Abstract
The phenotypic and genotypic diversity of the plant growth promoting Bacillus genus have been widely investigated in the rhizosphere of various agricultural crops. However, to our knowledge this is the first report on the Bacillus species isolated from the rhizosphere of Calendula officinalis. 15 % of the isolated bacteria were screened for their important antifungal activity against Fusarium oxysporum, Botrytis cinerea, Aspergillus niger, Cladosporium cucumerinium and Alternaria alternata. The bacteria identification based on 16S r-RNA and gyrase-A genes analysis, revealed strains closely related to Bacillus amyloliquefaciens, B. velezensis, B. subtilis sub sp spizezenii and Paenibacillus polymyxa species. The electro-spray mass spectrometry coupled to liquid chromatography (ESI-LC MS) analysis showed that most of the Bacillus isolates produced the three lipopeptides families. However, the P. polymyxa (18SRTS) didn’t produce any type of lipopeptides. All the tested Bacillus isolates produced cellulase but the protease activity was observed only in the B. amyloliquefaciens species (9SRTS). The Salkowsky colorimetric test showed that the screened bacteria synthesized 6–52 μg/ml of indole 3 acetic acid. These bacteria produced siderophores with more than 10 mm wide orange zones on chromazurol S. The greenhouse experiment using a naturally infested soil with Sclerotonia sclerotiorum showed that the B. amyloliquefaciens (9SRTS) had no significant (P > 0.05) effect on the pre-germination of the chickpea seeds. However, it increased the size of the chickpea plants and reduced the stem rot disease (P < 0.05).These results suggested that the Bacillus strains isolated in this work may be further used as bioinoculants to improve the production of C. officinalis and other crop systems.
Keywords: Bacillus, C. officinalis rhizosphere, Cell-wall degrading enzymes, IAA, Lipopeptides, Siderophores
Introduction
The demand for medicinal plants is increasing worldwide due to the growing recognition of biological products, being non-toxic, having no side effects and affordable prices [23]. Calendula officinalis is an important medicinal herb used in Europe, China, US and India. C. officinalis has numerous medicinal properties among which it is worth mentioning the anti-inflammatory and antioedematous activities, the antibacterial and antifungal activities, the anticancer and lymphocyte activation dual activities, the anti-HIV activity, the immunostimulant activity, the antioxidant activity, the hepatoprotective activity, the wound healing activity and the antiviral activity [17]. Several phytopathogenic fungi were detected on C. officinalis seeds such Alternaria alternata, Alternaria porri, Botrytis cinerea, Drechslera (Cochliobolus) hawaiiensis, Fusarium avenaceum, Fusarium culmorum and Sclerotonia sclerotiorum. These pathogens lead to significant yield losses of C. officinalis crops [21]. The application of agrochemicals is necessary to increase crop yields but these products have several negative side effects [27]. The plant growth-promoting rhizobacteria (PGPR) can be used as an attractive alternative to the use of such xenobiotic compounds [6]. It has been noted by many workers that the bacterial genera such as Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcous, Pseudomonas and Serratia belongs to PGPR, showed synergistic effects on plant growth [9]. The PGPRs mediate biological control indirectly by the production of antimicrobial molecules [19, 20], siderophores and eliciting induced systemic resistance against a number of plant diseases One of the most important direct ways that those bacteria affect growth and development is by producing Indole-3-acetic acid (IAA) that this hormone is led to plant root system development and subsequently nutritional uptake increase by plant [26].
The Bacillus species offer several advantages over the other genera because of their capacity to produce spores in unfavorable environmental conditions. This characteristic facilitates the conversion of spore suspensions to powder formulations without killing bacteria [16]. Thus, a number of Bacillus and Paenibacillus spp. have been commercially developed as biological fungicides, insecticide and nematicides or generic plant growth promoters, and their use in agriculture has recently been reviewed. The well studied and applied organisms are members of Bacillus subtilis spp. group [3, 7]. Such organisms have almost identical 16S rDNA sequences (99.2–99.6 % sequence similarity). Several molecular techniques were applied to assess the bacterial diversity and to analyze the genetic relationships between Bacillus species, i.e., DNA–DNA re-association studies, Rep-PCR, protein coding genes and internal transcribed spacers (ITS) sequences analysis, restriction fragment length polymorphism (RFLP) and Bacillus species-specific signature [22].
