Abstract
Biodiversity is spatially organized by climatic gradients across elevation and latitude. But do other gradients exist that might drive biogeographic patterns? Here, we show that rainforest's vertical strata provide climatic gradients much steeper than those offered by elevation and latitude, and biodiversity of arboreal species is organized along this gradient. In Philippine and Singaporean rainforests, we demonstrate that rainforest frogs tend to shift up in the rainforest strata as altitude increases. Moreover, a Philippine-wide dataset of frog distributions shows that frog assemblages become increasingly arboreal at higher elevations. Thus, increased arboreality with elevation at broad biogeographic scales mirrors patterns we observed at local scales. Our proposed ‘arboreality hypothesis’ suggests that the ability to exploit arboreal habitats confers the potential for larger geographical distributions because species can shift their location in the rainforest strata to compensate for shifts in temperature associated with elevation and latitude. This novel finding may help explain patterns of species richness and abundance wherever vegetation produces a vertical microclimatic gradient. Our results further suggest that global warming will ‘flatten’ the biodiversity in rainforests by pushing arboreal species towards the cooler and wetter ground. This ‘flattening’ could potentially have serious impacts on forest functioning and species survival.
Keywords: vertical stratification, gradients, canopy, biodiversity, arboreal, climate
1. Introduction
Changing distributions of species richness and abundance across environmental gradients such as elevation and latitude are fundamental features of life on the Earth [1]. Mechanisms behind these patterns are largely attributed to gradients of temperature and moisture [2]. But large-scale elevational and latitudinal gradients are not the only ones evident. In tropical rainforest, strong gradients in temperature and moisture occur from the forest floor to the canopy [3,4]. Patterns of species richness and abundance may organize along this vertical gradient in the same way that they do along the shallower gradients driven by elevation and latitude. If so, then vertical climate gradients may play a powerful role in structuring biodiversity [5].
Tropical rainforests are the most biodiverse communities on the Earth [1], and one reason for this high diversity is the great number and variety of niches afforded by the complex vertical structure of rainforest environments [6]. In rainforests, the three major climatic gradients (across latitude, elevation and height) may interact to drive species’ distributions. For example, a species that is highly arboreal at cooler high elevations may be much less so at warmer low elevations. Most rainforest animals are ectotherms and are thought to behaviourally exploit microclimatic mosaics within these complex forests to optimize temperature and water balance [7–9], but data on the vertical structuring of ectotherm communities, especially over large spatial gradients, are extremely limited [10]. This is owing to the difficulty in accessing and studying canopy habitats as well as the overall cryptic nature of ectothermic vertebrates [10]. As a consequence, the interaction among these three major environmental gradients in the structuring of rainforest communities has remained a relatively unexplored dimension in biodiversity science.
If vertical stratification of species assemblages changes across elevation, it suggests that patterns of diversity are indeed subject to the interaction of the vertical climatic gradient (height) with the much shallower one of elevation. Incorporating the impact of this underappreciated spatial dimension (height) on community structure could uncover important relationships that help explain (in concert with other biogeographic principles such as mid-domain effects [11]) distributional patterns of richness and abundance [12] and possibly reveal important patterns, not only in space but also in time. For example, as climate change progresses (i.e. time), plant and animal species alter their distributions as they track suitable climates through space [13]. Because the Earth is warming and in some areas becoming drier [14], species’ distributions are generally moving uphill or towards the poles, following thermal and moisture gradients associated with latitude and/or elevation [15–17]. The vertical partitioning of species in rainforest driven by a steep microclimatic gradient may provide some level of compensation against changing microclimates, by allowing species to shift vertically within the forest. If so then climate change will probably trigger small-scale downward shifts in height that precede distributional shifts poleward or to higher elevations.
Herein, we propose an ‘arboreality hypothesis’ which suggests that species’ distributions may adjust vertically in the rainforest strata to compensate for broad-scale shifts in climate associated with elevation. We explored these ideas by censusing frogs from the ground to canopy levels along an elevational gradient (and therefore a temperature and moisture gradient) in Philippine (900–1900 m) and Singaporean (approx. 10 m) rainforests. Along this gradient, we sampled frogs during both day and night within 67 individual trees, using ascenders to climb from ground level to nearly the uppermost canopy (Material and methods). We also placed 60 data loggers within the forest canopy and understory to measure temperature and moisture across the height and elevation gradient. To further explore how physical conditions might affect frog usage in the canopy, we used a biophysical WETAIR model [9] to show the effect of body mass, moisture and temperature on frog water loss. Lastly, we compiled a dataset for all frogs of the Philippines to explore how arboreality in frog assemblages might respond to changing climate across elevation at larger spatial scales.
2. Material and methods
(a). Study areas
In the Philippines, we surveyed a community of largely endemic frog species on Mount Banahaw in southern Luzon. The site is characterized by lowland dipterocarp forest up to 800 m elevation, dipterocarp and montane forest from 900 to 1700 m elevation, and mossy and Pinus forest above 1700 m elevation. Our study was not conducted below 900 m, because at lower elevations (less than 800 m) agriculture has replaced forest [18]. We allowed 100 m of elevation to buffer any potential effects from these disturbances. The climate is marked by the absence of a distinct dry season with annual rainfall of around 3100 mm yr−1 and 85% relative humidity on average [19]. We observed that rainfall and cloud cover for our Philippine study site varied with elevation, both of which increased at higher elevations.
In Singapore, our study area consisted of primary and older secondary lowland dipterocarp forest. Most areas on the island receive more than 2000 mm rainfall yr−1 with no apparent dry season. The average high temperature year round is around 30°C [20].
(b). Vertical stratification of frogs across an elevation gradient
In the Philippines, from May to October 2011, we conducted 118 ground-to-canopy surveys across a gradient of elevation at 900, 1100, 1300, 1500, 1700, 1900 and 2100 m above sea level. Each survey was centred on a single canopy tree. Tree selection was randomized at each elevation; however, each tree had to meet safety standards for arborist single-rope climbing [21]. Selected trees were at least 100 m apart at each elevation. We surveyed a total of 59 trees for adult frogs (14 trees at 900 m, five at 1100 m, 13 at 1300 m, five at 1500 m, 11 at 1700 m, five at 1900 m and six at 2100 m elevation).
