Abstract
Purpose
In patients with localised neuroblastoma without adverse genetic aberrations, observational treatment is justified. Therapy is required when organ or respiratory functions have become compromised. As the outcome is good, side effects of treatment should be prevented. The aim of this retrospective study was to evaluate response and outcome in patients treated with 131I-metaiodobenzylguanidine (MIBG) for unresectable localised neuroblastoma, with compromised organ functions.
Methods
Patients with localised neuroblastoma [median age 1.6 years (0–5.5 years)] diagnosed between 1989 and 2008 were included in this retrospective study (n = 21). Primary tumours were unresectable and there was a compromised organ or respiratory function. Diagnosis and staging were performed according to the International Neuroblastoma Staging System. Fixed doses of 131I-MIBG therapy (50–200 mCi) were given. The median number of infusions was two (range one to seven). Response was graded according to the International Neuroblastoma Response Criteria.
Results
Of the 21 patients, 14 did not need any chemotherapy. Patients were treated with 131I-MIBG therapy and, in most cases, with additional surgery and/or chemotherapy. Sixteen achieved complete response (CR), three very good partial response (VGPR), one partial response (PR) and one progressive disease (PD). Two patients died of PD after having achieved CR initially and due to surgical complications a few months after resection. Ten-year overall survival and event-free survival were 90.5 %. The median follow-up was 8.5 years (range 0.4–19.6 years).
Conclusion
131I-MIBG therapy is an effective treatment modality for unresectable localised neuroblastoma with compromised organ functions. However, this was a small and heterogeneous cohort and further studies are needed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00259-013-2455-2) contains supplementary material, which is available to authorized users.
Keywords: Neuroblastoma, 131I-MIBG therapy, Localised, Unresectable
Introduction
Neuroblastoma is the most common extracranial solid tumour of childhood. Arising from the neural crest, primary tumours can be localised in the adrenal glands or anywhere along the sympathetic side chain. Important prognostic factors are age, stage, MYCN amplification (MNA), loss of heterozygosity of chromosome 1p (1pLOH), chromosomal 11q aberration and DNA ploidy [1]. Approximately 25 % of patients present with localised disease without distant metastases.
Treatment modality and intensity of treatment in patients with localised disease, stage 1–3 [International Neuroblastoma Staging System (INSS)], are based upon risk stratification and defined by age, stage and biological features. The prognosis is good and the majority of patients can be cured by surgery alone or by a combination of surgery and chemotherapy [2].
If the primary tumour causes or is in danger of causing organ or respiratory dysfunction, as in paravertebral tumours with spinal cord compression, (urgent) treatment is needed. In these circumstances, volume reduction is necessary to reduce symptoms, usually with (neuro-) surgical resection (i.e. laminectomy). In these patients, treatment with chemotherapy is often recommended [3]. However, as the prognosis of localised neuroblastoma is good, toxicity and late effects should be minimised. Targeted radiotherapy with 131I-metaiodobenzylguanidine (MIBG) is an effective treatment modality for neuroblastoma and rarely causes severe toxicity [4, 5].
The use of 131I-MIBG as initial therapy in patients with localised neuroblastoma has not been reported thus far. The aim of this retrospective study was to investigate the effect of 131I-MIBG therapy on resectability and outcome in patients with unresectable localised neuroblastoma causing or in danger of causing organ or respiratory dysfunction.
Materials and methods
Patients
In this retrospective cohort study we included all (21) neuroblastoma patients (0–18 years) diagnosed with INSS stage 1–3, treated initially with 131I-MIBG therapy, with inoperable MIBG-positive lesions that were causing or were in danger of causing organ dysfunction. The study was performed in the Emma Children’s Hospital/Academic Medical Centre (EKZ/AMC) in Amsterdam between 1989 and 2008. Patients were treated with 131I-MIBG therapy if volume reduction was necessary to reduce symptoms of compromised organ or respiratory function and if tumour resection was not possible. Nine patients have been described before [6].
Staging
The diagnostic workup consisted of imaging, analysis of urinary catecholamines, bone marrow biopsies/trephines, serum lactate dehydrogenase (LDH) and ferritin. Extension of the tumour was visualised with ultrasound (US), 123I-MIBG scintigraphy, computed tomography (CT) and/or magnetic resonance imaging (MRI). The diagnosis was confirmed histologically, with tissue obtained either by fine-needle aspiration, Tru-Cut biopsy or excisional biopsy. Staging was performed according to the INSS criteria [7].
