Abstract
The minD gene encoding an inhibitor cell division MinD homolog from Lactobacillus acidophilus VTCC-B-871 was cloned. We showed that there were 97 % homology between minD genes of L. acidophilus VTCC-B-871 and Lactobacillus rhamnosus GG and Lactobacillus rhamnosus Lc705. Based on the analysis of the DNA sequence data from the L. rhamnosus genome project and sequenced minD gene of L. acidophilus VTCC-B-871, a pair of primers was designed to identified the different minD genes from L. acidophilus ATCC 4356, L. rhamnosus ATCC 11443. Besides, the polymerase chain reaction product of minD gene was also obtained in L. rhamnosus PN04, a strain was isolated from Vietnamese Hottuynia cordata Thunb. In addition, we performed a phylogenetic analysis of the deduced amino acid sequence of MinD homologs from L. acidophilus VTCC-B-871 with the other strains and compared the predicted three-dimension structure of L. acidophilus VTCC-B-871 MinD with Escherichia coli MinD, there are similarity that showed evolution of these strains. The overexpression of L. acidophilus VTCC-B-871 MinD in E. coli led to cell filamentation in IPTG and morphology changes in different sugar stresses, interestingly. The present study is the first report characterizing the Lactobacilus MinD homolog that will be useful in probiotic field.
Keywords: Cell division inhibitor, Morphology change, Lactobacillus, Comparative analyses, Hottuynia cordata Thunb.
Introduction
In Escherichia coli, the proper placement of the cell division site generates two equally sized daughter cells which are maintained by the MinC, MinD and MinE proteins encoded by the min locus. In this system, the MinC and MinD protein acts as the inhibitors of cell division by blocking septum formation at all potential division sites (polar and mid sites) [1]. The MinE protein gives topological specificity to the MinCD division inhibitors by restricting its activity to polar division sites, thus ensuring that separation is limited to the proper division site at midcell. MinE binds to the trailing edge of MinD and stimulating its ATP hydrolysis, which results in the realease of MinD, and thus MinC and MinE, from the membrane [2–4]. In the other hand, Bacillus subtilis contains MinCD homologues and DivIVA acts topologically, but not MinE [5]. It was also noticed that the entire nucleotide sequences of the Streptomyces genomes of Streptomyces coelicolor and Streptomycesavermitilis have recently reported, and this strain carried the MinD homolog, but not MinC or MinE [6, 7]. The MinD homolog harbored by Streptomyces lavendulae ATCC25233 has also been characterized and this strain did not carry MinC and MinE [8]. Since the genus Streptomyces consists of filamentous bacteria, minD in S. lavendulae ATCC 25233 may have a role other than cell division.
Lactic acid bacteria are one of the most commonly used probiotics. The role of prebiotics in improving human health has attracted global attention and the research is mostly focused on the strains belonging to Lactobacillus [9]. The survival of Lactobacillus probiotics was usually lower than the amounts noted in probiotic label in their products. Therefore, to find out the roles of Min system in Lactobacillus that made a wide genus with the different survival rates that relate to the cell division are necessary. From the stated reasons, we cloned and tested whether the Lactobacillus acidophilus MinD protein is functional in E. coli cells by overexpression. By analysis the minD gene from L. acidophilus VTCC-B-871, the minD genes from L. acidophilus ATCC 4356 and L. acidophilus ATCC 11443 Lactobacillus rhamnosus PN04, a strain isolated and identified from Vietnamese Hottuynia cordata Thunb. were identified from which a method for determination of L. acidophilus and L. rhamnosus will be applied so far.
Materials and Methods
Plasmids, Bacterial Strains, Growth Conditions
The pUC19 and pGEM-T vectors used for molecular cloning and E. coli JM109, BL21(DE3)pLysS were purchased by Promega. The pET28 (a+) used for overexpression was purchased by Novagen. L. rhamnosus GG, L. rhamnosus ATCC 11443, L. acidophilus ATCC 4356, L. acidophilus VTCC-B-871 purchased by Vietnam type culture collection (VTCC). E. coli JM109 was used as a host to clone LactobacillusminD genes. E. coli BL21(DE3)pLysS was used as an expression strain. Lactobacillus strains were grown on MRS for 72–96 h at 30 °C. E. coli strains were grown in Luria–Bertani for 18–24 h at 37 °C with shaking at 200 rpm. When required, antibiotics were added to media in the following concentrations: 100 μg of ampicillin/ml, 10 μg of chloramphenicol/ml, 50 μg of kanamycin/ml for E. coli.
