Abstract
In this paper, we review the recent progress in the development of low-cost microfluidic devices based on multifilament threads and textiles for semi-quantitative diagnostic and environmental assays. Hydrophilic multifilament threads are capable of transporting aqueous and non-aqueous fluids via capillary action and possess desirable properties for building fluid transport pathways in microfluidic devices. Thread can be sewn onto various support materials to form fluid transport channels without the need for the patterned hydrophobic barriers essential for paper-based microfluidic devices. Thread can also be used to manufacture fabrics which can be patterned to achieve suitable hydrophilic-hydrophobic contrast, creating hydrophilic channels which allow the control of fluids flow. Furthermore, well established textile patterning methods and combination of hydrophilic and hydrophobic threads can be applied to fabricate low-cost microfluidic devices that meet the low-cost and low-volume requirements. In this paper, we review the current limitations and shortcomings of multifilament thread and textile-based microfluidics, and the research efforts to date on the development of fluid flow control concepts and fabrication methods. We also present a summary of different methods for modelling the fluid capillary flow in microfluidic thread and textile-based systems. Finally, we summarized the published works of thread surface treatment methods and the potential of combining multifilament thread with other materials to construct devices with greater functionality. We believe these will be important research focuses of thread- and textile-based microfluidics in future.
INTRODUCTION
Diagnostic Laboratories are limited to non-existent in the developing world. In order to effectively control disease in developing countries, sensitive, portable, and simple analytical devices need to be built on low-cost materials for providing rapid diagnosis of human diseases and detection of environmental contaminants. The use of low-cost materials makes the large-scale deployment of such devices to developing regions economically viable. The recent rise of paper-based microfluidics as a platform for health and environmental monitoring is an example of using low-cost technology to take on the medical and environmental challenges faced by the developing regions of the world.1, 2, 3
As part of a continuous effort to seek new materials for making low-cost diagnostic devices, threads and fabrics have been investigated as a group of inexpensive materials for the fabrication of disposable microfluidic devices.4, 5, 6, 7, 8 Thread normally refers to twisted fibers and can be combined to make a yarn. The voids between these fibres form capillary channels and facilitate liquid flow without the requirement of external pumping, make them suitable for fabricating rapid and inexpensive point-of-care (POC) diagnostics. Thread- and fabric-based microfluidic devices require a few microliters of reagents and analyte sample solutions to perform chemical and biochemical analyses.4, 5, 6, 7, 8, 9, 10, 11 Although the porous fibre structures in threads provide capillary channels, some natural fibres are coated waxes composed of long fatty acid chains; the natural plant wax reduces or even stops the liquid wicking within threads.4, 6, 8, 12 Wettability of these fibres can be enhanced by removing the natural waxes from the fibre surface, allowing wicking by capillary action to occur. Combined with liquid wicking ability with other properties such as flexibility and wet-strength, thread can be used with other materials to fabricate light-weight and embeddable systems.4, 6 Threads can be easily made in white colour which provides desirable background reference for colorimetric assays. The facile colorimetric assay approach can be traced back to the work by George Oliver, who demonstrated the “Test Strips” using paper as well as cloth as substrates to perform multiple-analyte assays of human urine.13 To date, several published diagnostic studies using thread and textile have demonstrated using of colorimetric measurements to form calibration curves and detect and quantify analytes in samples; these include pH of human sweat, human blood type, glucose and protein in simulated urine sample.
The growing body of recent work in this field has demonstrated that multifilament thread- and textile-based microfluidic devices can also meet the “ASSURED” criteria set forth by the World Health Organisation (WHO) for diagnostic platforms to be affordable, sensitive, specific, user-friendly, rapid, equipment-free, and deliverable to the users.14
However, the development of multifilament thread and textile-based microfluidic diagnostic devices is still in its early stages; it requires the accumulation of a larger body of innovation, knowledge and skills in fabrication techniques and detection methods in the near future to enable this platform to reach maturity. In addition, a strong need to be able to accurately characterize liquid transport behaviour within threads and textile materials exists. Furthermore, the ability to embed such devices for direct sample collection remains an exciting prospect which deserves further investigation and would be useful for applications in both developed and developing regions.
In this paper, the advantages and limitations of multifilament thread and textile-based microfluidic devices are discussed which can be considered for further development of these devices in future work. We also summarised some of the wetting and mixing modulation methods demonstrated in some devices. Finally, we review the currently employed material treatment, fabrication, and detection techniques using thread and fabric materials.
ADVANTAGES AND LIMITATION OF CURRENT MULTIFILLAMENT THREAD- AND TEXTILE-BASED MICROFLUIDIC DEVICES
Several advantages and limitations of multifilament thread and textile-based microfluidic devices exist. The major advantages of thread as a substrate for low-cost microfluidic diagnostics over existing alternatives such as paper are (1) greater tensile strength and flexibility, (2) the ability to easily form 3D structures, (3) the choice of different fibre materials to make thread, (4) simplicity of combining threads into wearable materials, (5) in certain designs, thread microfluidics do not require precise control of sample volumes being introduced into the sensor in order to get a quantitative or semi-quantitative results, (6) that thread can be easily modified to form nitrocellulose thread and make detection zones, etc. On the other hand, limitations include the following: (1) the pores which form flow channels on threads and fabrics display a wide variety of inter fibre gap sizes and weave/twist interstitial porosities and spaces. Therefore, their characterization is dependent on many factors. While the interfibre channels can be correlated with the Washburn equation, it is not easy to do precise modeling, as some channels are fully enclosed by surrounding fibres, while others are not. (2) Thread is a “one-dimensional” material with limited surface area; it is difficult to use various designing and fabrication techniques such as printing. However, fabrics are typically two-dimensional and can be made three-dimensional, therefore have the potential to use unconventional assay reporting techniques such as text reporting demonstrated by Li et al. on paper-based sensors,15 although no such report has been published to date. In the future, new innovation will be required to overcome these limitations and expand upon the advantages of these substrates over alternatives.
