Abstract
Equisetum plants (horsetails) reproduce by producing tiny spherical spores that are typically 50 µm in diameter. The spores have four elaters, which are flexible ribbon-like appendages that are initially wrapped around the main spore body and that deploy upon drying or fold back in humid air. If elaters are believed to help dispersal, the exact mechanism for spore motion remains unclear in the literature. In this manuscript, we present observations of the ‘walks’ and ‘jumps’ of Equisetum spores, which are novel types of spore locomotion mechanisms compared to the ones of other spores. Walks are driven by humidity cycles, each cycle inducing a small step in a random direction. The dispersal range from the walk is limited, but the walk provides key steps to either exit the sporangium or to reorient and refold. Jumps occur when the spores suddenly thrust themselves after being tightly folded. They result in a very efficient dispersal: even spores jumping from the ground can catch the wind again, whereas non-jumping spores stay on the ground. The understanding of these movements, which are solely driven by humidity variations, conveys biomimetic inspiration for a new class of self-propelled objects.
Keywords: Equisetum, spores, locomotion, hygroscopic, elastic energy
1. Introduction
Plants show a broad spectrum of movements with a wide variety of underlying mechanisms. Spore dispersion mechanisms are widely varied in plants and fungi. However, one common physical process is rapid ejection achieved by the sudden rupture of material. For example, spore dispersal in fungus relies on the sudden rupture of the junction between the spore and its stalk after condensation of a water droplet [1,2], or after the rupture of the fluid-filled stalk under osmotic stress [3]. In sphagnum mosses, the spore-containing capsules break when the drying stress is excessively large, which expels a jet of spores [4]. These motions can also be compared with the explosive dispersal of seeds [5,6]. In ferns, the sudden collapse of water (cavitation) in the flexible arm of the sporangium catapults the spores [7].
Like ferns, Equisetum is a very ancient plant with robust adaptations [8]. Their spores present four elaters that respond to humidity variations [9,10]. The elaters function is to increase dispersal because they push the spores out of the plant and increase the aerodynamic drag in the wind [11]. Contrary to other types of spores mentioned earlier, the dispersal does not involve any rupture of material.
2. Hygroscopic response
To understand the locomotion and dispersion mechanisms of Equisetum spores, we performed a detailed microscopic study of the shape of the spores under various humidity conditions. We observed that the elaters clearly change their shape (figure 1). At high humidity levels, the elaters spiral around the spherical body. At less than 75% humidity, the elaters begin to unfurl and become straight at approximately 50% of relative humidity (RH), at which point they are fully extended. At less than 50% RH, the elaters curl up, and the maximum span of the spores is slightly reduced.
Figure 1.
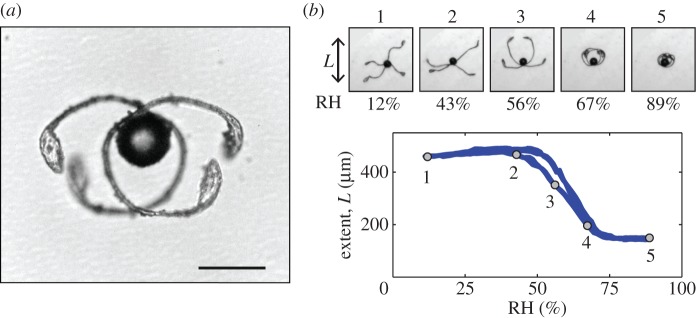
(a) The four elaters of a spore unfurling in a dry atmosphere. Scale bar, 50 µm. (b) Elater extent as a function of the RH. Curves obtained by varying the humidity at a rate of 1% RH per minute. Circles 1–5 on the curve correspond to the five pictures. (Online version in colour.)
These changes are fully reversible, and only slight hysteresis was observed when increasing the humidity, suggesting that the spore shape reflects the current humidity. Humidity variations that induce changes in the elater shape are typical in the Equisetum habitat where humidity is high but can decrease periodically, e.g. owing to wind or sun exposure.
The change in the curvature of the elaters can be understood from a structural perspective, as the elaters have a bilayer structure [12]. The inner layer consists of dense longitudinal cellulose microfibrils (similar to that of higher plant cell walls), whereas the outer layer is less dense. We can infer from this structure that the outer layer is highly porous to water and changes in volume with variations in humidity. The differential volume change of one layer with respect to the other is responsible for the marked curvature changes. Thus, elaters are an example of natural ‘hygromorphs’ [13]. Such hygromorphs include certain types of seeds [14].