DNA and RNA Isolation
Genomic DNA was isolated from Lactobacillus strains that had been grown for 72–96 h in MRS. The samples were incubated in MRS according to standard protocols. Total RNA was purified according to manufacturer’s instructions (Takara).
Isolation of the Homologous DNA minD Probe from Lactobacillus rhamnosus GG
Genomic DNA from L. rhamnosus GG was amplified by PCR using a sense primer OMR1(5′-GAATGCGACCGGGGCGGCTGACGGTGCGA-3′) and an anti-sense primer OMR2 (5′-TCAACGGCACGCTATCACCTAGTAACCGGC-3′) which was homologous to sequences between 391 and 739 nt of the minD gene (Gene ID: 8422477). The PCR was done under the following conditions: an initial 2 min at 95 °C; then, 29 cycles of 1 min at 95 °C followed by 30 s at 55 °C; and 30 s at 72 °C, finally, an extension period of 30 s at 72 °C. A PCR product of 349 bp corresponding to minD fragment was ligated into the pGEM-T vector and introduced into E. coli JM109 from the TA Cloning kit (Promega).
Cloning, Sequencing and DNA Analysis
The genomic DNA from L. acidophilus VTCC-B-871 strain was digested with restriction enzymes supplied by Takara (Japan). The digestion was followed as instructions of the company. Southern hybridization was performed by using a Hybond-N+ (Amersham Biosciences) membrane. Probe labeling, hybridization and detection were performed with AlkPhos Direct Labeling and Detection System (Amersham Biosciences) according to the protocol supplied by the manufacturer.
The cloning minD from L. acidophilus was performed [10]. DNA sequencing was performed with the ABI PRIZM 310 genetic analyzer using the BigDye terminator cycle sequencing ready reaction kit according to the manufacturer’s protocols. The Lactobacillus minD genes was determined and analyzed using Fasta. The protein molecular mass, pI were calculated on an ExPASy Proteomics Server. The sequence data obtained in this study has been submitted to the DDBJ.
Overexpression Studies of MinD and Light Microscopy
The L. acidophilusminD was amplified by PCR with a sense primer BHE1 (5′-CATATGGGGACAGCGTTAGTAGTGACTTC-3′) (the NdeI site is underlined) and an antisense BHE2 (5′-CTCGAGGATGGCGATGGAACAATTTTGAC-3′) (the XhoI site is underlined). The amplified minD was subcloned into pGEM-T vector and then was checked by DNA sequencing. The minD fragment was cut out from pGEM-T vector by NdeI and XhoI double-digestion and inserted into the same sites of pET-28(a+) to produce pET-28(a+)/minD. E. coli BL21(DE3)pLysS transformed with pET-28(a+)/minD was grown in LB medium supplemented with appropiate antibiotics at 37 °C to OD600 = 0.5, after which 0.5 mM IPTG or 1 % glucose, 1 % saccharose, 1 % manitol was added to culture to induce at 28 °C from 5 to 24 h. Light microscopy was used to observed the morphological changes in E. coli.
Isolation and Identification of Lactobacillus rhamnosus from Hottuynia Cordata Thunb.
Hottuynia Cordata Thunb. samples were collected in the Southern of Vietnam. No specific permits were required for the described field studies. The leaves were incubated in MRS for 72–96 h at 30 °C. The culture was used to spread onto MRS agar that was incubated in MRS for 72–96 h at 30 °C. The purified colonies were tested by microscopic examination with gram stain and catalase negative [11]. The isolated strains were identified by biochemical characterization based on the ability of the isolates to utilize different carbon sources, which determined by API CHL 50 system (bioMérieux, Lyon, France) and 16S rRNA sequencing analysis.
Phylogenetic Analyses, Protein Homology Modelling and Analysis
Phylogenetic analyses were performed on the MinD deduced amino acid sequences that were previously reported. Protein sequences were aligned with ClustalW software and clustered by using the un-weighted pair group method for the arithmetic mean. The tertiary structures of the deduced amino acid sequences of MinD were predicted by homology modelling using the Swiss-Model Server [12, 13] and MinD from E. coli (PDB: 3q9l) was used as template. The structural parameters and prediction quality of the modeled structures were evaluated using the program QMEAN4 with respect to score obtained for high-resolution experimental structures solved by X-ray crystallography [14].