ANALYSIS OF WICKING PROPERTY IN THREAD AND TEXTILE
Multifilament threads and textiles have been used in a variety of applications such as clothing and cleaning. Long before the use of these materials for microfluidics, different methods were reported for characterizing and analysing the performance of thread and fabrics for transporting liquids via their capillary structure. Research in this field shows that capillary pressure and permeability calculations can be modeled using Darcy's law and the Lucas-Washburn equation for use in textile technology.16 In order to have the ability to control the flow of fluids in microfluidic devices built with thread and textiles, liquid wicking phenomena in these materials need to be characterized to enable more precise estimation of the time required for sample fluids to transport for given distances.5, 17, 18, 19, 20
Working towards meeting this need, Benltoufa et al. investigated capillary kinetics in micro (Eqs. 1, 3) and macro pores (Eqs. 2, 4) of a cotton jersey knitting to develop two mathematical models. They stated that the capillary rise in micro pores () can be described by the Washburn equation by considering the geometry of the jersey loop lengths formed by fibers. However, in macro pores (), they considered the capillaries as two parallel plates by applying the Washburn style analysis,
| (1) |
| (2) |
| (3) |
| (4) |
where and are capillary kinetics in micro and macropores when , , τ, η, and ρ are the surface tension, tortuosity of capillary tube, viscosity, and density of liquid and g, θ, , and are gravitational acceleration, contact angle, radius of microfibres (microtubes), and parallel-plate width, respectively. They validated this model with a relatively large number of experiments, which showed good correlation with their theoretical models.21
Wang et al. studied liquid wicking properties of polyester yarns and presented results showing that the wicking rate could be increased to a certain level by increasing the number of twists per unit length in the yarn. However, further increases in twisting after this level decreased the liquid flow rate.20 They have also estimated the maximum wicking height by studying capillary force vs gravity according to the Washburn equation, by neglecting the hydrostatic pressure in the following equation:
| (5) |
where is the maximum wicking height reached by the liquid, Rs is the mean static radius of the pores, ∝ and ρ are the surface tension and density of the liquid, θ is the advancing contact angle of the liquid on the solid, and g is the gravitational acceleration. This equation states that the wicking height is related to the pore size of yarn and the physicochemical characteristics of the aqueous sample. In their modelling work, Wang et al. further indicated that the cross sectional geometry of the fibres in a thread has significant influence to the wicking rate.20
Further study by Bhandari et al. had shown that the number of twists per inch (TPI) is an effective modulator of the wicking rate of yarns. The wicking rates of low (∼3 TPI), medium (∼20 TPI), and high (∼50 TPI) yarns were examined and it was observed that the wicking rate was increased by increasing the twist/inch.7
Das et al. modeled liquid wicking of polyester yarns and textile against gravity using the Laplace and the Hagen–Poiseuille equations, and considering the effect of gravity. In their study, pore geometry, liquid contact angle with fibre, number of fibres in a yarn, fibre denier, fibre cross-sectional shape, yarn denier, and twist level in the yarn were considered. They developed a mathematical model of wicking height to show that an increase in the number of fibres in a yarn provides higher wicking rate, whereas an increase in the number of twists per length in the yarns will provide lower wicking rate.22 Their results were somewhat in disagreement with those of Wang et al. as no increase in wicking height was observed for low levels of twist. This difference may be the result of using alternative techniques for twisting and securing the yarns.
In addition, Owens et al. defined a series of parallel and orthogonal microfluidic channels on twisted fabric woven from hydrophobic (poly(ethylene terephthalate)) and hydrophilic (copolyester) spun yarns. Fluid flow from reservoirs was captured and analysed by video microscopy and displayed that inter-yarn microchannels and the geometry of the yarn contacts significantly increased wicking rates.19
Ballerini et al. have estimated the capillary rise of the testing fluid against gravity in single and twined threads. They used the Washburn equation to estimate the relationship between capillary rise height and time. They assumed that r is the equivalent radius of the interfibre cross section in the yarns. Furthermore, the Laplace pressure and the hydraulic pressure due to gravity lead combinely to the capillary driving force for the liquid rise. From this, they obtained the following equation:
| (6) |
where h is the fluid penetration distance along the thread, η, γ, ρ, and θ are the fluid surface tension, viscosity, density, and contact angle, respectively. The first term within the brackets represents the pressure that provides the liquid rising driving force; the second term, the gravity effect, represents the gravitational acceleration.18, 20 This equation shows that gravity influence must be considered for vertical capillary force. This relationship was also reported by Wang et al.20
Safavieh et al. studied liquid wicking in threads without gravity influence, but considered fluid loss via evaporation from the yarn surface, which would be a real problem in open-thread designed systems, as it would result in a decrease of the wicking rate. They estimated the volumetric flow rate of water () by neglecting the air flow using Eq. 7, where A is the surface area of water in contact with air, is the latent heat of water at the specific temperature, and and are the saturation vapour pressures at the water temperature and air dew point, respectively,5
| (7) |
In order to estimate the evaporation loss of water from the yarn's surface, they multiplied the wetted length by the apparent circumference of the yarn which shows a lower limit in comparison to the reality.5
Parikesit et al. also investigated the wetting dynamics of fabric by expanding the Washburn equation23 which estimates the capillary-driven rise only in narrow, hydrophilic, and vertically orientated tubes.17 They vertically placed and monitored two kinds of fabrics with different weaving models (grid and seat cover cloth). They captured images of rising dye solution in the fabrics and processed the data with an image software package (http://rsbweb.nih.gov/ij/) to study liquid rising dynamics in these samples. Parikesit et al. reported that the flow dynamics of the fabrics qualitatively follow Washburn's equation; however, the quantitative values differ significantly. In addition, their investigation of the wettability variation demonstrated that the liquid wetting dynamics on fabric are influenced by the fabric geometry (roughness) and fibre surface property (hydrophilicity).17 Therefore, in order to modulate the wetting property of microfluidic fibre-based channels, a variety of parameters such as fibre type, fibre surface property, and weaving patterns should be considered.17
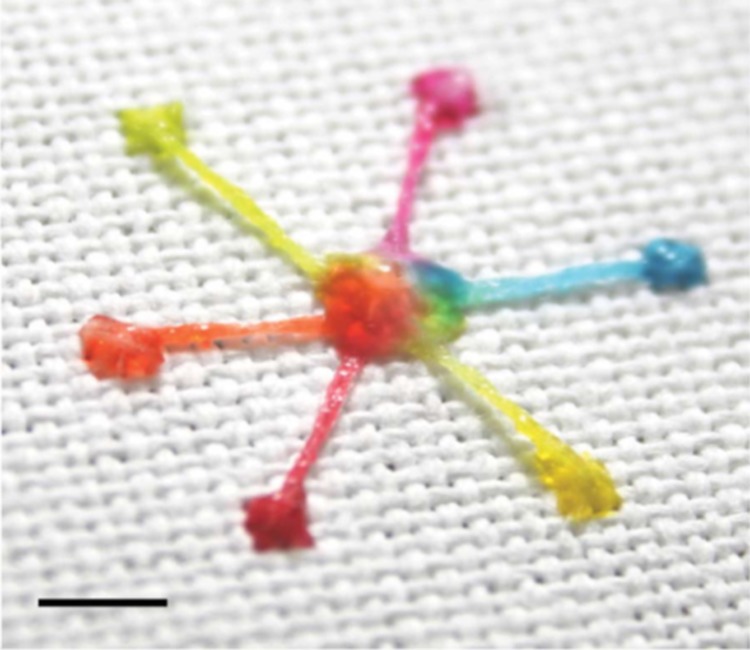
Most recently, Xing et al. fabricated micro-patterns on super hydrophobic textile (MST) for transporting microliter volumes of fluids by means of capillary wicking.24 They used a hydrophilic cotton yarn stitched into superhydrophobic fabric to create an interfacial transport model by considering the lengths of the yarn and bundle arrangement (Fig. 1). Therefore, the wetting contrast enables the aqueous sample to completely deposit along the hydrophilic channels where the internal pressure () is related to sample volume (V). They have obtained Eq. 8, Eq. 9 derived from the Laplace's Law to predict the and V,
| (8) |
| (9) |
where, h, D, and γ are the height of the droplet, size of the hydrophilic pattern in term of diameter, and the surface tension, respectively.
Figure 1.