3. Random walks
The elaters can open periodically in response to repeated humidity cycles. The position of the centre of mass oscillates but does not return to the same position at the end of a cycle (figure 2a,b). As a result, the spore ‘walks’ in random directions. The amplitude of the steps of these walks is variable (see also figure 2c), as a spore can stay in the same position for multiple cycles before experiencing a large step but with a well-defined average.
Figure 2.
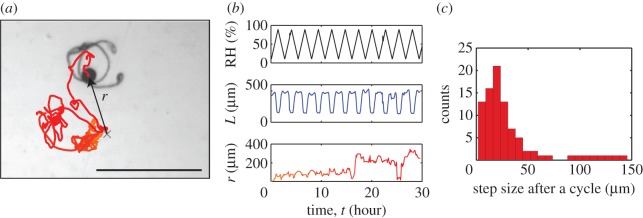
Walks under repeated humidity oscillations. (a) The trajectory of the centre of mass of the spore (see also the electronic supplementary material, movie S1), and (b) measurement of the extent, L, and the total distance, r, travelled. (c) Distribution of step sizes during a trajectory. The step size is defined as the distance between positions at the beginning of each humidity cycle. The average step size here is 31 µm. (Online version in colour.)
We interpret these random steps to be a consequence of friction with the ground. More precisely, kinetic friction (as opposed to static friction) occurs during the opening/closing cycles when the elaters slide on the ground. Kinetic friction creates irreversible changes because the position of static contact is displaced. These repeated displacements induce steps. The variability in the orientation of the steps is attributed to the changes in the points of contact of this complex ‘tetrapod’ during the opening/closing cycles.
The tracking of many spores using image analysis showed that the spores diffuse in space (figure 3a,b). The average squared distance travelled by each spore follows a diffusion law 〈r2〉 = 4Dt, where t is the time elapsed from a starting position, and D is the two-dimensional diffusion coefficient. The average is taken over several trajectories and over different starting points. The value of the diffusion coefficient corresponds to an effective step of amplitude l = 28 µm repeated with a period τ given by the cycle duration Tcycle (using D = l2/2τ). The average distance travelled was calculated for many spores and for various starting positions, which is typical when tracking mobile agents for instance swimming microorganisms [15].
Figure 3.
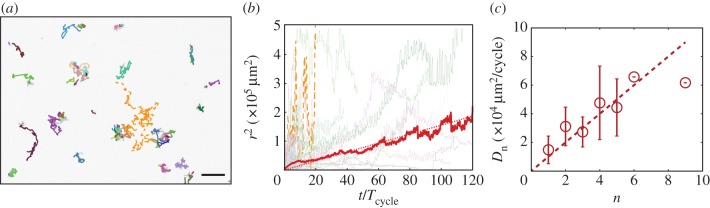
(a) Motion of spores under a succession of five humidity cycles (10% RH for 15 min and 90% RH for 15 min, Tcycle = 30 min). Scale bar is 500 µm. (b) Travelled distance as a function of time for several trajectories. The thick line is the average of all trajectories (around one hundred) and over time, and the dotted line shows diffusion with r2 = 4Dt. The dashed line shows the diffusion of a cluster (comprising five spores on average). (c) Diffusion coefficient as a function of the n number of spores in a cluster. (Online version in colour.)
At dense concentrations, spores can temporarily aggregate in mobile clusters. Surprisingly, these larger clusters diffuse at a higher speed, which we may call anti-Brownian behaviour. Indeed, standard Brownian motion of inert particles in a liquid is slower for larger objects. We attribute this effect to the larger number of elaters per cluster, which leads to a greater frequency of random steps at each cycle. Therefore, we assume that the time interval τ between steps is divided by the number of spores, so that the diffusion coefficient (of the order of l2/τ) is proportional to the number of spores. Measurements of the diffusion coefficient of clusters of different sizes (figure 3c) indeed shows a diffusion that increases proportionally to the number of spores (up to six spores), which is consistent with the assumption. This proportional trend should be less pronounced for very large heaps, because elaters on top of the heap do not touch the ground.
