Abstract
The homeodomain transcription factor Orthopedia (Otp) is an important regulator for specification of defined subsets of neuroendocrine cells and dopaminergic neurons in vertebrates. In zebrafish, two paralogous otp genes, otpa and otpb, are present in the genome. Neither complete loss of Otp activity nor differential contributions of Otpa and Otpb to specification of defined neuronal populations have been analyzed in detail. We characterized zebrafish embryos and early larvae mutant for null alleles of otpa, otpb, or both genes to determine their individual contributions to the specification of th expressing dopaminergic neuronal populations as well as of crh, oxt, avp, trh or sst1.1 expressing neuroendocrine cells. otpa mutant larvae show an almost complete reduction of ventral diencephalic dopaminergic neurons, as reported previously. A small reduction in the number of trh cells in the preoptic region is detectable in otpa mutants, but no significant loss of crh, oxt and avp preoptic neuroendocrine cells. otpb single mutant larvae do not display a reduction in dopaminergic neurons or neuroendocrine cells in the otp expressing regions. In contrast, in otpa and otpb double mutant larvae specific groups of dopaminergic neurons as well as of crh, oxt, avp, trh and sst1.1-expressing neuroendocrine cells are completely lost. These observations suggest that the requirement for otpa and otpb function during development of the larval diencephalon is partially redundant. During evolutionary diversification of the paralogous otp genes, otpa maintained the prominent role in ventral diencephalic dopaminergic and neuroendocrine cell specification and is capable of partially compensating otpb loss of function. In addition, we identified a role of Otp in the development of a domain of somatostatin1-expressing cells in the rostral hindbrain, a region with strong otp expression but so far uncharacterized Otp function. Otp may thus be crucial for defined neuronal cell types also in the hindbrain.
Introduction
Patterning and neuronal differentiation in the vertebrate brain are controlled by a diverse group of transcription factors highly conserved throughout evolution. The Orthopedia homeodomain transcription factor encoding genes were initially discovered in both Drosophila and mouse based on their homeobox sequence, and characterized for their expression in the central nervous system [1]. Analysis of Otp mutant mice, which die shortly after birth, revealed that Otp contributes to patterning in the hypothalamus and preoptic region, and is required for differentiation of specific oxytocin (OT), arginine vasopressin (AVT), corticotropin-releasing hormone (CRH) and somatostatin (SS) expressing cells in the paraventricular, supraoptic, anterior periventricular, and arcuate nuclei [2], [3]. It was further revealed that Otp acts in parallel with the transcription factor Sim1 and both of them are required to maintain Brn2 expression for terminal differentiation of neurosecretory cells in the mouse hypothalamus [3]. Otp expression in the hypothalamus was shown to be highly conserved across tetrapods [4]. Further, already in ascidian embryos an otp gene is expressed in the hypothalamus adjacent to the sensory vesicle [5], which may derive from a proto-neuroendocrine territory in a chordate ancestor [6]. In humans, OTP is also expressed in the hypothalamus [7]. The expression of otp in the preoptic region (PO) is also highly conserved in chordates [4].
Work in zebrafish revealed that Otp is also required for the development of a specific subset of dopaminergic (DA) neurons in the hypothalamus and posterior tuberculum in zebrafish and of the homologous A11 group DA neurons in the dorsal hypothalamus of mice [8]. Based on their relevance to human diseases, including Parkinson’s and schizophrenia, intensive research efforts have been focused on ventral midbrain DA neurons [9]–[11]. In contrast, DA neurons in other parts of the brain [12], specifically the ventral diencephalon, have received relatively little attention. The small size and transparent nature of the larvae in combination with the genetics have made zebrafish a good system to investigate DA development [12]–[16]. In zebrafish, the Otp-dependent DA neurons are of particular interest because they represent the most prominent far projecting DA system in larval zebrafish [17], and are the only DA group sending projections ascending to the telencephalon, descending to hindbrain and spinal cord, as well as contributing to endohypothalamic circuitry [18]. Work in mammals showed that the Otp-dependent A11 DA group appears to have a projection pattern very similar to zebrafish [19]. In zebrafish, mutations in only one of the two paralogous otpa and otpb genes have been previously reported [8]. otpa mutants show a reduction in four posterior tubercular and hypothalamic DA groups, termed DC 2, 4, 5 and 6 based on the nomenclature proposed by Rink and Wullimann [20], but not a total loss of these DA neurons [8].
While the contribution of Otp to neuroendocrine development has been studied in detail in mice [2], [3], this function and potentially evolutionary conserved aspects are less well understood in the zebrafish system. As in mammals, two major types of neuroendocrine systems can be distinguished in the hypothalamus in zebrafish [21], the parvocellular and the magnocellular systems. Parvocellular neuroendocrine cells send projections to the adenohypophysis (anterior lobe of pituitary) and release several peptides, which include thyrotropin-releasing hormone (TRH), corticotropin-releasing hormone (CRH) and somatostatin (SST). Magnocellular neurons synthesize oxytocin (oxt; previously termed isotocin neurophysin itnp [22]) and arginine vasopressin- like (avp; also termed vasotocin neurophysin vsnp [23]) and project to the neurohypophysis (posterior lobe of the pituitary). Similar to mammals, in fish otp together with arnt2 and its binding partner sim1 were shown to be a core component of a conserved transcriptional network for neuroendocrine cells [24]. The expression of otp in the preoptic region is highly conserved in tetrapods and is assumed to be plesiomorphic among chordates [4]. In zebrafish, it was reported that otp is necessary for oxt- and avp-producing cells in the PO [23], [25]. In a recent study, a contribution of Otp activity to development of caudal hypothalamic Vasoactive intestinal peptide hormone secreting cells was also shown [26].
Here, we investigated in detail the contributions of the paralogous otpa and otpb genes to neuroendocrine development in zebrafish. We found that both genes act in a partially redundant manner regarding DA neuron and neuroendocrine cell specification. While Otpb appears dispensable for DA neuron and neuroendocrine cells specification in the presence of otpa, loss of otpb enhances the otpa mutant phenotype, resulting in the complete loss of specific neuronal groups. Surprisingly, we found that otp genes are also important for the development of a group of somatostatin1.1-expressing cells in the hindbrain. Otp is strongly expressed in the hindbrain in both mammals and zebrafish [1]–[3], [8], but its requirement during development of specific neurons in the hindbrain has not previously been reported.
Materials and Methods
Zebrafish Husbandry
Zebrafish breeding and maintenance were carried out under standard conditions at 28.5°C [27]. Experiments were performed with otpam866 [8] and otpbsa115 (obtained from Sanger Zebrafish Mutation Project) mutant alleles. To inhibit pigmentation, embryos were incubated in egg water containing 0.2 mM 1-phenyl-2-thiourea. All the experimental procedures were in accordance with the German laws for animal care.
Genotyping
The genotype of otp alleles was determined by genomic PCR using dCAPS assays [28]. The otpam866 mutant fish were genotyped using the following primer pair: otpa-m866-F1 5′-ggtcacagggaggcattaaa-3′ and otpa-m866-R1 5′-gatagtgggttttggcgaag-3′. The 310 bp PCR product was then digested with Hpy188III. Upon restriction, the wild type allele results in two fragments of 170 bp and 140 bp. The m866 mutation abolishes the restriction site, therefore the mutant allele is not cut by restriction with Hpy188III. For genotyping of otpbsa115 mutants the following primer pair was used: otpb-sa115-F1 5′-aggtcaacgccaaagaccaa-3′ and otpb-sa115-R1 5′-gcgatcggaaacatatttga-3′. The 399 bp PCR product was then digested with BbvI. Restriction results in two fragments (374 bp and a 25 bp) for the wild type allele and in one uncut fragment for the mutant allele.
In situ Hybridization
Larvae were fixed in 4% paraformaldehyde in phosphate-buffered saline at three days post-fertilization. Standard colorimetric whole-mount in situ hybridization (WISH) and fluorescent WISH were performed as previously described [29]. The following digoxigenin-labeled riboprobes were synthesized: th [13], crh, trh, sst1.1, oxt/itnp [24], avp/vsnp [30] and gad2/gad65 and gad1b/gad67 [31]. A mixture of antisense digoxigenin-labeled riboprobes against vglut2a/slc17a6b and vglut2b/slc17a6a [32] were used to detect glutamatergic neurons and a glyt2/slc6a5 probe [32] for glycinergic neurons.