Image of a multi-inlet–single-outlet design fabricated using hydrophilic cotton yarn on superhydrophobic textile (scale bar: 5 mm). The hydrophilic channels are able to transfer aqueous liquids using the interfacial transport concept. Reprinted with permission from Xing et al., Lab Chip 13(10), 1937 (2013). Copyright 2010 Royal Society of Chemistry.
The above-mentioned work on liquid wicking rate in thread and textile materials showed that liquid wicking obeys qualitatively the Washburn equation, i.e., the wicking distance is proportional to the square root of time. The quantitative description of wicking dynamics, however, depends on parameters such as fibre properties, yarn properties, and the fabrics' weaving patterns and models. A serious of issue that requires further research will be to quantify or to predict liquid loss during transport on threads and textiles in order to gain more precise control of liquids in sensing devices made of these materials.
TREATMENT METHODS AND WICKING RATE
Natural and some synthetic threads require surface treatment to enhance their liquid wicking property. An example is thread made from natural cotton fibres, which have wax on the fibre surface and are not readily wettable by water. Several methods have been employed by different groups to remove the fibre surface wax. Li et al. utilized vacuum plasma to treat cotton thread to increase the fibre surface oxygen concentration and polarity. This treatment strongly improved the wettability of the cotton yarn, making it suitable for building thread-based microfluidic devices. Li et al. characterized the surface of the treated and untreated cotton threads using X-ray photoelectron spectroscopy (XPS) and demonstrated, expectedly, that plasma treatment significantly oxidizes the fibre surface, generating O-C-O, C = O, and O-C = O groups which enhance surface polarity. They further characterized the apparent surface energy of the treated and untreated threads by employing a surface tension matching method.4, 25 The apparent surface energy characterization enabled the prediction from the liquid's surface tension of its wettability to a thread.4 For thread made from plant fibres, mercerization is an effective way to remove wax from the fibre surface. Mercerization of cotton thread usually involves scouring the thread with NaOH solution followed by neutralization in an acid bath to remove the wax from the fibres. Apart from increasing the wettability of cotton thread, mercerization also improves the mechanical strength of thread.
Following the work by Li et al., Reches et al. reported an empirical study of the liquid wicking rates of nine types of threads (cotton, rayon, hemp, nylon, polyester, wool, 50% cotton/50% acrylic, acrylic, and natural silk) for fabricating thread-based microfluidic devices.6 They showed that plasma treatment improved aqueous liquid wicking rates on natural as well as synthetic fibres, but the chemical composition of the thread surface before and after the plasma treatment and the surface energies of the thread were not characterized. Table TABLE I. shows the results of liquid wicking in different threads, where Rwb and Rwa are the average of wicking rate before and after plasma oxidation, respectively.
TABLE I.
Wicking rate for different types of threads. Reprinted with permission from Reches et al., ACS Appl. Mater. Interface 2(6), 1722 (2010). Copyright 2010 American Chemical Society.
| Threads | Rwb (cm/s) | Rwa (cm/s) |
|---|---|---|
| Rayon | 0.29 ± 0.06 | 1.01 ± 0.69 |
| Hemp | 0.02 ± 0.01 | 0.55 ± 0.55 |
| Nylon | 0.03 ± 0.00 | 0.04 ± 0.01 |
| Cotton | 0.23 ± 0.04 | 1.89 ± 0.52 |
| Polyester | 0.13 ± 0.03 | 1.98 ± 0.79 |
| Wool | Did not wick | 2.20 ± 0.40 |
| 50% Cotton, 50% Acrylic | Did not wick | 2.11 ± 0.30 |
| Acrylic | Did not wick | 1.91 ± 0.42 |
| Natural silk | Did not wick | 0.60 ± 0.21 |
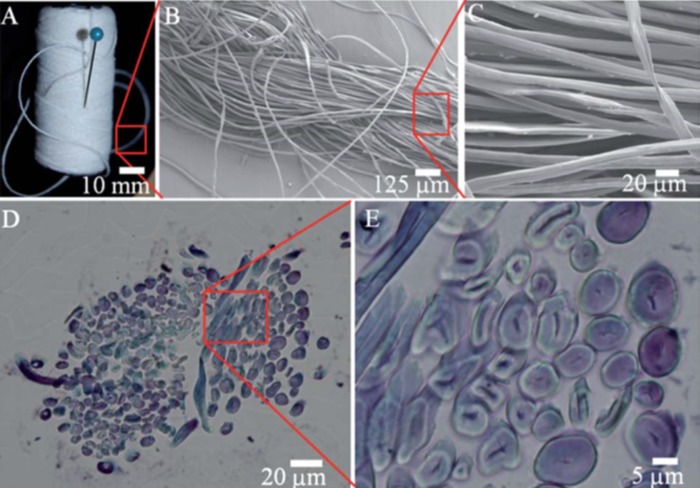
Safavieh et al. have also shown that the capillary action of threads made from natural fibres provides slow wicking rate, which is sensitive to humidity of the environment, but could be improved by plasma activation and hydrophilization. In addition, they have demonstrated that both the gaps between the fibres and the porous structures of the fibres themselves contribute to liquid wicking along the thread and can be filled with aqueous solution (Fig. 2).5 Furthermore, they have shown that knots can be utilized as effective mixers and applied for fabrication of branched microfluidic devices.5
Figure 2.
Images of cotton yarn across a range of different scales. (a) Cotton yarn, (b) and (c) SEM images of the yarn in two different scales. (d) The cross-section of cotton yarn and (e) enlarged view demonstrating lumen contribution to fluidic flow. Reprinted with permission from Safavieh et al., Lab Chip 11(15), 2618 (2011). Copyright 2011 Royal Chemical Society.
Bhandari et al. treated silk yarn by boiling and immersing it in a blocking solution. Afterwards, the wetting and wicking properties of treated and untreated yarns were evaluated by dropping microliter volumes of dye solution onto the yarns. These authors demonstrated that the procedure had made the silk more hydrophilic and enabled it to wick a predefined volume of coloured dye solution.7
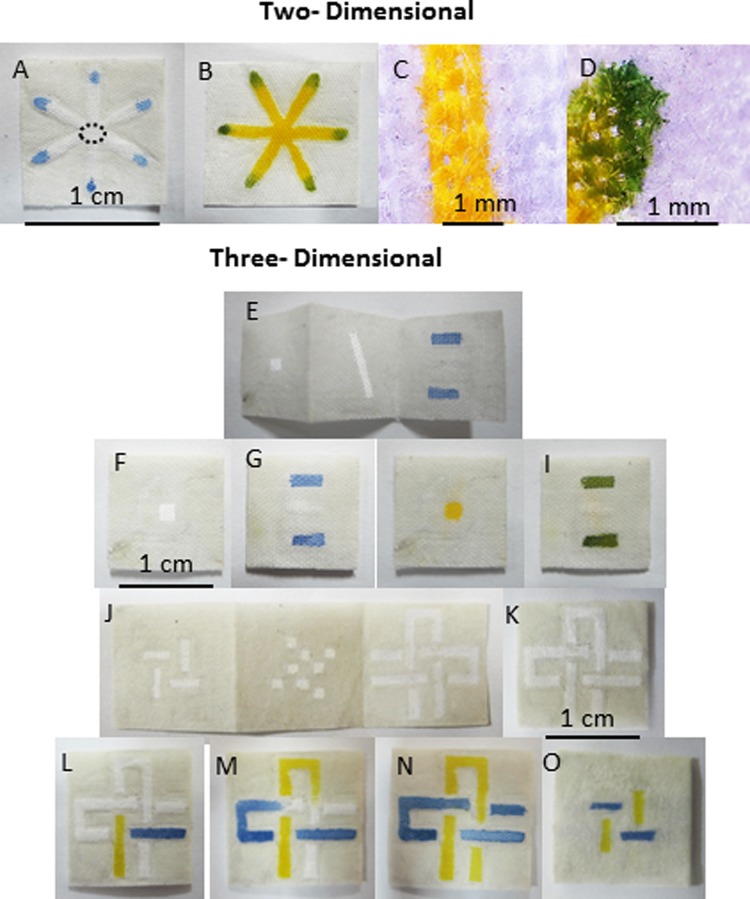
Nilghaz et al. have used sewn cotton cloth which is inherently wettable and hydrophilic due to the porous structure of cloth formed by the gaps between the woven threads. They demonstrated that this porous structure is sufficient for providing capillary force and rapid wetting and wicking. However, they have enhanced the wettability of the cotton cloth by performing a scouring treatment using Na2CO3 hot bath which was both safer and more error-tolerant in comparison with NaOH scouring (mercerization).8 The procedure included boiling the cloth in dilute Na2CO3 solution before washing with an ample amount of water to remove any remaining Na2CO3. Then, the scoured and un-scoured cotton cloths were characterized by XPS which indicated that wax in natural cotton fibres was removed from the fibre surface after treatment. Nilghaz et al. have also confirmed that the capillary wicking rates of threads scoured with Na2CO3 has increased (Fig. 3).8
Figure 3.