Results and Discussion
Analysis of Genomic DNA of Lactobacillus acidophilus VTCC-B-871
L. rhamnosus GG genome was used as a template to prepare a 349 bp-DNA probe for Southern blotting and colony hybridization. A 4.0 kb HindIII-PvuII fragment was cloned from L. acidophilus VTCC-B-871 chromosomal DNA. A minD gene of 798 bp was sequenced and was deposited in the DDBJ database under accession no. AB725356. The MinD protein encoded by L. acidophilus VTCC-B-871 consists of 265 amino acids with a calculated pI of 5.07 and Mw of 28857.17 kDa. Having 100 % identity, the protein exhibits highest similarity to MinD (EHJ35458) from L. rhamnosus ATCC 21052 and 99 % identity to MinD (YP_00317015, gene ID: 8422477) from L. rhamnosus GG.
By comparison of nucleotide sequences of the minD gene from L. acidophilus VTCC-B-871 with L. rhamnosus strains, there are 779/798 (98 %) identity to L. rhamnosus ATCC 8530 and 777/798 (97 %) identity to Lc 705 and strain GG. Interestingly, the analyses the nucleotide sequence showed that there was 100 % similarity of the 40 nt at 5′ end and 40 nt at 3′ end of minD gene in L. acidophilus VTCC-B-871 and L. rhamnosus ATCC 8530, Lc 705 as well as GG. Clearly, minD gene will be identified easily and used in the identification of L. acidophilus or L. rhamnosus.
Conservation of MinD Proteins from Various Bacteria and Phylogenic Analysis
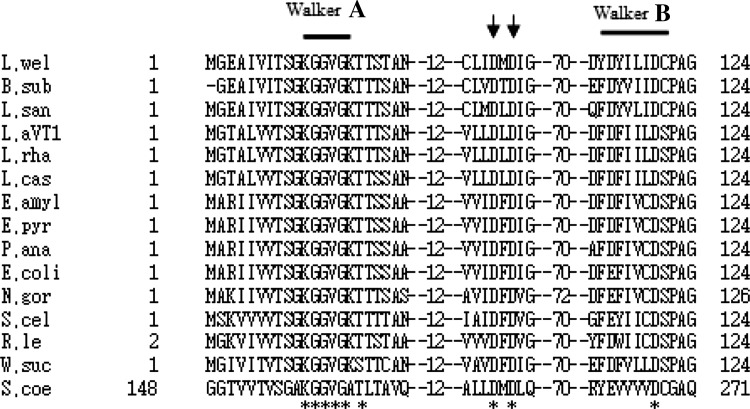
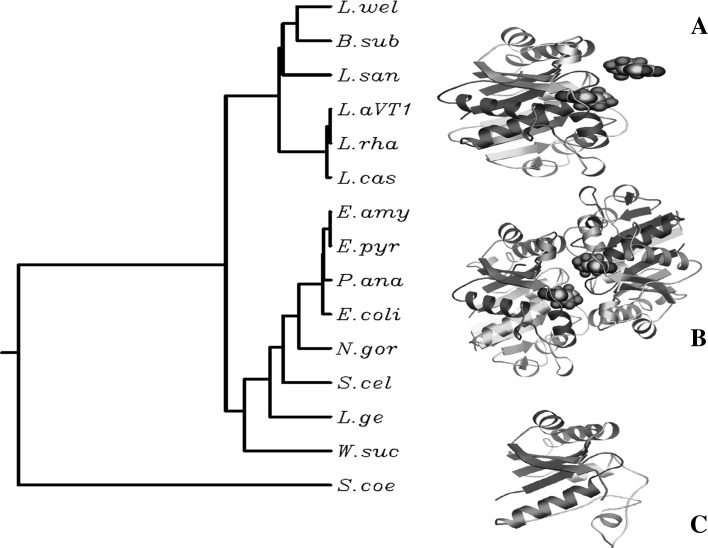
Figure 1. shows the alignment of amino acid sequences of MinD proteins from Lactobacillus strains with 15 strains listed in Fig. 1. The Walker A and B motifs and the two Asp residues (Asp38, Asp40) located between them are conserved in all sequences, suggesting that the Lactobacillus MinD protein possess an ATPase activity like that of E. coli MinD. Although L. acidophilus and L. rhamnosus strains are gram positive, the consensus nucleotide binding sequence of the Walker A motif is known as G/A-X-X-G-X-G-K-T/S that overlap with Walker A in gram negative E. coli, and Neiserria gonorrhoea [15] and that is distinguishable with Streptomyces which are gram-positive and the seventh Lys is replaced by Ala or Thr [8]. The results elucidated the relationship of minD from gram negative and gram positive. The protein or deduced amino acid MinD sequences from 15 strains were used to generate phylogenetic trees. Clustal alignment used for phylogenetic analysis allowed to determine the location of amino acids expected to have a catalytic role in MinD (Fig. 1). The combination with the tree analysis showed the early separation between amino acid sequences from the aligned strains can be explained in terms of the evolution of MinD for different purposes (Fig. 2). On this background, the results of the phylogenetic analyses based both on amino acid sequence similarities as well as their structural features would be strengthen the phylogenetic analysis and to establish a relationship between the genes encoding MinD with their three-dimensional structures involved in ATP binding (Fig. 2). By the comparison, the MinC and MinE homologs might have been eliminated in the process of the evolution.
