Abstract
Acetic acid bacteria (AAB) are obligately aerobic bacteria within the family Acetobacteraceae, widespread in sugary, acidic and alcoholic niches. They are known for their ability to partially oxidise a variety of carbohydrates and to release the corresponding metabolites (aldehydes, ketones and organic acids) into the media. Since a long time they are used to perform specific oxidation reactions through processes called “oxidative fermentations”, especially in vinegar production. In the last decades physiology of AAB have been widely studied because of their role in food production, where they act as beneficial or spoiling organisms, and in biotechnological industry, where their oxidation machinery is exploited to produce a number of compounds such as l-ascorbic acid, dihydroxyacetone, gluconic acid and cellulose. The present review aims to provide an overview of AAB physiology focusing carbon sources oxidation and main products of their metabolism.
Keywords: Acetic acid bacteria, Oxidative fermentation, Cellulose, Acetobacter, Gluconacetobacter, Gluconobacter oxydans
Introduction
Acetic acid bacteria (AAB) are strictly aerobic bacteria occurring in sugary, alcoholic and acidic niches such as fruits, flowers and particularly fermented beverages [1–4]. Although foods are the most known sources of AAB, they play role as plant-associated bacteria (N2 fixing), symbionts of insects and human pathogens [5, 6].
The metabolic potential of AAB in these environments is expressed by the partial oxidation of carbohydrates releasing the corresponding products (aldehydes, ketones and organic acids) into the surrounding media. Through processes called “oxidative fermentations”, AAB perform specific oxidation reactions and channel the released electrons to molecular oxygen. Due to these ability they are known since a long time especially for their role in vinegar production [7].
Vinegar, an aqueous solution of acetic acid (AcOH) that is produced by AAB from a dilute ethanol (EtOH) solution [8], was the first investigated environment concerning the biological formation of AcOH. Early researches [9] allowed to recognize that the surface layer during vinegar formation, commonly known as “mother of vinegar”, was a mass of living microorganisms causing AcOH production.
AAB are also involved in the production of other foods, like palm wine [10], cocoa powder [11], nata de coco (a fermented food from coconut), pulque (a beverage from agave) and kombucha, a slightly acid and sparkling beverage obtained from tea fermentation by a symbiotic culture of AAB and yeasts [8].
They can spoil fermented beverages such as wine, cider and beer, where the production of AcOH is undesired [12], whereas in others foods, such as sourdough for bread production [13], AAB can occasionally occur contributing to the acidification of dough.
Besides fermented foods, some AAB are used as biocatalysts for the industrial production of a range of compounds, making them important biocatalysts for the development of eco-friendly fermentation processes as an alternative to the chemical synthesis. Strains of Gluconobacter oxydans produce enzymes involved in amino acids synthesis e.g. glutamic and aspartic acids thanks to the incomplete set of tricarboxylic acid (TCA) enzymes which could function primarily for glutamate, aspartate and succinate biosynthesis [14].
One of the most important biotechnological application of G. oxydans is the production of l-ascorbic acid (vitamin C) precursors such as l-sorbose from d-sorbitol and 2-keto-l-gluconic acid from 2,5-diketo-d-gluconic acid or l-sorbosone [15].
Strains of G. oxydans are also exploited for the microbial production of dihydroxyacetone (DHA) that is used in the pharmaceutical industry as a cosmetic tanning agent and also as an intermediate for the synthesis of various organic chemicals and surfactants [16].
Among organic acids, gluconic acid, used as a bulk chemical in the food, textile, medical and construction industries can be produced by G. oxydans which oxidize glucose to gluconate by the membrane bound glucose dehydrogenase [17].
Other applications of G. oxydans are the production of miglitol’s precursors, used as a therapeutic drug for the treatment of non-insulin-dependent diabetes mellitus; d-tagatose, used as a bulking agent in food and a non-calorific sweetener; and shikimate, which is a key intermediate for a number of antibiotics [16].
Species of Acetobacter and Gluconacetobacter genera, besides vinegar, are of interest in agricultural field, where especially strains of Ga. diazotrophicus have been proved useful for their role as N2 fixing bacteria [5]. Finally Ga. xylinus is well known for the ability to produce high amount of pure cellulose [18].
On the basis of the increasing prospective on AAB for food, beverages and other biotechnological applications, this work aims to review physiology of AAB including carbon sources oxidation and metabolites production.
General Phenotypic Characteristics and Taxonomic Aspects of Acetic Acid Bacteria
AAB are gram negative or gram-variable, non-spore forming, ellipsoidal to rod-shaped cells that can occur in single, pairs or in short chains. They could be motile due to the presence of peritrichous or polar flagella. Catalase positive and oxidase negative, AAB have an obligate aerobic metabolism, with oxygen as the terminal electron acceptor. The optimum pH for the growth is 5–6.5, while they can grow at lower values (3–4) [1]. Their optimum temperature vary between +28 and +30 °C although some species are recognized as thermotolerant [19, 20]. They can produce pigments and also different kinds of exopolysaccharides [21]. Main distinctive traits of AAB are reported in Table 1.
Table 1.