To our knowledge, this is the first study to characterize Bacillus isolates from the rhizosphere of C. officinalis. The main objectives were to: (I) isolate the predominant antifungal Bacillus species; (II) study their phylogenetic diversity based on 16S r-RNA and gyr-A genes analysis; and (III) evaluate their in vitro and in vivo biocontrol and plant growth-promoting traits, in order to further use them as bio-inoculant strains.
Materials and Methods
Bacillus Isolation and Antagonism Test
Bacillus strains were isolated from the rhizosphere of C. officinalis, using a procedure involving a heat treatment at 80 °C during 12 min. The antifungal activity of the Bacillus isolates was investigated on PDA Petri dishes by the dual culture technique. The tested phytopathogenic fungi were Fusarium oxysporium, B. cinerea, Aspergillus niger, C. cucumerinium and A. alternata. Mycelia growth inhibition was calculated as the reduction percentage of mycelia expansion compared with control plates without bacteria [25]. Mean values and standard errors were calculated from three replicates used for each fungal strain.
Spore Yields Determination
The Bacillus isolates were grown in the opt liquid medium described by Jacques et al. [12], for 72 h at 30 °C and agitated flasks (180 rpm). Spores concentration in Bacillus cultures was investigated using a thermal chock technique [8]. All experiments were performed as three replicates.
Identification of the Bacillus Strains and Phylogenetic Analysis
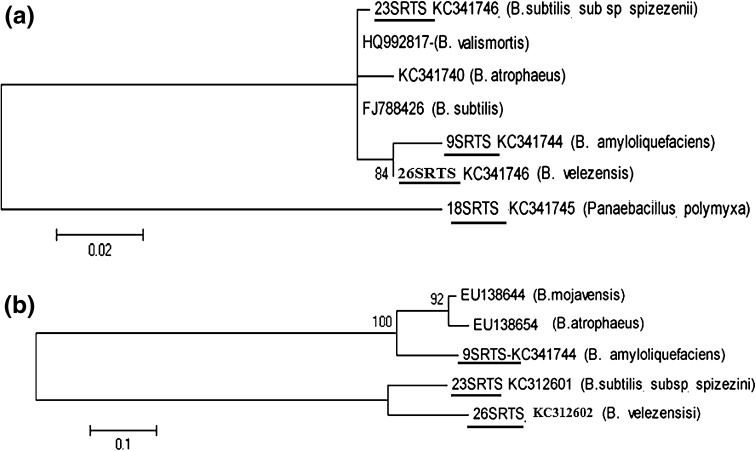
The total DNA was extracted from Bacillus liquid cultures by the wizard genomic DNA purification kit (Promega), using the manufacturer’s instructions. The primers used for the PCR amplification were the universal primers 16SP0 and 16SP6 for the 16S r-RNA gene [2] and gyr-A.f and gyr-A.r for the gyr-A gene [11]. The purification of the PCR products was achieved using the GFX PCR DNA and Gel Band Purification Kit. The amplified genes were sequencing using the same primers sited above and the obtained sequences were corrected by the Bio-edit program. The obtained sequences were deposited in Genbank database and the accession numbers were obtained (Fig. 1). To identify the Bacillus isolates, the DNA sequences were compared to those previously published in Genbank using the BLASTN program. The taxonomic position of the Bacillus isolates studied in this work was investigated by analyzing the 16S r-RNA and gyr-A genes sequences. The phylogenetic trees were constructed by the MEGA 5 program, using maximum-likelihood (ML) method based on the Jukes-Cantor model.
Fig. 1.
Phylogenetic trees of the Bacillus strains isolated from C. officinalis rhizosphere, based on a 16S r-RNA and bgyr-A genes sequences analysis. The bacteria isolated in this work were underlined and followed by the accession number provided by Genbank
Production of Cell-Wall Degrading Enzymes and Lipopeptides
The enzymatic activities were assessed in a qualitative way through a halo formation on solid media containing colloidal chitin, milk powder and carboxymethyl cellulose substrates to reveal chitinase, protease and cellulase activities respectively [4]. The lipopeptides were analyzed by mass spectrometry coupled to HPLC. The Bacillus strains were grown in agitated flasks (180 rpm) containing the opt medium at 30 °C for 72 h. Cultures were centrifuged at 15,000×g for 20 min. The supernatant samples were loaded on C18 solid-phase extraction cartridges (900 mg, Alltech) and lipopeptides were desorbed with 100 % ACN. The resulting samples were analyzed by reverse phase HPLC coupled with single quad mass spectrometer (HPLC Waters Alliance 2695/diode array detector, coupled with Waters SQD mass analyzer) on a X-terra MS (Waters) 150 × 2.1 mm, 3.5 μm column as previously described by Nihorimbere et al. [18]. In this work, a single elution gradient allowing the simultaneous measurement of all three lipopeptides families was used. The water acidified with 0.1 % formic acid (A) and acetonitril (ACN) acidified with 0.1 % formic acid (B) were used as a mobile phase. The flow rate was maintained at 0.5 ml min−1 and the column temperature at 40 °C, with a gradient of 35 min (43–80 %, vol/vol ACN in 18 min; 100 %, vol/vol ACN for 9 min, and 43 %, vol/vol ACN in 8 min). Compounds were identified on the basis of their retention times compared to purified standards. The identity of each homologue was confirmed on the basis of the masses detected in the SQD by setting electrospray ionization conditions in the MS as source temp., 130 °C; desolvation temp., 250 °C; nitrogen flow, 500 l/h; cone voltage, 70 V. The positive ion mode was used for analysis of all three families because a higher signal/background ration was obtained compared to negative ion recording.
