms for which no neurological explanation could be found (n=14). From two of these patients there was not enough CSF left for further analysis. In addition, 49 patients with probable or definite ALS (for three no serum and for one no CSF sample was available) [26], probable Alzheimer’s disease (AD) [27], or a Guillain-Barré syndrome (GBS) (for one no serum sample was available) (n=20 each) were included (Table 1). The analyst was blinded to all clinical data.
Table 1. Demographic characteristics of healthy controls, control patients and patients.
| HC | CP | AD | GBS | ALS | |
|---|---|---|---|---|---|
| N | 67 | 68 | 20 | 20 | 49 |
| Females (n [%])a | 38 (56.7) | 41 (60.3) | 13 (65.0) | 10 (50.0) | 14 (28.6) |
| Age [years, median (IQR)]b | 35.0 (28.0-42.0) | 38.3 (27.5-46.4) | 72.5 (70.1-80.2) | 59.6 (39.1-71.7) | 62.7 (54.5-70.7) |
aThere were less female ALS patients compared to HC (p=0.004), CP (p=0.001) and AD (p=0.007).
bHC and CP, were younger compared to AD, GBS and ALS (p<0.0001, respectively). HC: Healthy controls; CP: Control patients; AD: Alzheimer’s disease; GBS: Guillain-Barré syndrome; ALS: Amyotrophic lateral sclerosis; IQR: Interquartile range.
Analytical procedure
The 96-well plates (Multi-Array® plates, Meso Scale Discovery, Gaithersburg, MD) include integrated screen-printed carbon ink electrodes on the bottom of the wells. Coating was done overnight with 30 µl of capture antibody (mAB 47:3,1.25 µg/ml) diluted in PBS (pH 7.4) at 4°C. All following incubation steps were done on a plate shaker (800 rpm) and were preceded by three wash steps with 200 µl of TBS, containing 0.1% Tween 20 (pH 7.5) per well. Non-specific binding sites were blocked with 100 µl of TBS, containing 3% BSA, per well for 1h. After washing, 25 µl of TBS containing 1% BSA and 0.1% Tween 20 was added as sample diluent to each well. 25 µl of standard, control or serum/CSF sample was then added in duplicate and the plate incubated at room temperature (RT) for 2h. After washing, 25 µl of the secondary antibody (mAB 2:1, 0.5 µg/ml) diluted in TBS containing 1% BSA and 0.1% Tween 20 was added to each well and the plate incubated for 1 h at RT. After washing, MSD SULFO-TAGTM labelled streptavidin (0.25 µg/ml), diluted in TBS containing 1% BSA and 0.1% Tween 20, was added and incubated for 1h at RT. Following a final wash, 150 µl of ECL read buffer (MSD) diluted 1:2 with distilled water was added and the ECL signal, detected by photodetectors, measured using the MSD Sector Imager 2400 plate reader. A four-parameter weighted logistic fit curve was generated, sample concentrations extrapolated and analysed using the Discovery Workbench 3.0 software (MSD). If required, samples were appropriately diluted to fall in the range of the standard curve. Non measurable NfL samples were reported as 0 pg/ml.
Statistical analysis
Continuous variables were described by their median and interquartile range (IQR), and categorical variables by numbers and percentages. Comparison of demographic data was performed using the Kruskal-Wallis test, and pairwise post-hoc comparisons using Dunn’s post-test or chi-square test as appropriate. Serum and CSF levels of NfL were log-transformed to achieve a normal distribution for subsequent analysis. To control for age as a potential confounding factor, an analysis of covariance with age as covariate and disease group as fixed factor, was performed [7]. Group-specific levels of NfL were expressed as geometric means with 95%-confidence intervals. For log-normal variables, the geometric mean equals the median. Correlations were computed by determining the Spearman rank correlation coefficient (r). The cut-off (upper reference range of normal) providing optimal sensitivity and specificity in distinguishing ALS from HC by serum NfL was defined by receiver operating characteristic (ROC) curve analysis. Proportions above and below this cut-off were compared with the Chi-Square test. A two-sided p-value < 0.05 was considered as significant. P-values of post-hoc comparisons were adjusted using a Bonferroni correction. All statistical analyses and graphs were performed using SPSS (Version 15.0 SPSS, Chicago, IL) and Graph Pad Prism 5.02 for Windows (GraphPad Software, San Diego, CA).