Distinctive characteristics of acetic acid bacteria genera
| Characteristic | G. | A. | Ga. | Ac. | As. | K. | Sw. | Sa. | N. | Gr. | Am. | T. | Ne. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production of AcOH | + | + | + | + | − | + | + | v (w/−) | + | v (w/−) | + | − | − |
| Oxidation of | |||||||||||||
| Acetate to CO2 and H2O | − | + | + | + | w | w | w | − | − | w | + | − | − |
| Lactate to CO2 and H2O | − | + | + | v (−/w) | w | w | w | − | + | w | − | − | |
| Growth on 0.35 % AcOH (pH 3.5) | + | + | + | + | − | + | + | − | + | nd | + | + | − |
| Growth in the presence of 1 % KNO3 | − | − | − | + | − | − | + | nd | − | nd | − | − | − |
| Production of keto-d-gluconic acid from d-glucose | |||||||||||||
| 2,5-Diketo-d-gluconic acid | v | − | v | − | − | − | nd | nd | nd | nd | − | + | nd |
| 5-Keto-d-gluconic acid | + | v | v | − | + | + | nd | + | + | nd | + | + | nd |
| 2-Keto-d-gluconic acid | + | v | v | − | + | + | nd | + | + | nd | + | + | nd |
| Production of DHA from glycerol | + | v | v | − | v | + | + | − | w | − | w | + | − |
| Growth on methanol as carbon source | − | v | − | + | − | − | − | − | − | + | vw | − | nd |
| Production of water soluble brown pigment (s) | v | − | v | − | − | − | + | − | − | nd | − | + | − |
| Production of γ-pyrones from | |||||||||||||
| d-glucose | v | − | v | nd | − | − | nd | nd | nd | nd | nd | nd | nd |
| d-fructose | + | − | − | nd | v (+/w) | v | nd | nd | nd | nd | nd | nd | nd |
| Acid production from | |||||||||||||
| l-Arabinose | + | v | v | + | + | + | + | + | + | nd | nd | nd | nd |
| d-arabinose | + | − | − | v | + | v | nd | − | w | nd | nd | nd | nd |
| d-xylose | + | v | v | + | + | + | v | + | + | − | nd | nd | nd |
| l-Rhamnose | − | − | − | − | v | − | − | − | w | nd | nd | nd | nd |
| d-glucose | + | v | + | + | + | + | + | + | + | w | nd | nd | nd |
| d-galactose | + | v | + | + | + | + | + | + | + | nd | nd | nd | nd |
| d-mannose | + | v | v | + | + | + | + | + | + | nd | nd | nd | nd |
| d-fructose | + | − | + | − | + | − | v | v | + | nd | nd | nd | nd |
| l-Sorbose | + | − | v | nd | + | − | nd | − | − | nd | nd | nd | nd |
| Melibiose | + | − | − | v | + | + | nd | + | + | nd | nd | nd | nd |
| Sucrose | + | − | − | − | + | v | nd | + | + | − | nd | nd | nd |
| Raffinose | − | − | − | − | − | + | nd | − | + | nd | − | w | − |
| d-mannitol | + | − | v | − | v | − | − | + | w | − | − | − | − |
| d-sorbitol | + | − | − | − | v | − | + | − | + | − | − | − | − |
| Dulcitol | − | − | − | − | v | − | v | − | w | − | − | − | − |
| Glycerol | + | − | + | + | + | + | + | − | + | v (w/−) | w | + | − |
| EtOH | + | + | + | + | − | + | + | − | + | + | + | + | − |
| Production of cellulose | − | − | v | − | − | − | nd | − | nd | nd | nd | nd | nd |
| Production of levan-like mucous substance (s) from sucrose | − | v | − | − | − | + | nd | − | − | nd | − | − | − |
| Growth in the presence of 30 % d-glucose | − | v | v | + | + | − | nd | + | + | nd | − | + | + |
| Motility and flagellation | N-m (mostly) or po | N-m or pe | N-m or pe | N-m or pe | N-m or pe | N-m | pe | N-m | N-m | N-m | pe | N | N |
| Major ubiquinone | Q10 | Q9 | Q10 | Q10 | Q10 | Q10 | Q10 | Q10 | Q10 | nd | Q10 | Q10 | Q10 |
| G + C content (mol%) | 54–64 | 52–64 | 56–67 | 62–63 | 59–61 | 56–57 | 57–60 | 52–53 | 63.1 | 59 | 66.0 | 65.6 | 56.8 |
Adapted from [24, 56, 57]. G., Gluconobacter; A., Acetobacter; Ga., Gluconacetobacter; Ac., Acidomonas; As., Asaia; K., Kozakia; Sw., Swaminathania; Sa., Saccharibacter; N., Neoasaia; Gr., Granulibacter; Am.; Ameyamaea; T., Tanticharoenia; N., Neokomagataea; + positive; − negative; w weak; v variable; nd not determined; N-m non-motile; po polar; pe peritrichous
AAB are assigned to the order Rhodospirillales as part of the Alphaproteobacteria, within the family Acetobacteraceae. At present they are represented by the following genera: Acetobacter, Acidomonas, Ameyamaea, Asaia, Gluconacetobacter, Gluconobacter, Granulibacter, Kozakia, Neoasaia, Neokomagataea, Saccharibacter, Swaminathania and Tanticharoenia.
The most updated data on valid published species of each genus are reported by the List of Prokaryotic names with standing in nomenclature [22].
Since their first description as “vinegar bacteria”, about 150 years ago [9], classification of AAB has undergone robust changes, with scission, renaming, restoration and emendation of genera and species [23].
