Abstract
Many groups show higher species richness in tropical regions but the underlying causes remain unclear. Despite many competing hypotheses to explain latitudinal diversity gradients, only three processes can directly change species richness across regions: speciation, extinction and dispersal. These processes can be addressed most powerfully using large-scale phylogenetic approaches, but most previous studies have focused on small groups and recent time scales, or did not separate speciation and extinction rates. We investigate the origins of high tropical diversity in amphibians, applying new phylogenetic comparative methods to a tree of 2871 species. Our results show that high tropical diversity is explained by higher speciation in the tropics, higher extinction in temperate regions and limited dispersal out of the tropics compared with colonization of the tropics from temperate regions. These patterns are strongly associated with climate-related variables such as temperature, precipitation and ecosystem energy. Results from models of diversity dependence in speciation rate suggest that temperate clades may have lower carrying capacities and may be more saturated (closer to carrying capacity) than tropical clades. Furthermore, we estimate strikingly low tropical extinction rates over geological time scales, in stark contrast to the dramatic losses of diversity occurring in tropical regions presently.
Keywords: amphibians, diversification, extinction, latitudinal gradients, speciation, species richness
1. Introduction
A negative latitudinal gradient in species richness (i.e. higher tropical diversity) is one of the most well-known patterns in biology [1] and has been documented across many organisms, time scales and geographical regions [2]. However, the underlying causes of this pattern remain unclear and highly controversial. Dozens of explanations have been proposed [3,4], which have previously been classified into various categories [5], including ecological (e.g. more niches in tropical regions), evolutionary (e.g. higher speciation rates in tropical clades) and temporal (e.g. longer time-for-speciation in tropical regions) factors. However, many of these explanations are not mutually exclusive (i.e. the tropics may simultaneously have more niches, promote higher speciation rates and have been inhabited longer). Most importantly, any complete explanation for high tropical richness must incorporate at least one of the three processes that directly change species richness in a region: speciation, extinction and dispersal [6] (here, we use ‘dispersal’ synonymously with colonization and range expansion, following standard practice).
Many ecological factors clearly differ between tropical and temperate regions (e.g. temperature, ecosystem energy) but such factors can only affect species richness by influencing rates and patterns of speciation, extinction and dispersal, including the timing of the first successful colonization of a region [7]. Thus, these ecological factors may be associated with changing rates of diversification (speciation and extinction; [8–12]), limitations on dispersal among regions [13–15], or differences in the timing of colonization of different regions and subsequent time-for-speciation and accumulation of richness [13,16–18]. These processes of speciation, extinction and dispersal can be estimated most powerfully using a phylogenetic approach, and numerous studies have begun to address the causes of latitudinal richness gradients using phylogenetic information [e.g. 8,9,15–22].
Previous phylogenetic studies of the latitudinal diversity gradient have so far been limited to two major aspects, however. First, most have focused on relatively small taxonomic groups (e.g. families; [13,17,23]) or sister species-pairs [19,24]. Second, previous studies have not examined the role of all three processes in generating richness patterns. For example, some have shown latitudinal gradients in diversification rates but did not untangle speciation and extinction [8,16,21,25], did not address how ecological factors influence rates [9,19,26], or did not consider the roles of time and dispersal between regions [8,9,19]. Overall, these studies were limited mostly by the data (e.g. smaller phylogenies) and methods available at the time.
Recent analytical advances in phylogenetic comparative methods now allow for the use of large-scale phylogenies (e.g. thousands of species) to disentangle the roles of speciation, extinction and dispersal in driving the latitudinal gradient in diversity. For example, the GeoSSE method [27] tests if the biogeographic distribution of clades (e.g. occurring in temperate versus tropical regions) influences rates of speciation and extinction, and simultaneously estimates rates of dispersal between regions. The related QuaSSE method [28] tests whether a continuous variable (e.g. a measure of climatic niche) is related to rates of speciation and extinction. However, these methods have yet to be applied to examine the latitudinal diversity gradient in a widespread, species-rich group.