4. Jumps
When drying from a fully hydrated state, spores can suddenly leave the ground at speeds of approximately 1 m s−1 and reach elevations of up to one centimetre (figure 4a,b). This height is very large compared with the spore size. These jumps were rarely observed in the walking experiment described above with a maximum of 90% RH. We noted that a high humidity is necessary, here obtained in a saturated atmosphere at 100% RH or by immersion in a tiny droplet, in order to sufficiently entangle the elaters prior to drying. Spores could jump several times, after a humidification/drying cycle. The probability of the spores to jump depends on the nature of the ground surface. On a hydrophobic surface, the proportion of spores jumping (34%) is much larger than on a hydrophilic glass (12%), see Material and methods. We interpret this result as a consequence of a larger adhesion energy of wet spores with glass. However, the height of jumps did not change much from surface to surface.
Figure 4.
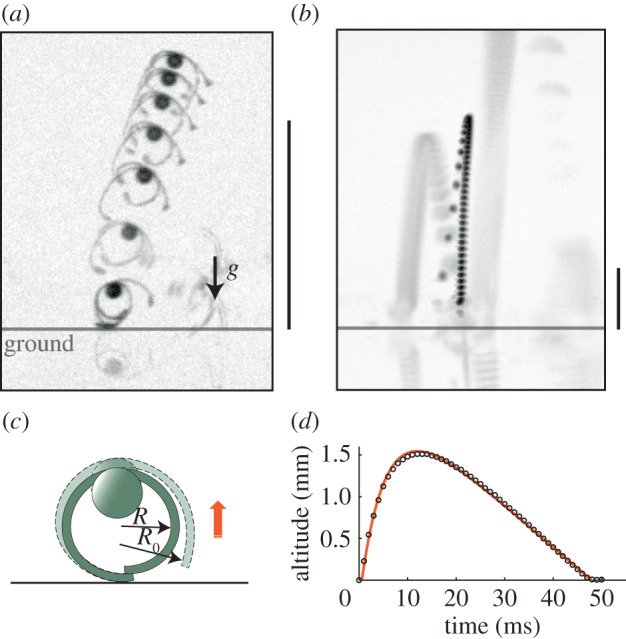
Side view of jumps. (a) Superposition of images immediately after the jump with an interval of 0.57 ms. The take-off velocity is 0.35 m s−1. (b) Larger view with images taken every 1 ms. The take-off velocity is 0.4 m s−1 for the jump in the middle and 0.8 m s−1 for the jump starting on the right. On both images, the scale bar is 500 µm and the ground position is indicated by an horizontal line. (c) Jumping mechanism of a spore (only two elaters are drawn). Elaters can be stuck by friction. The released elaters, when stresses overcome static friction, are drawn with dashes. (d) The altitude of the spore (middle trajectory of figure 4b showing a dark spore). Circles represent measurements, and the line represents the model prediction with an initial velocity of 0.41 m s−1 and a drag radius of 21.2 µm. (Online version in colour.)
Careful observations led us to hypothesize that pairs of elaters can be temporarily stuck by friction during opening and store an elastic energy that is released when the forces are sufficiently large. The friction is localized between the elaters (figure 4c). The upward thrust is obtained when the body is pushed upwards by the elater that is still in contact with the ground, this elater acting like a compressed spring releasing its energy.
Subsequently, we evaluated the energies at play during this motion to assess this hypothesis. The elastic energy is stored when the elaters are stuck with a given curvature c = 1/R (radius of curvature R = 60 µm in the sequence of figure 4a), while the drying process changes the spontaneous curvature c0 of the elaters. Observations of other loose elaters allow us to monitor the evolution of the spontaneous curvature: it changes to c0 = 1/R0, with R0 around 83 µm immediately prior to ejection. The elastic energy, mainly caused by bending, can be modelled by assuming that the elater is a thin strip plate of length Lel (approx. 160 µm) according to the following equation:
where E is the Young's modulus, Ih = h3w/12 is the bending moment, h (approx. 3 µm) is the thickness and w (approx. 7 µm) is the width.
The kinetic energy after ejection at velocity ve is
where the mass of the spore is that of the central body m =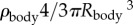 , using Rbody = 20 µm, and a density, ρbody, similar to that of water. Here, we neglect any rotational kinetic energy: the majority of jumps showed little rotation during ejection.