Fig. 1.
Alignment of conserved motifs in MinD from Lactobacillus strains and other species. Alignment was carried out with the Clustal W program. Listed proteins are from the following strains: L. wel, Listeria welshimeri; B. sub, Bacillus subtilis; L. san, Lactobacillus sanfranciscensis; L. aVT1, Lactobacillus acidophilus VTCC-B-871, L. rha, Lactobacillus rhamnosus; L. cas, Lactobacillus casei, E. amyl, Erwinia amylovora; E. pyr, Erwinia pyrifoliae; P. ana, Pantoea ananatis; E. coli, Escherichia coli; N. gor, Neisseria gonorrhoeae; S. cel, Sorangium cellulosum; R. le, Rhizobium leguminosarum; W. suc, Wolinella succinogenes; S. coe, Streptomyces coelicolor. The conserved motifs (Walker A and B) and the two Asp residues are indicated by bars and arrow heads, respectively. Asterisks below the sequences show the conserved residues in all sequences
Fig. 2.
The phyogenic tree was used by the un-weighted pair group method using the arithmetic mean and clustering the three-dimensional structures of MinD. a and c Three-dimensional structure of MinD from Lactobacillus acidophilus VTCC-B-871 and S. coelicolor respectively, predicted by homology modelling using the Swiss-Model Server. b Three-dimensional structure of MinD from E. coli (PDB: 3q9l)
Protein Homology Modelling and Comparisons of Protein Structures
Once the tertiary structure of MinD was predicted, these results strongly support the notion that there is a close relationship between the tertiary structure of MinD and the lifestyle of the microorganisms. Comparative analyses of three-dimensional structures have been utilized for different purposes in searching for putative biological functions, drug design, protein–protein interaction studies [14]. However, to our knowledge, the study that uses a comparative analysis of protein structure in combination with a phylogenetic analysis to explore the evolution of lifestyle. Using Swiss-model server, the structure of L. acidophilus was predicted, using the template of E. coli (Fig. 2) with the final total energy of −8877.295 kJ/mol. The quality of the structure prediction was estimated by QMEAN4 (Table 1). The structures showed the alpha helix and beta sheet in L. acidophilus MinD and E. coli MinD occuring in the region of 2–249 amino acids of L. acidophilus (Fig. 2) with the ligand models of 2 ATP and 1Mg2+ molecules while Streptomyces MinD showed modelling homology in the region of 149–245 amino acids with no ligand model in this structure that corresponds to the sequences aligned in the Fig. 1. Although E. coli MinD exhibited a dimer that indicated the self-interaction [4] and L. acidophilus MinD predicted in monomer, the monomers of these structures are highly similar.
Table 1.
QMEAN4 data for model quality estimation
| Scoring function term | Raw score | Z-score |
|---|---|---|
| C-beta interaction energy | −138.89 | 0.13 |
| All-atom pairwise energy | −6408.44 | −0.7 |
| Solvation energy | −26.27 | −0.06 |
| Torsion angle energy | −55.07 | −0.85 |
| QMEAN4 score | 0.721 | −0.90 |
The results meant the comparative analysis can be an important tool for studying the proteins of microorganisms but also for the evolution of microorganisms and their proteins, since structural differences may reflect other important properties such as substrate specificity and others that can not be inferred from the analysis of amino acid sequences only. Therefore, minD gene might also participate in the evolution in microorganisms.