Images of two-(2D) and three-dimensional (3D) μCADs fabricated by wax patterning on cloth. (a)-(d) Blue and yellow dye solutions were utilized to prove 2D microfluidic concepts. (e)-(o) Fabrication of 3D μCADs created by folding method. Images of the 3D devices (e) before and (f)-(i) after assembly designed for multiple detection. (j)-(o) Photographs showing fluids flowing into two microfluidic channels which cross each other vertically and horizontally without mixing. (l) and (m) Top and (o) bottom layers of the device. Reprinted with permission from Nilghaz et al., Lab Chip 12, 209 (2012). Copyright 2011 Royal Society of Chemistry.
FABRICATION METHODS
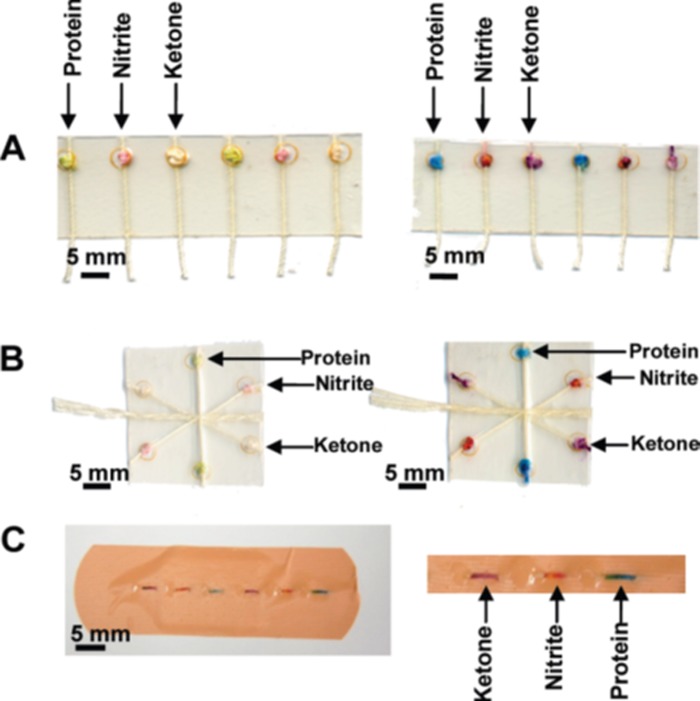
A variety of methods have been reported for the fabrication of multi-filament thread/textile-based microfluidic devices in recent years. The majority of hydrophilic sample wicking channels have been created by two efficient and inexpensive manufacturing techniques. The first involves the use of hydrophilic threads and yarns to build microfluidic channels. Li et al., Reches et al., and Safavieh et al. have explored the use of cotton and polyester threads for fabrication of microfluidic channels.4, 5, 6, 26 An advantage of thread-based microfluidic channel over a paper-based one is that the former does not required barriers to define channels. Li et al. stitched threads through a translucent polymer film using needles or household sewing machines where each stitch was used as a reaction zone. In addition, they demonstrated liquid flow control concept and sample mixing concepts using hydrophilic and hydrophobic cotton threads and by twisting threads together.4 They have also combined threads with punched filter paper discs which were used as reaction zones to form microfluidic devices.4 The Whitesides Group fabricated a “woven array” by encapsulating loom woven threads with knots (as reservoirs for chemical reagents) into transparent and water-impermeable adhesive tape (Fig. 4). The work took advantage of machine looms to speed up the fabrication process, making it suitable for large scale production,6 although the need for encapsulation between layers of tape remained. Safavieh et al. made their microfluidic devices of yarns and knots to have better control on combining, mixing, and splitting of fluidic flow.5
Figure 4.
Multiple colorimetric detection of protein, nitrite and ketone using the (a) woven array device, (b) branching device, and (c) sewn array design. Reprinted with permission from Reches et al., ACS Appl. Mater. Interface 2(6), 1722 (2010). Copyright 2010 American Chemical Society.
The second technique of making sample wicking channels involves the use of hydrophilic woven fabrics. Bhandari et al. introduced woven fabric as a substrate for microfluidic devices. They showed that silk fabric-based microfluidic devices can be produced using a handloom machine which would be suitable for scaling up for use as a unified manufacturing platform.7 Contrasting hydrophilic (boiled and treated with blocking solution) and hydrophobic (brass coated) silk yarns were woven together with a hand-loom to create long hydrophilic channels enclosed by hydrophobic yarn. A variety of 2D microfluidic chips of different size can be designed and constructed using this method,7 although no techniques for fabricating 3-D fabric-based devices were presented.