, using Rbody = 20 µm, and a density, ρbody, similar to that of water. Here, we neglect any rotational kinetic energy: the majority of jumps showed little rotation during ejection.
By assuming that all of the bending energy is released as kinetic energy, the jump velocity can be predicted and is directly given by the unknown Young's modulus of the elater. To match the measured jumping velocity, ve = 0.4 m s−1 (figure 4), we determine that Young's modulus should have a value of 100 MPa, as a lower bound. This value seems reasonable because it is much less than that of wood (approx. 10 GPa [16]), as it contains soft hygroscopic tissue, but is much larger than that of parenchymatous tissue (approx. 10 MPa [17]). Independent measurements of the bending modulus based on their vibration frequency (around 41 kHz for dry elaters, see Material and methods) yielded a modulus that was of the same order of magnitude.
In conclusion, the elastic energy stored in the bending of the very thin elaters is sufficient to propel the spores. In the absence of air friction, all of the initial kinetic energy could be converted to gravitational potential energy, and a jump would reach a height H, such that mgH = Ec, which implies that 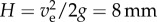 . However, this height cannot be attained owing to air drag. Therefore, we have included air drag in the model (see Material and methods). The experimental trajectory, with a maximum height of 1.5 mm, closely fits the model (figure 4d).
. However, this height cannot be attained owing to air drag. Therefore, we have included air drag in the model (see Material and methods). The experimental trajectory, with a maximum height of 1.5 mm, closely fits the model (figure 4d).
5. Discussion and perspectives
Jumps enhance the dispersion of spores and provide a way to overcome adhesion to the plant or to neighbouring spores. They are a natural occurrence of the phenomenon of Levy flights [18] in statistical physics that greatly increase the diffusion compared with a random walk with limited step amplitude. Figure 5a illustrates how a small jump is enough for the spore to be entrained by wind. The jump allows the spore to exit the ground where the air velocity is reduced, and to enter the higher velocity wind current. These spores are then more likely to travel than are immobile spores. Indeed, non-jumping spores stayed on the ground of a wind tunnel for velocities of less than 5 m s−1 and were just gliding on the flat ground for higher velocities.
Figure 5.
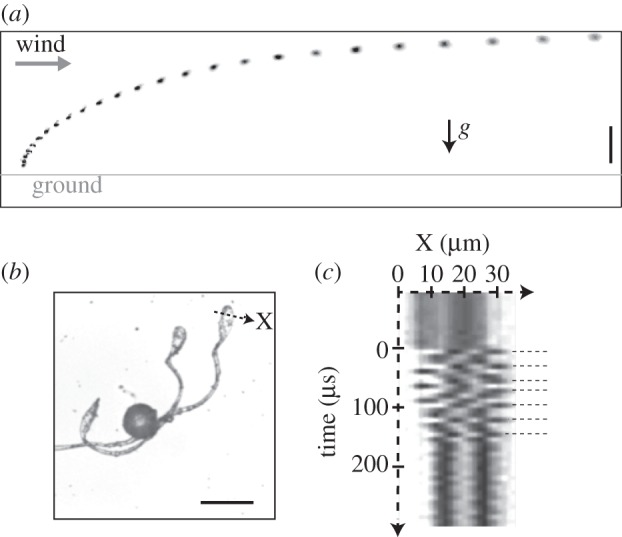
(a) Spore jump in a wind tunnel (superposition of images at 0.4 ms intervals). The wind speed far away from the ground is 4.85 m s−1, corresponding to a ‘gentle breeze’ of Beaufort number 3. The scale bar is 500 µm. (b) An elater was set into vibration using a tungsten tip mounted on a micrometric translation stage. High-speed recordings of the vibration at 121 000 images s−1 were performed. The camera was recording a line of pixels (dotted line). Scale bar, 50 µm. (c) A spatio-temporal diagram showing six periods of oscillations is plotted.
The Equisetum habitat is usually wet, which provides opportunities for the spores to contract into the entangled state quite frequently, and therefore also jump frequently. The jump mechanism can be repeated many times because it does not involve any irreversible fracture, in contrast to the movement of other species. Note that as the jumps occur upon drying, they provide an escape from dry locations and thus increase the chances of finding a more humid environment.