Tree surveys lasted for 1 h and were conducted during the day and repeated at night to account for species with diurnal and nocturnal activity. We alternated surveys along the elevation gradient (low-to-high-to-low elevations) to avoid temporal bias in sampling. We recorded the maximum height climbed and tree height for each survey. Following each canopy survey, we used a laser distance metre (Leica Geosystems, Leica Disto D2; http://www.leica-geosystems.ca) to record tree height from the top of the tree to the base of the tree. Climbing on the top of trees is dependent on suitable branches that allow for safe access. Thus, we could not always ascend to 100% of the total tree height. We accounted for this in our analyses (see below).
We conducted ‘canopy surveys’ for adult frogs—a single 10 min visual survey for the ground (base of tree), sub-canopy (approx. half the maximum height climbed) and canopy (maximum height climbed). Ten minute ground surveys were confined to a randomly selected 4 × 4 m plot, and consisted of thoroughly searching leaf litter, logs and other microhabitats that may harbour animals. Visual-encounter surveys are expected to be the most comprehensive when attempting to locate both ground and arboreal animals [22]. Both the middle and canopy surveys were confined to approximately four vertical metres of above-ground habitat. Thus, we attempted to standardize the search area across our three survey locations. Because of limited above-ground surface area, we consider our sub-canopy and canopy surveys to be conservative, as we probably surveyed more area on the ground than above ground. For above-ground surveys, we searched for arboreal frogs in tree holes, moss, epiphytes and other microhabitat structures. We conducted ground surveys first to account for the potential bias of having frogs jump out of the tree while conducting arboreal surveys, and thereby inflating ground-survey abundances.
(c). Surveys in Singapore
In February 2011, we surveyed eight trees from ground to upper canopy for amphibians, using identical canopy-survey methods described earlier. Surveys were conducted within the primary lowland dipterocarp forests of Gnee Soon Swamp (one tree) and Bukit Timah Nature Preserve (two trees), and within the mature second-growth forest of Kent Ridge (two trees), Bukit Batok (one tree), and Labrador Park (two trees) preserves.
(d). Environmental temperatures
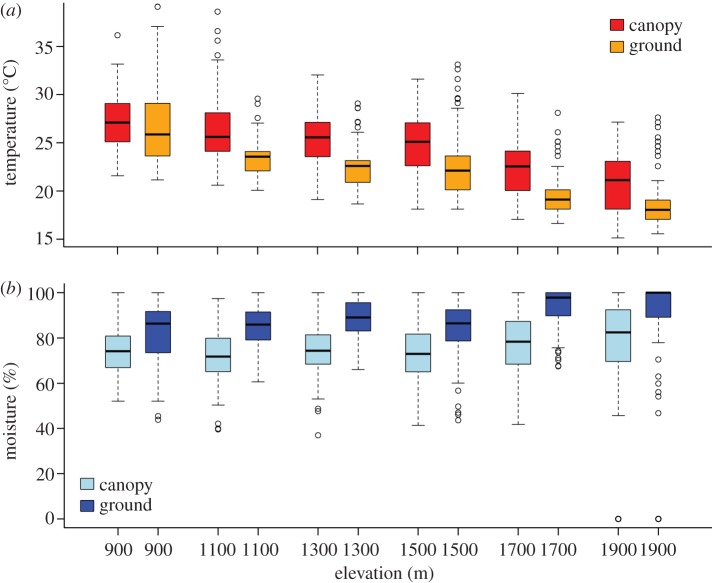
From May to September 2011, we used temperature and moisture loggers (Maxim Hygrochron ibutton Model DS1923; http://www.maxim-ic.com/) to determine the thermal and moisture profiles of forests in the Philippines. To identify the maximum potential ambient-air temperature as well as minimum moisture for our study area, we placed data loggers in the upper canopy of five trees per elevation (900–1900 m elevation), each paired with an identical logger suspended 1 m above ground. We chose to record maximum temperature and minimum moisture as these two variables are important to frog survival (e.g. high temperature and low moisture can negatively affect frog survival). Data were recorded every 15 min. Canopy and near-surface loggers were suspended under a plastic funnel and were thereby sheltered from direct solar radiation and precipitation. We used box-and-whisker plots to display maximum daily temperature and minimum daily moisture (figure 1).
Figure 1.
Temperature and moisture differ between canopy and ground as well as across elevation. Canopy and ground daily maximum temperature and minimum moisture profiles from sub-montane-to-montane rainforests in the Philippines. Temperature and moisture were collected from May to September 2011.
(e). Elevation gradient of species richness and arboreality in the Philippines
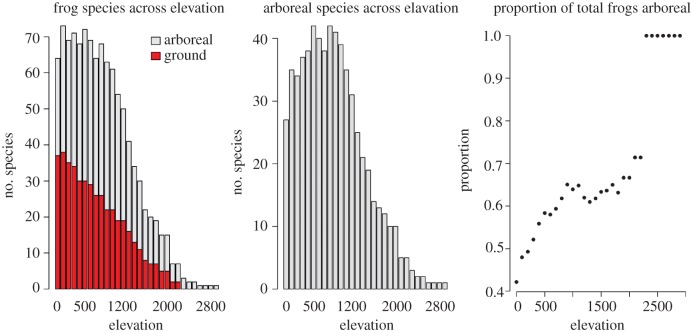
We compiled a database of frog distributions across elevation, taken from Diesmos & Brown [23]. For each of the 107 species, we recorded it as being in either one of two possible habitat niches: arboreal or ground-dwelling. A species was defined as arboreal, if it is capable of climbing and using above-ground habitats. By contrast, non-arboreal species were those that lacked grasping toe-pads and are thus less likely to exploit above-ground habitats. Alternatively, a species is considered ground-dwelling, if it is confined to the ground 100% over the course of its life. We examined patterns of arboreal richness across elevation by (i) plotting total species richness for each 100 m elevation band and (ii) plotting the proportion of species that are arboreal and ground-dwelling across 100 m elevation bands. Lastly, we plot total arboreal and ground-dwelling species richness by genus to explore taxonomic relationships across the elevation gradient.
3. Data analysis
(a). Kernel-density estimation
We used univariate kernel density to estimate the distributions of amphibians across vertical forest strata [24,25]. Kernel-density estimation is a procedure for generating a smoothed histogram of data, with the advantage that the area under the smoothed histogram integrates to one. Thus, the smoothed line represents the probability density of the data. In our study, we estimate the probability density of the height at which frogs were observed. Although not an absolute density of animals, the absolute density of animals should scale directly with the probability density (assuming animal detectability is invariant with height), so the probability density we calculate here can be thought of as the relative density of animals with height.