Treatment
Patients were treated with 131I-MIBG according to the protocol previously described by de Kraker et al. [4]. Fixed doses were given; the first dose was either 200 or 150 mCi (7,400 or 5,550 MBq), and the second dose was lower, 150 or 100 mCi (5,550 or 3,700 MBq). Doses were adjusted based on age and stage. 131I-MIBG was infused slowly (100 mCi/h) with an interval of 4 weeks. Patients remained in radioprotective isolation until the exposure rate was less than 20 μSv/h, measured with a counter at a distance of 1 m from the patient [4].
The parents were extensively informed about the nature and precise elements of the treatment and the differences from other strategies and consent was obtained prior to treatment.
Response
Response was graded according to the International Neuroblastoma Response Criteria (INRC) [7]. Response of the tumour was evaluated 3–4 weeks after each course. Patients underwent multiple 131I-MIBG therapies if treatment was tolerated well, if 131I-MIBG uptake in the tumour was sufficient and if response was ongoing, but the tumour remained unresectable. Gross total resection was defined as removal of more than 95 % of the tumour. Partial resection was defined by the presence of macroscopic remnants.
Overall survival (OS) was measured as the time from start of treatment to death by any cause. Event-free survival (EFS) was defined as the time from start of treatment to a first event (progression, relapse or death).
Statistical analysis
OS and EFS were estimated by the Kaplan-Meier method. Patients were censored in the EFS analysis if they were lost to follow-up. The median follow-up was 8.5 years (range 0.4–19.6 years).
Results
Patients
Between 1989 and 2008, 21 patients (8 male, 13 female) with localised neuroblastoma (INSS stage 1–3) were treated with 131I-MIBG in the EKZ/AMC. The median age at diagnosis was 1.6 years (range 0–5.5 years); nine patients (43 %) were 1 year or younger (Table 1, Supplementary Table 1). According to the INSS staging criteria, four neuroblastomas were classified as stage 1, three as stage 2 and 14 as stage 3. Tumours were localised in the abdomen in 11 patients. Other localisations were thoracic (n = 5), pelvic (n = 4) and the neck (n = 1).
Table 1.
Patient characteristics at diagnosis
| Number (%) | |
|---|---|
| n | 21 |
| Sex | |
| Male | 8 (38) |
| Female | 13 (62) |
| Age at diagnosis (years) | |
| Median (range) | 1.6 (0–5.5) |
| ≤1 year | 9 (43) |
| INSS stage | |
| 1 | 4 (19) |
| 2 | 3 (14) |
| 3 | 14 (67) |
| Histology | |
| Neuroblastoma | 17 (81) |
| Ganglioneuroblastoma | 4 (19) |
| Localisation | |
| Neck | 1 (5) |
| Thoracic | 5 (24) |
| Abdominal | 11 (52) |
| Pelvic | 4 (19) |
| Intraspinal component | 7 (33) |
| Genetic aberrations | |
| MNA | 2/18 |
| 1pLOH | 1/18 |
| Both | 1/18 |
| Urinary catecholamines | |
| Elevated | 18/21 |
| Normal | 3/21 |
| LDH | |
| 0–1 year (≥400 U/l) | 3/9 |
| 1–17 years (≥300 U/l) | 3/12 |
| Ferritin | |
| ≥143 ng/ml | 4/18 |
n number of patients, INSS International Neuroblastoma Staging System, MNA MYCN amplification, 1pLOH chromosome 1p loss of heterozygosity, LDH lactate dehydrogenase
Seventeen patients had a neuroblastoma and four a ganglioneuroblastoma. Genetic aberrations were analysed in 18 patients: MNA was present in two tumours, 1pLOH in one and both aberrations in another patient. Urinary catecholamines were elevated in 18 patients; 3 patients with ganglioneuroblastoma had normal excretion (stage 1). LDH levels were elevated in 6 patients; ferritin was elevated in 4 of 18 patients.
131I-MIBG therapy
Primary resection of the tumour was not feasible in all patients and the tumour caused or was in danger of causing organ or respiratory dysfunction (Table 2, Supplementary Table 2). Tumour extension into the spinal canal was the indication for 131I-MIBG therapy in seven patients. This caused neurological deficits in six patients. Indications for 131I-MIBG therapy in the remaining 14 patients were: compression of the trachea, close relationship to the kidney, encasement of major vessels, attachment to the crus of the diaphragm, partial intestinal obstruction or hydronephrosis.