Sequence Alignments
Otp protein sequences were aligned and analyzed with Clustal X2 [33] and CLC Genomics Workbench 5 (http://www.clcbio.com).
Microscopy, Cell Quantification and Image Analysis
Transmitted light images were acquired using a Zeiss Axioskop compound microscope. For quantification of cell numbers from WISH experiments (Figure S2), an Axio Examiner.D1 microscope (Carl Zeiss) with the transmitted-light differential interference contrast (DIC) illumination technique and a high numerical aperture 20× NA 1.0 lens was used to count cells at single-cell resolution in WISH stained embryos (Figure S3). The high numerical aperture lens enabled optical sectioning to obtain cellular resolution even when cells were densely packed in clusters. With DIC illumination best images were acquired with the iris diaphragm completely open. A Z-stack (1 µm steps) of images was recorded. Zen software was used to mark and count WISH stained cells in each stack. NIH ImageJ software and Adobe Photoshop were used to compose figures. DOG 1.0 [34] and Inkscape [www.inkscape.org] software were used for schematic drawings.
Statistical Analysis
Cell numbers from the different genotypes analyzed were compared with wildtype larvae using the Wilcoxon–Mann–Whitney rank-sum non-parametric test. Statistical analysis were performed with the help of the Excel add-in MegaStat (http://glencoe.mcgraw-hill.com/sites/0010126585/student_view0/megastat.html).
Results
otpbsa115 Mutant Otpb Protein Lacks the Highly Conserved Homeodomain
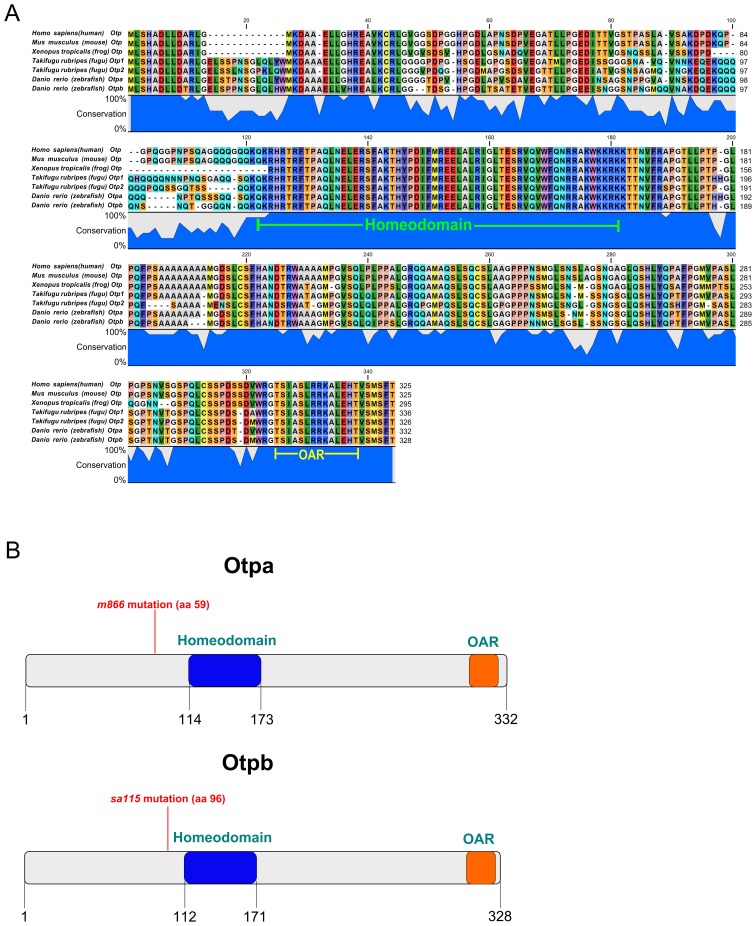
The Otp homeodomain is extremely well conserved from fish to human, and both zebrafish Otpa and Otpb proteins share this high conservation among vertebrates (Figure1 A). In addition, more carboxyterminal regions are highly conserved, including the OAR (otp, aristaless, rax) potential interaction domain present in several paired-like homeodomain proteins. While mutations in the otpa locus have been previously characterized [8], a mutant allele for the paralogous gene otpb has not been reported so far, and information concerning the phenotype of complete loss of Otp function is limited to Morpholino knockdown studies [8], [35]. Recently, the potential null allele otpbsa115 was made available by the Sanger Zebrafish Mutation Project. The otpbsa115 allele carries a base-pair deletion at amino acid 96 of the otpb ORF, which causes a premature stop codon before the highly conserved homeodomain (Figure 1B, bottom). This otpb mutation likely results in the production of a short, nonfunctional protein lacking the entire Otp homeodomain. Therefore, similar to otpam866, otpbsa115 is likely a null allele with complete loss of function (Figure 1B, top).
Figure 1. Otpbsa0115 mutants lack the highly conserved Homeodomain.
(A) The Otp protein and specifically the homeodomain sequence (green label) are highly conserved in vertebrates. The conserved OAR domain present in all Otp proteins in vertebrates is also depicted (yellow label). (B) Schematic representation showing both Otpa and Otpb protein structure and position of stop codons caused by mutations. In the otpam866 allele the mutation results in a frameshift and a premature stop codon after additional 59 amino acids. In the otpbsa0115 allele a base-pair deletion results in a premature stop codon 96 amino acids downstream of the start codon. Both mutations generate smaller proteins which completely lack the highly conserved homeodomain (in blue). The conserved OAR (otp, aristaless, rax), potential interaction domain present in several paired-like homeodomain proteins and all Otp proteins in vertebrates is also depicted (orange).
otpa and otpb are Differentially Required for the Development of Dopaminergic Neurons in the Ventral Diencephalon
Although otpa and otpb expression domains show a high degree of overlap (e.g. in the preoptic region and hindbrain; Figure 2), there are regions where just one of the otp genes is expressed (e.g. otpa in the medial periventricular area of the caudal hypothalamus, otpb in the more lateral caudal hypothalamus; Figure 2). This suggests that specific neuronal populations may differentially rely on otpa or otpb activity. To investigate the contributions of otpa and otpb to development of zebrafish DA neurons, we analyzed otpa and otpb homozygous single mutants and generated otpa;otpb double mutant embryos. While otpa homozygous as well as otpb homozygous fish are adult viable, otpa;otpb double mutant embryos and larvae develop morphologically normal and may form swim bladders, but die during late larval stages before juvenile ages. Due to the lethality at larval stages, the function of both otp genes for the specification of specific neuronal populations in adult zebrafish could not be addressed using double mutants.
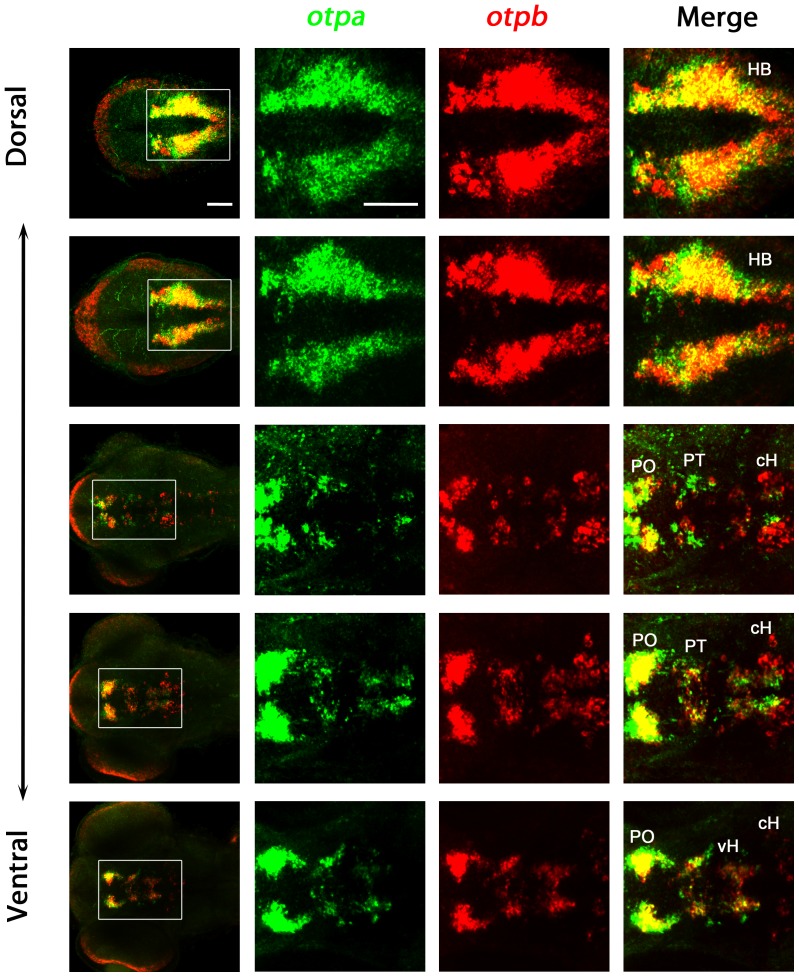
Figure 2. Expression of otpa and otpb in wildtype larvae.