We attempted to estimate the true probability density of animals with height by integrating our results across the distribution of tree heights in the forest (estimated using our data on climb height for each tree). Thus, we estimated a kernel bandwidth (using Silverman's rule of thumb; see Silverman [24]) for our total dataset, but then executed a kernel-density estimate, using this bandwidth independently for each tree in our dataset. We then combined these treewise kernels using a weighted mean, where the weighting for each tree was taken from the kernel describing the distribution of tree heights. As no animals can be observed at negative height, we used a modified version of the density function in the R (v. 2.12.2) statistical package [26] to generate left-bounded univariate kernel-density estimates by reflecting the density falling below zero back into the positive domain of our estimated kernel [24].
We generated a composite distribution based on data collected from ground, sub-canopy and canopy surveys to reflect an aggregate distribution for amphibians. To explore the presence of an upward shift in vertical positioning across elevation, we generated distributions for all arboreal frogs for three elevational zones (900–1100, 1300–1500 and 1700–1900 m). We examined only arboreal frogs in this analysis as non-arboreal species, which lack grasping toe-pads, are incapable of exploiting above-ground habitats. Finally, we compared these trends to arboreal frog distributions in Singapore.
To identify the potential impacts of arboreal frogs shifting downwards towards ground communities, we quantified the proportion of the total community (both ground and arboreal frogs), which was found above ground. Specifically, as the total area under the curve equals one, we identified the cumulative distribution of all frogs found above 1 m height (i.e. the area occurring above 1 m on the curve) across all elevations in the Philippines.
(b). Dehydration and arboreality
Frogs that exploit canopy habitats are often away from water for extended periods of time, making them vulnerable to desiccation. Body mass, moisture and temperature are all factors that affect the rates at which an individual loses water and thus its ability to use canopy habitats [9]. To further explore whether there is support for decreased arboreality by elevation in frogs, we used a biophysical WETAIR model [9] to show the effect of body mass, moisture and temperature on frog water loss. In this theoretical exercise, all parameters but body mass were held constant, whereas mass (ranging from 0.1 to 10 g) and temperature were allowed to increase. This analysis was repeated with increasing mass and moisture. Mass selection from 1 to 10 g was based on a range of masses representative of species found in our study area. The smaller masses (i.e. 0.1 and 0.5 g) are indicative of young-of-the-year/metamorphs for species in our study area. The output for models was time (h) to 30% desiccation. Specifically, the variables held constant were cutaneous resistance (Rc) at 1.64 (averaged from Wygoda [27]), relative humidity at 72% and elevation at 450 m elevation (the mid-point of the elevational difference between Singapore and the Philippines). We then repeated this exercise for the same range of body masses as above but used temperature, moisture and elevation derived from our study area to determine the time to 30% desiccation specifically for our study areas. Lastly, we used the WETAIR models to display desiccation under three climate scenarios across elevation to identify climate scenarios that are favourable and unfavourable for arboreality: (i) high temperature (35°C–28°C) and high moisture (95–100%), (ii) low temperature (22°C–15°C) and low moisture (42–47%), and (iii) high temperature (35°C–28°C) and low moisture (42–47%).
(c). Alternative hypotheses
We considered three additional variables that represent structural components of the forest that may affect arboreality in frogs across elevation. It is possible that tree height influences patterns of frog arboreality as taller trees may offer greater height for frogs to use. Tree density and basal area (cross-sectional area of all stems per transect) are indicative of structure and habitat for frogs. Therefore, frog arboreality may correlate with increasing tree height, stem density and basal area of the local environment across elevation.
To examine this relationship, we documented tree height, stem density and basal area in order to characterize the local environment surrounding our tree surveys. We counted all trees (i.e. density), greater than 4 cm diameter-at-breast-height (dbh), along a 2 m wide and 20 m long transect. The direction of each transect was chosen at random and each transect was centred on a survey tree. Height and dbh was recorded for each tree recorded. We determined basal area by multiplying 0.00007854 by dbh to the power of 2 [28]. For each transect, basal area was summed for all trees and divided by the transect area (40 m2).
(d). Linear models
We explored temperature and moisture across our elevation gradient. We modelled temperature across elevation by running an analysis of covariance (ANCOVA) with temperature as a response variable and elevation and position (ground or canopy) as predictors. A second ANCOVA was performed with the same predictors, but moisture was used instead of temperature as the response variable.
We examined whether the proportion of frog arboreality changed with elevation in the Philippines. To do this, we used linear regression with the proportionate of total frogs that are arboreal as our response variable and elevation as our predictor variable.
To explore the relationship between animal height and elevation, we performed an ANCOVA with our response variable as height (m) and predictor variables as elevation and species. To properly assess animal height by elevation, we only used species for which we had occurrence data at three or more elevations. Therefore, Platymantis luzonensis was not included in this analyses as it occurred only at 900 and 1100 m elevation. Lastly, to determine whether height in canopy predicts body mass, we used a second ANCOVA with two covariates. Our response variable was mass (g) and the predictor variables were height in forest stratum, elevation and species. Both body mass and height were log-transformed to normalize data. In both cases, we initially tested for first-order interactions between height and elevation, but removed the term because it was not statistically significant.
We explored tree height, tree density and total basal area as alternative predictors of arboreality across elevation. We performed three linear regressions with tree height, tree density and total basal area as response variables and elevation as a predictor variable. Data were log-transformed to achieve normality.
All models were checked for heteroscedasticity via the studentized Breusch–Pagan test. Both our mass and height and height and elevation models were non-heteroscedastic. Of our three alternative hypotheses models, both the basal area and elevation, and tree density and elevation models were non-heteroscedastic. We did not log-transform data in our ANCOVA analyses of temperature and moisture, as these analyses were primarily conducted to derive a slope for temperature and moisture across elevation. We corrected for heteroscedasticity using White's robust standard errors [29].
(e). Diagram of height and elevation shifts
To display the options an animal may have to remain at an optimum temperature and moisture under climate warming, we created a temperature-by-height-by-elevation contour figure derived from our temperature data and a moisture-by-height-by-elevation contour figure derived from our moisture data.
4. Results
Microclimatic gradients in temperature and moisture are significantly steeper than elevational gradients. In our rainforests, temperature decreased by 1.4°C with every approximately 200 m increase in elevation but varied by 2.2°C over just approximately 20 m between the forest canopy and ground level (F2,1421 = 559.9, p < 0.001; ANCOVA; figure 1 and electronic supplementary material, table S1). Similarly, moisture increased by 1% with every approximately 200 m increase in elevation but differed by 11% over the approximately 20 m gradient between canopy and ground (F2,1421 = 107.8, p < 0.001; ANCOVA; figure 1 and electronic supplementary material, table S1).