During the last decades studies on AAB identity and their phylogenetic relationships have been achieved by polyphasic approaches combining phenotypic, chemotaxonomic and genotypic data of strains. Both the use in a polyphasic strategy of 16S rRNA gene as molecular marker, and the formulation of more suitable isolation media, reflect the increased number of new described genera and species.
However, identification at the species level is often difficult due to the low resolution power of phenotypic characterization and the high sequence similarity (≥99.5 %) of 16S rDNA of phylogenetically closely related species [24].
Weaknesses of phenotypic characterization are generated by a difficult standardisation of tests, in some cases by low number of discriminant characters and by instability of physiological traits of preserved strains [25].
For instance physiological deficiencies caused by inactivation of enzymes, such as the membrane-bound alcohol dehydrogenase (ADH) and cellulose synthase, deriving from genetic instability, can affect reliability of phenotypic assays. The main source of genetic instability has been attributed to mobile genetic elements, mainly transposons, widely distributed in the genome. Insertion sequences (IS) responsible for EtOH oxidation deficiency, like those of the family IS 12528, were found in the chromosome of A. pasteurianus NCIB 7214 (5 copies) [26], A. aceti 1023 (1 copy) and G. oxydans IFO 12528 (10 copies). Likewise 100 copies of IS 1380 occur in A. aceti 1023 and 74 copies in A. pasteurianus NBRC 3283 [1, 27]. Also spontaneous cellulose deficient mutants due to the IS 1031 were detected in Ga. xylinus ATCC 23769 [28].
Advances in taxonomy of AAB derive from the availability of full genome sequences especially of type strains, that allowed the application of new genomic approaches.
To solve ambiguities of phylogenetically closely related species, recently the use of different genes as phylogenetic markers, such as housekeeping genes (dnaK, groEL and rpoB) has been proved useful for AAB species differentiation [29]; whereas protein-coding genes, such as those involved in the metabolism of AcOH, have been applied to investigate phylogenetic relationships among Acetobacter, Gluconacetobacter and Gluconobacter genera [30]. Since multigene analysis can resolve ambiguities in phylogenetic reconstructions when a single gene is not enough, it is expected that the availability of more complete genome sequences will increase the application of these approaches.
Isolation and Cultivability
Isolation and cultivation of AAB strains, especially from fermented beverages, have been described to be problematic, giving rise to an underestimation of species richness when culture dependent methods are applied [31].
Low recovery of strains due to the fraction of population that could reach a viable but non-culturable (VBNC) state has been stated. For instance in wine it was shown that VBNC status of spoiling AAB is induced by O2 deprivation [32]. Whereas in vinegars some studies developed by no culture approaches, such as PCR/DGGE, revealed higher species diversity respect to that detected by culturing methods [3, 33, 34].
Limitations of culturing were in part overcame with the formulation of appropriate media, which allowed the cultivation of no growing strains or slow growing strains.
A number of conventional culture media to isolate AAB from different sources are reported in literature, which carbon source are mainly glucose, mannitol and EtOH. Early researches proposed a variety of media containing yeast-glucose agar (pH 5.5–6.0), peptone-glucose agar fortified with yeast extract and tomato juice, and media containing EtOH (1.5 %) as single carbon source, yeast extract (0.5 %) and agar (2.5 %) [35]. To control the growth of other bacteria and yeasts, these media can be acidified and/or supplemented with antibiotics and cycloheximide [36].
A medium that allows successful isolation from different niches is glucose yeast extract carbonate (GYC) composed by d-glucose, 10 %; yeast extract, 0.5 %; peptone 0.3 %; CaCO3, 0.12 %; and agar, 0.12 % [37]. After incubation, colonies of AAB are recognized by the surrounding zones of CaCO3 clearing. CaCO3 neutralizes AcOH generated by AAB, preventing physiological stress and cell death [25]. The last edition of Bergey’s manual of systematic bacteriology [1] proposed standard medium for enrichment and isolation of AAB, with the exception of Ga. europaeus (which strains are AcOH-dependent), containing: d-glucose, 0.05 %; EtOH, 1.5 %; yeast extract, 0.5 %; peptone, 0.3 %; cycloheximide, 0.01 %; and agar, 0.12 %.
Important advances in recovering AAB strains from industrial vinegar have been reached introducing a double agar layer (0.5 % in the lower layer and 1 % in the upper layer) and media containing EtOH and AcOH, in an attempt to simulate the environment of the acetification tanks. Using this approach the new species Ga. europaeus isolated from industrial wine vinegar was described [38].
It was also stated that isolates from cider or wine vinegar grew readily in reinforced AE-medium (RAE), containing d-glucose, 4.0 %; peptone, 1.0 %; yeast extract, 1 %; citric acid, 0.15 %; disodium hydrogen phosphate (Na2HPO4), 0.38 %; glacial AcOH, 1 ml; absolute EtOH, 1 ml; agar, 0.5 % upper layer; 1 % bottom layer); pH 3.8. The simpler AE medium (glucose, 0.5 %; yeast extract, 0.3 %; peptone, 0.4 %; agar, 0.9 %; absolute EtOH, 3 ml; glacial AcOH, 3 ml) has been proved suitable for the isolation of strains from spirit vinegars [39, 40].