Expression of otpa and otpb were detected by double fluorescent whole mount in situ hybridization of wildtype larvae fixed at 3 dpf. From the whole confocal image stack, sub-stacks ranging from dorsal hindbrain image planes to ventral forebrain planes were used to generate a series of dorso-ventral Z-projections. The data reveal that otpa and otpb have overlapping expression but also non-overlapping domains. Dorsal view, anterior at left. Abbreviations: cH, caudal hypothalamus; HB, hindbrain; PO, preoptic region; PT, posterior tuberculum; vH, ventral hypothalamus. Scale bar is 50 µm.
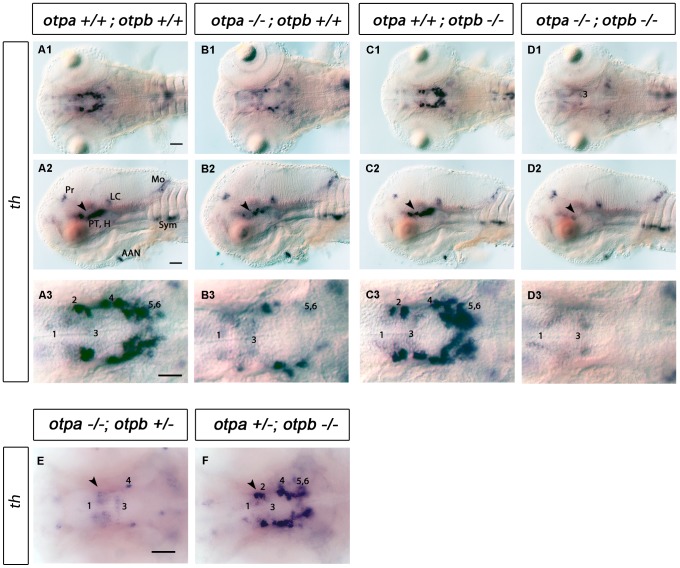
otpbsa115−/− mutant larvae develop DA neurons in normal numbers and anatomical positions, indistinguishable from wildtype siblings (compare Figure 3 A1–3 with C1–3). This is in contrast to the clear reduction of specific DA neurons in otpa mutants (Figure 3 B1–3) [8].
Figure 3. Analysis of DA neurons by expression of th in otpa and otpb single and double mutant larvae.
(A–D) Whole-mount in situ hybridization of 3 dpf larvae reveals reduction of th expression in the posterior tuberculum of otpa and total loss of the expression in otpa;otpb double mutants (arrowhead). Other th expressing domains are not affected. (A1–D1, A3–D3) Dorsal views, anterior at left; (A2–D2) lateral views, dorsal up. Scale bar is 50 µm. (E,F) Whole-mount in situ hybridization of 3 dpf larvae reveals reduction of th expression in the posterior tuberculum of otpa mutant, otpb heterozygous larvae (E) (arrowhead). No clear reduction is detected in the posterior tuberculum of otpb mutant, otpa heterozygous larvae (F) (arrowhead). Dorsal view, anterior at left. Scale bar is 50 µm. Abbreviations: AAC, arch associated cluster; DC, diencephalic cluster; H, hypothalamus; LC, locus coeruleus; MO, medulla oblongata; Pr, pretectum; PT, posterior tuberculum. Numbers indicate dopaminergic neurons in the ventral thalamic cluster (1) and posterior tuberculum/hypothalamus (2–6) according to [20]. Scale bar is 50 µm.
Complete loss of Otp activity in otpa;otpb double mutants caused a more severe phenotype than otpa mutants alone. The double mutants displayed a complete loss of DA groups 2, 4, 5 and 6 in the ventral diencephalon (Figure 3 D1–3). We also noticed that fish homozygous mutant for otpa and heterozygous for otpb show a stronger reduction of DA neurons in the posterior tuberculum (Figure 3E), while larvae homozygous mutant for otpb and heterozygous for otpa showed no evident phenotype (Figure 3F). This suggests that most of the Otp activity required for DA differentiation in the PT/vDC is provided by otpa, while otpb activity makes a minor, albeit significant contribution.
otpa and otpb Control Development of crh Expressing Cells in Preoptic and Ventral Diencephalic Regions
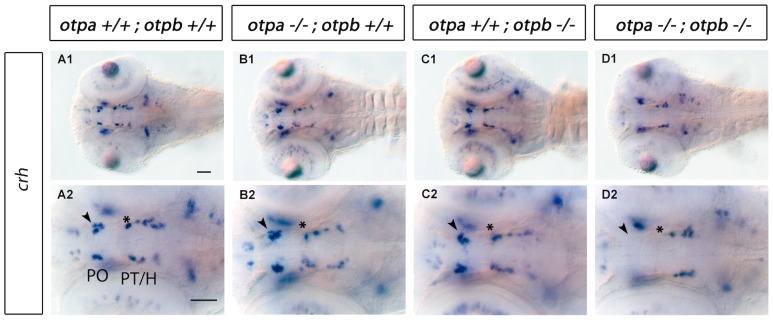
Corticotropin-releasing hormone (CRH) has been described as being secreted by the paraventricular nucleus (PVN) of the hypothalamus in response to stress [36]–[38]. crh is expressed in several regions of the embryonic zebrafish brain, including telencephalon, hypothalamus, posterior tuberculum, thalamus, retina and hindbrain [39]. crh positive neurons in the posterior tuberculum and hypothalamus were shown to be intermingled with DA neurons of the ventral diencephalic groups [24], [39]. In otpa or otpb single mutants we did not detect any significant changes in number or location of crh-expressing cells compared to wildtype siblings (Figure 4A–C, for cell counts see Figure S2E). In contrast, otpa;otpb double mutants showed a complete loss of crh-expressing cells in defined crh neuronal clusters in the PO region (Figure 4D1,D2 arrowhead) and a clear reduction of crh-expressing cells in the most anterior PT domain (Figure 4D1,D2 asterisk, for cell counts see Figure S2F). This suggests that both otpa and otpb genes act functionally redundant during specification of crh-expressing neurons in zebrafish (see also Figure S1G,H). Interestingly, only a defined subset of crh neuronal groups depends on Otp activity, while others, including the more caudal hypothalamic crh neurons, are apparently specified by Otp-independent mechanisms.
Figure 4. Expression of crh in otpa and otpb single and double mutant larvae.
Whole-mount in situ hybridization of 3 dpf larvae reveals loss of crh expression in the preoptic region and posterior tuberculum of otpa;otpb double mutant larvae (arrowhead and asterisk, respectively). Dorsal view, anterior at left. Scale bar is 50 µm. Abbreviations: H, hypothalamus; PO, preoptic region; PT, posterior tuberculum.
otpa and otpb are Required for Development of oxt, avp and trh Neurons in the Preoptic Region
Oxytocin (oxt, in fish previously named isotocin-neurophysin itnp) and arginine vasopressin (avp, previously vsnp) influence several behavioral and physiological processes such as reproductive, maternal, and aggression behaviors, as well as learning and memory [40]–[42]. Thyrotropin-releasing hormone (trh) has several important roles including regulation of energy homeostasis, feeding behavior and locomotion activation [43].