Even if the walk participates in only small distances of locomotion, it enables the spores to make key steps. First, with humidity variations or rain, groups of spores make large steps, which helps their fall from the sporangium. It is an additional contribution to the spilling out of spores owing to the volume increase of clusters when spores unfold. Second, the random steps reorient the spores and change its contact points with the ground, which result in different entanglements at each humidity cycle, and increases the probability to block the elaters and store elastic energy. Third, the reorientation also helps to obtain jumps in random directions, maximizing the explored area.
As a perspective, we believe that this study will inspire new biomimetic classes of self-propelled objects, based on many appendices that open/close and that undergo friction with the ground. The comprehension of the velocity of walk and directionality of motion as a function of their geometry suggest a whole scope of investigation. Concerning their energy source, these hygroscopic objects have the interesting property to be able to move in response to humidity changes only, a free resource that occurs naturally.
Acknowledgements
We thank Salima Rafaï for her insight on the tracking of spores and the computation of the diffusion coefficients, and Nicolas Mordant for his help in operating the wind tunnel. Automated tracking was performed using the routines of Erik Weeks. We also thank Tobias Haushan for suggesting literature on spore anatomy, and Zi Chen and Catherine Quilliet for helpful discussions on elaters.
Appendix A. Material and methods
(a). Material
The experiments were performed using fresh spores obtained by collecting sporangium shoots of Equisetum arvense at Saint Martin d'Hères in the early spring. The spores were placed in a Petri dish and imaged from the bottom or from the side using a binocular microscope. Image acquisition was performed either with a slow-motion CCD camera (Marlin, Allied Vision Technology) or with a high-speed camera (Miro 4, Vision Research) at 7000 images per second for full images and up to 120 000 images per second for reduced regions of interest. The humidity was controlled by a programmable humidity generator (Wetsys, Setaram Instrumentation) with air blowing at a low speed above the Petri dish. To obtain rapid changes in humidity, the spores were covered for several minutes by a cap containing wet paper to obtain vapour saturation, and the cap was then quickly removed by the operator.
(b). Measurement of the bending modulus of an elater
Recordings of the vibrations of an elater in a dry atmosphere (figure 5b,c) yielded vibration frequencies in the ultrasonic range, f = 41 ± 4 kHz, for an elater of length Lel = 159 ± 10 µm.
According to Rocard [19], the vibration frequency of a cantilever having a round cross-section is 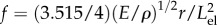 . By assuming that the density of the elater is similar to that of water, and because the elater is twisted into an effective round cross-section of r = 5.7 µm, which is the cubic mean of h and w, we predict that Young's modulus is 400 MPa. This value is on the order of magnitude estimated based on the jump ejection velocity. The factor of four difference may be observed because here the elater is drier, which tends to increase the elastic modulus. In addition, there may be a certain amount of energy lost by friction during the jump, leading to smaller jumps than expected based on the stored energy.
. By assuming that the density of the elater is similar to that of water, and because the elater is twisted into an effective round cross-section of r = 5.7 µm, which is the cubic mean of h and w, we predict that Young's modulus is 400 MPa. This value is on the order of magnitude estimated based on the jump ejection velocity. The factor of four difference may be observed because here the elater is drier, which tends to increase the elastic modulus. In addition, there may be a certain amount of energy lost by friction during the jump, leading to smaller jumps than expected based on the stored energy.
(c). Jump trajectory with air drag, in still air
The equation of motion for the spore having a vertical position z and vertical velocity vz = dz/dt is as follows:
where the last term is the Stokes drag force owing to the dynamic viscosity of the air, ηair (≈1.95 × 10−6 Pa.s), and Rdrag is the effective radius of a sphere given the same amount of drag. Stokes drag formula holds because the Reynolds number, ρairνeRdrag/ηair, is low (approx. 1) at the maximum velocity. This equation can be integrated twice to give the evolution of the altitude
where τ = m/6πηairRdrag is the deceleration time owing to the viscosity. Fitting of the trajectory, shown in figure 4d, yielded a deceleration time of 5 ms.
(d). Jumping statistics
The spores were spread on two kinds of surfaces: clean glass (hydrophilic, contact angle around 17°) or polystyrene Petri dishes (much less hydrophilic, contact angle around 88°). The contact angle of the water interface with respect to the ground was measured on photographs of tiny droplets of deionized water gently deposited on these surfaces. Three behaviours were observed using an inverted microscope with a 4× objective and classified in three categories: (i) clear jumps, (ii) start of jumps, exhibiting a sudden motion without take-off, and (iii) continuous unfolding. The percentages given in the main text consider the first two classes. See the electronic supplementary materials, figure S2 for detailed statistics.