Results
Reproducibility of the standard curve
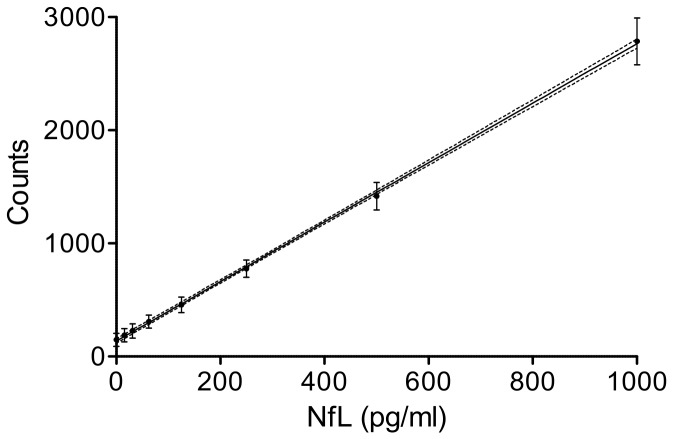
Figure 1 shows the mean raw counts of 20 consecutive standard curves in the range of 0-1,000 pg/ml and the resulting regression line. Individual standard curves showed a high degree of linearity (R2 = 0.99) (Figure 1).
Figure 1. Reproducibility of the standard curve.
Reproducibility of 20 consecutive standard curves. The graph shows the mean counts (dots) ± SD (bars), linear regression line and 5% and 95% confidence interval curves (broken lines) (R2=0.99).
Precision and accuracy
Reproducibility (intra-assay variability) and repeatability (inter-assay variability) of the assay was evaluated with native serum samples in 10 consecutive assays on independent days. In four independent samples of native serum the mean coefficients of variation (CV) of duplicates (intra-assay precision) for NfLUmea47:3 were 4.9% (12.1 pg/ml, sample 1), 5.5% (39.6 pg/ml, sample 2), 4.1% (83.1 pg/ml, sample 3) and 3.8% (103 pg/ml, sample 4, average: 4.6%). In CSF the mean intra-assay CVs were 6.0% (569 pg/ml, sample 1), 6.4% (3,645 pg/ml, sample 2), 2.7% (7,501 pg/ml, sample 3) and 6.8% (12,762 pg/ml, sample 4) averaging at 5.5%. Inter-assay CVs for serum were 23.6% (sample 1), 16.9% (sample 2), 8.5% (sample 3), and 10.9% (sample 4, average: 15.0%). In CSF inter-assay CVs were 10.3% (sample 1), 10.4% (sample 2), 6.7% (sample 3) and 11.7% (sample 4, average: 9.8%).
Recovery rates were tested in 6 serum samples from healthy volunteers. Recovery of NfL (serum spiked with 50 pg/ml of HPLC purified bovine NfL) was 72% and 114%. For serum spiked with 100 pg/ml of NfL it was 81% and 96%, and for 1,000 pg/ml of NfL recovery was 82% and 116%.
Analytical sensitivity and stability of the analyte
Sensitivity (lowest standard above blank) was calculated as blank signal plus three standard deviations (SD) from 32 assays. The mean blank signal was 138 counts (SD 20.9 counts). The mean signal of the lowest standard (15.6 pg/ml) was 184.5 counts (SD 23.2): accordingly analytical sensitivity was defined to be 15.6 pg/ml. We tested the stability of NfL at room temperature (RT), 4 °C and compared this to samples stored at -80 °C. Four aliquoted serum samples were frozen at -80 °C. The aliquots were thawed on days 0, 3 hours before measurement, days 1, 4 and 8 and stored at RT or 4 °C until analysis. The measured signals were normalised to the signal of the day 0. There was no significant change in signal in samples stored at RT and at 4 °C (RT: day 8: 1.06 ± 0.08 (mean ± SD), p = 0.4063 and 4 °C: day 8: 1.01 ± 0.09, p = 0.1721). Four serum samples were analysed for stability during freeze-thawing cycles. The samples underwent 1, 2, 3, 4 or 5 freeze-thawing cycles and the signal was normalised to the sample freeze-thawed once, without any relevant effect of freeze-thawing on the measured signals (5 freeze-thawing cycles: 1.03 ± 0.03, p = 0.5076).