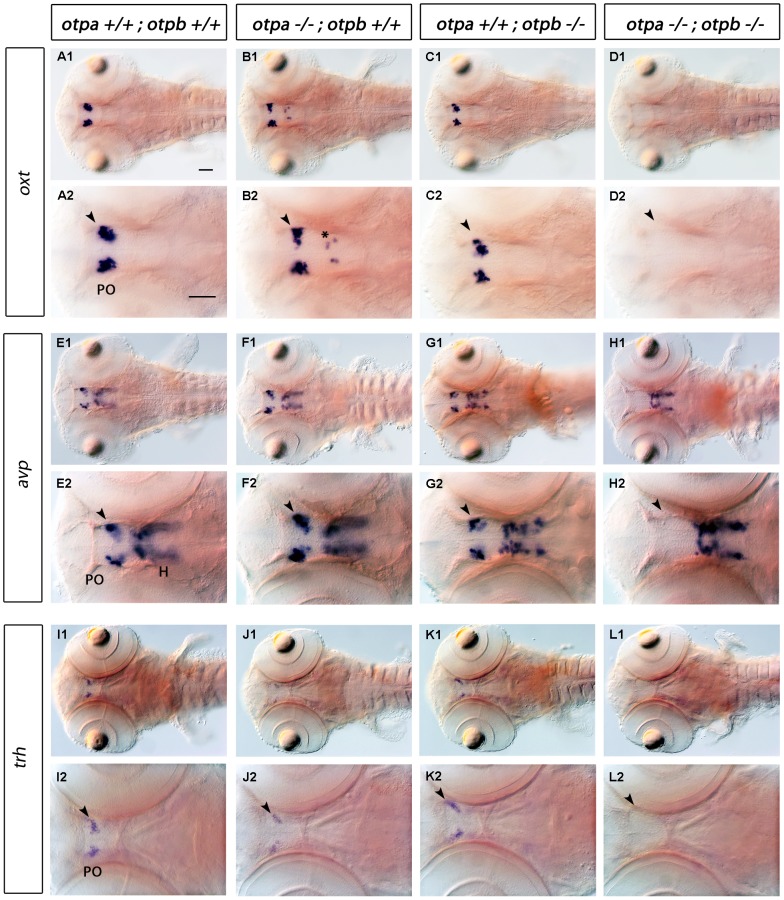
Based on otpb Morpholino knockdown it was previously postulated that otpb is necessary for oxt- and avp-producing cells in the PO [23], [25]. In contrast to these reports, we did not observe a significant reduction in cells expressing oxt and avp in otpbsa0115 mutants (Figure 5 A, C, E, G, for cell counts see Figure S2A and C). We also did not observe a phenotype affecting trh expressing cells in otpbsa0115 mutants (Figure 5 K, compare with I, for cell counts see Figure S2D). Moreover, in otpam866 single mutants we did not detect a clear reduction in oxt and avp expressing cells in the PO region (Figure 5 B, F). However, we detected oxt-expressing cells at ectopic locations within the diencephalon in otpa mutants (Figure 5 B2, asterisk, for cell counts see Figure S2B). The number of cells expressing trh in the PO region was significantly reduced in otpam866 mutants when compared to wild-type or the otpb mutant (Figure 5 J2, arrowhead, for cell counts see Figure S2D). When analyzing the expression of oxt, avp and trh in otpa;otpb double mutants, we observed a complete loss of these cell types in the PO region (Figure 5 D, H, L, arrowheads). The analysis of otpa−/−;otpb+/− and otpa+/−;otpb−/− mutants revealed that for all three neuronal types, otpa has a more prominent contribution to neuronal specification than otpb, because in each case the otpa−/−;otpb+/− phenotype was stronger (Figure S1A-F and Figure S2). In summary, these data reveal a crucial activity of Otp in oxt, avp and trh cell specification and show the partially redundant nature of otpa and otpb activity.
Figure 5. Expression of oxt, avp and trh in otpa and otpb single and double mutant larvae.
Whole-mount in situ hybridization reveals loss of oxt, avp and trh expression in the preoptic region (arrowhead) of otpa;otpb double mutant larvae at 3 dpf. We detected oxt-expressing cells at ectopic locations within the diencephalon in otpa mutants (B2, asterisk). A reduction of trh-expressing cells in the preoptic region in otpa single mutants is detectable, (J2, arrowhead). Dorsal view, anterior at left. Scale bar is 50 µm. Abbreviations: H, hypothalamus; PO, preoptic region.
otpa and otpb are Required for the Development of Hindbrain somatostatin1.1-expressing Cells
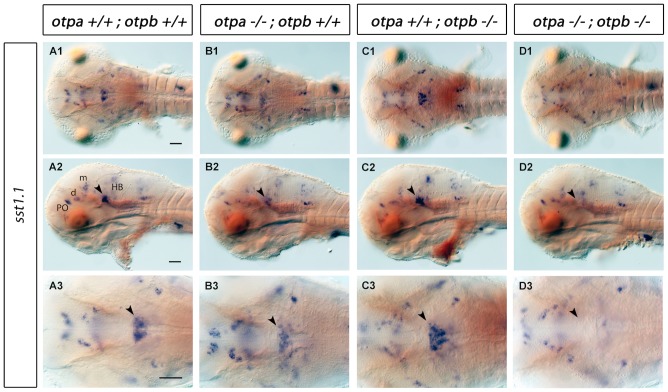
Somatostatins play important roles for negative regulation of endocrine secretion and regulation of growth in vertebrates. Most notably they also act as neuromodulators in the central nervous system, mediating motor, cognitive and sensory effects [44], [45]. somatostatin1.1 (sst1.1) expression in the brain was previously characterized in zebrafish [46.]. In both arnt2m1055 mutants and sim1a morphants, sst1.1 expression was shown to be reduced in the PO region [24]. Surprisingly, we did not observe a strong reduction of sst1.1 expression in the PO region in otpa and otpb single or double mutants (Figure 6). However, sst1.1 expression was reduced in the rostral hindbrain of otpa mutant larvae (Fig. 6 B1–B3, arrowhead, for cell counts see Figure S2G). This sst1.1 expression domain was not affected in otpb mutants (Figure 6 C1–C3), whereas in otpa;otpb double mutants it was completely lost (Figure 6 D1–D3, arrowhead). Other sst1.1-expressing domains appear not to rely on Otp function.
Figure 6. Expression of sst1.1 in otpa and otpb single and double mutant larvae.
Whole-mount in situ hybridization reveals reduction of sst1.1 expression (arrowhead) in the rostral hindbrain of otpa mutants and total loss of the expression in otpa;otpb double mutant larvae at 3 dpf. (A1–D1, A3–D3) Dorsal view, anterior at left; (A2–D2) lateral view, dorsal up. Scale bar is 50 µm. Abbreviations: d, diencephalon; HB, hindbrain; m, mesencephalon; PO, preoptic region.
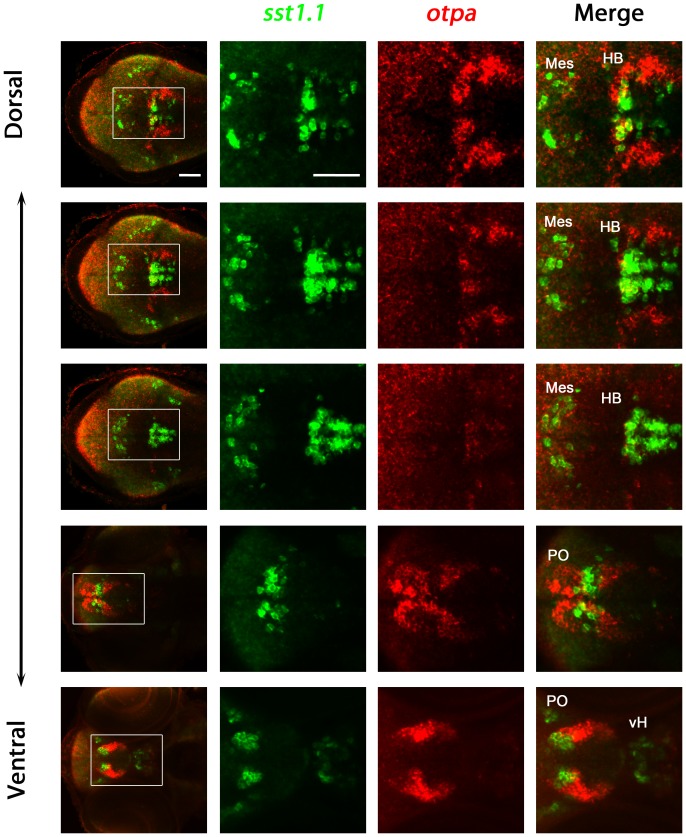
The results suggest that sst1.1 and otpa may be coexpressed in some domains but not in others. Therefore, we performed double fluorescent whole-mount in situ hybridization for sst1.1 and otpa and compare results between the hindbrain and the preoptic region domains (Figure 7). sst1.1 and otpa coexpression analysis revealed that cells expressing sst1.1 and otpa in the hindbrain are intermingled and some may coexpress both genes, while we could not observe any coexpression of sst1.1 and otpa in cells of the preoptic region at 3 day post fertilization (Figure 7). Analysis of sst1.1 expression and otpa mutants together suggest that sst1.1 expression may only be affected in areas of coexpression with otpa. However, we cannot exclude that sst1.1 and otpa may be coexpressed in other domains at different developmental stages, not analyzed in this study.