Our data show that, with increasing elevation, total species richness generally decreased and the degree of assemblage arboreality increased (figure 2). Specifically, ground-dwelling species richness decreased from low-to-high elevations and arboreal species richness peaked between 600 and 800 m. Our linear regression model suggests that the proportion of all frogs that are arboreal increases with elevation (F1,28 = 112, R2 = 0.793, p < 0.001)—a 10% increase in assemblage arboreality for every 500 m rise in elevation. Assemblages above 300 m are comprised more than 50% arboreal species, whereas they are 100% arboreal above 2300 m (figure 2). The number of arboreal species is lower in the lowlands, with approximately 42% of the total assemblage being arboreal at sea level. Multiple genera are present across the elevation gradient for both ground and arboreal species, suggesting that a single genus is not driving the observed biogeographic trends (see the electronic supplementary material, figures S1 and S2).
Figure 2.
(left) Changes in frog species richness across elevation in the Philippines (n = 107) separated by ground-dwelling and arboreal species. (right) Frog assemblages become increasingly arboreal with elevation. Assemblages above 300 m are more than 50% arboreal. Above 2300 m, species richness is low, so our percentages in these altitudinal bands are derived from only one to three frog species.
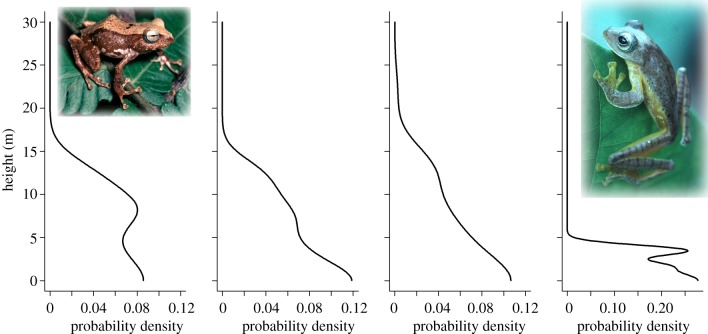
We documented three ground and five arboreal species in the Philippines and seven ground and four arboreal species in Singapore (see the electronic supplementary material, table S2). There was a significant relationship between elevation and height of frogs in forest canopies across our Philippine landscape (F4,51 = 4.50, p = 0.003; ANCOVA; figures 3 and 4; electronic supplementary material, table S3); a trend that was consistent across all species for which we had sufficient data (interaction terms were not found to be significant (p > 0.29 for all factor levels), and so were dropped from the final reported model). In the lowland forests of Singapore, all frogs were located either on the ground or below 3.5 m in height (figure 3). Increased arboreality in frogs is evidenced by the second hump in the kernel-density estimate distribution (figure 3), which is missing at 900–1100 m elevation but becomes more apparent at higher elevations. Across the entire elevational range (900–1900 m) in the Philippines, 88% of all frogs are found above 1 m in height (see the electronic supplementary material, figure S3).
Figure 3.
Vertical stratification of arboreal frogs from higher (left) to lower (right) elevations (n = 20 at 1900 and 1700 m; n = 33 at 1500 and1300 m; n = 17 at 1100 and 900 m and n = 5 at 0 m; respectively). These data show a clear decrease in arboreality from cooler/moister (high elevation) to warmer/drier (low elevation) climate. Curves are derived from kernel-density estimation techniques and can be interpreted as the relative density of animals with height [24] (see Material and methods). (Photos of Platymantis montanus and Rhacophorus pardalis in the Philippines by Rafe Brown.)
Figure 4.
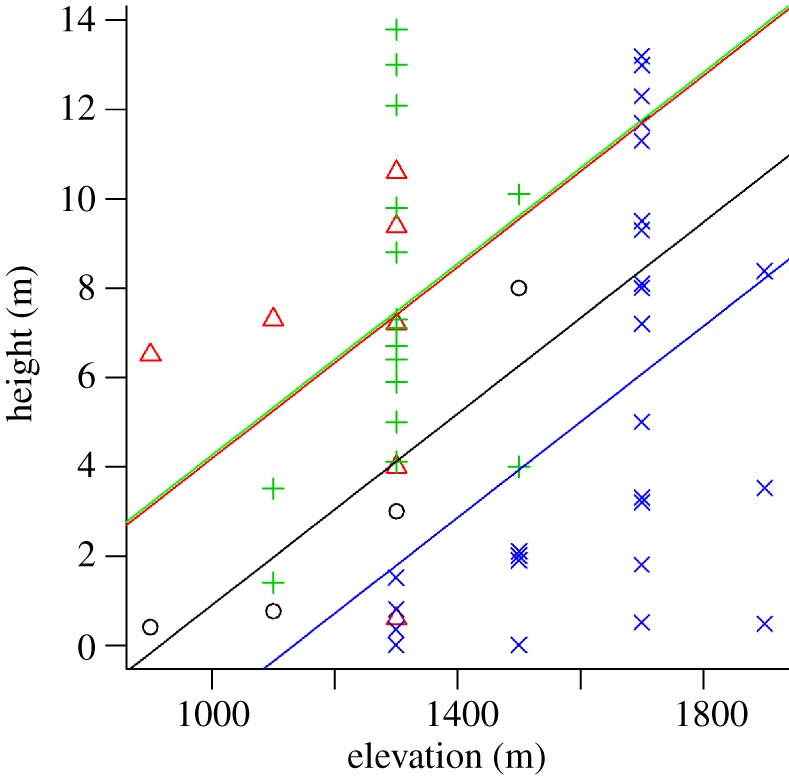
Height in forest stratum increases with elevation for arboreal frog species. According to ANCOVA models, elevation significantly predicts height in forest canopy at which frogs were observed (n = 56; electronic supplementary material, table S3). Plus symbols, Platymantis banahao; crosses, Platymantis montanus; circles, Kaloula kalengensis; triangles, Philautus surdus.