Actually, some of the most widely used isolation media are: GYC, AE; YPM medium (yeast extract, 0.5 %; peptone, 0.3 %; mannitol, 2.5 %; agar, 1.2 %) and MYA medium (malt extract, 1.5 %; yeast extract, 0.5 %; agar, 1.5 %; and EtOH, 60 ml) [41].
Carbon Sources
Ethanol Oxidation
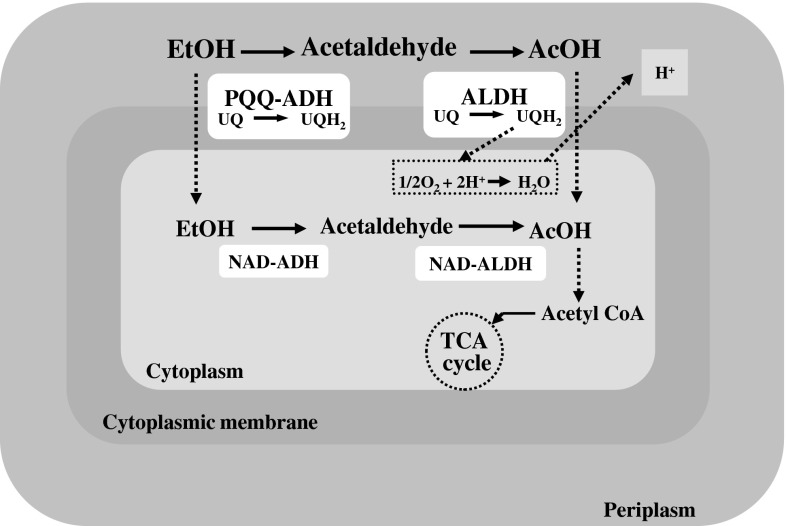
AAB partially oxidize EtOH by two successive catalytic reactions of the ADH and a membrane-bound aldehyde dehydrogenase (ALDH) that are bound to the periplasmic side of the cytoplasmic membrane. The complete oxidation of EtOH occurs at cytoplasmic level by a NAD-ADH and NAD-ALDH. The AcOH produced can be further utilized by acetyl CoA synthase and via TCA cycle (Fig. 1).
Fig. 1.
Ethanol oxidation by PQQ-ADH and ALDH at the outer surface of cytoplasmic membrane and by NAD-ADH and NAD-ALDH in the cytoplasm
The membrane-bound ADH and ALDH complexes are tightly linked to the respiratory chain, which transfers electrons via ubiquinone (UQ) and a terminal ubiquinol oxidase to oxygen as final electron acceptor [42]. ADH oxidizes EtOH to acetaldehyde, which is further oxidized to AcOH by ALDH as follows:
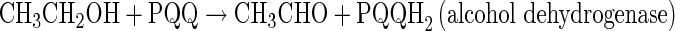 |
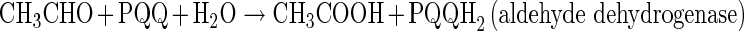 |
ADH of many AAB is composed by three subunits. Subunit I is the largest (72–78 kDa) and it is encoded in the adhA gene. It possesses a heme c and a pyrroloquinoline quinone (PQQ) as cofactors and requires Ca2+ to be active, according to the catalytic mechanism given by Anthony [43] and Goodwin and Anthony [44]. The subunit II which molecular size range from 44 to 45 kDa contains three heme c moieties and it is encoded in the adhB gene. The third and smallest subunit (20 kDa), encoded in the adhS, helps the two functional subunits with their association to the membrane protecting the catalytic subunit from proteolysis and it contributes to the correct conformation of the ADH complex for electron transport on the periplasmic surface [26, 45]. Oxidation of EtOH occurs at PQQ site that acts as two-electron redox mediator; electrons are initially transferred to UQ, which will be re-oxidized by a membrane-associated oxidase. Eventually, oxygen is the final electron acceptor, resulting in formation of H2O and a proton motive force necessary for energy production through a membrane-bound ATPase.
AAB possesses also an inactive form of ADH with the same subunit composition of the active form, but having 10 times lower Q-1 reductase activity. However, it exhibits an ubiquinol:ferricyanide oxidoreductase activity, an ethanol:Q-1 and ethanol: ferricyanide oxidoreductase activities. The ubiquinol:ferricyanide oxidoreductase activity is higher in the inactive ADH than in the active enzyme suggesting that it play a role for the regulation of redox levels of UQ/ubiquinol in the cytoplasmic membrane contributing to the functionality of AAB under acidic and high aeration conditions [45].
The ALDH complex of AAB is composed of two or three subunits of different molecular masse organized as an operon. It has been reported that the ALDH enzymes from A. aceti and Ga. europaeus contain three subunits, whereas two subunits were detected in G. suboxydans, A. rances, and A. polyoxogenes [1]. Its optimum pH is between 4 and 5, although it can catalyse the oxidation of acetaldehyde to acetate at lower pH values [46]. ALDH is sensitive to oxygen concentrations, and when these are low its activity decreases, accumulating acetaldehyde into the media. It is also more sensitive to the presence of EtOH than ADH [47].
Sugars Oxidation
AAB are known to have a high oxidative ability against sugars, mainly glucose but also arabinose, fructose, galactose, mannose, ribose, sorbose and xylose (Table 1) [48]. They can catabolize sugars through the cytoplasmic hexose monophosphate pathway (Warburg-Dickens pathway) [36]. The Entner-Doudoroff pathway occurs only in cellulose-synthesizing Acetobacter and Gluconacetobacter strains, where it appears to be more active than the hexose monophosphate cycle [49].