Figure 7. Analysis of coexpression of otpa and sst1.1 in wildtype larvae.
Expression of otpa and sst1.1 were detected by double fluorescent whole mount in situ hybridization of wildtype larvae fixed at 3 dpf. From the whole confocal image stack, sub-stacks ranging from dorsal hindbrain image planes to ventral forebrain planes were used to generate a series of dorso-ventral Z-projections. The data reveal that sst1.1 and otpa expression domains overlap in the rostral hindbrain in wildtype larvae at 3 dpf, and some cells appear to coexpress both genes. Dorsal view, anterior at left. Abbreviations: cH, caudal hypothalamus; HB, hindbrain; Mes, mesencephalon; PO, preoptic region; vH, ventral hypothalamus. Scale bar is 50 µm.
otpa and otpb are not Required for the Development of Hindbrain Gabaergic, Glycinergic, and Glutamatergic Neurons
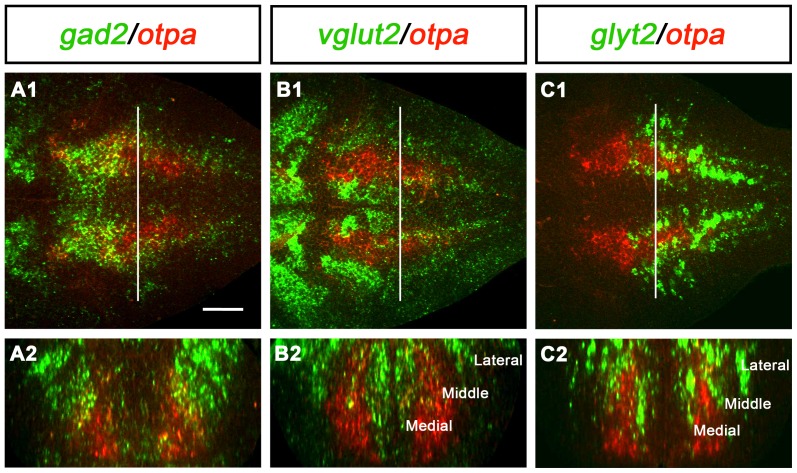
Given that both otpa and otpb are expressed broadly in the hindbrain [8], [47], we analyzed potential effects of the loss of Otp function on the development of other neuronal populations in this region. Given the longitudinal extent of the otp expression domains in the hindbrain, we expected that otp may not be regulated in rhombomeric patterns, but correlate with some of the rostrocaudal hindbrain neurotransmitter stripes previously characterized in zebrafish [32], [48]. These publications distinguished three longitudinal stripes (medial, middle and lateral) on each side for glutamatergic and glycinergic neurons. We therefore analyzed coexpression of markers for gabaergic, glycinergic, and glutamatergic neurons with otpa expression in the hindbrain (Figure 8). There was no one-to-one correlation of otpa expression with any of these three transmitter types in the hindbrain. However, it appeared that a significant portion of the otpa expressing cells in the rostral hindbrain expressed gabaergic markers (Figure 8A). In contrast, in the medial and caudal hindbrain, only the lateral portion of the longitudinal otpa expression domain may be gabaergic. The otpa expression domain is limited medially and overlaps with the medial glutamatergic stripe, and appears to contain the middle glutamatergic stripe (Figure 8B). Similarly, coexpression of otpa and glyt2 was detected in parts of the medial and middle glycinergic stripe (Figure 8C). To investigate whether loss of Otp activity affects any of these transmitter stripes, we analyzed the development of gabaergic (expression of gad1b/gad2), glycinergic (expression of glyt2) and glutamatergic (expression of vglut2) neurons in otpa;otpb double mutants. Surprisingly, we could not detect any significant differences in neurotransmitter-specific expression domains when otpa;otpb double mutants were compared to wildtype (Figure 9). However, these findings do not exclude that Otp may affect other aspects of differentiation of these neurons. These observations also suggest that the requirement for Otp activity during specification of sst1.1-expressing cells in the hindbrain is a specific function of Otp proteins, and is not caused by more global potential patterning defects of the hindbrain in otpa;otpb double mutants. Other potential roles of the broad Otp expression in the hindbrain still remain to be elucidated.
Figure 8. Expression of otpa in relation to gabaergic, glutamatergic and glycinergic markers in the hindbrain of wildtype larvae.
Potential coexpression of otpa with gabaergic (gad2, A), glutamatergic (vglut2, B) and glycinergic (glyt2, C) markers was analyzed by double whole mount FISH at 3 dpf. A1, B1, and C1 are single plane dorsal views of the hindbrain, anterior is to the left. A2, B2, and C2 are cross-sections at the level of the hindbrain indicated by the white line in A1, B1, and C1, respectively. The orthogonal view cross sections were obtained from dorsal confocal stacks using the TransformJ Turn plugin of the ImageJ software. Scale bar is 50 µm.
Figure 9. Expression of gad1b/2, glyt2 and vglut2 in wildtype and in otp;otpb double mutant larvae.
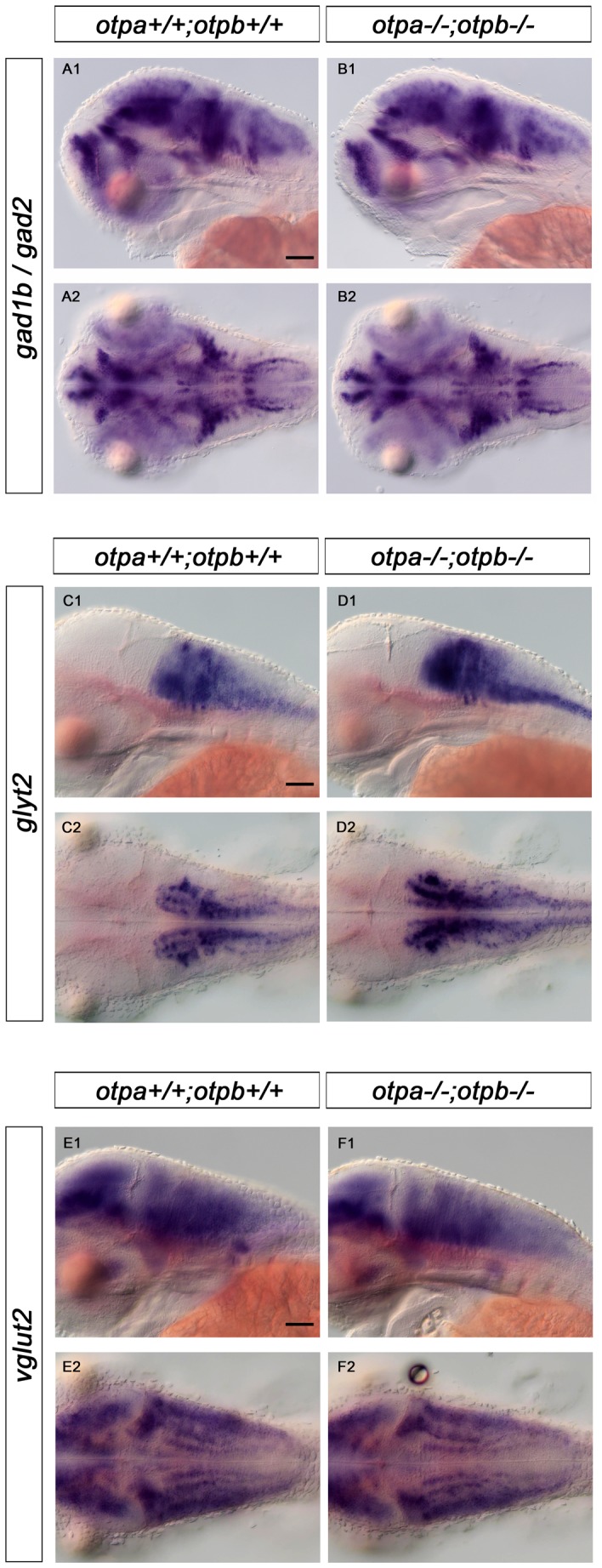
Whole-mount in situ hybridization reveals no changes in expression of gabaergic (gad1b/gad2), glycinergic (glyt2)and glutamatergic (vglut2) in the hindbrain of otpa;otpb double mutant larvae at 3 dpf. (A1, B1, C1, D1, E1, F1) lateral view, dorsal up; (A2, B2, C2, D2, E2,F2) dorsal view, anterior at left. Scale bar is 50 µm.