(e). Wind tunnel experiments
The spores were deposited on the lower flat surface of a wind tunnel (cross-section of air stream: 250 × 250 mm), the spores being initially wetted within a water droplet. The spores were seen to jump after complete evaporation of the droplets.
References
- 1.Noblin X, Yang S, Dumais J. 2009. Surface tension propulsion of fungal spores. J. Exp. Biol. 212, 2835–2843 (doi:10.1242/jeb.029975) [DOI] [PubMed] [Google Scholar]
- 2.Dumais J, Forterre Y. 2012. Vegetable dynamics: the role of water in plant movements. Annu. Rev. Fluid Mech. 44, 453–478 (doi:10.1146/annurev-fluid-120710-101200) [Google Scholar]
- 3.Yafetto L, et al. 2008. The fastest flights in nature: high-speed spore discharge mechanisms among fungi. PLoS ONE 3, e3237 (doi:10.1371/journal.pone.0003237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker DL, Edwards J. 2010. Sphagnum moss disperses spores with vortex rings. Science 329, 406 (doi:10.1126/science.1190179) [DOI] [PubMed] [Google Scholar]
- 5.Evangelista D, Hotton S, Dumais J. 2011. The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae). J. Exp. Biol. 214, 521–529 (doi:10.1242/jeb.050567) [DOI] [PubMed] [Google Scholar]
- 6.Armon S, Efrati E, Kupferman R, Sharon E. 2011. Geometry and mechanics in the opening of chiral seed pods. Science 333, 1726–1730 (doi:10.1126/science.1203874) [DOI] [PubMed] [Google Scholar]
- 7.Noblin X, Rojas NO, Westbrook J, Llorens C, Argentina M, Dumais J. 2012. The fern sporangium: a unique catapult. Science 335, 1322 (doi:10.1126/science.1215985) [DOI] [PubMed] [Google Scholar]
- 8.Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD. 2001. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409, 618–622 (doi:10.1038/35054555) [DOI] [PubMed] [Google Scholar]
- 9.Beer R. 1909. The development of the spores of Equisetum. New Phytol. 8, 261–266 (doi:10.1111/j.1469-8137.1909.tb05531.x) [Google Scholar]
- 10.Duckett JG. 1970. Spore size in the genus Equisetum. New Phytol. 69, 333–346 (doi:10.1111/j.1469-8137.1970.tb02432.x) [Google Scholar]
- 11.Newcombe FC. 1888. Spore-dissemination of Equisetum. Bot. Gaz. 13, 173–178 (doi:10.1086/326297) [Google Scholar]
- 12.Uehara K, Kurita S. 1989. An ultrastructural study of spore wall morphogenesis in Equisetum arvense. Am. J. Bot. 76, 939–951 (doi:10.2307/2444515) [Google Scholar]
- 13.Reyssat E, Mahadevan L. 2009. Hygromorphs: from pine cones to biomimetic bilayers. J. R. Soc. Interface 6, 951–957 (doi:10.1098/rsif.2009.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbaum R, Zaltzman L, Burgert I, Fratzl P. 2007. The role of wheat awns in the seed dispersal unit. Science 316, 884–886 (doi:10.1126/science.1140097) [DOI] [PubMed] [Google Scholar]
- 15.Garcia M, Berti S, Peyla P, Rafaï S. 2011. Random walk of a swimmer in a low-Reynolds-number medium. Phys. Rev. E 83, 035301 (doi:10.1103/PhysRevE.83.035301) [DOI] [PubMed] [Google Scholar]
- 16.Ponomarenko A. 2012. Ecoulements critiques et plantes. PhD thesis, University Paris 6, Paris, France [Google Scholar]
- 17.Niklas KJ. 1988. Dependency of the tensile modulus on transverse dimensions, water potential, and cell number of pith parenchyma. Am. J. Bot. 75, 1286–1292 (doi:10.2307/2444450) [Google Scholar]
- 18.Mandelbrot B. 1982. The fractal geometry of nature. San Francisco, CA: W. H. Freeman [Google Scholar]
- 19.Rocard Y. 1949. Dynamique générale des vibrations. Paris, France: Masson & Cie [Google Scholar]