Table 2.
131I-MIBG therapy characteristics
| Number (%) | |
|---|---|
| n | 21 |
| Reasons for 131I-MIBG therapy | |
| Compromised organ functions | 14 (67) |
| Intraspinal | 7 (33) |
| Number of 131I-MIBG-infusions | |
| 1 | 3 (14) |
| 2 | 15 (71) |
| 3 | 1 (5) |
| 4 | 0 (0) |
| 5 | 1 (5) |
| 6 | 0 (0) |
| 7 | 1 (5) |
| First 131I-MIBG-dose in mCi (Mbq) | |
| 50 (≈1,850) | 2 (10) |
| 100 (≈3,700) | 4 (19) |
| 150 (≈5,550) | 7 (33) |
| 200 (≈7,400) | 6 (28) |
| Unknown | 2 (10) |
| Second 131I-MIBG-dose in mCi (Mbq) | |
| 50 (≈1,850) | 1 (5) |
| 100 (≈3,700) | 7 (39) |
| 150 (≈5,550) | 7 (39) |
| 200 (≈7,400) | 0 |
| Unknown | 3 (17) |
n number of patients, MIBG metaiodobenzylguanidine, mCi millicurie, MBq megabecquerel
The median number of 131I-MIBG therapies given was two (range one to seven). Administered doses varied from 5.8 to 20.6 mCi/kg (median 13.4 mCi/kg) during the first treatment and from 5.9 to 16.3 mCi/kg (median 12.4 mCi/kg) during the second treatment. 131I-MIBG therapy was combined with topotecan in two patients according to the protocol that was used during that period.
Response
Delivered therapy and response are presented in Fig. 1. Treatment with solely 131I-MIBG therapy without additional chemotherapy (group A) in two patients resulted in complete response (CR) in one patient and a very good partial response (VGPR) in the other. This last patient was considered inoperable after 131I-MIBG therapy as the residual tumour was located against the crus of the diaphragm. CR was achieved in seven patients after 131I-MIBG therapy followed by surgery (group B). 131I-MIBG therapy, surgery and chemotherapy (group C) were given in three patients. One of them received two cycles of vincristine, etoposide, carboplatin and ifosfamide (VECI) after surgery because of 1pLOH and achieved CR. 1pLOH was not yet incorporated in the neuroblastoma risk stratification at that time, but increased risk was suspected based on preliminary data. In a second patient high-risk (HR) neuroblastoma (MNA and 1pLOH) was diagnosed and treated according to the HR protocol following 131I-MIBG therapy; subsequently CR was achieved. The third patient was treated with one VECI cycle while waiting for 131I-MIBG therapy; after 131I-MIBG therapy disease progressed and HR treatment was initiated. This patient died of progressive disease (PD).
Fig. 1.
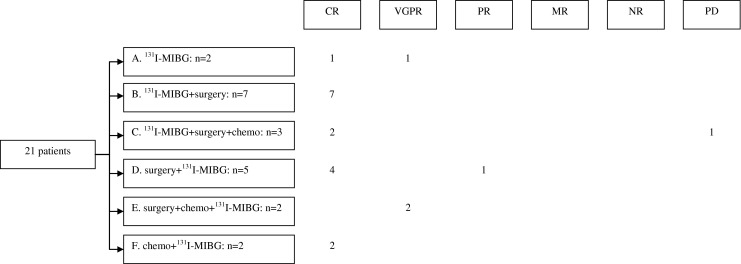
Flow chart response. MIBG metaiodobenzylguanidine, chemo chemotherapy, CR complete response, VGPR very good partial response, PR partial response, MR mixed response, NR no response, PD progressive disease
Nine patients had treatment prior to 131I-MIBG therapies. Surgery was the treatment preceding 131I-MIBG therapy (group D) in five patients. A laminotomy was performed in one patient because of tumour progression; this patient continued with 131I-MIBG therapy and achieved CR. Another patient underwent a laminectomy to relieve symptoms caused by an intraspinal tumour. The tumour recurred 4 months later and the patient was re-operated. A second relapse was treated with two cycles of 131I-MIBG therapy and CR was achieved. An emergency laminectomy and laminotomy were necessary in a third patient because of intraspinal extension of the tumour and this patient achieved CR after 131I-MIBG therapies. A presacral tumour in a fourth patient was treated with 131I-MIBG therapy after incomplete surgery. This patient achieved partial response (PR) after five cycles of 131I-MIBG therapy and the residual lesion remained stable during follow-up. The fifth patient was an infant at diagnosis and had a partial resection of a retroperitoneal presacral tumour in order to delay 131I-MIBG therapy. This patient achieved CR after 131I-MIBG therapy.