Parallelism
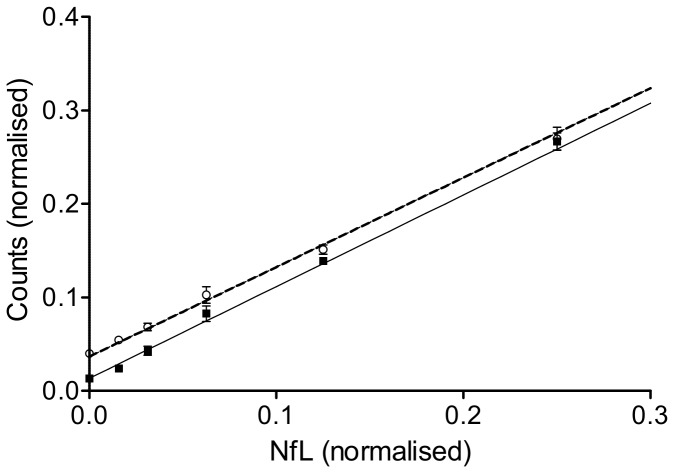
We studied parallelism between standards and samples by reciprocal dilutions of three serum samples and three standard curves. The obtained signals were normalised to the highest value within this series (100%). The parallel relationship is demonstrated in Figure 2, suggesting the absence of aggregate formation or endogenous binding between NfL and other blood substrates [28] (Figure 2).
Figure 2. Parallelism between standards and serum dilutions.
Parallelism for NfL between standards (open line, open dots) and serum (closed line, black squares). The linear regression lines, mean (open dots or black squares) and ±SD are shown.
Reference populations
NfL was determined in serum of 67 HC (56.7% females, median age 35.0 years) and in serum of 68 and CSF of 66 CP (60.3% female, median age: 38.3 years) (Table 1). Serum levels between HC (3.3 pg/ml, 2.0-5.3) and CP (4.4 pg/ml, 2.4-8.1) did not differ (p=1.0) and did not correlate with either age or gender. Conversely, CSF levels in CP correlated with age (r=0.68, p<0.0001).
Neurological disease population
A. Serum
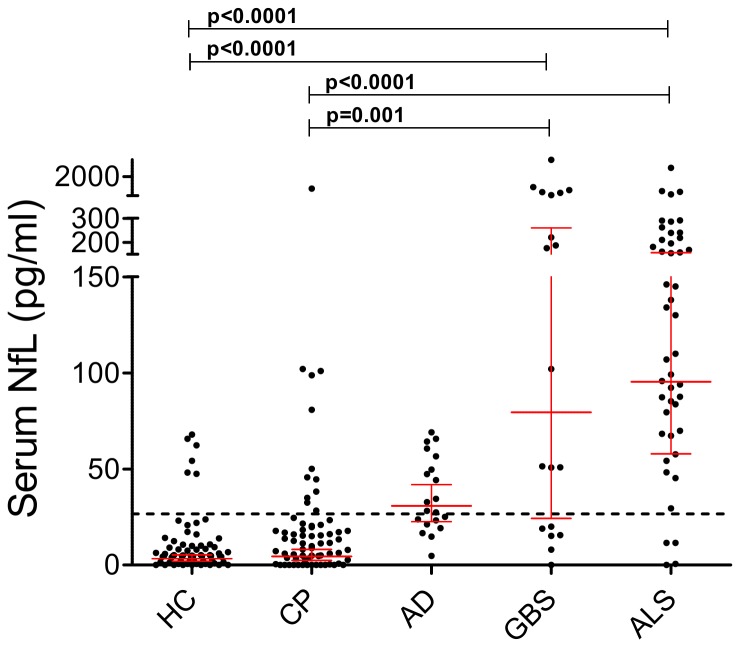
AD (30.8 pg/ml, 22.6-41.9), GBS (79.4 pg/ml, 24.3-259.6) and ALS (95.4 pg/ml, 57.9-157.0) had higher serum NfL levels compared with HC and CP (AD versus CP: p=0.002 all other comparisons versus HC and CP: p<0.0001). NfL levels correlated with age in GBS (r=0.48, p=0.038) and ALS (r=0.30, p=0.04). In the age-corrected group comparisons versus HC and CP differences remained significant, except for the comparisons against AD (Figure 3).
Figure 3. Serum NfL levels in the two reference groups (healthy controls, HC and control patients, CP) and neurological disease groups.
Patients with a Guillain-Barré syndrome (GBS) (79.4 pg/ml) or Amyotrophic lateral sclerosis (ALS) (95.4 pg/ml) had higher values compared with HC (3.3 pg/ml; p<0.0001, respectively) and CP (4.4 pg/ml, p<0.0001 and p=0.001). Significances for comparisons between patients with Alzheimer’s disease (AD) and HC (p<0.0001) and AD and CP (p=0.002) were lost after age corrections. The horizontal dotted line represents the upper reference range (cut-off value) of 26.6 pg/ml. Geometric mean and 95% CI are displayed. Dots represent individual samples. P-values are adjusted for age and corrected by Bonferroni method.
A cut-off level of 26.6 pg/ml (Figure 3) for serum NfL resulted in a sensitivity of 91.3% and a specificity of 91.0% for differentiating ALS versus HC. A higher proportion (p<0.0001 for all comparisons) of patients had serum NfL values above this cut-off: 16/20 (80.0%) in AD, 13/19 (68.4%) in GBS, 42/46 (91.3%) in ALS, compared to HC (6/67, 9.0%).