Production of Indole 3 Acetic Acid (IAA)
The indole acetic acid production was assayed calorimetrically by using the Salkowski reagent (0.01 M FeCl3 in 36 % H2SO4) as described by Benduzi et al. [5]. The test was achieved in duplicate.
Production of Siderophores
The Bacillus isolates were streaked on azurol S medium (CAS-medium) as described by Husen [10] and siderophores production was indicated by the formation of yellow-orange halos around the colonies after incubation. This test was achieved in three replicates.
Effects of the Soil Treatment with the Biocontrol Agent (9SRTS) on Chickpea Plant Size; Damping-Off and Stem Rot Diseases Under Greenhouse Conditions
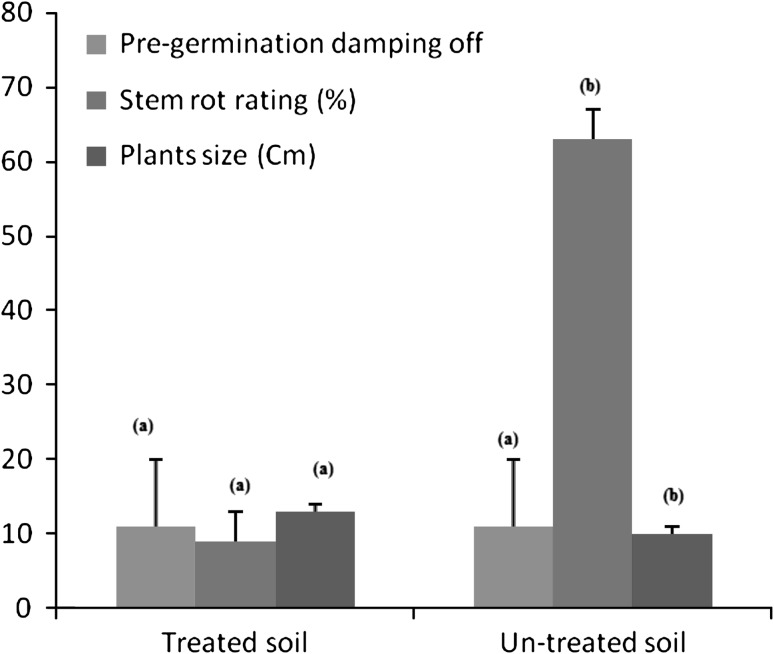
The B.amyloliquefaciens (9SRTS) was produced under optimized industrial conditions in a 500 L bioreactor in the society Artechno S.A (Belgium). The fermentation was stopped at the time of almost full sporulation, centrifuged and lyophilized to yield a highly concentrated stable powder. This product was resuspended in sterile distilled water to obtain the final desired spore concentration (107 spores/ml). The chickpea seeds (CV. Flipe 13 90) were sown in a naturally infested soil with S. sclerotiorum. The treatment was carried out by spraying the bacterial suspension on the soil. Two replicates were used; each replicate consisted of three pots (4 seeds/pot). Data were recorded for damping-off; size and stem rot rating disease (percentage of discoloration leaves per plant) after 30 days of sowing. The SAS software (SAS Institute 2000) was used for all statistical analysis. The soil treatment effect on the studied parameters was assessed by a general linear model (GLM). Least square means (LSM) and standard errors were calculated, allowing ranking of treated and control lots according to Duncan’s procedure (Fig. 2).
Fig. 2.