Discussion
Neuroendocrine and neuromodulatory systems of the hypothalamus, including the DA systems, are central to the control of basic behavior patterns and physiology, but molecular mechanisms controlling their neuronal differentiation are not well understood. Otp is a transcription factor crucial for development of several neuronal types in the hypothalamus and preoptic region [2], [3], [8], [25], [47]. While zebrafish are an excellent model to study neuronal development, a genome duplication at the base of teleost evolution resulted in two paralogous copies of many genes [49], including otp. Here we have genetically dissected the contributions of the paralogous genes otpa and otpb to the specification of neuroendocrine and DA neuron types in the zebrafish larval brain. The analysis of the otpb single mutant and otpa;otpb double mutant phenotype has only now become possible through a potential null allele for the otpb gene in zebrafish isolated by the Sanger Zebrafish Mutation Project. This mutation causes a truncated protein that completely lacks the homeodomain. While both single otpa and otpb mutants are viable and develop into fertile adults, with no abnormal morphological phenotype, otpa;otpb double mutant embryos die at larval stages without any obvious morphological defect (data not shown).
We analyzed the role of Otp during the development of th, crh, oxt, avp, trh and sst1.1-expressing cells. In zebrafish both otp paralogous genes act partially redundant to accomplish a function equivalent to the single OTP gene in mice. Our experiments with otpa;otpb double mutants regarding DA neurons are in agreement with this view. Double mutants display a complete loss of DA groups 2, 4, 5 and 6 in the ventral diencephalon which is similar to what is observed in the Otp−/− mice, which lack all the neurons belonging to the A11 group [8].
For DA neuron specification in the posterior tubercular region otpa appears to provide most of Otp activity, since otpa mutants have a drastically reduced number of DA neurons, whereas otpb mutants do not. It has been previously reported that knocking down otpb alone by morpholino approach leads to a strong reduction of DA neurons in the ventral diencephalon [35], [47]. However, our analysis of the otpbsa0115 mutant allele and our previous study using an otpb specific Morpholino [8] did not reveal a significant DA phenotype. We compared the sequences of the otpb Morpholino used in two publications [25], [47] with otpa and otpb sequences, and found that the otpb morpholino used by Del Giacco et al. (2006) may bind with only three mismatches also to otpa, while the otpb morpholino used by Eaton et al. (2006) may bind with four mismatches also to otpa. Thus, the results reported in these manuscripts for otpb knockdown may in part be attributed to the otpb morpholino used in these studies binding also to the otpa paralog. Our data contradicts the previously published hypothesis that otpb would be more relevant than otpa for diencephalic neurodifferentiation [50]. The different results may arise by non-specific effects of morpholino injections to knock down otpb gene function. Many of the common problems using morpholinos were reviewed in detail recently [51] and our results once more emphasize the need for a careful experimental setup when making conclusions from morpholino experiments, especially when addressing the function of paralogous genes during zebrafish development. Fortunately, gene-specific mutations are now more readily available through TILLING screens [52] and TALEN site specific mutagenesis [53].
A previous study has also reported RT-PCR data indicating that otpb may be expressed maternally and otpb mRNA deposited in to the egg [47]. This would raise the possibility that maternal otpb message may attenuate the zygotic otpb mutant phenotype. However, there are three lines of evidence suggesting that there is no significant maternal contribution of otpb: (1) systematic microarray analysis of expression mRNA profiles from zygote to late gastrula stages demonstrate that there is no specific otpb mRNA signal at blastula or gastrula stages [54]; (2) we have not been able to detect otpb message by whole mount in situ hybridization (unpublished data); (3) Del Giacco et al. (2006) also reveal in their manuscript that they were not able to detect otpb mRNA by WISH before the 3-somite stage [47]. We therefore conclude that if any maternal otpb mRNA persists beyond zygote stage, the amount is so low that it likely does not affect the phenotype. We further investigated the possibility that otpa and otpb may mutually contribute to regulation of their expression by analyzing otpa expression in otpb mutants and otpb expression in otpa mutants (Figure S4). We could not detect any influence of loss-of-function in one otp paralog on expression of the other paralog.
Our analysis of otpa;otpb double mutant embryos clearly demonstrates the requirement for Otp activity by defined subsets of crh, avp, trh and sst1.1 neuroendocrine cells in the posterior tubercular/hypothalamic, hindbrain as well as preoptic regions, and for essentially all preoptic oxt neuroendocrine cells at zebrafish larval stages. These findings are summarized schematically in Figure 10. For the specification of crh, oxt and avp neuroendocrine cells both otp paralogous genes appear to act mutually redundant, as no significant reduction in the number of cells is detectable in otpa or otpb single mutant embryos. However, ectopic oxt-expressing cells are apparent in otpa mutant embryos. The observation of ectopic oxt-expressing cells in otpa mutants resembles the phenotype caused by sim1a Morpholino knockdown [24]. This paper also reports a reduction of oxt, avp and trh expression in the PO region in arnt2m1055 mutant and sim1a morpholino knockdown embryos [24]. trh expressing cells in the PO region are also reduced in otpa single mutants, but not in otpb single mutants.
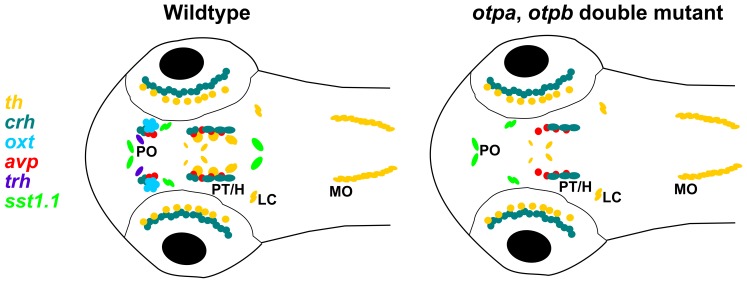
Figure 10. Schematic representation of neuroendocrine and dopaminergic cell groups affected in otpa/otpb double mutant larval zebrafish at 3 dpf.
Schematic diagram showing the expression of the th, sst1.1, trh, oxt, avp, and crh neuronal groups analyzed in this study at 3 dpf in zebrafish larvae. For simplicity, the schematic representation does not include the entire expression patterns of all these 7 genes, but focuses on anatomical regions with Otp expression. The comparison of wildtype and otpa;otpb double mutant larvae reveals groups dependent on Otp activity. H, hypothalamus; LC, locus coeruleus; MO, medulla oblongata; PO, preoptic region; PT, posterior tuberculum.
Our findings that several neuroendocrine populations are completely lost in the PO of otpa;otpb double mutants is similar to what has been reported for Otp−/− mice. Otp in mice is expressed in the paraventricular (PVN), supraoptic (SON), anterior periventricular (aPV), and arcuate (ARN) nuclei, as well as in other parts of the central nervous system [2], [3]. In Otp−/− mice, TRH and CRH expression is completely lost in the PVN. AVP and OT (also known as OXT) expression is completely lost in the SON. In Otp−/− mice SS (also known as SST) has been shown to be absent in the aPV and in the ARN. In contrast, we could not detect a reduction in the number of sst1.1 positive cells in the PO of otp mutants at 3 dpf. However, we have identified a group of sst1.1 expressing neurons in the rostral hindbrain that depend on Otp activity in zebrafish embryos. Otp has been shown to be strongly expressed in the hindbrain in mice [3], but so far no abnormalities in hindbrain expression of SS have been reported for Otp−/− mice.