B. CSF
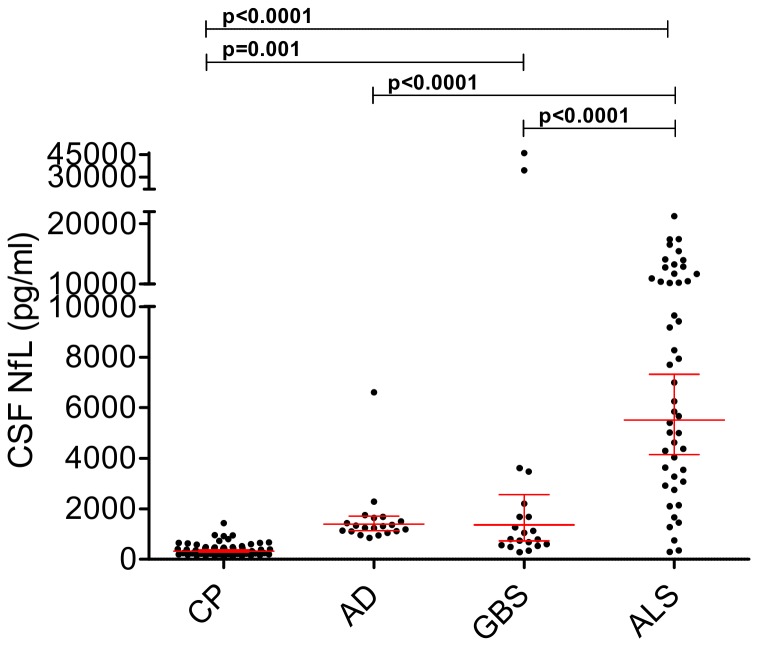
NfL levels in AD (1396 pg/ml, 1139-1711), GBS (1361 pg/ml, 726-2554) and ALS (5513 pg/ml, 4151-7323) were higher than in CP (324 pg/ml, 282-372, p<0.0001 for all), and CSF NfL concentrations in ALS were higher than in AD and GBS (p<0.0001, respectively).
Similar to the serum results, CSF levels of NfL correlated with age in GBS (r=0.65, p=0.002) and ALS (r=0.30, p=0.048). After correction for age, a significant difference remained between GBS (p=0.001) and ALS (p<0.0001), but not AD (p=1.0) versus CP. Similarly, we confirmed the higher levels in ALS as compared to AD and GBS (p<0.0001, for both comparisons) (Figure 4).
Figure 4. CSF NfL levels in the reference group (control patients, CP) and neurological disease cohorts.
Patients with Amyotrophic lateral sclerosis (ALS) (5513 pg/ml) or a Guillain-Barré syndrome (GBS) (1361 pg/ml) had higher levels than CP (324 pg/ml, p<0.0001 and p=0.001). In addition ALS had higher levels than patients with Alzheimer’s disease (AD) (1361 pg/ml) and GBS (p<0.0001, respectively). Geometric mean and 95% CI are displayed. Dots represent individual samples. P-values are adjusted for age and corrected by Bonferroni method.
C. CSF – serum relationship
Overall geometric mean levels in CSF (1,142 pg/ml, 906-1,439) were 96.8-fold higher than in serum (11.8 pg/ml, 8.5-16.5, p<0.0001; fold-increase in CSF versus serum: CP: 73.6, AD: 45.3, GBS: 17.1, ALS: 57.8, p<0.0001, respectively).