Both chemotherapy and surgery preceding 131I-MIBG therapy (group E) were applied in two patients. A laminotomy followed by a laminectomy and chemotherapy (CADO: cyclophosphamide, vincristine and doxorubicin) were performed in one patient. Because of PD during chemotherapy this patient received two cycles of 131I-MIBG therapy and subsequently achieved VGPR with a residual primary lesion that remained stable during follow-up. The other patient was treated with two cycles of vindesine, VP-16 and cisplatin followed by decompressive surgery in her home country preceding 131I-MIBG therapy achieving VGPR. Four months after the third course of 131I-MIBG therapy, she underwent a (macroscopic complete) surgical resection in the home country, despite the presence of surgical risk factors. As a complication of surgery, she became tetraplegic and died a few months later of an infection.
Modified cycles of chemotherapy were started before 131I-MIBG therapy (group F) in two patients. The first patient was treated in the home country with CADO before coming to our centre. The second patient was suspected to have a Wilms’ tumour and was pretreated with vincristine and actinomycin; both achieved CR. No toxic deaths related to 131I-MIBG therapy occurred.
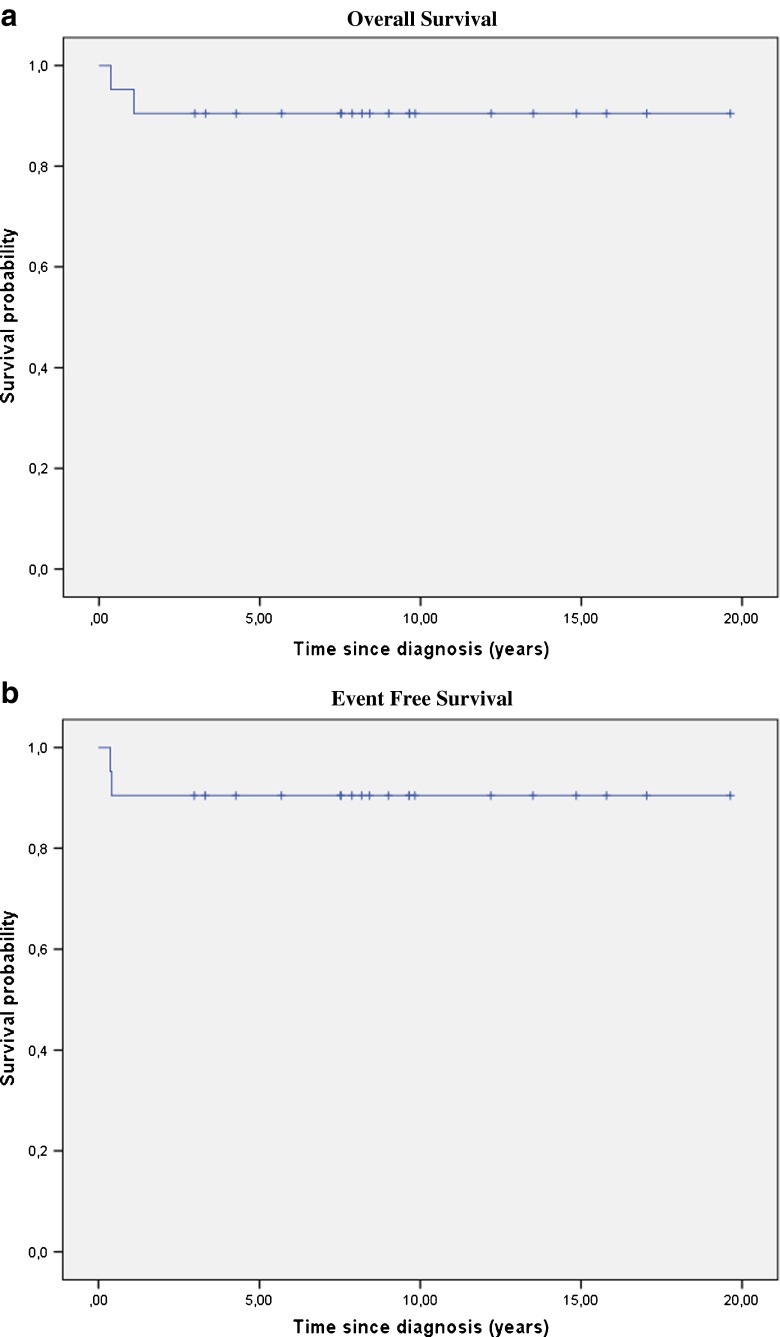
In conclusion, 16 of 21 patients achieved CR after treatment. Residual but stable disease (three VGPR, one PR) was achieved in four patients. One patient had PD and consequently died. The 10-year OS and EFS were both 90.5 % (Fig. 2). The median follow-up was 8.5 years (range 0.4–19.6 years).