The effect of the B. amyloliquefaciens (9SRTS) on the pre-germination damping-off, the size of chickpea plants and the stem rot disease rating after 1 month of seeds sowing. Different letters above histograms corresponding to the same parameter mention that the control pots are significantly different from the treated ones (P < 0.05)
Results and Discussion
Twenty-six Bacillus strains were isolated from the rhizosphere of C. officinalis. Four isolates (15 %) were screened for their ability to inhibit growth of some phytopathogenic fungi such as F. oxysporium, B. cinerea, A. niger, C. cucumerinium and and A. alternata (Table 2). These bacteria were identified based on 16S r-RNA and gyr-A gene sequences analysis as B. amyloliquefaciens, B. subtilis sub sp spizezenii, B. velezensis and P. polymyxa. Approximate results were found by Martina Köberl et al. [14] where the Bacillus strains isolated from the rhizosphere and endorhiza of the medicinal plants (Matricaria chamomilla, C. officinalis and Solanum distichum) had an antagonistic effect against Rhizoctonia solani, Verticillium dahliae and F. culmorum. The isolated bacteria in this same study were identified based on 16S r-RNA sequences analysis as B. subtilis, B. vallismortis, B. amyloliquefaciens and B. atrophaeus [13]. In this study, the average similarity values of the 16S r-RNA sequences from Bacillus spp. was 99.1 % and the isolates were indistinguishable from one another. However, the gyr-A gene sequences analysis clarified further the identification of the Bacillus spp. Isolates. The gyr-A based tree clearly delineated three distinct clusters, cluster 1 contained B. atrophaeus and B. mojavensis, cluster 2 contained B. amyloliquefaciens and cluster 3 contained B. velezensis and B. subtilissub sp spizezenii (Fig. 1b). Comparatively, the 16S r-RNA gene-based tree yielded three clusters, cluster 1 contained strains of B. subtilis, B. vallismortis, B. atrophaeus and B. subtilissub sp spizezenii, cluster 2 contained B. amyloliquefaciens and B. velezensis and cluster 3 contained P. polymyxa. These results were similar to that previously found by Jongsik and Kyung [13]. The P. polymyxa (18SRTS) strain had a very low sporulation yields. However, the other Bacillus isolates (9SRTS, 23SRTS and 26SRTS) had high sporulation levels which varied between 0.8 × 109 and 2.5 × 109 spores/ml (Table 1). Previously, the spore yields in submerged optimized cultivation were lower and estimated at 8.35 × 108 spores/ml [15]. In this work, the Bacillus isolates showed important growth inhibition percentages against F. oxysporium and B. Cinerea i.e., 39–83 % (Table 2). The detected antifungal activity can be explained by the capacity of the Bacillus isolates to produce the cyclic lipopeptides (cLPs) and the cell-wall degrading enzymes as previously sowed [19, 25]. Indeed, all screened bacteria here produced cellulase but the protease activity was found only in the B. amyloliquefaciens species (9SRTS) and non strain produced chitinase (Table 2). The LC–MS analysis showed that most of Bacillus isolates produced surfactin and iturin. Two types of iturin were produced, the iturin (A) and the Bcillomycin D. However, the P. polymyxa (18SRTS) didn’t produce any type of lipopeptides. The B. velezensis (26SRTS) was the only strain producing fengycins. In previous works, it has been mentioned that a very limited number of strains are reported to co-produce fengycin homologues [19]. The production of the phytohormone (IAA) and siderophores by Bacillus species has been investigated in many studies. The IAA stimulates the plant growth and siderophores chelate iron (Fe) and deprive the phytopathogenic fungi of it [5–24]. Here, the Bacillus spp. isolates (9SRTS, 23SRTS and 26SRTS) produced low concentrations of IAA (7–14 μg/ml) and high levels of siderophores (more than 10 mm yellow-orange zone diameter). However, the P. polymyxa (18SRTS) didn’t produce siderophores and produced higher concentrations of IAA which reached 53 μg/ml (Table 3). The in vivo test carried here showed that the B. amyloliquefaciens (9SRTS) had no significant effect on the pre-germination of chickpea seeds (P > 0.05). However, it increased the size of the chickpea plants and reduced the stem rating disease (P < 0.05). The B. subtilis and B. megaterium species decreased the pre-germination damping-off and the stem rot rating disease of Giza variety of chickpea in the study carried out by Abel-Monaim [1]. To conclude, the Bacillus strains isolated from the rhizosphere of C. officinalis have interesting in vitro and in vivo biocontrol and plant growth promotion characteristics and high spore yields which enable them to be a feasible product that can be further used to improve the production of C. officinalis and other crop systems.