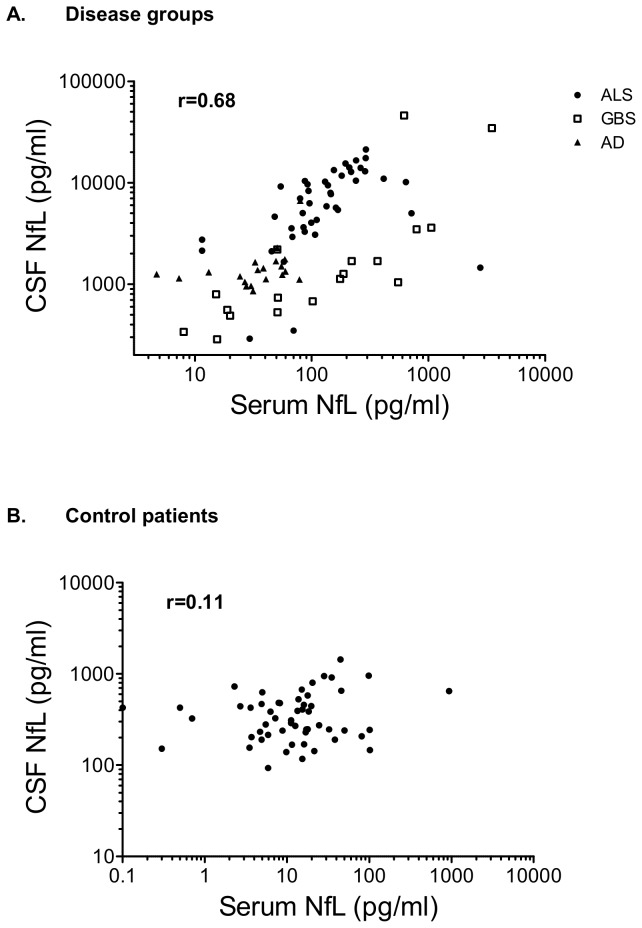
Serum and CSF measurements of NfL correlated in the disease groups (Figure 5a): AD (r=0.48, p=0.033), GBS (r=0.79, p<0.0001) and ALS (r=0.70, p<0.0001), conversely this was not seen in CP (r=0.11, p=0.3739) (Figure 5b).
Figure 5. Correlation of serum and CSF NfL measurements.
Serum and CSF measurements of NfL correlated in the disease groups (A): Alzheimer’s disease (AD) (r=0.48, p=0.033), Guillain-Barré syndrome (GBS) (r=0.79, p<0.0001) and Amyotrophic lateral sclerosis (ALS) (r=0.70, p<0.0001): overall: r=0.68, p<0.001. Conversely this was not seen in the control patients (CP) (r=0.11, p=0.3739) (B).
Discussion
A highly sensitive method for the detection of a clinically relevant biomarker of neurodegeneration has been developed. Importantly, our method allows us to make use of readily available longitudinal patient blood samples, instead of being restricted to ethically difficult to obtain CSF samples. One potential clinical application for serum NfL levels is demonstrated by the diagnostic sensitivity of 91.3% for ALS, a rapidly progressive neurodegenerative disease [29,30].
We present the first ECL based solid phase immunoassay for the NfL protein in blood based on two non-competitive, monoclonal antibodies. These antibodies have been widely used and validated in a commercial ELISA for CSF measurements of NfL (NF-light® assay) [5,24,31]. NfL is considered to represent the most abundant and also most soluble Nf subunit [1].
The optimised ECL-NfL assay protocol proved to be highly accurate (intra-assay CV < 6%, inter-assay CV < 24%), sensitive (sensitivity 15.6 pg/ml) and demonstrated linearity and parallelism (Figures 1 and 2) over a wide analytical range (15.6-10,000 pg/ml). In addition we found NfLUmea47:3 to be stable in serum [25]. This is relevant for a potential value to monitor drug effects by serum NfL in ALS where Nf aggregate formation is a key pathological finding [28]. In contrast to NfHSMI34 and NfHSMI35, no such aggregates were found for NfLUmea47:3, essentially overcoming the limitations of the Nf “hook effect” (matrix effect) [28]. In this context a more than 20-fold elevation of serum NfLUmea47:3 levels in ALS compared to HC cannot be overestimated. Interestingly, the fold-differences between disease groups and CP for serum NfLUmea47:3 was higher compared to the respective CSF levels (serum/CSF: ALS: 21.7/17.0; AD: 7.0/4.3 and GBS: 18.0/4,2).
An important and unresolved question is whether or not there is a relevant correlation between Nf levels and age. If present, such a relationship would require age dependent cut-off values [7]. A major limitation to all studies in this field to date [6,7,11,32–34] is that they have not been powered to investigate this potential correlation in the CSF, due to lack of samples from a sufficiently large healthy control group across all age categories. Again, the availability of the present method to investigate this in readily available serum samples is highly relevant. Importantly, we did not find a correlation between serum NfLUmea47:3 levels and age in either HC or CP. Whether or not a possible relationship with age exists for ALS, GBS or AD is questionable, as older patients are often more severely affected and higher age is the most important prognostic factor in either condition and therefore not independent of the neurodegeneration related release of NfLUmea47:3.