Nilghaz et al. fabricated 3-D microfluidic cloth-based devices (μCADs) with confined detection zones by restricting flow within the cotton fabric to microfluidic channels formed through the deposition of hydrophobic wax.8 Although μCADs created by Nilghaz et al. have similarities to μPADs, they retain the flexibility and mechanical strength of fabrics. As shown in Fig. 3, this approach has advantages such as having specific detection zones as well as being more compact and embeddable for 3-D devices.8, 27, 28 They fabricated 2-D microfluidic channels on a single layer of cloth using hydrophobic wax resist. This structure conveyed liquid within the hydrophilic channels, allowing the fabrication of distinct detection zones which could be functionalized with colorimetric reagents. This wax-patterned cotton cloth could also be folded and pressed along a predetermined folding line to allow the creation of complex 3-D structures without the need for layers of plastic tape. This functionality was achieved by folding the wax patterned layers together and has the benefit of producing more compact devices.8
Most recently, Xing et al. created 3-D MST by stitching and tightening the mercerized cotton thread into a cotton fabric treated with a superhydrophobic coating. In their devices, fluids have been transported using an interfacial principle along the hydrophilic thread in autonomous, controllable, and continues models.24
FLOW CONTROL CONCEPT
The natural flow patterns that occur in threads is quite special, since it includes fibres of varying lengths and interfibre bonding, as well as great differences in the types of gaps and voids found in the structures of threads, yarns, and fabrics.5, 8 Liquid wicks one dimensionally along a thread. This is very different from the other cellulosic materials such as paper, where liquid penetration is radial, which results in the need for barriers when fabricating paper microfluidics.29 The one-dimensional wicking of liquid in thread has two implications: One is that liquid penetration along the thread is faster than across the thread; this property has been used to design the fabrics patterns using textile technology that can efficiently control the liquid penetration direction in the textile products. The second is that there is no need to accurately measure the analyte sample in order to perform a semi-quantitative analysis, since the liquid penetration length is linear in low volume (≤0.4 μl).11 These properties have been applied by different research groups to have better fluidic control on thread and fabrics.
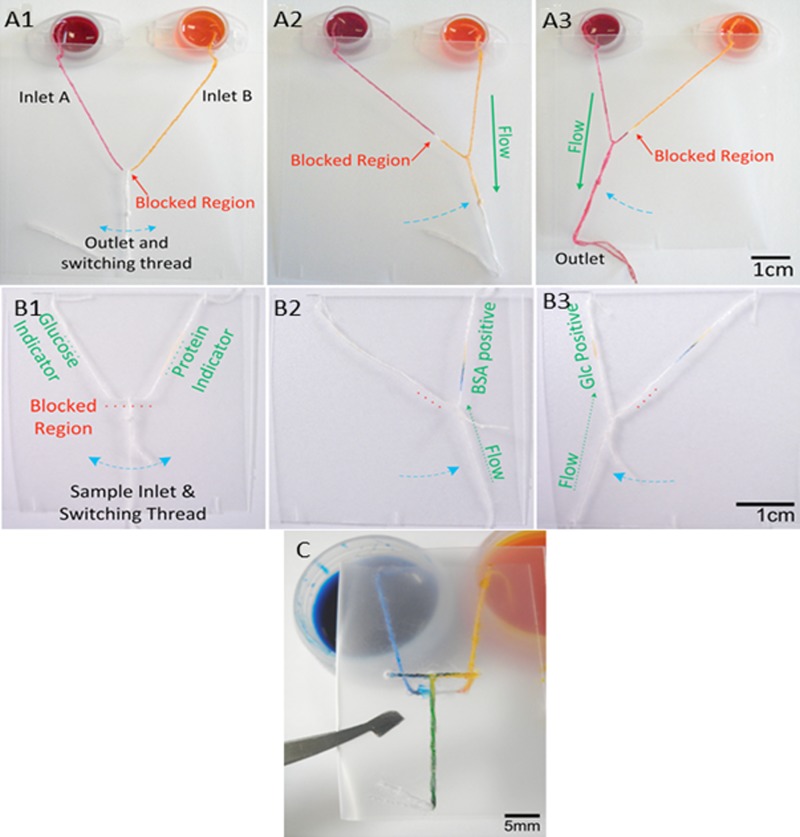
In order to improve the functionality of thread-based microfluidic devices, Ballerini et al. explored various functional elements, such as a binary on/off style switch, selector switches and “micromixers.” They utilized a commonly available adhesive glue as a blocking solution fill the interfibre gaps of a thread, thus restricted capillary flow of the liquid at certain points of the thread channel, creating possibilities to control and direct the liquid flow.18 The binary or on/off type switch was fabricated by tying a sliding knot near a portion of the thread that had been blocked using glue. When in the “off” position, the knot was placed on the blocked portion inhibiting liquid flow along the thread, but it could be activated by sliding the knot away from the blocked portion to allow contact with hydrophilic areas. The selector was fabricated by separating a thread into two parts by applying glue to the thread, then tying a separate thread to the adhesive-blocked section of the original thread with a loose sliding knot. By dragging the knotted thread along the other thread, away from the blockage, the user can select between two different pathways, achieving selectivity of fluid flow. A mixing device was also fabricated using a folding polymer support film, which enabled the user to combine two inlet flows into a single outlet at the time they desired (Fig. 5).18
Figure 5.
Images of differing microfluidic switches to prove the concepts of flow control: (a1)-(a3) simple On/Off switches; (b1)-(b3) the selector switches for choosing between two competing inlets and outlets: (c) mixing switch for combining two inlet flows into a single outlet. Reprinted with permission from Ballerini et al., Biomicrofluidics 5, 014105 (2011). Copyright 2011 American Institute of Physics.
Safavieh et al. also made use of different types of knots for combining, mixing, and splitting fluid flows. They showed that different knots (single and double-stranded knots) provide different levels of flow resistance. Therefore, the mixing ratio of two inlet streams can be controlled by tying different knots, where they prepared a serial dilutor made of double-stranded knots in yarns.5
Working towards the enhancement of fluid control on thread, Li et al. also investigated the liquid penetration along hydrophilic threads to demonstrate the relationship between the volume of aqueous sample and the wicking length of liquid. Their experiments showed that the liquid penetration length along thread increases linearly for small volumes of samples which allow the colour intensity to correlate to this volume range. Therefore, semi-quantitative assays on thread can be carried out without having to precisely control the sample volume when working in this linear range.11
Additionally, work by Nilghaz et al. fabricated 3-D μCADs by patterning a cotton fabric with hydrophobic wax to achieve hydrophilic/hydrophobic contrast which creates barriers to confine fluid flow, creating channels. The patterned fabric then was folded to create 3 layers, where solution can wick along the hydrophilic microchannels but also between channels in adjacently folded layers. In this design, the middle layer controls the fluid flow between top and bottom layers. This method can allow two hydrophilic channels to cross over each other vertically and horizontally without mixing their contents (Fig. 3).8
DIAGNOSTIC AND SYNTHETIC APPLICATIONS
Several research groups have used thread- or textile-based microfluidic devices to conduct biomedical and environmental assays. In this review, we have considered several of these works, listing them by type of substrate, treatments methods, diagnostic applications, etc, in Table TABLE II.. Li et al. conducted a semi-quantitative assay of NO2− on stitches of an indicator-treated cotton thread sewn into a polymer film support. They used a desk top scanner to convert the assay results into electronic images and measured the colour density of the stitches of thread and established calibration curves using serially diluted NO2− solutions (0, 125, 250, 500, 1000 μmol/L).4 Li et al. also fabricated devices using cotton thread and filter paper to perform nitrite assays of simulated human urine. In their study, the punched paper disks of 2 mm in diameter were treated using nitrite indicator reagent and dried at room temperature. Analyte samples wicked along the threads towards the paper discs, where they reacted with the stored reagents to produce a visible pink colour. The intensity of each colour was proportional at low concentrations and asymptotic at high concentrations of nitrite.4 Interpretation of results has been carried out both with the naked eye and also by employing computer-interfaced scanners or cameras and transferring to Microsoft Excel to enable the result to be determined digitally. Paper disks provided larger assay result display area; assay results can be much more easily processed using a scanner and computer software or transmitted electronically. A systematic comparison of linear range, sensitivity, and limit of detection between paper- and thread/fabric-based microfluidic devices has not been reported up to date, although sporadic reports could be found in literature. Li et al. used same colorimetric protocols for detecting nitrite on both paper and thread. According to their work, the linear range obtained for the nitrite assay on thread covered the clinical range and performed even better than on paper. The linear ranges for detecting nitrite are 0–900 and 0–500 μM on thread and paper, respectively.4
TABLE II.