Table 2.
Antifungal activity, lipopeptides and cell-wall degrading enzymes production
| Fungal growth inhibition (%) | Lipopeptide homologues production | Cell-wall degrading enzymes production | |||||
|---|---|---|---|---|---|---|---|
| Bacillus isolates | F. oxysporium | B. cinerea | Iturin | Fengycin | Surfactin | Protease activitya | Cellulase activityb |
| 9SRTS | 83 ± 2 | 65 ± 2 | It A+ | – | + | + | ++ |
| 18SRTS | 66 ± 3 | 66 ± 4 | – | – | − | – | ++ |
| 23SRTS | 39 ± 2 | 48 ± 2 | It A+ | – | + | – | + |
| 26SRTS | 60 ± 2 | 61 ± 2 | It B.D+ | + | + | – | ++ |
aIn vitro protease activity (plate assay): +represents hydrolysis; − represents no hydrolysis
bIn vitro cellulase activity: + represents 10–15 mm wide clear zone; ++ represents 15–20 mm wide clear zone; +++ represents >20 mm wide clear zone
Table 1.
Geographical origin, identification and spore yields in flasks cultivation of Bacillus strains isolated from the rhizosphere of C. officinalis
| Sampled site | Antagonistic Bacillus strains | Spore yields (×108 spores/ml) |
|---|---|---|
| C. officinalis rhizosphere (greenhouse, Setif-Eastern Algeria) | (9SRTS) B. amyloliquefaciens | 25 ± 1 |
| (18SRTS) P. polymyxa | Not determined | |
| (23SRTS) B. subtilis sub sp spizezenii | 8 ± 2 | |
| (26SRTS) B. velezensis | 20 ± 1 |
Table 3.
The production of the phytohormone (IAA) and siderophores
| Bacillus isolates | IAA (μg/ml) | Siderophores productiona |
|---|---|---|
| 9SRTS | 7 ± 2 | +++ |
| 18SRTS | 53 ± 2 | – |
| 23SRTS | 14 ± 1 | +++ |
| 26SRTS | 6 ± 1 | +++ |
aIn vitro siderophores production: − represents the absence of siderophores production, +++ represents >10 mm wide yellow-orange zone
Acknowledgments
The authors are grateful to Dr. Sabri Ahmed for his help and guidance in DNA sequences analysis and to Dr Nassim Moula for the statistical analysis of the in vivo test data with SAS program. This study was financed by a fellowships European program Erasmus Mundus External Cooperation Window-consortium AVERROES.
Contributor Information
Asma Ait Kaki, Phone: +003243662861, FAX: +003243662862, Email: a.aitkaki@student.ulg.ac.be.
Noreddine Kacem Chaouche, Phone: +00213560516072, Email: nkacemchaouche@yahoo.fr.
Laid Dehimat, Phone: +00213560516072, Email: dehimat2000@yahoo.fr.
Asma Milet, Phone: +00213560516072, Email: esma_lmd@yahoo.fr.
Mounia Youcef-Ali, Phone: 00213560516072, Email: mouniayoucefali@yahoo.fr.
Marc Ongena, Phone: +003281622256, FAX: +003243662862, Email: marc.ongena@ulg.ac.be.
Philippe Thonart, Phone: +003243662861, FAX: +003243662862, Email: p.thonart@ulg.ac.be.