The absence of the Nf hook-effect is an important analytical advantage for quantification of the ECL based serum NfLUmea47:3 assay compared to the serum NfHSMI34 and NfHSMI35 ELISA, as there is no necessity for a time-consuming pre-incubation step with urea [28]. Given the important prognostic information that NfH levels provide on a number of clinical conditions, we anticipate NfLUmea47:3 to be relevant for future studies. Serum NfLUmea47:3 bears the potential for predicting disease progression in ALS [15,35,36] and MS [17,18], detecting particularly disabling acute episodes of optic neuritis or relapses in MS [16], identifying primary and secondary brain damage in stroke [22,37], SAH [13], TBI [19,38] and in the emerging concept of chronic traumatic encephalopathy (CTE) [20,38]. Like serum NfHSMI35, serum NfLUmea47:3 may also be exploited as a safety biomarker for recognising neurotoxicity [21]. There is already data that serum NfL levels are of comparable prognostic value to NfHSMI35 levels following cardiac arrest [19,23]. Of note there were no controls and no analytical validation data from the NfL assay used in one study [23].
Similar to our previous findings for NfHSMI35 in CSF, a bimodal distribution of serum NfL levels was seen in patients with GBS [6]. There are no previous studies on Nf in blood from patients with GBS. We have earlier shown that CSF levels of NfH are higher in patients with evidence of axonal damage compared to those with purely demyelinating GBS, with CSF NfH levels predictive of outcome [9,39]. Future prospective studies incorporating detailed longitudinal clinical and electrophysiological assessments, and sampling are clearly warranted. These studies will also shed light on the role of proximal versus more distal axonotmesis and secondary axonal peripheral degeneration and the relationship of increased blood NfL levels [40].
Blood levels of Nf have similarly not been investigated in patients with dementia. In our study the differences in serum and CSF NfL levels in AD compared to HC and CP (p<0.0001 and p=0.002) lost significance after age and Bonferroni correction. This is in line with previous investigations where CSF NfHSMI35 levels were increased, but diagnostic sensitivity, and hence potential for clinical use of NfH SMI35 was not superior to that of the benchmark biomarkers total tau, phospho tau, or amyloid beta 1-42 [41,42]. To explore these questions further we are currently expanding our database in a larger and well characterised cohort of AD and control patients.
In summary, we developed and validated a sensitive and reliable assay for measurements of NfL in human blood samples. For the first time, we were able to demonstrate increased blood NfL levels in patients with ALS and GBS. These differences were more pronounced for the ECL-NfLUmea 47:3 assay than those reported in ALS for NfH in previous reports [15,36]. Our data support further studies of serum NfL in well-defined longitudinal cohorts of neurodegenerative diseases. These studies will show if serum NfL measurements can be used as a biomarker for disease progression and as an outcome measure in clinical trials.
Acknowledgments
J. Kuhle’s work is supported by an ECTRIMS Research Fellowship Programme and by the “Forschungsfonds” of the University of Basel, Switzerland. R. Dobson is funded by an ABN/MS Society of Great Britain Clinical Research Fellowship.
Funding Statement
The authors have no support or funding to report.
References
- 1. Petzold A (2005) Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233: 183-198. doi:10.1016/j.jns.2005.03.015. PubMed: 15896809. [DOI] [PubMed] [Google Scholar]
- 2. Brettschneider J, Petzold A, Junker A, Tumani H (2006) Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult Scler 12: 143-148. doi:10.1191/135248506ms1263oa. PubMed: 16629417. [DOI] [PubMed] [Google Scholar]
- 3. Deisenhammer F, Egg R, Giovannoni G, Hemmer B, Petzold A et al. (2009) EFNS guidelines on disease-specific CSF investigations. Eur J Neurol 16: 760-770. doi:10.1111/j.1468-1331.2009.02595.x. PubMed: 19475759. [DOI] [PubMed] [Google Scholar]
- 4. Giovannoni G (2010) Cerebrospinal fluid neurofilament: the biomarker that will resuscitate the 'Spinal Tap'. Mult Scler 16: 285-286. 16/3/285 [PII]; doi:10.1177/1352458510361358 [DOI] [PubMed] [Google Scholar]
- 5. Gunnarsson M, Malmeström C, Axelsson M, Sundström P, Dahle C et al. (2011) Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 69: 83-89. doi:10.1002/ana.22247. PubMed: 21280078. [DOI] [PubMed] [Google Scholar]