What may cause the slightly different effects of otpa and otpb on different neuroendocrine and DA populations? Hypothetically, this could be differences in Otpa and Otpb protein function, or differences in spatial and temporal expression patterns of both paralogous genes. Given the high conservation of Otpa and Otpb proteins (Fig. 1) as well as the observed partial functional redundancies, we favor the second option as cause for the differences. otpa and otpb have similar but in some regions slightly spatially shifted expression domains (Figure 2) [8], making different neuronal populations differentially sensitive to reduction of otpa and otpb activity.
While modulation of Otp activity levels in single mutant or transheterozygous otpa and otpb mutations has little effect on crh expression, Otp may have functions in behavioral physiology not detected in normal developmental assays. In a recent study an antibody against CRH protein was used to evaluate CRH expression, and under standard conditions no phenotype was detected in otpa mutants versus wild-type during development [55]. However, in the presence of a stressor stimulus, crh transcription was significantly induced in wildtype, but not in otpam866 mutant larvae. Similarly, the same study showed that transgene driven enhanced Otp expression caused increased crh transcription, suggesting that Otp may have a role in physiological stress related control of crh expression.
Given the complexity of neuronal phenotypes in the hypothalamus and preoptic region, additional types of neurons likely depend on Otp activity in this region. In a recent report, we could show that opn4a expressing cells in a specific cluster in the preoptic region are reduced in otpa mutants and absent in otpa and otpb double mutants [56]. This was surprising, as opn4a cells are supposed to be light sensing, adding them to the repertoire of neurosecretory and neuromodulatory neurons specified by Otp. A similarly interesting finding in our current study is the dependence of a large group of sst1.1 expressing neurons in the rostral hindbrain on Otp activity, which provides the first evidence that the strong expression domains of otpa and otpb are indeed involved in neuronal differentiation in the hindbrain of vertebrates. It is likely that future studies on otpa and otpb function in zebrafish will identify additional neuronal groups and potentially neuronal circuits depending on Otp activity.
Supporting Information
Expression of oxt, avp, trh, crh and sst1.1 in otpa and otpb mutant larvae. Whole-mount in situ hybridization of 3 dpf larvae reveals changes of oxt, avp, trh and crh expression in the preoptic region (arrowhead in A, C, E, G) and reduction of sst1.1 expression (arrowhead in I) in the hindbrain of otpa−/− mutant, otpb+/− heterozygous larvae. In contrast, no obvious change is detected in the preoptic region (arrowheads in B, D, F, H) and hindbrain (J) of otpb−/− mutant, otpa+/− heterozygous larvae. Dorsal view, anterior at left. Scale bar is 50 µm. H, hypothalamus; PO, preoptic region; PT, posterior tuberculum.
(TIF)
Quantification of oxt, avp, trh, crh and sst1.1 cell numbers in otp mutants. Histogram illustrating the average number of oxt (A), oxt ectopic cells (B), avp (C), trh (D), crh preoptic region (E), crh anterior posterior tuberculum (F) and sst1.1 rostral hindbrain (G) neurons. Y-axis gives number of stained neurons per embryo and anatomical group. Numbers in histogram bars provide the number of embryos imaged and analyzed. To evaluate differences for statistical significance, cell numbers from the different genotypes analyzed were compared with wildtype larvae using the Wilcoxon–Mann–Whitney rank-sum test. * P<0.05 one-tailed, ** P<0.01 one-tailed. Error bars indicate standard error of the mean.
(TIF)
High-resolution imaging of neuroendocrine cells for cell counting. Example of 3 dpf wildtype embryos imaged at single-cell resolution for quantification of cell numbers. (A) oxt, (B), avp, (C) trh, (D) crh expression analysis by WISH. For this figure, from the whole image stack with images at 1 µm spacing, sub-stacks of planes were used to generate a series of dorso-ventral Z-projections containing the region of interest. A higher magnification of regions of interest is shown on the right panel. Dorsal view, anterior at left. Scale bar is 100 µm. H, hypothalamus; HB, Hindbrain; PO, preoptic region; PT, posterior tuberculum.
(TIF)
Expression of otp genes is not altered in otp mutant larvae. Whole-mount in situ hybridization of 3 dpf larvae reveals no obvious changes of otpb expression in the preoptic region (A1, B1) and hindbrain (A2, B2) in otpa mutants. Similarly, no obvious changes in otpa expression were detected in the preoptic region (C1, D1) and hindbrain (C2, D2) expression domains in 3 dpf otpb mutants. Scale bar is 100 µm.
(TIF)
Acknowledgments
For providing the zebrafish knockout allele otpbsa0115 allele we thank the Sanger Institute Zebrafish Mutation Resource, sponsored by the Welcome Trust (grant number WT 077047/Z/05/Z). We thank Mattanja Sonn for excellent technical support, Dr. Aristides Arrenberg, Dr. Jörn Schweitzer and Martha Manoli for discussions and comments on manuscript, the zebrafish community for sharing reagents, and Sabine Götter for expert zebrafish care.
Funding Statement
The work was supported by the following grants to W.D.: Excellence Initiative of the German Federal and State Governments (Centre for Biological Signalling Studies EXC 294; www.dfg.de & www.bioss.uni-freiburg.de), the German Research Foundation (DFG-SFB780-B6; www.dfg.de), and the EU ZF-HEALTH project (Grant number 242048; http://cordis.europa.eu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Simeone A, D’Apice MR, Nigro V, Casanova J, Graziani F, et al. (1994) Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron 13: 83–101. [DOI] [PubMed] [Google Scholar]
- 2. Acampora D, Postiglione MP, Avantaggiato V, Di BM, Vaccarino FM, et al. (1999) Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev 13: 2787–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang W, Lufkin T (2000) The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol 227: 432–449. [DOI] [PubMed] [Google Scholar]
- 4. Bardet SM, Martinez-de-la-Torre M, Northcutt RG, Rubenstein JL, Puelles L (2008) Conserved pattern of OTP-positive cells in the paraventricular nucleus and other hypothalamic sites of tetrapods. Brain Res Bull 75: 231–235. [DOI] [PubMed] [Google Scholar]
- 5. Moret F, Christiaen L, Deyts C, Blin M, Vernier P, et al. (2005) Regulatory gene expressions in the ascidian ventral sensory vesicle: evolutionary relationships with the vertebrate hypothalamus. Dev Biol 277: 567–579. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto K, Vernier P (2011) The evolution of dopamine systems in chordates. Front Neuroanat 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin X, State MW, Vaccarino FM, Greally J, Hass M, et al. (1999) Identification, chromosomal assignment, and expression analysis of the human homeodomain-containing gene Orthopedia (OTP). Genomics 60: 96–104. [DOI] [PubMed] [Google Scholar]
- 8. Ryu S, Mahler J, Acampora D, Holzschuh J, Erhardt S, et al. (2007) Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr Biol 17: 873–880. [DOI] [PubMed] [Google Scholar]
- 9. Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30: 194–202. [DOI] [PubMed] [Google Scholar]
- 10. Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30: 188–193. [DOI] [PubMed] [Google Scholar]
- 11. Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63: 187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smeets WJ, Gonzalez A (2000) Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Brain Res Rev 33: 308–379. [DOI] [PubMed] [Google Scholar]
- 13. Holzschuh J, Ryu S, Aberger F, Driever W (2001) Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev 101: 237–243. [DOI] [PubMed] [Google Scholar]
- 14. Ma PM (2003) Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J Comp Neurol 460: 13–37. [DOI] [PubMed] [Google Scholar]
- 15. McLean DL, Fetcho JR (2004) Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol 480: 38–56. [DOI] [PubMed] [Google Scholar]
- 16. Filippi A, Jainok C, Driever W (2012) Analysis of transcriptional codes for zebrafish dopaminergic neurons reveals essential functions of Arx and Isl1 in prethalamic dopaminergic neuron development. Dev Biol 369: 133–149. [DOI] [PubMed] [Google Scholar]
- 17. Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W (2010) Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J Comp Neurol 518: 439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W (2011) Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun 2: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barraud Q, Obeid I, Aubert I, Barriere G, Contamin H, et al. (2010) Neuroanatomical study of the A11 diencephalospinal pathway in the non-human primate. PLoS One 5: e13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rink E, Wullimann MF (2002) Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res Dev Brain Res 137: 89–100. [DOI] [PubMed] [Google Scholar]
- 21. Lohr H, Hammerschmidt M (2011) Zebrafish in endocrine systems: recent advances and implications for human disease. Annu Rev Physiol 73: 183–211. [DOI] [PubMed] [Google Scholar]
- 22. Unger JL, Glasgow E (2003) Expression of isotocin-neurophysin mRNA in developing zebrafish. 3: 105–108. [DOI] [PubMed] [Google Scholar]
- 23. Eaton JL, Holmqvist B, Glasgow E (2008) Ontogeny of vasotocin-expressing cells in zebrafish: selective requirement for the transcriptional regulators orthopedia and single-minded 1 in the preoptic area. Dev Dyn 237: 995–1005. [DOI] [PubMed] [Google Scholar]
- 24. Lohr H, Ryu S, Driever W (2009) Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages. Development 136: 1007–1017. [DOI] [PubMed] [Google Scholar]
- 25. Eaton JL, Glasgow E (2007) Zebrafish orthopedia (otp) is required for isotocin cell development. Dev Genes Evol 217: 149–158. [DOI] [PubMed] [Google Scholar]
- 26. Wolf A, Ryu S (2013) Specification of posterior hypothalamic neurons requires coordinated activities of Fezf2, Otp, Sim1a and Foxb1.2. Development 140: 1762–1773. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield M (2000) The zebrafish book: a guide to the laboratory use of zebrafish (Danio rerio). Eugene: Univ. of Oregon Press.