Study the minD Genes in Lactobacillus acidophilus ATCC 4356 and Lactobacillus rhamnosus ATCC 11443
To find out the relation, the MinD homologs were identified in L. acidophilus ATCC 4356 and L. rhamnosus ATCC 11443. As discussed above, a pair of primers with a sense primer OMS (5′-ATGGGGACAGCGTTAGTAGTGACTTC-3′) and an antisense OMS1 (5′-GATGGCGATGGAACAATTTTGAC-3′) was designed from L. acidophilus VTCC-B-871 to identify minD gene in L. acidophilus ATCC 4356 and L. rhamnosus ATCC 11443. After isolation, a minD gene from L. acidophilus ATCC 4356 was sequenced. A minD gene of 798 bp was sequenced and deposited in the DDBJ under accession no. AB725355. Similarity, a minD gene of 798 bp from L. rhamnosus ATCC 11443 was deposited in the DDBJ under accession no. AB725357. With the understanding of minD in sequences, the role of the survival of different strains will be discovered.
Isolation and Identification of Lactobacillus rhamnosus from Hottuynia Cordata Thunb.
To make a sure of the minD existence and aid for identification, an isolation of L. rhamnosus from H. cordata Thunb. was done and checked by biochemical tests using ABI 50CHL and 16S rRNA sequencing. By using the API 50CHL (BioMerieux), a isolated strain showed the result of L. rhamnosus (Data not shown). By Blast search, the 16S rRNA sequence of L. rhamnosus shows 99 % identity to L. rhamnosus NT10. The isolated strain was named L. rhamnosus PN04 and the 16S rRNA sequence was deposited in DDBJ (accession number: AB738399). Using the OMS sense and OMS1 antisense primers, a PCR product of minD gene was detected, indeed (Fig. 3). The results also pointed the relationship between L. acidophilus and Lactobacillus rhamnosus.
Fig. 3.
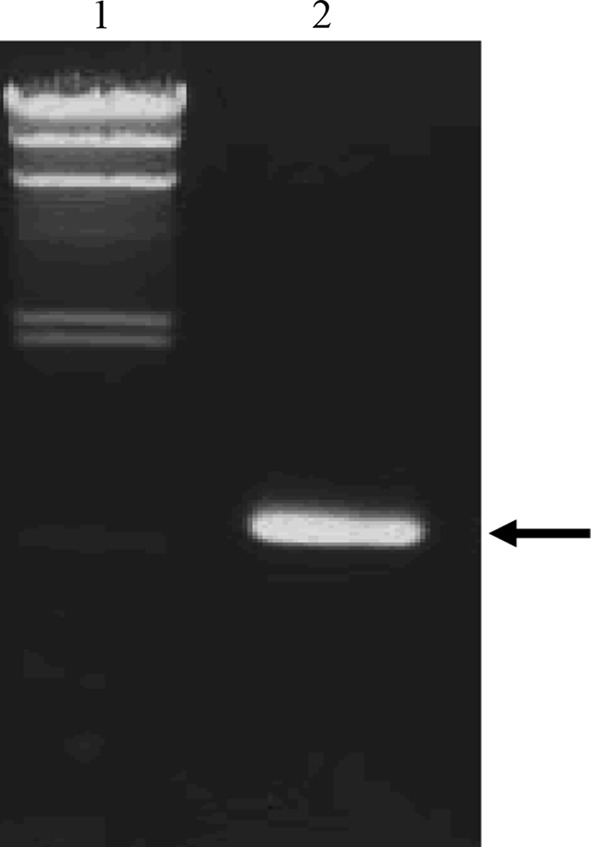
The PCR product of minD gene from isolated Lactobacillus rhamnosus. From left to right: 1, λ/HindIII marker; 2, PCR product. The arrow shows the PCR product
Overexpression of minD Gene and Light Microscopy
To test whether the L. acidophilus MinD protein is functional in E. coli cells, the E. coli BL21(DE3)plysS was introduces with plasmid pET-28(a+) containing minD. After expression, cells transformed with the pET-28(a+) exhibited the normal rod-shaped morphology (Fig. 4a), while the same strain transformed with pET-28(a+)/minD exhibited a mixed phenotype of long filaments (Fig. 4b). The result of filamentous phenotype may have occurred because Lactobacillus MinD enhanced MinC-mediated inhibition of cell division at all potential division sites in E. coli cells. Indeed, it has also been reported that the overexpression of Neissheria MinD in E. coli cells leads to filamentation [15]. This result indicated that Lactobacillus MinD is functional across species. The cells transformed with the pET-28(a+)/minD were also tested to grow in glucose, saccharose and manitol. Interestingly, under the saccharose stress, the cells become long and curled shape (Fig. 4c). The IPTG inducer was used in pET vector system because of T7 promoter. However, under the sugar stresses, the morphology was changeable. The hypothesis was posed whether the interaction between MinD and sugar. The results were the first reports in the morphological differentiation of E. coli carrying minD gene of Lactobacillus.