The oxidative pentose-phosphate pathway was reported to be the most important route for phosphorylative breakdown of sugars and polyols to CO2 in G. oxydans. Therefore it was predicted that G. oxydans has the capability to take up and to channel many polyols, sugars and sugar derivatives into the oxidative pentose phosphate pathway: polyols are first oxidized by soluble dehydrogenases; these products, other ketoses and aldoses are further modified by isomerases and epimerases. Finally, the compounds are phosphorylated by specific or unspecific kinases forming intermediates of the oxidative pentose phosphate pathway [50].
Acetobacter species can use sugars through the hexose monophosphate pathway and also through the Embden-Meyerhof-Parnas and Entner-Doudoroff pathways [51]. Sugars are further metabolised to CO2 and H2O via the TCA pathway, which is not functional in Gluconobacter. Sugar is more preferred as carbon source by Gluconobacter than by Acetobacter because the species of this genus can obtain energy more efficiently by the metabolisation of the sugars via pentose phosphate pathway [48]. The most characteristic reaction is the direct oxidation of glucose into glucono-δ-lactone, which is oxidized into gluconic acid. This reaction is particularly active in Gluconobacter growing at high concentrations of sugars. d-gluconate can be further oxidized to 2-ketogluconate and 2,5-diketogluconate by the gluconate dehydrogenase and 2-ketogluconate dehydrogenase [52].
At industrial scale, massive gluconic acid production by G. oxydans requires high glucose concentrations, low pH and high aeration rate. The further oxidation to ketogluconic acids is potentially undesirable reaction when using Gluconobacter strains for gluconic acid production. Suppression of ketogluconates formation has been achieved performing processes at low pH values [53].
Sugar Alcohols Oxidation
AAB can oxidize several sugar alcohols like glycerol to DHA, d-mannitol to d-fructose, d-sorbitol to l-sorbose, d-arabitol to xylulose, d and meso-erythritol to l-erythrulose [1, 7]. Particularly in winemaking, AAB can use glycerol as carbon source which is converted into DHA by the glycerol dehydrogenase; an enzyme bound with the cellular membrane inducing a high accumulation of DHA in the media. In wine, DHA can react with proline producing a “crust-like” aroma and it can bind SO2 reducing its anti-microbial activity [36].
In G. oxydans it was reported that oxidization of glycerol is catalized by the membrane-bound glycerol/sorbitol dehydrogenase. This quinoprotein is considered the main polyol-dehydrogenase of G. oxydans that exhibits a broad substrate specificity. It catalyzes the oxidation of d-sorbitol, gluconate, and glycerol to l-sorbose, 5-ketogluconate and DHA, respectively [54].
The metabolic pathway of glycerol in A. pasteurianus, predicted from Ga. xylinus, showed that glycerol utilization is accompanied by the formation of DHA, cellulose, CO2, and small amounts of acetate. In this species, DHA phosphate from glycerol can be produced by two pathways, one is via DHA catalyzed by glycerol dehydrogenase and the other one is via glycerol 3-phosphate by glycerol kinase. The DHA phosphate may be converted to d-glyceraldehyde 3-phosphate by triosephosphate isomerase and thus enter into the gluconeogenesis pathway [27].
Organic Acid Oxidation
The ability of Acetobacter and Gluconacetobacter strains to oxidize AcOH (Fig. 1) generating the so-called acetate overoxidation occurs via TCA cycle [42]. Other acids such as lactic, pyruvic, malic, succinic, citric, and fumaric are similarly metabolized. In contrast, strains of Gluconobacter do not have a functional TCA cycle because of deficiencies in the two key enzymes, alpha-ketoglutarate dehydrogenase and succinate dehydrogenase. Consequently, they are unable to metabolize AcOH and other organic acids. Although the optimum pH for the oxidation of organic acids by AAB is near 6.0, there is evidence that it occur at lower values (3.5–4.0). In vinegar, for instance, Acetobacter species exhibits a biphasic growth curve, where the first corresponds to an EtOH oxidation with AcOH production, and the second to an overoxidation [42].
Several strains of Acetobacter and Gluconobacter, particularly strains of A. pasteurianus, can oxidize lactate to acetoin. Acetoin has a characteristic “butter-like” aroma and flavor occurring in spoiled wine [36].
Production of Exopolysaccharides
Dextrans, levans and cellulose are the main exopolysaccharides produced by AAB glucose metabolism [1]. Ga. xylinus species have been regarded as model system for the study of biochemistry and genetics of cellulose biogenesis. The rate of cellulose production in Ga. xylinus is proportional to the rate of cell growth, and the yield is dependent on the carbon sources. Activators for bacterial cellulose production are compounds like caffeine and related xanthines [1]. Ga. xylinus synthesizes a cellulose mat that covers the surface of the growth medium in static cultures, whereas round balls of cellulose are formed in shaking cultures. The key enzyme in cellulose synthesis by Ga. xylinus is the membrane bound cellulose synthase which uses UDP-glucose as substrate. The pathway from glucose to cellulose consists of the following four enzymatic steps:
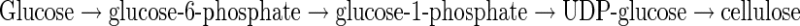 |
Cells of cellulose-producing AAB are entrapped in the polymer matrix, supporting the population at the liquid–air interface. This facilitates oxygen and nutrient supply, since the concentration of nutrients in the cellulose matrix is enhanced by its absorptive properties, in contrast to the surrounding aqueous environment. Aeration of cultures gives rise to the formation of spontaneous non-cellulose-producing mutants. Most Ga. xylinus and Ga. intermedius strains produce besides the water-insoluble cellulose also a water-soluble polysaccharide called “acetan,” a heteropolymer containing glucose, mannose, glucuronic acid and rhamnose in a molar ratio of 4:1:1:1. Acetan formation seems to influence the degree of polymerization and crystallinity of the cellulose fibrils [55].