Larger frogs were found higher in the canopy. That frog body size varies significantly with height in the forest (F6,51 = 8.27, p < 0.001; ANCOVA; figure 5 and electronic supplementary material, table S4) supports the notion that temperature and moisture are key drivers of frog arboreality. Biophysical WETAIR models reveal that frogs desiccate faster with higher temperatures, lower moisture, and when they are smaller bodied (see the electronic supplementary material, figure S4a–c). The WETAIR models, which include environmental parameters of our study sites, suggest a frog of 3 g can remain in the canopy at the highest elevations during the hottest hours of the day for approximately 8 h before reaching lethal dehydration, whereas an equivalent frog in the lowland forest canopy of Singapore has about half as much time. To that end, we also modelled three climate scenarios using WETAIR models: (i) high temperature and high moisture, (ii) low temperature and low moisture, and (iii) high temperature and low moisture. Desiccation rates for high temperature and low moisture were three to four times greater than for low temperature and low moisture and six to seven times greater than for high temperature and high moisture (see the electronic supplementary material, figure S4d).
Figure 5.
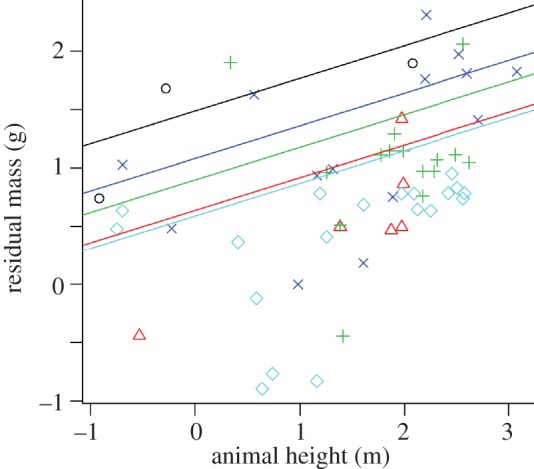
Larger arboreal frogs occur higher in forest stratum than smaller frogs. According to linear regression analysis, animal height in forest significantly predicts body mass (n = 58; electronic supplementary material, table S4). Residual mass is mass corrected for the influence of elevation. Mass and height were log-transformed. Plus symbols, Platymantis banahao; circles, Kaloula kalengensis; crosses, Platymantis luzonensis; triangles, Philautus surdus; diamonds, Platymantis montanus.
We considered three alternative hypotheses in which elevation-related changes in forest structure might cause the observed changes in arboreality, but none was supported. Specifically, linear regression models suggest that neither tree height (F1,469 = 1.06, R2 = 0.00, p = 0.31), basal area (F1,48 = 0.25, R2 = −0.02, p = 0.62), nor tree density (F1,48 = 0.35, R2 = −0.01, p = 0.56) significantly change from 900 to 1900 m elevation (see the electronic supplementary material, tables S5 and S6).
5. Discussion
Our study shows conclusively that arboreality (or ‘vertical stratification’) plays a role in determining patterns of rainforest species richness and abundance, especially for ectothermic and hydrophilic species. Specifically, the vertical distributions of frogs shift upwards into the forest strata with increasing elevation. The distribution of animals with height (their ‘vertical distribution’) is rarely examined relative to the large body of research on animal distributions across elevation and latitude [15–17,30]. Moreover, given the logistical difficulties of ascending to upper forest strata, species’ vertical distributions, when examined, are rarely studied at multiple sites, as we have done here. To our knowledge, ours is the first study to examine comprehensively how the vertical dimension of faunal distributions changes with elevation. From these observations, our proposed ‘arboreality hypothesis’ suggests that arboreality offers flexibility in finding optimal microclimatic conditions across a wider range of macro-climates. Thus, we propose that species would have a narrower geographical range in the absence of their ability to exploit microclimates offered by vertical habitat structure.
We propose that adult frogs, which use two key habitat niches—arboreal and terrestrial—are physiologically constrained on opposite ends of the elevation gradient. Specifically, arboreality is constrained in the lowlands from conditions that are excessively hot and dry in the canopy, whereas terrestriality is constrained in the uplands because ground temperatures are too cold. Our data support this proposition. First, our canopy surveys show that frogs become more arboreal as canopy conditions improve with increasing elevation. Second, our Philippines-wide dataset shows that species richness of ground-dwelling frogs decreases linearly with increased elevation (as ground conditions become too cold), whereas the proportion of the total frog assemblage that is arboreal increases with elevation (as canopy conditions become more favourable, transitioning from being hot in the lowlands to being warm or cool in the uplands).
These patterns suggest that species richness of frogs and other temperature- and desiccation-sensitive fauna should be highest at some intermediate elevation where both canopy and ground-level temperatures are most optimal. Our data show a peak in frog species richness at around 600–800 m elevation (well below the 1400 m mid-domain in this system) and a linear decrease in the proportion of ground-dwelling species as elevation increases. Mid-elevation peaks of richness and abundance are found across various taxonomic groups [2,12,30] and are generally thought to be driven by temperature and moisture [12] as well as the mid-domain effect [11,12]. Although it is difficult to disentangle mid-domain effects from other potential drivers, the strong shift from ground-dwelling to arboreal life-histories with elevation argues strongly that the interaction between the climatic gradients of height and elevation is a powerful mechanism driving patterns of richness in this system.
The inference that high temperatures and lower moisture greatly constrain frog arboreality [31] is supported by our observation that frog mass increased with height above-ground (figure 4), a pattern predicted by our WETAIR models. These models indicate that larger frogs are substantially more resistant to evaporative water loss [9], which is an increasing problem in the warmer and drier upper forest strata [32] as well as at lower elevations (see the electronic supplementary material, figure S4a–c).
Our novel height dimension to biogeography may broadly apply to other taxonomic groups. For example, some species of ground carabid beetles from southeast Asia appear to increase in arboreality with elevation [33,34]. Our findings, however, may not apply to non-ectothermic communities. The general applicability of our hypothesis to other non-anuran animal groups with variable physiological requirements should be further tested.
Interestingly, the vertical stratification of body size in our study is opposite to that expected under Bergmann's rule [35,36], in which large-bodied animals are generally found in cooler habitats. Opposing patterns to Bergmann's rule are, however, routinely observed in ectotherms, possibly because of the effects of body size on thermal inertia and water loss as captured by our WETAIR model [36]. Indeed, ants and other arthropods are closely tied to temperature and energy within ecosystems [37] and therefore may mirror the same stratification patterns as we found with frogs [38]. Similar non-Bergmann ‘body size’ clines have also been observed in Asplenium bird's nest ferns in Malaysia, where individual plant biomass of ferns increases with height in the forest canopy [39] and in arthropods in Indonesia and Australia where mean arthropod body size decreased from treetop down to soil [38,40]. In short, the mechanisms driving the vertical distribution of frogs in our study might well apply to a broad suite of taxa.