Fig. 4.
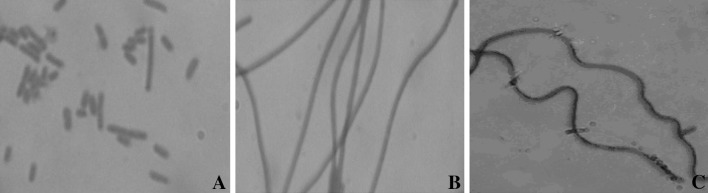
Morphology of Escherichia coli harboring the minD gene from Lactobacillus acidophilus. Escherichia coli BL21(DE3)plysS cells harboring pET 28(a+)/minD and pET 28(a+) were analyzed by light microscopy. aEscherichia coli BL21(DE3)plysS cells harboring pET 28(a+). bEscherichia coli BL21(DE3)plysS cells harboring pET 28(a+)/minD in IPTG. cEscherichia coli BL21(DE3)plysS cells harboring pET 28(a+)/minD in saccharose. The scale bar is 5 μm
Acknowledgments
Thanks to the grant supplied by the National Foundation of Science and Technology Development of Vietnam (Nafosted) and the support of Hochiminh City International University by which this work has been fullfiled.
References
- 1.Park KT, Wu W, Lovell S, Lutkenhaus J. Mechanism of the asymetric activation of the MinD ATPase by MinE. Mol Microbiol. 2012;85(2):271–281. doi: 10.1111/j.1365-2958.2012.08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou H, Schulze R, Cox S, Saez C, Hu Z, Lutkenhaus J. Analysis of minD mutations reveals residues required for minE stimulation of the minD ATPase and residues required for minC interaction. J Bact. 2005;187(2):629–638. doi: 10.1128/JB.187.2.629-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma L, King GF, Rothfield L. Positioning of the MinE binding site on the MinD surface suggests a plausible mechanism for activation of the Escherichia coli MinD ATPase during division site selection. Mol Microbiol. 2004;54(1):99–108. doi: 10.1111/j.1365-2958.2004.04265.x. [DOI] [PubMed] [Google Scholar]
- 4.Lutkenhaus J, Sundaramoorthy M. MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation. Mol Microbiol. 2003;48(2):295–303. doi: 10.1046/j.1365-2958.2003.03427.x. [DOI] [PubMed] [Google Scholar]
- 5.Stahlber H, Kutejova E, Muchova K, Gregorini M, Lustig A, Muller SA, Olivieri V, Engel A, Wilkinson AJ, Barak I. Oligomeric structure of Bacillus subtilis cell division protein DivIVA determined by transmission electron microscopy. Mol Microbiol. 2004;52(5):1281–1290. doi: 10.1111/j.1365-2958.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 6.Bently SD, Chater KF, Cerdeno-Tárraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, et al. Compete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda H, Ishikawa J, Hanomoto A, Shinose M, kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen HKT, Kumagai T, Matoba Y, Suzaki T, Sugiyama M. Molecular cloning and functional analysis of minD gene from Streptomyces lavendulae ATCC 25233. J Biosci Bioeng. 2008;106(3):303–305. doi: 10.1263/jbb.106.303. [DOI] [PubMed] [Google Scholar]
- 9.Chithra M, Muralikrishna G. Prebiotic activity of purified xylobiose obtained from ragi (Eleusine coracana, Indaf-15) Bran. Indian J Microbiol. 2012;52(2):251–257. doi: 10.1007/s12088-011-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratories; 2001. [Google Scholar]
- 11.Seema P, Avishek M, Arun G. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52(1):3–12. doi: 10.1007/s12088-011-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold K, Bordoli L, Kopp J, Schwede T. The swiss-model workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 13.Schwede T, Kopp J, Guex N, Peitsch MC. Swiss-model: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27(3):343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parti RP, Biswas D, Helgeson S, Michael FS, Cox A, Dillon JA. Attenuated virulence of min operon mutants of Neisseria gonorrhoeae and their interactions with human urethral epithelial cells. Microbes Infect. 2011;13(6):545–554. doi: 10.1016/j.micinf.2011.01.018. [DOI] [PubMed] [Google Scholar]