Fig. 2.
a OS: 10-year OS = 90.5 %, median follow-up = 8.5 years (range 0.4–19.6 years). Time since diagnosis: years. b EFS: 10-year EFS = 90.5 %, median follow-up = 8.5 years (range 0.4–19.6 years). Time since diagnosis: years
Discussion
We conclude that 131I-MIBG therapy is an effective treatment modality for unresectable localised neuroblastoma causing or in danger of causing organ or respiratory dysfunction. Of the 21 patients, 14 did not need chemotherapy.
In patients with localised neuroblastoma, without MNA or 1pLOH, observation or surgery is usually recommended [8]. Since the start of 131I-MIBG therapy in the 1980s, several reports have described its efficacy and toxicity proven to be mild and infrequent [4]. Phase I/II studies in patients with refractory or relapsed neuroblastoma showed a response rate of 10–56 %. Persistent thrombocytopenia was the main toxicity observed in these extensively pretreated patients [9]. Because 131I-MIBG therapy was effective with limited toxicity, the patients with localised neuroblastoma in our cohort were treated with 131I-MIBG therapy, rather than chemotherapy when urgent treatment was indicated.
In this report, 131I-MIBG therapy proved to be a good alternative for chemotherapy for at least 14 of 21 (66.7 %) patients. Tumours regressed completely; residual lesions became resectable or remained stable. One exception was a HR tumour with MNA and 1pLOH that progressed during HR treatment and the patient eventually died.
The 10-year OS was 90.5 %. However, compared to other cohorts treated with chemotherapy and/or surgery, our cohort is small and heterogeneous. It included infants as well as older children with different risk profiles and different pre- and post-treatment modalities.
Since the prognosis of localised neuroblastoma is good, toxicity and late effects should be avoided if possible. Hence, Rubie et al. reported a 5-year OS of 99 % for a heterogeneous patient group of 120 infants. This group consisted of patients with and without threatening symptoms and different treatment strategies. The study stated that low-dose chemotherapy without anthracyclines was effective in 62 % of infants with an unresectable neuroblastoma and no MNA [3]. No treatment-related deaths were observed. However, PD and recurrence occurred in 12 of 120 patients. Two patients in our cohort died. These deaths were not related to 131I-MIBG therapy but to progressive HR disease and to complications of surgery.
131I-MIBG therapy is mainly reported in patients with refractory or relapsed neuroblastoma. These patients have been treated with multiple preceding treatment modalities and therefore it is difficult to report which late effects are caused by 131I-MIBG therapy alone.
Thyroid toxicity is a known side effect of 131I-MIBG therapy. Although thyroid protection during 131I-MIBG therapy mostly prevents 131I thyroid uptake, late effects of 131I-MIBG therapy on thyroid function cannot be ruled out. Recently, our group reported two patients with differentiated thyroid carcinoma following 131I-MIBG therapy [10]. These patients received adequate thyroid protection during 131I-MIBG therapy, and no 131I-MIBG uptake was seen in their thyroids on MIBG imaging. So, although no clear evidence is available thus far, patients should be checked regularly for thyroid problems after 131I-MIBG therapy. None of the patients described in this report developed a thyroid carcinoma.
In current protocols genetic aberrations such as MNA, 1pLOH and 11q aberration are included in risk classification systems [1]. Three patients from our cohort were treated before genetic aberrations were implemented in treatment protocols. MNA and 1pLOH were present in one patient with a stage 3 neuroblastoma. This patient was eventually treated as a HR patient after three courses of 131I-MIBG therapy and is still alive 11 years after treatment. MNA was present in two other patients (stage 2 and 3). These two patients received two courses of 131I-MIBG therapy followed by a complete resection and are still alive after 12.2 and 7.5 years of follow-up respectively.