In the frogs we studied, eight of every 10 individuals were found above ground (see the electronic supplementary material, figure S3), echoing patterns of species abundance in other taxa, such as insects [41]. Yet, these patterns seem to be highly dependent on locality and taxa under consideration: beetle assemblages from the Australian wet tropics are equally abundant and diverse in both canopy and ground habitats [40], whereas arthropod densities in a Cameroon rainforest were approximately three times higher in the canopy than understory shrub layers [42]. Future research should also consider the role that keystone micro-habitats play in vertical stratification. For example, a single Asplenium bird's nest fern in Bornean rainforests contains twice the invertebrate biomass as its entire host tree [43]. Thus, the distribution of select keystone micro-habitats across elevation may be an important feature governing vertical stratification of select animal communities.
(a). Research caveats and future prospects
We highlight the following possible caveats to our study and research prospects for further exploration of the arboreality hypothesis:
— a limitation to our study as well as many previous studies on canopy communities is sample size [10]. Although we conducted over 120 ground-to-canopy surveys over several months, we documented only 87 individual frogs. We suggest testing the ‘arboreality hypothesis’ with a more abundant study organism such as beetles, spiders and/or ants as a priority;
— our study is confined to southeast Asia. Are patterns of frog arboreality also prevalent in other tropical regions such as the Neotropics? Currently, no other research has explored our hypothesis (although see [33,34]) so until such work is undertaken, caution should be used when generalizing our trends to other habitats and geographical regions;
— cloud cover was not examined in our study area but may vary by topography and location and its presence could intensify moisture over small spatial scales [44], therefore, cloud cover may interact with the degree of change in arboreality with elevation;
— arboreality may vary by season. For example, vertical stratification of some arthropod species varies in areas with strong seasonal variation in climate [45]. Interestingly, the impacts of future climate change on arboreal communities might be deduced from examining seasonal variation in arboreality (see below for further discussion);
— at our local Philippine site, we observed that frogs at 1900 m were primarily arboreal, whereas no frogs were observed at our highest elevation of 2100 m. One plausible explanation for these trends is that the climate in the canopy at 1900 m was marginally suitable for frogs and thus even canopy habitats may be rendered unsuitable at colder and wetter elevations;
— frogs are one of the most threatened animal groups on the Earth largely as a result of the fungal disease, Chytridiomycosis [46]. Some species believed to have gone extinct have recently been rediscovered [47], but in hotter and drier habitats than normal [46]. Hot habitats dry frog's skin making them more resilient to fungal infection. Therefore, chytrid fungus may be less prevalent in arboreal than ground-dwelling frog species, especially those at higher elevations, as canopy frogs are exposed to warmer and drier conditions than the ground; and
— variation in thermal tolerances (i.e. the minimum and maximum temperatures lethal to an individual) by forest height is unknown and only a few studies have examined ecotypic variation in thermal tolerances along elevation gradients (e.g. [48]). Lowland frogs are assumed to operate only within a narrow thermal range, whereas high elevation frogs are assumed to operate over a much larger range of temperature [49]. Despite different thermal ranges, all species will have a thermal optimum, and we would expect abundances to track this optimum. Thus, although the ground at high elevations may not be ‘as cold’ for montane frogs (with broad tolerances) as they might be for lowland frogs (with narrower tolerance), we would still expect abundances to track gradients in temperature across height and altitude.
(b). Arboreality under climate change
That the vertical distributions of arboreal species shift with elevation are important not only for understanding biogeographic patterns, but for projecting how rainforest biodiversity might respond to future climatic change. Under a changing climate, shifts in both temperature and moisture could strongly influence species distributions (see the electronic supplementary material, figure S4) [50]. Mean temperatures and moisture in rainforests followed a steep gradient from the upper canopy to the forest floor, spanning more than 2°C and 11% relative humidity over a distance of just approximately 20 m (figure 1). Similar changes in temperature and moisture across elevation require movements of over 300 m and 2000 m, respectively. Thus, these vertical temperature and moisture gradients are orders of magnitude steeper than those associated with elevation or latitude. This steep climate-height gradient is the principle driver of stratification patterns in our study and suggests that, in a warming and sporadically drier world, amphibians and perhaps other temperature-sensitive ectotherms will probably adjust their vertical distributions downward within the forest (figure 3 and electronic supplementary material, figure S5). The steepness of the vertical gradient suggests that this will happen long before they shift to cooler and wetter conditions at higher elevations or latitudes [51]. Indeed, this downward shift might be the only one available to arboreal species in the world's vast lowland rainforests, such as the Amazon and Congo Basins, where elevational gradients are virtually absent. If patterns of arboreality in frogs are globally coherent, climate change may exacerbate the vulnerability of this critically threatened animal group [52].
Most alarmingly, our findings suggest that rising temperatures and severe drying events, such as the major Amazonian droughts in 2005 and 2010 [14], could create an ‘extinction zone’ for ectotherms in rainforests that progressively widens (see the electronic supplementary material, figure S5) as one moves towards the ground and uphill into mountain habitats. Species will be pushed off the top of mountains, but they will also be pushed to the ground. A downward shift in arboreal communities would squeeze together species that normally avoid interacting via vertical niche partitioning [53]. For example, during hot, dry El Niño events in Papua New Guinea, significantly more frogs were encountered on the ground of which 78% of individuals were arboreal species [53]. Thus, this effect could be dramatic; in our Philippines sample, for example, 88% of frogs were found more than 1 m above the ground (see the electronic supplementary material, figure S3). If these individuals were compressed downward (see the electronic supplementary material, figure S5), it would inflate ground densities of animals and potentially promote an array of negative interactions for affected species [15] such as intensified interspecific and intraspecific competition [54,55], and increased density-dependent mortality from predators, pathogens and parasites [13]. Thermal stress and altered community interactions might interact synergistically, increasing extinction risk for vulnerable species [56,57].
Hence, our findings suggest the downward movement of many arboreal species in rainforests could be a rapid response to warming climates and should be carefully monitored in the future. Such movements could serve as an early warning that rich arboreal communities in rainforests are in danger of reorganization or collapse.
Acknowledgements
We thank N. Stork, J. Malcolm, T. Evans and two anonymous reviewers for comments on earlier versions of this manuscript.
Research permits were provided by PAWP-DENR and the local government unit of the Municipality of Majajay to the National Museum of the Philippines (by virtue of Republic Act 10066, “National Cultural Heritage Act of 2009”). All procedures were approved by the National University of Singapore's Institutional Animal Care and Use Committee (IACUC) (protocol # B01/10).