Production of exopolysaccharides especially cellulose from AAB seems to be a promising area of application because of the increasing need of pure cellulose in medical and engineering fields [18].
Conclusion
AAB are food-associated microorganisms that have a long history in oxidative fermentation processes, where they are spoiling or beneficial organisms. The physiological uniqueness of AAB is due to their ability to partially oxidize carbon sources and quantitatively excrete the corresponding compounds in the surrounding media. This feature, besides vinegar, is exploited for the industrial production of a number of compounds from alcohols, sugar and sugar alcohols oxidation. Moreover they are considered promising for the production of the pure form of cellulose. Although the valuable potential of AAB in biotechnological applications, their industrial exploitation is not full developed.
Perspectives to enhance their role as biocatalysts include the availability of genetic stable strains and further advance on their metabolic potential, in order to obtain high carbon substrates conversion efficiency.
References
- 1.Sievers M, Swings J. Family Acetobacteraceae. In: Garrity GM, editor. Bergey’s manual of systematic bacteriology. 2. New York: Springer; 2005. pp. 41–95. [Google Scholar]
- 2.Wu J, Gullo M, Chen F, Giudici P. Diversity of Acetobacter pasteurianus strains isolated from solid-state fermentation of cereal vinegars. Curr Microbiol. 2010;60:280–286. doi: 10.1007/s00284-009-9538-0. [DOI] [PubMed] [Google Scholar]
- 3.Mamlouk D, Hidalgo C, Torija MJ, Gullo M. Evaluation and optimization of bacterial genomic DNA extraction for no-culture techniques applied to vinegars. Food Microbiol. 2011;28:1374–1379. doi: 10.1016/j.fm.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Gullo M, Giudici P. Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol. 2008;125(1):46–53. doi: 10.1016/j.ijfoodmicro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Pedraza RO. Recent advances in nitrogen-fixing acetic acid bacteria. Int J Food Microbiol. 2008;125(1):25–35. doi: 10.1016/j.ijfoodmicro.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 6.Kim EK, Kim SH, Nam HJ, Choi MK, Lee KA, Choi SH, Seo YY, You H, Kim B, Lee WJ. Draft genome sequence of Gluconobacter morbifer G707T, a pathogenic gut bacterium isolated from Drosophila melanogaster intestine. J Bacteriol. 2012;194(5):1245. doi: 10.1128/JB.06670-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi O, Moonmangmee D, Toyama H, Yamada M, Shinagawa E, Matsushita K. New developments in oxidative fermentation. Appl Microbiol Biotechnol. 2003;60(6):643–653. doi: 10.1007/s00253-002-1155-9. [DOI] [PubMed] [Google Scholar]
- 8.Kersters K, Lisdiyanti P, Komagata K, Swings J. The family Acetobacteraceae: the genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter and Kozakia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The prokaryotes. 3. New York: Springer; 2006. pp. 163–200. [Google Scholar]
- 9.Pasteur L. Mémoire sur la fermentation acétique. Annales Scientifiques de l’E.N.S Paris. 1864;1:113–158. [Google Scholar]
- 10.Swings J, De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977;41:1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camu N, De Winter T, Verbrugghe K, Cleenwerck I, Vandamme P, Takrama JS, Vancanneyt M, De Vuyst L. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl Environ Microbiol. 2007;73(6):1809–1824. doi: 10.1128/AEM.02189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartowsky EJ, Henschke PA. Acetic acid bacteria spoilage of bottled red wine-a review. Int J Food Microbiol. 2008;125(1):60–70. doi: 10.1016/j.ijfoodmicro.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Gullo M, Romano AD, Pulvirenti A, Giudici P. Candida humilis-dominant species in sourdoughs for the production of durum wheat bran flour bread. Int J Food Microbiol. 2003;80(1):55–59. doi: 10.1016/S0168-1605(02)00121-6. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield S, Claus GW. Nonfunctional tricarboxylic acid cycle and the mechanism of glutamate biosynthesis in Acetobacter suboxydans. J Bacteriol. 1972;112:1295–1301. doi: 10.1128/jb.112.3.1295-1301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Singh VK, Qazi GN, Kumar A. Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol. 2001;3(3):445–456. [PubMed] [Google Scholar]
- 16.De Vero L, Gullo M, Giudici P. Acetic acid bacteria, biotechnological applications. In: Flickinger MC, editor. Encyclopedia of industrial biotechnology: bioprocess bioseparation and cell technology. New York: Wiley; 2010. pp. 9–25. [Google Scholar]
- 17.Singh OV, Kumar R. Biotechnological production of gluconic acid: future implications. Appl Microbiol Biotechnol. 2007;75(4):713–772. doi: 10.1007/s00253-007-0851-x. [DOI] [PubMed] [Google Scholar]