References
- 1.Abdel-Monaim MF. Integrated management of damping-off, root and/or stem rot diseases of chickpea and efficacy of the suggested formula. Not Sci Biol. 2011;3:80–88. [Google Scholar]
- 2.Abdlwareth AA, Xie GL, Tian WX, Xu LH, Zhang GQ, Ibrahim M. Characterization and evaluation of Bacillus isolates for their potential plant growth and biocontrol activities against tomato bacterial wilt. Afr J Biotechnol. 2011;11:7193–7201. [Google Scholar]
- 3.Abulreesh HH, Osman GEH, Assaeedi ASA. Characterization of insecticidal genes of Bacillus thuringiensis strains isolated from arid environments. Indian J Microbiol. 2012;52:500–503. doi: 10.1007/s12088-012-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariffin H, Abdullah N, Umi Kalsom MS, Shirai Y, Hassan MA. Production and characterisation of cellulase produced by Bacillus pumilus EB3. Int J Eng Technol. 2006;3:47–53. [Google Scholar]
- 5.Beneduzi A, Peres D, Da Costa PB, Zanettini MHB. Genetic and phenotypic diversity of plant growth- promoting bacilli isolated from wheat fields in southern Brazil. Res Microbiol. 2008;159:244–250. doi: 10.1016/j.resmic.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 7.Cawoy H, Bettiol W, Fickers P Ongena M (2011) Bacillus-Based Biological Control of Plant Diseases. Agricultural and biological sciences. In: Stoytcheva M (ed) Pesticides in the modern world—pesticides use and management, Chap 13, pp 273–302
- 8.Chen ZM, Li Q, Liu HM, Na Y, Xie TJ, Yang MY, Shen P, Chen XD. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol. 2010;85:1353–1360. doi: 10.1007/s00253-009-2162-x. [DOI] [PubMed] [Google Scholar]
- 9.Gray EJ, Smith DL. Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem. 2005;37:395–412. doi: 10.1016/j.soilbio.2004.08.030. [DOI] [Google Scholar]
- 10.Husen E. Screening of soil bacteria for plant growth promoting activities in vitro. Indones J Agric Sci. 2003;4:27–31. [Google Scholar]
- 11.Izumi S, Aranishi F. Relationship between gyrA mutations and quinolone resistance in Flavobacterium psychrophilum isolates. Appl Environ Microb. 2004;70:3968–3972. doi: 10.1128/AEM.70.7.3968-3972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacques P, Hbid C, Destain J, Razafindralambo H, Paquot M, De Pauw E, Thonart P. Optimization of biosurfactant lipopeptides production from Bacillus subtilis S499 by Plackett–Burman design. Appl Biochem Biotechnol. 1999;l77:223–233. doi: 10.1385/ABAB:77:1-3:223. [DOI] [Google Scholar]
- 13.Jongsik C, Kyung SB. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek. 2000;78:123–127. doi: 10.1023/A:1026555830014. [DOI] [PubMed] [Google Scholar]
- 14.Köberl1 M, Zachow1 C, Müller1 H, Ramadan EM, Bauer R, Berg G (2013) Biological control agents for combating soil-bornes pathogens in Egypt. Retrieved from http://ebookbrowse.com/bi/biological-control-agents
- 15.Liu BL, Tzeng YM. Optimization of growth medium for the production of spores from Bacillus thuringiensis using response surface methodology. Bioprocess Eng. 1998;18:413–418. [Google Scholar]
- 16.Lolloo R, Maharaih D, Görgens J, Gardiner N. A downstream process for production of a viable and stable Bacilluscereus aquaculture biological agent. Appl Microbiol Biotechnol. 2010;86:499–508. doi: 10.1007/s00253-009-2294-z. [DOI] [PubMed] [Google Scholar]
- 17.Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review. Trop J Pharm Res. 2009;5:455–465. [Google Scholar]
- 18.Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol. 2012;79:176–191. doi: 10.1111/j.1574-6941.2011.01208.x. [DOI] [PubMed] [Google Scholar]
- 19.Nithya V, Halami PM. Novel whole-cell reporter assay for stress based classification of antibacterial compounds produced by locally isolated Bacillus spp. Indian J Microbiol. 2012;52:180–184. doi: 10.1007/s12088-012-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ongena M, Adam A, Jourdan E, Paquot M, Brans A, Joris B, Arpigny JL, Thonart P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol. 2007;9(1084):1090. doi: 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 21.Pieta D. Mycoflora of Calendula officinalis L. seeds. Acta Agrobot. 1991;44:1–2. [Google Scholar]
- 22.Porwal S, Lal S, Cheema S, Kalia VC. Phylogeny in aid of the present and novel microbial lineages: diversity in Bacillus. PLoS ONE. 2009;4(2):e4438. doi: 10.1371/journal.pone.0004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekar S, Kandavel D. Interaction of plant growth promoting rhizobacteria (PGPR) and endophytes with medicinal plants-new avenues for phytochemicals. J Phytol. 2010;7:91–100. [Google Scholar]
- 24.Subhash Y. Diversity and phylogeny of plant growth-promoting bacilli from moderately acidic soil. J Basic Microbiol. 2011;51:98–106. doi: 10.1002/jobm.201000098. [DOI] [PubMed] [Google Scholar]
- 25.Toure Y, Ongena M, Jacques P, Guiro A, Thonart P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol. 2004;96:1151–1160. doi: 10.1111/j.1365-2672.2004.02252.x. [DOI] [PubMed] [Google Scholar]
- 26.Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA. Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob. 2011;3:34–40. [Google Scholar]
- 27.Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52:487–511. doi: 10.1093/jexbot/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]