Data accessibility
Data will be made available 1 year after publication.
Funding statement
We thank the local community of Mount Banahaw for supporting our research and Rafe, Warren, P. A. Buenavente, A. Barnuevo, B. Brunner, B. Harris, S. Ramirez, R. Willis and M. Wise for assistance in the field. Support was provided by the Singapore International Graduate Award, Wildlife Reserves Singapore Conservation Fund, Australian Government National Environment Research Program and the Australian Research Council.
References
- 1.Gaston KJ. 2000. Global patterns in biodiversity. Nature 405, 220–227 (doi:10.1038/35012228) [DOI] [PubMed] [Google Scholar]
- 2.McCain CM. 2009. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 18, 346–360 (doi:10.1111/j.1466-8238.2008.00443.x) [Google Scholar]
- 3.Johansson D. 1974. Ecology of vascular epiphytes in West African rain forest. Uppsala, Sweden: Acta Phytogeographica Suecica 59 [Google Scholar]
- 4.Fetcher N, Oberbauer SF, Strain BR. 1985. Vegetation effects on microclimate in lowland forest in Costa Rica. Int. J. Biometeor. 29, 145–155 (doi:10.1007/BF02189035) [Google Scholar]
- 5.Schulze CH, Linsenmair KE, Fiedler K. 2001. Understory versus canopy: patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. Plant Ecol. 153, 133–152 (doi:10.1023/A:1017589711553) [Google Scholar]
- 6.Ozanne CMP, et al. 2003. Biodiversity meets the atmosphere: a global view of forest canopies. Science 301, 183–186 (doi:10.1126/science.1084507) [DOI] [PubMed] [Google Scholar]
- 7.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: incorporating the buffering roles of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracy CR, Christian KA, Tracy CR. 2010. Not just small, wet, and cold: effects of body size and skin resistance on thermoregulation and arboreality of frogs. Ecology 91, 1477–1484 (doi:10.1890/09-0839.1) [DOI] [PubMed] [Google Scholar]
- 10.Kays R, Allison A. 2001. Arboreal tropical forest vertebrates: current knowledge and research trends. Plant Ecol. 153, 109–210 (doi:10.1023/A:1017585622940) [Google Scholar]
- 11.Colwell RK, Rahbek C, Gotelli NJ. 2004. The mid-domain effect and species richness patterns: what we have learned so far? Am. Nat. 163, E1–E23 (doi:10.1086/382056) [DOI] [PubMed] [Google Scholar]
- 12.McCain CM. 2010. Global analysis of reptile elevational diversity. Glob. Ecol. Biogeogr. 19, 541–553 (doi:10.1111/j.1466-8238.2010.00528.x) [Google Scholar]
- 13.Patrick DA, Harper EB, Hunter ML, Jr, Calhoun AJK. 2008. Terrestrial habitat selection and strong density-dependent mortality in recently metamorphosed amphibians. Ecology 89, 2563–2574 (doi:10.1890/07-0906.1) [DOI] [PubMed] [Google Scholar]
- 14.Lewis SL, Brando PM, Phillips OL, van der Heijden GMF, Nepstad D. 2011. The 2010 Amazon drought. Science 331, 554 (doi:10.1126/science.1200807) [DOI] [PubMed] [Google Scholar]
- 15.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–667 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- 16.Colwell RK, Brehm G, Cardelus CL, Gilman AC, Longino JT. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 17.Raxworthy CJ, Pearson RG, Rabibisoa N, Rakotondrazafy AM, Ramanamanjato J, Raselimanana AP, Wu S, Nussbaum RA, Stone DA. 2008. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Glob. Change Biol. 14, 1703–1720 (doi:10.1111/j.1365-2486.2008.01596.x) [Google Scholar]
- 18.Peh KSH, Soh MCK, Sodhi NS, Laurance WF, Ong DJ, Clements R. 2011. Up in the clouds: is sustainable use of tropical montane cloud forests possible in Malaysia? BioScience 61, 27 (doi:10.1525/bio.2011.61.1.8) [Google Scholar]
- 19.Banaticla MCM, Buot IE. 2005. Altitudinal zonation of pteridophytes on Mt. Banahaw de Lucban, Luzon Island, Philippines. Plant Ecol. 180, 135–151 (doi:10.1007/s11258-004-2494-7) [Google Scholar]
- 20.Chia LS, Foong SF. 1991. Climate and weather. In The biophysical environment of Singapore (eds Chia LS, Rahman A, Tay DBH.), pp. 13–29 Singapore: Singapore University Press [Google Scholar]
- 21.Jepson J. 2000. The tree climber's companion. USA: Beaver Tree Publishing [Google Scholar]
- 22.Doan TM. 2003. Which methods are most effective for surveying rain forest herpetofauna? J. Herpetol. 37, 72–81 (doi:10.1670/0022-1511(2003)037 [0072:WMAMEF]2.0.CO;2) [Google Scholar]
- 23.Diesmos AV, Brown RM. 2011. Diversity, biogeography and conservation of Philippine amphibians. In Biology and conservation of tropical Asian amphibians (eds Das I, Haas A, Alek Tuen A.), pp. 26–49 Sarawak, Malaysia: Universiti Malaysia [Google Scholar]
- 24.Silverman BW. 1986. Density estimation for statistics and data analysis. London, UK: Chapman and Hall [Google Scholar]
- 25.Venables WN, Ripley BD. 2002. Modern applied statistics with S. Berlin, Germany: Springer [Google Scholar]
- 26.R Development Core Team 2011. The r project for statistical computing, version 2.11. 1. See http://www.R-project.orgæ
- 27.Wygoda ML. 1984. Low cutaneous evaporative water loss in arboreal frogs. Physiol. Zool. 57, 329–337 [Google Scholar]
- 28.Husch B, Miller C, Beers T. 1972. Forest mensuration. New York, NY: Ronald Press Company [Google Scholar]
- 29.MacKinnon JG, White H. 1985. Some heteroskedastic-consistent covariance matrix estimators with improved finite sample properties. J. Econ. 29, 305–325 (doi:10.1016/0304-4076(85)90158-7) [Google Scholar]
- 30.Williams SE, Shoo LP, Henriod R, Pearson RG. 2010. Elevational gradients in species abundance, assemblage structure and energy use of rainforest birds in the Australian wet tropics bioregion. Austral Ecol. 35, 650–664 (doi:10.1111/j.1442-9993.2009.02073.x) [Google Scholar]