- 18.Czaja W, Young D, Kawecki M, Brown RM. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8:1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 19.Saeki A, Theeragol G, Matsushita K, Toyama H, Lotong N, Adachi O. Development of thermotolerant acetic acid bacteria useful for vinegar fermentation at higher temperatures. Biosci Biotechnol Biochem. 1997;61:138–145. doi: 10.1271/bbb.61.317. [DOI] [Google Scholar]
- 20.Ndoye B, Lebecque S, Dubois-Dauphin R, Tounkara L, Guiro AT, Kere C, Diawara B, Thonart P. Thermoresistant properties of acetic acids bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enzyme Microb Technol. 2006;39:916–923. doi: 10.1016/j.enzmictec.2006.01.020. [DOI] [Google Scholar]
- 21.De Ley J, Gillis M, Swings J (1984) Family VI. Acetobacteraceae. In: Krieg NR, Holt JG. (eds.) Bergey’s manual of systematic bacteriology, baltimore, pp 267–278
- 22.Euzéby JP (1997) List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Bacteriol 47:590–592 (List of prokaryotic names with standing in nomenclature). http://www.bacterio.net [DOI] [PubMed]
- 23.Ludwig W. Nucleic acid techniques in bacterial systematics and identification. Int J Food Microbiol. 2008;125:1–12. doi: 10.1016/S0168-1605(08)00293-6. [DOI] [PubMed] [Google Scholar]
- 24.Cleenwerck I, de Vos P. Polyphasic taxonomy of acetic acid bacteria: an overview of the currently applied methodology. Int J Food Microbiol. 2008;125(1):2–14. doi: 10.1016/j.ijfoodmicro.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Gullo M, Mamlouk D, De Vero L, Giudici P. Acetobacter pasteurianus strain AB0220: cultivability and phenotypic stability over 9 years of preservation. Curr Microbiol. 2012;64:576–580. doi: 10.1007/s00284-012-0112-9. [DOI] [PubMed] [Google Scholar]
- 26.Kondo K, Horinouchi S. Characterization of the genes encoding the three-component membrane-bound alcohol dehydrogenase from Gluconobacter suboxydans and their expression in Acetobacter pasteurianus. Appl Environ Microbiol. 1997;63:1131–1138. doi: 10.1128/aem.63.3.1131-1138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma Y, Hosoyama A, Matsutani M, Furuya N, Horikawa H, Harada T, Hirakawa H, Kuhara S, Matsushita K, Fujita N, Shirai M. Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Res. 2009;37(17):5768–5783. doi: 10.1093/nar/gkp612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coucheron DH. An Acetobacter xylinum insertion sequence element associated with inactivation of cellulose production. J Bacteriol. 1991;173:5723–5731. doi: 10.1128/jb.173.18.5723-5731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleenwerck I, De Vos P, De Vuyst L. Phylogeny and differentiation of species of the genus Gluconacetobacter and related taxa based on multilocus sequence analyses of housekeeping genes and reclassification of Acetobacter xylinussubsp. sucrofermentans as Gluconacetobacter sucrofermentans (Toyosaki et al. 1996) sp. nov., comb. nov. Int J Syst Evol Microbiol. 2010;60(10):2277–2283. doi: 10.1099/ijs.0.018465-0. [DOI] [PubMed] [Google Scholar]
- 30.Matsutani M, Hirakawa H, Yakushi T, Matsushita K. Genome-wide phylogenetic analysis of Gluconobacter, Acetobacter, and Gluconacetobacter. FEMS Microbiol Lett. 2011;315(2):122–1228. doi: 10.1111/j.1574-6968.2010.02180.x. [DOI] [PubMed] [Google Scholar]
- 31.Kittelman M, Stamm WW, Follmann H, Truper HG. Isolation and classification of acetic acid bacteria from high percentage vinegar fermentations. Appl Bicrobiol Biotechnol. 1989;30:47–52. [Google Scholar]
- 32.Millet V, Lonvaud-Funel A. The viable but non-culturable state of wine microorganisms during storage. Lett Appl Microbiol. 2000;30:136–141. doi: 10.1046/j.1472-765x.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 33.De Vero L, Gala E, Gullo M, Solieri L, Landi S, Giudici P. Application of denaturing gradient gel electrophoresis (DGGE) analysis to evaluate acetic acid bacteria in traditional balsamic vinegar. Food Microbiol. 2006;23(8):809–813. doi: 10.1016/j.fm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Gullo M, De Vero L, Giudici P. Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl Environ Microbiol. 2009;75:2585–2589. doi: 10.1128/AEM.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupuy P. Recherche d’une technique d’isolement des Acetobacter du vin. Ann Technol Agric. 1952;1:107–112. [Google Scholar]
- 36.Drysdale GS, Fleet GH. Acetic acid bacteria in winemaking: a review. Am J Enol Vitic. 1988;39(2):143–154. [Google Scholar]
- 37.Carr JG, Passmore SM. Methods for identifying acetic acid bacteria. In: Skinner FA, Lovelock DW, editors. Identification methods for microbiologists. UK: Academic Press; 1979. pp. 33–47. [Google Scholar]