- 6. Kuhle J, Regeniter A, Leppert D, Mehling M, Kappos L et al. (2010) A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol 220: 114-119. S0165-. doi:10.1016/j.jneuroim.2010.01.004. PubMed: 20117845. [DOI] [PubMed] [Google Scholar]
- 7. Kuhle J, Leppert D, Petzold A, Regeniter A, Schindler C et al. (2011) Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 76: 1206-1213. doi:10.1212/WNL.0b013e31821432ff. PubMed: 21346223. [DOI] [PubMed] [Google Scholar]
- 8. Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ (2003) A specific ELISA for measuring neurofilament heavy chain phosphoforms. J Immunol Methods 278: 179-190. doi:10.1016/S0022-1759(03)00189-3. PubMed: 12957406. [DOI] [PubMed] [Google Scholar]
- 9. Petzold A, Brettschneider J, Jin K, Keir G, Murray NM et al. (2009) CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain-Barre syndrome. Muscle Nerve 40: 42-49. doi:10.1002/mus.21239. PubMed: 19533642. [DOI] [PubMed] [Google Scholar]
- 10. Norgren N, Rosengren L, Stigbrand T (2003) Elevated neurofilament levels in neurological diseases. Brain Res 987: 25-31. doi:10.1016/S0006-8993(03)03219-0. PubMed: 14499942. [DOI] [PubMed] [Google Scholar]
- 11. Teunissen CE, Iacobaeus E, Khademi M, Brundin L, Norgren N et al. (2009) Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 72: 1322-1329. doi:10.1212/WNL.0b013e3181a0fe3f. PubMed: 19365053. [DOI] [PubMed] [Google Scholar]
- 12. Norgren N, Sundström P, Svenningsson A, Rosengren L, Stigbrand T et al. (2004) Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 63: 1586-1590. doi:10.1212/01.WNL.0000142988.49341.D1. PubMed: 15534240. 63/9/1586 [PII]. [DOI] [PubMed] [Google Scholar]
- 13. Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G (2008) Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 28: 1261-1271. doi:10.1038/jcbfm.2008.12. PubMed: 18319731. [DOI] [PubMed] [Google Scholar]
- 14. Ganesalingam J, An J, Shaw CE, Shaw G, Lacomis D et al. (2011) Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem 117: 528-537. doi:10.1111/j.1471-4159.2011.07224.x. PubMed: 21418221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boylan KB, Glass JD, Crook JE, Yang C, Thomas CS et al. (2013) Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg, Psychiatry 84: 467-472. doi:10.1136/jnnp-2012-303768. PubMed: 23117489. [DOI] [PubMed] [Google Scholar]
- 16. Petzold A, Rejdak K, Plant GT (2004) Axonal degeneration and inflammation in acute optic neuritis. J Neurol Neurosurg, Psychiatry 75: 1178-1180. doi:10.1136/jnnp.2003.017236. PubMed: 15258226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petzold A, Brassat D, Mas P, Rejdak K, Keir G et al. (2004) Treatment response in relation to inflammatory and axonal surrogate marker in multiple sclerosis. Mult Scler 10: 281-283. doi:10.1191/1352458504ms1021sr. PubMed: 15222692. [DOI] [PubMed] [Google Scholar]
- 18. Petzold A, Plant GT (2012) The diagnostic and prognostic value of neurofilament heavy chain levels in immune-mediated optic neuropathies. Mult Scler Int, 2012: 217802. doi:10.1155/2012/217802. PubMed: 23316360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rundgren M, Friberg H, Cronberg T, Romner B, Petzold A et al. (2012) Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Crit Care 16: R45 PubMed: 22410303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tisdall M, Petzold A (2012) Comment on "chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model". Sci Transl Med 4: 157le8. doi:10.1126/scitranslmed.3004403. [DOI] [PubMed] [Google Scholar]
- 21. Petzold A, Mondria T, Kuhle J, Rocca MA, Cornelissen J et al. (2010) Evidence for acute neurotoxicity after chemotherapy. Ann Neurol 68: 806-815. doi:10.1002/ana.22169. PubMed: 21194151. [DOI] [PubMed] [Google Scholar]
- 22. Singh P, Yan J, Hull R, Read S, O’Sullivan J et al. (2011) Levels of phosphorylated axonal neurofilament subunit H (pNfH) are increased in acute ischemic stroke. J Neurol Sci 304: 117-121. doi:10.1016/j.jns.2011.01.025. PubMed: 21349546. [DOI] [PubMed] [Google Scholar]
- 23. Rana OR, Schroder JW, Baukloh JK, Saygili E, Mischke K et al. (2012) Neurofilament light chain as an early and sensitive predictor of long-term neurological outcome in patients after cardiac arrest. Int J Cardiol: S0167-S5273. doi:10.1016/j.ijcard.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 24. Norgren N, Karlsson JE, Rosengren L, Stigbrand T (2002) Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics 21: 53-59. doi:10.1089/15368590252917647. PubMed: 11991817. [DOI] [PubMed] [Google Scholar]