- 31.Stewart MM. 1995. Climate driven population fluctuations in rain forest frogs. J. Herpetol. 29, 437–446 (doi:10.2307/1564995) [Google Scholar]
- 32.Bohlman SA, Matelson TJ, Nadkarni NM. 1995. Moisture and temperature patterns of canopy humus and forest floor soil of a montane cloud forest, Costa Rica. Biotropica 27, 13–19 (doi:10.2307/2388898) [Google Scholar]
- 33.Stork NE, Brendell MJD. 1990. Variation in the insect fauna of Sulawesi trees in season, altitude and forest type. In Insects and the Rain Forests of South East Asia (Wallacea). A special Project Wallace Symposium (eds Knight WJ, Holloway JD.), pp. 173–190 London, UK: The Chameleon Press [Google Scholar]
- 34.Stork NE. 1988. Adaptations of arboreal Carabids to life in trees. Acta Phytopathologica Hungarica 22, 273–291 [Google Scholar]
- 35.Bergmann C. 1847. About the conditions of the thermal economy of animals to their size. Göttingen Stud. 3, 595–708 [German Transl.] [Google Scholar]
- 36.Sears MW, Angilletta MJ., Jr 2004. Body size clines in Sceloporus lizards: proximate mechanisms and demographic constraints. Integr. Comp. Bio. 44, 433–442 (doi:10.1093/icb/44.6.433) [DOI] [PubMed] [Google Scholar]
- 37.Kaspari M, Ward PS, Yuan M. 2004. Energy gradients and the geographic distribution of local ant diversity. Oecologia 140, 407–413 (doi:10.1007/s00442-004-1607-2) [DOI] [PubMed] [Google Scholar]
- 38.Stork NE, Blackburn TM. 1993. Abundance, body size and biomass of arthropods in tropical forest. Oikos 67, 483–489 (doi:10.2307/3545360) [Google Scholar]
- 39.Fayle TM, Chung AYC, Dumbrell AJ, Eggleton P, Foster WA. 2009. The effect of rain forest canopy architecture on the distribution of epiphytic ferns (Asplenium spp.) in Sabah, Malaysia. Biotropica 41, 676–681 (doi:10.1111/j.1744-7429.2009.00523.x) [Google Scholar]
- 40.Grimbacher PS, Stork NE. 2007. Vertical stratification of feeding guilds and body size in beetle assemblages from an Australian tropical rainforest. Austral Ecol. 32, 77–85 (doi:10.1111/j.1442-9993.2007.01735.x) [Google Scholar]
- 41.Stork NE. 1991. The composition of the arthropod fauna of Bornean lowland rain forest trees. J. Trop. Ecol. 7, 161–180 (doi:10.1017/S0266467400005319) [Google Scholar]
- 42.Bassett Y, Aberlenc H, Delvare G. 1992. Abundance and stratification of foliage arthropods in a lowland rain forest of Cameroon. Ecol. Entomol. 17, 310–318 (doi:10.1111/j.1365-2311.1992.tb01063.x) [Google Scholar]
- 43.Ellwood MDF, Foster WA. 2004. Doubling the estimate of invertebrate biomass in a rainforest canopy. Nature 429, 549–551 (doi:10.1038/nature02560) [DOI] [PubMed] [Google Scholar]
- 44.Cavelier J, Solis D, Jaramillo MA. 1996. Fog interception in montane forests across the Central Cordillera of Panamá. J. Trop. Ecol. 12, 357–369 (doi:10.1017/S026646740000955X) [Google Scholar]
- 45.Wagner T. 2001. Seasonal changes in the canopy arthropod fauna in Rinorea beniensis in Budongo Forest, Uganda. Plant Ecol. 153, 169–178 (doi:10.1023/A:1017514417913) [Google Scholar]
- 46.Puschendorf R, Hoskin CJ, Cashins SD, McDonald K, Skerratt LF, VanDerWal J, Alford RA. 2011. Environmental refuge from disease-driven amphibian extinction. Conserv. Biol. 25, 956–964 (doi:10.1111/j.1523-1739.2011.01728.x) [DOI] [PubMed] [Google Scholar]
- 47.Scheffers BR, Ding Li Y, Harris JBC, Giam X, Sodhi NS. 2011. The world's rediscovered species: back from the brink? PLoS ONE 6, e22531 (doi:10.1371/journal.pone.0022531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller K, Packard GC. 1974. Critical thermal maximum: ecotypic variation between montane and Piedmont chorus frogs (Pseudacris triseriata, Hylidae). Experientia 30, 355–356 (doi:10.1007/BF01921660) [DOI] [PubMed] [Google Scholar]
- 49.Navas CA. 1997. Thermal extremes at high elevations in the Andes: physiological ecology of frogs. J. Therm. Biol. 22, 467–477 (doi:10.1016/S0306-4565(97)00065-X) [Google Scholar]
- 50.McCain CM, Colwell RK. 2011. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 14, 1236–1245 (doi:10.1111/j.1461-0248.2011.01695.x) [DOI] [PubMed] [Google Scholar]
- 51.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 52.Sodhi NS, Bickford D, Diesmos AC, Lee TM, Koh LP, Brook BW, Sekercioglu CH, Bradshaw CJA. 2008. Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS ONE 3, e1636 (doi:10.1371/journal.pone.0001636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bickford D. 2005. Long-term frog monitoring by local people in Papua New Guinea and the 1997-98 EL Niño Southern Oscillation. In Ecology and evolution in the tropics: a herpetological perspective (eds Donnelly MA, Crother BI, Guyer C, Wake MH, White ME.), pp. 260–283 Chicago, IL: University of Chicago Press [Google Scholar]
- 54.Gifford ME, Kozak KH. 2012. Islands in the sky or squeezed at the top? Ecological causes of elevational range limits in montane salamanders. Ecography 35, 193–203 (doi:10.1111/j.1600-0587.2011.06866.x) [Google Scholar]
- 55.Jankowski JE, Robinson SK, Levey DJ. 2010. Squeezed at the top: interspecific aggression may constrain elevational ranges in tropical birds. Ecology 91, 1877–1884 (doi:10.1890/09-2063.1) [DOI] [PubMed] [Google Scholar]
- 56.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 57.Laurance WF, Useche DC. 2009. Environmental synergisms and extinctions of tropical species. Conserv. Biol. 23, 427–1437 (doi:10.1111/j.1523-1739.2009.01336.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available 1 year after publication.