Property comparisons of the reported multifilament- and textile-based devices.
| Substrate | Cotton | Polyester | Silk |
|---|---|---|---|
| Chemical composition | Cellulose fibre8 | Polyethylene terephthalate (PET)39 | Fibroin40 |
| Treatments for increasing hydrophilicity | Plasma oxidation4 | Plasma oxidation30 | Boiling7 |
| Mercerization6 | |||
| Washing with sodium carbonate8 | As received36 | ||
| Boiling in sodium carbonate (1.5 wt%) and hand soap solution24 | |||
| Treatment toward increasing hydrophobicity | Wax8 | Nail polish, glue18 | Brass coating7 |
| Superhydrophobic fluoropel24 | |||
| Nail polish6 | |||
| Form of material | Thread4, 36 | Thread30, 37 | Thread36 |
| Yarn5, 24 | |||
| Fabric patch8 | |||
| Stich4, 24 | |||
| Mixing with paper4 | Yarn18 | Fabric patch7 | |
| Diagnostic Applications | Colorimetric assay (nitrite, glucose, protein and alkaline phosphate assays)4, 6, 8 | Electrochemical detection37 | Colorimetric assay (glucose and ferric hydroxide assay)36 |
| Immunochromatographic assay31 | Blood grouping30 | Immunoassay7 | |
| pH detection34 | |||
| Functional device | Splitter5 | Micromixer18 | Synthesis substrate36 |
| Mixer5 | Microselector18 | ||
| Mixing ratio controller5 | On/Off switch18 | ||
| Human body fluid (simulated/real) | Urine6, 8 | Whole blood30, 36 | Blood plasma36 |
| Sweat34, 24 | |||
| Blood plasma36 |
In addition, Reches et al. demonstrated qualitative multiplexed colorimetric assays using a thread-based device to analyze artificial urine. They immersed cotton threads into colorimetric indicator reagents for the detection of protein, nitrite, and ketones which are indicators of kidney dysfunction, infection, and diabetic ketoacidosis, respectively (Fig. 4). Reches et al. also demonstrated enzymatic-based detection by combining cotton thread with polyacrylamide gels which were impregnated with the required reagents and enzymes for the detection of glucose and alkaline phosphatase in simulated urine and plasma, respectively. The end of the thread was used as an inlet point for absorbing the sample analyte. They showed that when using these gel particles, reagents remained active for at least 5 days at ambient temperatures.6
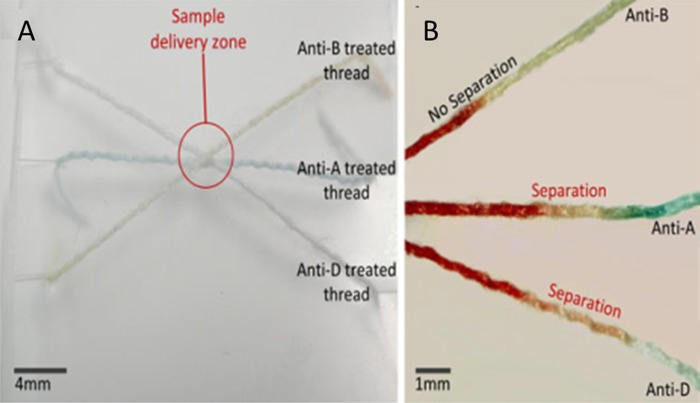
Ballerini et al. also used thread as a flexible and inexpensive substrate for the rapid ABO/rhesus (RhD) grouping of human blood.30 As shown in Fig. 6, they successfully determined the ABO and RhD group of a 2 μl sample of whole blood obtained from a finger prick. This work demonstrated the capability of thread for carrying out biomedical assays by reducing sample and reagent volumes.30
Figure 6.
Blood grouping test performed on threads using agglutinating antibodies reagents (anti-A, anti-B, and anti-D) and whole blood. (a) The device created by tiding antibody treated threads together. (b) The separation of red blood cells and plasma shows that the blood is of type A+. Reprinted with permission from Ballerini et al., Anal. Bioanal. Chem. 399(5), 1869 (2011). Copyright 2011 Springer.
Work by Bhandari et al. demonstrated the possibility of performing direct immunoassays on fabric-based microfluidic devices. They utilized reagent coated yarns to fabricate a parent strip of 125 mm length and 5 mm width which could be cut into small strips and which had been designed as a platform for a polyclonal Goat Anti-Rabbit (GAR)-Rabbit IgG system.7 The yarn was pre-blocked prior to coating with detection conjugate (including GAR IgG conjugated to gold nano-particles). The predetermined test and control regions of yarns were coated with Rabbit IgG and Mouse Anti-Goat IgG, respectively. The analyte sample and buffer was wicked by the strip to react with reagents in the test and control zones.7
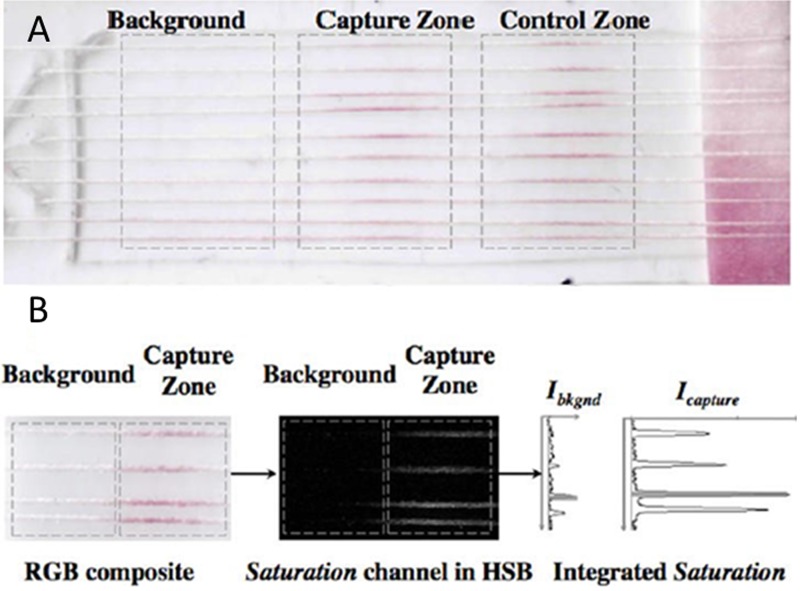
In other work, threads were used as hydrophilic channels to transfer and mix analyte samples for immunochromatographic assays. A cotton thread was knotted to a nylon fibre bundle which had been coated with antibodies which act against three different target analytes (CRP, osteopontin, and leptin proteins). This device was used for performing immunochromatographic assays on thread (ICAT) (Fig. 7).31, 32
Figure 7.