- 38.Sievers M, Sellmer S, Teuber M. Acetobacter europaeus sp. nov., a main component of industrial vinegar fermenters in central Europe. Syst Appl Microbiol. 1992;15:386–392. doi: 10.1016/S0723-2020(11)80212-2. [DOI] [Google Scholar]
- 39.Entani E, Ohmori S, Masai H, Suzuki KI. Acetobacter polyoxogenes sp. nov., a new species of an acetic acid bacterium useful for producing vinegar with high acidity. J Gen Appl Microbiol. 1985;31:475–490. doi: 10.2323/jgam.31.475. [DOI] [Google Scholar]
- 40.Sokollek SJ, Hertel C, Hammes WP. Cultivation and preservation of vinegar bacteria. J Biotechnol. 1998;60:195–206. doi: 10.1016/S0168-1656(98)00014-5. [DOI] [Google Scholar]
- 41.Gullo M, Caggia C, De Vero L, Giudici P. Characterization of acetic acid bacteria in traditional balsamic vinegar. Int J Food Microbiol. 2006;106:209–212. doi: 10.1016/j.ijfoodmicro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, Toyama H, Adachi O. Respiratory chains in acetic acid bacteria: membrane bound periplasmic sugar and alcohol respirations. In: Zannoni D, editor. Respiration in archaea and bacteria, advances in photosynthesis and respiration. Dordrecht: Springer; 2004. pp. 81–99. [Google Scholar]
- 43.Anthony C. Quinoprotein-catalysed reactions. Biochem J. 1996;320:697–711. doi: 10.1042/bj3200697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodwin PM, Anthony C. The biochemistry, physiology and genetics of PQQ and PQQ-containing enzymes. Adv Microb Physiol. 1998;40:1–80. doi: 10.1016/S0065-2911(08)60129-0. [DOI] [PubMed] [Google Scholar]
- 45.Yakushi T, Matsushita K. Alcohol dehydrogenase of acetic acid bacteria: structure, mode of action, and applications in biotechnology. Appl Microbiol Biotechnol. 2010;86(5):1257–1265. doi: 10.1007/s00253-010-2529-z. [DOI] [PubMed] [Google Scholar]
- 46.Adachi O, Matsushita K, Shinagawa E, Ameyama M. Crystallization and properties of NADP-dependent d-glucose dehydrogenase from Gluconobacter suboxydans. Agric Biol Chem. 1980;44:301–308. doi: 10.1271/bbb1961.44.301. [DOI] [Google Scholar]
- 47.Muraoka H, Watabe Y, Ogasawara N, Takahashi H. Trigger damage by oxygen deficiency to the acid production system during submerged acetic acid fermentation with Acetobacter aceti. J Ferment Technol. 1983;61:89–93. [Google Scholar]
- 48.De Ley J, Gillis M, Swings J. Family VI. Acetobacteraceae. In: Krieg NR, Holt JG, editors. Bergey’s manual of systematic bacteriology. 1. Baltimore: Williams and Wilkins Co; 1984. pp. 267–278. [Google Scholar]
- 49.White GA, Wang CH. The dissimilation of glucose and gluconate by Acetobacter xylinum. The origin and the fate of triose phosphate. Biochem J. 1964;90(2):408–423. doi: 10.1042/bj0900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deppenmeier U, Ehrenreich A. Physiology of acetic acid bacteria in light of the genome sequence of Gluconobacter oxydans. J Mol Microbiol Biotechnol. 2009;16:69–80. doi: 10.1159/000142895. [DOI] [PubMed] [Google Scholar]
- 51.Attwood M, van Dijken JP, Pronk JT. Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. J Ferment Bioeng. 1991;72(2):101–105. doi: 10.1016/0922-338X(91)90317-A. [DOI] [Google Scholar]
- 52.Klasen R, Bringer-Meyer S, Sahm H. Biochemical characterization and sequence analysis of the gluconate: NADP 5-oxidoreductase gene from Gluconobacteroxydans. J Bacteriol. 1995;177(10):2637–2643. doi: 10.1128/jb.177.10.2637-2643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roehr M, Kubicek CP, Kominek J. Gluconic acid. In: Roehr M, editor. Biotechnology. New York: VCH; 1996. pp. 348–362. [Google Scholar]
- 54.Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF, Ehrenreich A, Gottschalk G, Deppenmeier U. Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechonol. 2005;23:195–200. doi: 10.1038/nbt1062. [DOI] [PubMed] [Google Scholar]
- 55.Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Mol Biol Rev. 1991;55:135–158. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yukphan P, Malimas T, Muramatsu Y, Potacharoen W, Tanasupawat S, Nakagawa Y, Tanticharoen M, Yamada Y. Neokomagataea gen. nov., with descriptions of Neokomagataea thailandica sp. nov. and Neokomagataea tanensis sp. nov., osmotolerant acetic acid bacterium of the a Proteobacteria. Biosci Biotechnol Biochem. 2011;75(3):419–426. doi: 10.1271/bbb.100344. [DOI] [PubMed] [Google Scholar]
- 57.Yukphan P, Malimas T, Muramatsu Y, Takahashi M, Kaneyasu M, Potacharoen W, Tanasupawat S, Nakagawa Y, Hamana K, Tahara Y, Suzuki K, Tanticharoen M, Yamada Y. Ameyamaea chiangmaiensis gen. nov., sp. nov., an acetic acid bacterium in the alpha proteobacteria. Biosci Biotechnol Biochem. 2009;73(10):2156–2162. doi: 10.1271/bbb.90070. [DOI] [PubMed] [Google Scholar]