We conclude that 131I-MIBG therapy is an effective treatment modality for unresectable localised neuroblastoma causing or in danger of causing organ or respiratory dysfunction and offers a good alternative to chemotherapy if urgent treatment is needed. However, for the assessment of long-term toxicity, long-term follow-up for all patients treated with chemotherapy and/or with 131I-MIBG is needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Patient overview. Nr: patient number; Stage: INSS stage; Age: in years; MNA: MYCN amplification; 1pLOH: chromosome 1p loss of heterozygosity; 131I-MIBG therapy: the number of 131I-MIBG therapies the patient received; INRC: international neuroblastoma response criteria; CR: complete response; VGPR: very good partial response; PR: partial response; MR: mixed response; NR: no response; PD: progressive disease (DOC 52 kb)
Indications for treatment (DOC 27 kb)
Acknowledgments
Unfortunately Dr. J. de Kraker passed away during this study. This work was supported by Kika (Children Cancer Free Foundation) and Tom Voûte foundation. We would like to thank Dr. C. A. Hoefnagel for contributing data to this report.
Conflicts of interest
None.
Footnotes
Reineke A. Schoot and Gitta Bleeker contributed equally to this article.
Author contributions
Conception and design: Reineke A. Schoot, Gitta Bleeker, Berthe L. van Eck, Hugo A. Heij, Jan de Kraker, Godelieve A. M. Tytgat.
Collection and assembly of data: Reineke A. Schoot, Gitta Bleeker, Jan de Kraker.
Data analyses and interpretation: Reineke A. Schoot, Gitta Bleeker, Huib N. Caron, Jan de Kraker, Godelieve A. M. Tytgat.
Manuscript writing: Reineke A. Schoot, Gitta Bleeker, Huib N. Caron, Berthe L. van Eck, Hugo A. Heij, Jan de Kraker, Godelieve A. M. Tytgat.
Final approval of manuscript: Reineke A. Schoot, Gitta Bleeker, Huib N. Caron, Berthe L. van Eck, Hugo A. Heij, Godelieve A. M. Tytgat.
References
- 1.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289–97. [DOI] [PMC free article] [PubMed]
- 2.Perez CA, Matthay KK, Atkinson JB, Seeger RC, Shimada H, Haase GM, et al. Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children’s cancer group study. J Clin Oncol. 2000;18(1):18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Rubie H, De Bernardi B, Gerrard M, Canete A, Ladenstein R, Couturier J, et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: results of the prospective INES 99.1. J Clin Oncol 2011;29:449–55. [DOI] [PubMed]
- 4.de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer. 2008;44(4):551–556. doi: 10.1016/j.ejca.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 5.van Hasselt EJ, Heij HA, de Kraker J, Vos A, Voûte PA. Pretreatment with [131I] metaiodobenzylguanidine and surgical resection of advanced neuroblastoma. Eur J Pediatr Surg. 1996;6(3):155–158. doi: 10.1055/s-2008-1066495. [DOI] [PubMed] [Google Scholar]
- 6.de Kraker J, Hoefnagel CA, Caron H, Valdés Olmos RA, Zsiros J, Heij HA, et al. First line targeted radiotherapy, a new concept in the treatment of advanced stage neuroblastoma. Eur J Cancer. 1995;31A(4):600–602. doi: 10.1016/0959-8049(95)00063-O. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 8.Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel-Walter HG, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26(9):1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 9.DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol. 2008;35(Suppl 1):S35–S48. doi: 10.1016/j.nucmedbio.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Santen HM, Tytgat G, van de Wetering MD, van Eck-Smit BL, Hopman S, van der Steeg AF, et al. Differentiated thyroid carcinoma after (131)I-MIBG treatment for neuroblastoma during childhood: description of the first two cases. Thyroid 2012;22:643–6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient overview. Nr: patient number; Stage: INSS stage; Age: in years; MNA: MYCN amplification; 1pLOH: chromosome 1p loss of heterozygosity; 131I-MIBG therapy: the number of 131I-MIBG therapies the patient received; INRC: international neuroblastoma response criteria; CR: complete response; VGPR: very good partial response; PR: partial response; MR: mixed response; NR: no response; PD: progressive disease (DOC 52 kb)
Indications for treatment (DOC 27 kb)