Images of the ICAT and its signal quantification. (a) Three assay zones are demonstrated on multicolumn threads: “background,” “capture zone,” and “control zone.” (b) The image of the assay is analysed using ImageJ software in red-green-blue (RGB) and hue-saturation-brightness (HSB) mode. The intensity (I) is calculated by subtracting the Ibkgnd from Icapture. Reprinted with permission from Zhou et al., Anal. Chem. 84, 7736 (2012). Copyright 2012 American Chemical Society.
Coyle et al. worked on embedding wearable chemosensors into clothing which were an integration of chemical sensors into a textile matrix. They presented textile-based microfluidic devices which were embedded into wearable textile to collect and analyse sweat in-situ, providing real-time feedback to the subjects wearing them.33 After this work, Curto et al. further developed a device for monitoring sweat pH during cycling activity using wearable sensors. They also improved micro-fluidic chip functionality by combining with miniaturized electrical components.34
Vatansever et al. fabricated pH-sensitive sensors using pH-sensitive polymers such as PAA [poly(acrylic acid)] and P2VP [poly(2-vinyl pyridine)] that redirect aqueous liquids toward PAA channels at pH > 4 and toward both PAA and P2VP channels at pH < 4. These kinds of devices can be embedded as close as possible to wearable products to fabricate the smart cloth.35
Banerjee et al. demonstrated the fabrication and detection of ferric hydroxide and 2,4-dichloro-N-(2-morpholinoethyl) benzamide by colorimetric detection on both cotton and silk threads. Their method showed ∼84% and 72% for yield ferric hydroxide and 2,4-dichloro-N-(2-morpholinoethyl) benzamide, respectively, comparing to test tube-based method. They applied surface chemistry to avoid the adsorption of cations (Fe3+and NH4+) on hydroxyl groups of cellulose by using acidulated ferric chloride.36
Besides the many different instances of colorimetric detections on thread, Wei et al. developed a method of electrophoretic separation and electrochemical detection of ion samples using thread. In their study, plasma treated and untreated polyester threads were affixed on a PMMA chip using protruding sleeper structures. This device was used for separating the mixed ion samples (Cl−, Br−, and I−) and detecting potassium ferricyanide (K3Fe(CN)6) at a detection limit of 6.25 μM.37
FUTURE TRENDS
All of the aforementioned studies illustrate the efficacy of multifilament thread and textiles for creating effective and inexpensive platforms for performing a variety of multiplexed diagnostic tests. However, research in this area remains at an early stage of development and requires a variety of future investigations into diagnostic, POC, and environmental monitoring applications. There exists the capability for embedding thread into wearable clothing and products, putting diagnostic devices close to real subjects. The use of these embedded sensors is not restricted to underdeveloped areas, as convenient sample collection is desired equally in developed regions.35
The well-established textile industry will also significantly benefit from the future development and large-scale fabrication of thread and textile-based sensors. To date, most multifilament thread and textile-based microfluidic devices have been made using cotton; in future, other grades of natural and synthetic multifilament threads with unique properties may be used for different purposes such as detection of surface temperature, UV-radiation, or microorganisms.38
Understanding liquid transport behavior within multifilament thread and textiles is be essential for control fluid flow and estimate the required volumes of aqueous liquid samples and reagents. Liquid transport behavior on thread and fabric specifically for microfluidic diagnostics deserves further investigation and reconsideration, since the major investigations in this field have been performed by textile technology scientists working towards achieving very different goals and applications.
Furthermore, applying different fabrication and detection methods for creating thread-based microfluidic systems has been restricted due to its small thickness which is not suitable for colorimetric detection. Therefore, future works can apply thread for various identification methods.
Surface modification of fibres is important for making thread and fabric-based microfluidics. Pore structure in thread is very different from that found in paper. Synthetic characterization of the pore structure may further enrich our understanding of the advantages of thread and fabric materials over paper for certain specific tasks of microfluidic analysis. Surface modification of fibres in thread may substantially enhance the sample filtration and analyte detection abilities of thread and fabrics.
SUMMARY
Work to-date shows that there is a great potential for thread and fabrics to be further studied as materials for microfluidics. This includes investigation of fibre surface treatments and functionalization; more quantitative characterization of pore structures of threads and fabrics; and using the existing manufacturing capabilities of the textile industries to explore new technical possibilities of thread and fabric microfluidics. This paper reviewed the progress made in the use of multifilament fibres and threads as hydrophilic/hydrophobic platform for the fabrication of thread and textile-based microfluidic devices. Liquid wicking along a thread depends on the level of hydrophobicity of the thread surface, which can be modified by treatment. The voids which form microchannels between filaments and the weave/twist porosity of threads and fabrics enable strong wetting and wicking properties which make them appropriate for use as microfluidic conduits for fluid flow without the need of external pumps. Such devices may be mass-fabricated using well established and inexpensive mass-manufacturing techniques for spinning, twisting, and dying which make them appropriate for diagnostic applications in developing regions. These alternative materials also have the capability to be combined with other hydrophilic/hydrophobic materials. Several methods have been applied to form hydrophilic-hydrophobic contrast and liquid control for fabricating complete microfluidic systems. In addition, different diagnostic methods can be utilized in conjunction with these substrates such as well-established colorimetric detection techniques, immunoassay and electrochemical detection mechanisms, and many others.
On the other hand, the durability and flexibility of fibre-based substrates allow us to have more compact and embeddable devices which can be implemented as close as possible to real subjects, pushing the boundaries of what is possible for real-time sample collection and analysis across a range of applications. Although much progress has been made, there remains a strong need to better understand, model, and control the flow of reagents and samples within these devices.
ACKNOWLEDGMENTS
This work was supported by the Australian Research Council Grant (DP1094179). The research scholarships of The Monash University and the Department of Chemical Engineering are gratefully acknowledged. The authors would also like to extend their gratitude to the copyright owners of the many figures which were permitted to be reproduced within this work.
References
- Nie Z., Nijhuis C. A., Gong J., Chen X., Kumachev A., Martinez A. W., Narovlyansky M., and Whitesides G. M., Lab Chip. 10(4), 477 (2010). 10.1039/b917150a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. W., Phillips S. T., and Whitesides G. M., Proc. Natl. Acad. Sci. U.S.A. 105, 19606 (2008). 10.1073/pnas.0810903105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tian J., Nguyen T., and Shen W., Anal. Chem. 80(23), 9131 (2008). 10.1021/ac801729t [DOI] [PubMed] [Google Scholar]
- Li X., Tian J., and Shen W., ACS Appl. Mater. Interfaces 2(1), 1 (2010). 10.1021/am9006148 [DOI] [PubMed] [Google Scholar]
- Safavieh R., Zhou G. Z., and Juncker D., Lab Chip 11(15), 2618 (2011). 10.1039/c1lc20336c [DOI] [PubMed] [Google Scholar]
- Reches M., Mirica K. A., Dasgupta R., Dickey M. D., Butte M. J., and Whitesides G. M., ACS Appl. Mater. Interfaces 2(6), 1722 (2010). 10.1021/am1002266 [DOI] [PubMed] [Google Scholar]
- Bhandari P., Narahari T., and Dendukuri D., Lab Chip 11(15), 2493 (2011). 10.1039/c1lc20373h [DOI] [PubMed] [Google Scholar]
- Nilghaz A., Wicaksono D. H. B., Gustiono D., Abdul Majid F. A., Supriyanto E., and Abdul Kadir M. R., Lab Chip 12(1), 209 (2012). 10.1039/c1lc20764d [DOI] [PubMed] [Google Scholar]
- Ballerini D. R., Li X., and Shen W., Microfluid. Nanofluid. 13(5), 769 (2012). 10.1007/s10404-012-0999-2 [DOI] [Google Scholar]
- Ballerini D. R., Li X., and Shen W., in 5th European Conference of the International Federation for Medical and Biological Engineering 14 - 18 Sept. 2011, Budapest, Hungary, IFMBE Proceedings Vol. 37, edited by Jobbágy Á. (Springer, 2011), p. 1019.