- 25. Kuhle J, Plattner K, Bestwick JP, Lindberg RL, Ramagopalan SV et al. (2013) A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler; doi:10.1177/1352458513482374. [DOI] [PubMed] [Google Scholar]
- 26. Ross MA, Miller RG, Berchert L, Parry G, Barohn RJ et al. (1998) Toward earlier diagnosis of amyotrophic lateral sclerosis: revised criteria. rhCNTF ALS Study Group. Neurology 50: 768-772. doi:10.1212/WNL.50.3.768. PubMed: 9521272. [DOI] [PubMed] [Google Scholar]
- 27. McKhann G, Drachman D, Folstein M, Katzman R, Price D et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: 939-944. doi:10.1212/WNL.34.7.939. PubMed: 6610841. [DOI] [PubMed] [Google Scholar]
- 28. Lu CH, Kalmar B, Malaspina A, Greensmith L, Petzold A (2011) A method to solubilise protein aggregates for immunoassay quantification which overcomes the neurofilament "hook" effect. J Neurosci Methods 195: 143-150. S0165- doi:10.1016/j.jneumeth.2010.11.026. PubMed: 21134399 [DOI] [PubMed] [Google Scholar]
- 29. Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H (2006) Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66: 852-856. doi:10.1212/01.wnl.0000203120.85850.54. PubMed: 16567701. [DOI] [PubMed] [Google Scholar]
- 30. Otto M, Bowser R, Turner M, Berry J, Brettschneider J et al. (2012) Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph Lateral Scler 13: 1-10. doi:10.3109/17482968.2011.627589. PubMed: 22214350. [DOI] [PubMed] [Google Scholar]
- 31. Norgren N, Edelstam A, Stigbrand T (2005) Cerebrospinal fluid levels of neurofilament light in chronic experimental autoimmune encephalomyelitis. Brain. Res Bull 67: 264-268. doi:10.1016/j.brainresbull.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 32. Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C (1996) Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem 67: 2013-2018. PubMed: 8863508. [DOI] [PubMed] [Google Scholar]
- 33. Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM (2007) Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur J Neurol 14: 1329-1333. doi:10.1111/j.1468-1331.2007.01972.x. PubMed: 17903209. [DOI] [PubMed] [Google Scholar]
- 34. Khalil M, Enzinger C, Langkammer C, Ropele S, Mader A et al. (2012) CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult Scler, 19 1352458512458010 PubMed: 22917689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw G, Yang C, Ellis R, Anderson K, Parker MJ et al. (2005) Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun 336: 1268-1277. doi:10.1016/j.bbrc.2005.08.252. PubMed: 16176808. [DOI] [PubMed] [Google Scholar]
- 36. Boylan K, Yang C, Crook J, Overstreet K, Heckman M et al. (2009) Immunoreactivity of the phosphorylated axonal neurofilament H subunit (pNF-H) in blood of ALS model rodents and ALS patients: evaluation of blood pNF-H as a potential ALS biomarker. J Neurochem 111: 1182-1191. doi:10.1111/j.1471-4159.2009.06386.x. PubMed: 19765193. [DOI] [PubMed] [Google Scholar]
- 37. Sellner J, Patel A, Dassan P, Brown MM, Petzold A (2011) Hyperacute detection of neurofilament heavy chain in serum following stroke: a transient sign. Neurochem Res 36: 2287-2291. doi:10.1007/s11064-011-0553-8. PubMed: 21792676. [DOI] [PubMed] [Google Scholar]
- 38. Blennow K, Hardy J, Zetterberg H (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76: 886-899. doi:10.1016/j.neuron.2012.11.021. PubMed: 23217738. [DOI] [PubMed] [Google Scholar]
- 39. Petzold A, Hinds N, Murray NM, Hirsch NP, Grant D et al. (2006) CSF neurofilament levels: a potential prognostic marker in Guillain-Barre syndrome. Neurology 67: 1071-1073. doi:10.1212/01.wnl.0000237334.69665.92. PubMed: 17000982. [DOI] [PubMed] [Google Scholar]
- 40. Dujmovic I, Lunn MP, Reilly MM, Petzold A (2012) Serial cerebrospinal fluid neurofilament heavy chain levels in severe Guillain-Barre syndrome. Muscle Nerve. doi:10.1002/mus.23752. [DOI] [PubMed] [Google Scholar]
- 41. Brettschneider J, Petzold A, Schottle D, Claus A, Riepe M et al. (2006) The neurofilament heavy chain (NfH) in the cerebrospinal fluid diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord 21: 291-295. doi:10.1159/000091436. PubMed: 16484807. [DOI] [PubMed] [Google Scholar]
- 42. Petzold A, Keir G, Warren J, Fox N, Rossor MN (2007) A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neurodegener Dis 4: 185-194. doi:10.1159/000101843. PubMed: 17596713. [DOI] [PubMed] [Google Scholar]