- 28. Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18: 613–615. [DOI] [PubMed] [Google Scholar]
- 29. Filippi A, Mahler J, Schweitzer J, Driever W (2010) Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J Comp Neurol 518: 423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, et al. (2007) Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129: 1389–1400. [DOI] [PubMed] [Google Scholar]
- 31. Martin SC, Heinrich G, Sandell JH (1998) Sequence and expression of glutamic acid decarboxylase isoforms in the developing zebrafish. J Comp Neurol 396: 253–266. [PubMed] [Google Scholar]
- 32. Higashijima S, Mandel G, Fetcho JR (2004) Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol 480: 1–18. [DOI] [PubMed] [Google Scholar]
- 33. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 34. Ren J, Wen L, Gao X, Jin C, Xue Y, et al. (2009) DOG 1.0: illustrator of protein domain structures. Cell Res 19: 271–273. [DOI] [PubMed] [Google Scholar]
- 35. Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, et al. (2007) Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development 134: 4417–4426. [DOI] [PubMed] [Google Scholar]
- 36. Bale TL, Contarino A, Smith GW, Chan R, Gold LH, et al. (2000) Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414. [DOI] [PubMed] [Google Scholar]
- 37. Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, et al. (1998) Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20: 1093–1102. [DOI] [PubMed] [Google Scholar]
- 38. Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, et al. (1998) Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19: 162–166. [DOI] [PubMed] [Google Scholar]
- 39. Chandrasekar G, Lauter G, Hauptmann G (2007) Distribution of corticotropin-releasing hormone in the developing zebrafish brain. J Comp Neurol 505: 337–351. [DOI] [PubMed] [Google Scholar]
- 40. Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd (2009) Oxytocin: the great facilitator of life. Prog Neurobiol 88: 127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nair HP, Young LJ (2006) Vasopressin and pair-bond formation: genes to brain to behavior. Physiology (Bethesda) 21: 146–152. [DOI] [PubMed] [Google Scholar]
- 42. Caldwell HK, Lee HJ, Macbeth AH, Young WS 3rd (2008) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lechan RM, Fekete C (2006) The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153: 209–235. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Lu D, Zhang Y, Li S, Liu X, et al. (2010) The evolution of somatostatin in vertebrates. Gene 463: 21–28. [DOI] [PubMed] [Google Scholar]
- 45. Gahete MD, Cordoba-Chacon J, Duran-Prado M, Malagon MM, Martinez-Fuentes AJ, et al. (2010) Somatostatin and its receptors from fish to mammals. Ann N Y Acad Sci 1200: 43–52. [DOI] [PubMed] [Google Scholar]
- 46. Devos N, Deflorian G, Biemar F, Bortolussi M, Martial JA, et al. (2002) Differential expression of two somatostatin genes during zebrafish embryonic development. Mech Dev 115: 133–137. [DOI] [PubMed] [Google Scholar]
- 47. Del Giacco L, Sordino P, Pistocchi A, Andreakis N, Tarallo R, et al. (2006) Differential regulation of the zebrafish orthopedia 1 gene during fate determination of diencephalic neurons. BMC Dev Biol 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kinkhabwala A, Riley M, Koyama M, Monen J, Satou C, et al. (2011) A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proc Natl Acad Sci U S A 108: 1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, et al. (1998) Vertebrate genome evolution and the zebrafish gene map. Nat Genet 18: 345–349. [DOI] [PubMed] [Google Scholar]
- 50. Del Giacco L, Pistocchi A, Cotelli F, Fortunato AE, Sordino P (2008) A peek inside the neurosecretory brain through Orthopedia lenses. Dev Dyn 237: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 51. Eisen JS, Smith JC (2008) Controlling morpholino experiments: don’t stop making antisense. Development 135: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 52. Kettleborough RN, Bruijn E, Eeden F, Cuppen E, Stemple DL (2011) High-throughput target-selected gene inactivation in zebrafish. Methods Cell Biol 104: 121–127. [DOI] [PubMed] [Google Scholar]
- 53. Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, et al. (2012) In vivo genome editing using a high-efficiency TALEN system. Nature 491: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Onichtchouk D, Geier F, Polok B, Messerschmidt DM, Mossner R, et al. (2010) Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol 6: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amir-Zilberstein L, Blechman J, Sztainberg Y, Norton WH, Reuveny A, et al. (2012) Homeodomain protein otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron 73: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, et al. (2012) Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol 22: 2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of oxt, avp, trh, crh and sst1.1 in otpa and otpb mutant larvae. Whole-mount in situ hybridization of 3 dpf larvae reveals changes of oxt, avp, trh and crh expression in the preoptic region (arrowhead in A, C, E, G) and reduction of sst1.1 expression (arrowhead in I) in the hindbrain of otpa−/− mutant, otpb+/− heterozygous larvae. In contrast, no obvious change is detected in the preoptic region (arrowheads in B, D, F, H) and hindbrain (J) of otpb−/− mutant, otpa+/− heterozygous larvae. Dorsal view, anterior at left. Scale bar is 50 µm. H, hypothalamus; PO, preoptic region; PT, posterior tuberculum.
(TIF)
Quantification of oxt, avp, trh, crh and sst1.1 cell numbers in otp mutants. Histogram illustrating the average number of oxt (A), oxt ectopic cells (B), avp (C), trh (D), crh preoptic region (E), crh anterior posterior tuberculum (F) and sst1.1 rostral hindbrain (G) neurons. Y-axis gives number of stained neurons per embryo and anatomical group. Numbers in histogram bars provide the number of embryos imaged and analyzed. To evaluate differences for statistical significance, cell numbers from the different genotypes analyzed were compared with wildtype larvae using the Wilcoxon–Mann–Whitney rank-sum test. * P<0.05 one-tailed, ** P<0.01 one-tailed. Error bars indicate standard error of the mean.
(TIF)
High-resolution imaging of neuroendocrine cells for cell counting. Example of 3 dpf wildtype embryos imaged at single-cell resolution for quantification of cell numbers. (A) oxt, (B), avp, (C) trh, (D) crh expression analysis by WISH. For this figure, from the whole image stack with images at 1 µm spacing, sub-stacks of planes were used to generate a series of dorso-ventral Z-projections containing the region of interest. A higher magnification of regions of interest is shown on the right panel. Dorsal view, anterior at left. Scale bar is 100 µm. H, hypothalamus; HB, Hindbrain; PO, preoptic region; PT, posterior tuberculum.
(TIF)
Expression of otp genes is not altered in otp mutant larvae. Whole-mount in situ hybridization of 3 dpf larvae reveals no obvious changes of otpb expression in the preoptic region (A1, B1) and hindbrain (A2, B2) in otpa mutants. Similarly, no obvious changes in otpa expression were detected in the preoptic region (C1, D1) and hindbrain (C2, D2) expression domains in 3 dpf otpb mutants. Scale bar is 100 µm.
(TIF)