- Li X., Ballerini D., Tian J., and Shen W., in Proceedings of Sustainable Chemistry Conference, Antwerp, Belgium, 6 - 8 July 2011, edited by Reniers G. and Brebbia C. A. (Wessex Institute of Technology, UK, 2011), p. 233.
- Kunst L. and Samuels A. L., Prog. Lipid Res. 42(1), 51 (2003). 10.1016/S0163-7827(02)00045-0 [DOI] [PubMed] [Google Scholar]
- Hohenberger E. and Kimling H., Compendium Urinalysis with Test Strips (Roche Diagnostics, 2004). [Google Scholar]
- Mabey D., Peeling R. W., Ustianowskis A., and Perkins M. D., Nature 2, 231 (2004). 10.1038/nrmicro841 [DOI] [PubMed] [Google Scholar]
- Li M., Tian J., Al-Tamimi M., and Shen W., Angew. Chem., Int. Ed. 51(22), 5497 (2012). 10.1002/anie.201201822 [DOI] [PubMed] [Google Scholar]
- Washburn E. W., Phys. Rev. 17(3), 273 (1921). 10.1103/PhysRev.17.273 [DOI] [Google Scholar]
- Parikesit G. O. F., Prasetia F., Pribadi G. A., Simbolon D. C., Pradhana G. Y., Prastowo A. R., Gunawan A., Suryopratomo K., and Kusumaningtyas I., J. Text. Inst. 103(10), 1077 (2012). 10.1080/00405000.2012.660756 [DOI] [Google Scholar]
- Ballerini D. R., Li X., and Shen W., Biomicrofluidics 5(1), 014105 (2011). 10.1063/1.3567094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. L., Leisen J., Beckham H. W., and Breedveld V., ACS Appl. Mater. Interfaces 3(10), 3796 (2011). 10.1021/am201003b [DOI] [PubMed] [Google Scholar]
- Wang N., Zha A., and Wang J., Fibers Polym. 9(1), 97 (2008). 10.1007/s12221-008-0016-2 [DOI] [Google Scholar]
- Benltoufa S., Fayala F., and BenNasrallah S., J. Eng. Fibers Fabr. 3(3), 47 (2008); available at http://www.jeffjournal.org. [Google Scholar]
- Das B., Das A., Kothari V. K., and Fangueiro R., J. Text. Inst. 102, 14 (2011). 10.1080/00405000.2010.529281 [DOI] [Google Scholar]
- de Gennes P. G., Brochard-Wyart F., and Quéré D., Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves (Springer, New York, 2004). [Google Scholar]
- Xing S., Jiang J., and Pan T., Lab Chip. 13(10), 1937 (2013). 10.1039/c3lc41255e [DOI] [PubMed] [Google Scholar]
- Nisbet D., Pattanawong S., Ritchie N., Shen W., Finkelstein D., Horne M., and Forsythe J., J. Neural Eng. 4, 35 (2007). 10.1088/1741-2560/4/2/004 [DOI] [PubMed] [Google Scholar]
- Safavieh R., Mirzaei M., Qasaimeh M. A., and Juncker D., in MicroTAS 2009, Jeju, South Korea (Chemical and Biological Microsystems Society, 2009), p. 685. [Google Scholar]
- Nilghaz A., Wicaksono D. H. B., and Abdul Majid F. A., in Proceedings of 2011 2nd International Conference on Instrumentation Control and Automation (ICA 2011), Bandung, Indonesia (IEEE, 2011), p. 82. 10.1109/ICA.2011.6130134 [DOI]
- Nilghaz A., Wicaksono D. H. B., and Supriyanto E., in Proceedings of 2011 2nd International Conference on Instrumentation Control and Automation (ICA 2011), Bandung, Indonesia (IEEE, 2011), p. 266. 10.1109/ICA.2011.6130169 [DOI]
- Martinez A. W., Phillips S. T., Whitesides G. M., and Carrilho E., Anal. Chem. 82(1), 3 (2010). 10.1021/ac9013989 [DOI] [PubMed] [Google Scholar]
- Ballerini D. R., Li X., and Shen W., Anal. Bioanal. Chem. 399(5), 1869 (2011). 10.1007/s00216-010-4588-5 [DOI] [PubMed] [Google Scholar]
- Zhou G., Mao X., and Juncker D., Anal. Chem. 84, 7736 (2012). 10.1021/ac301082d [DOI] [PubMed] [Google Scholar]
- Zhou R. S. G. Z., Mao X., and Juncker D., in Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 2010 (Chemical and Biological Microsystems Society, 2010), p. 25. [Google Scholar]
- Coyle S., Benito-Lopez F., Byrne R., and Diamond D., in Wearable and Autonomous Biomedical Devices and Systems for Smart Environment, Lecture Notes in Electrical Engineering Vol. 75 (Springer, 2010), p. 177. [Google Scholar]
- Curto V. F., Coyle S., Byrne R., Diamond D., and Benito-Lopez F., Sensors Actuators B 175, 263 (2012). 10.1016/j.snb.2012.02.010 [DOI] [Google Scholar]
- Vatansever F., Burtovyy R., Zdyrko B., Ramaratnam K., Andrukh T., Minko S., Owens J. R., Kornev K. G., and Luzinov I., ACS Appl. Mater. Interfaces 4, 4541 (2012). 10.1021/am3008664 [DOI] [PubMed] [Google Scholar]
- Banerjee S. S., Roychowdhury A., Taneja N., Janrao R., Khandare J., and Paul D., Sens. Actuators B 186, 439 (2013). 10.1016/j.snb.2013.06.036 [DOI] [Google Scholar]
- Wei Y. C., Su S. Y., Fu L. M., and Lin C. H., in 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France 29 January-2 February 2012 (IEEE, 2012), p. 104. 10.1109/MEMSYS.2012.6170104 [DOI]
- Truong Y. B., Glattauer V., Briggs K. L., Zappe S., and Ramshaw J. A. M., Biomaterials 33(36), 9198 (2012). 10.1016/j.biomaterials.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Osipenko I. F., Lopatik D. V., Bondareva O. M., Levdanskii V. A., Gorbatenko A. P., and Karpova O. Y., Fibre Chem. 21(3), 228 (1990). 10.1007/BF00549653 [DOI] [Google Scholar]
- Marsh R. E., Corey R. B., and Pauling L., Biochim. Biophys. Acta 16, 1 (1955). 10.1016/0006-3002(55)90178-5 [DOI] [PubMed] [Google Scholar]