Here, we investigate the underlying causes of high tropical diversity in amphibians, one of the most diverse groups of terrestrial vertebrates (approx. 7000 species; [29]). Amphibians exhibit a strong latitudinal diversity gradient (figure 1a), with thousands of tropical species and only seven north of the Arctic Circle [29]. Amphibians are ancient and globally widespread, with numerous geographically distinct radiations [25,30]. We first use the GeoSSE and QuaSSE algorithms on a large-scale, time-calibrated phylogeny containing more than 40% of extant species (2871 taxa). We use algorithms accounting for the missing species, which simulations suggest are generally accurate (tested specifically for Binary State Speciation and Extinction (BiSSE); [31]). We estimate rates of speciation and extinction in temperate and tropical regions (and dispersal between them), and test for the influence of ecological variables related to climate and ecosystem energy on these rates. We then perform analyses of family-level clades and large-scale ecoregions to corroborate the tree-based analyses and incorporate additional factors such as time-for-speciation, geographical area, rates of climatic-niche evolution and diversity-dependent diversification.
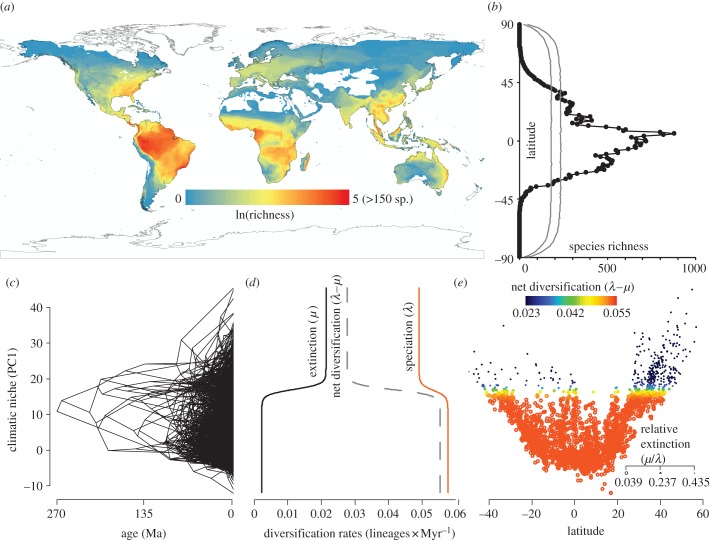
Figure 1.
(a) Global richness of 6117 amphibians on a log scale in 5° grid cells; (b) latitudinal distribution of species (black) compared to expectations under null models of geographical range-shuffling (grey lines showing 95% confidence intervals); (c) time-calibrated phylogeny of 2794 amphibian species with tip and node heights equal to the reconstructed or extant climatic-niche (PC1) value for each node or living species (see the electronic supplementary material); (d) response of speciation, extinction and net diversification rates to PC1 based on the sigmoidal model in QuaSSE; (e) plot of PC1 versus latitude for the species included in the phylogeny, with species points coloured by estimated net diversification rate (r) and sized by relative extinction fraction (ɛ; smaller circles indicate higher extinction) with estimates of r and ɛ from the QuaSSE results (see the electronic supplementary material), showing an increase in net diversification towards the equator.
We find that all three lines of evidence are generally concordant in supporting a simple and intuitive set of results. The latitudinal diversity gradient in amphibians is driven by higher speciation and lower extinction rates in the tropics, and lower speciation and higher extinction rates in temperate regions, and maintained by limited dispersal out of the tropics and higher dispersal into the tropics from temperate regions. These rates are strongly correlated with ecological factors related to climatic niche, such as temperature, precipitation and ecosystem energy. By contrast, other factors such as time-for-speciation in areas and rates of climatic-niche evolution have more limited effects.
2. Methods and material
(a). Primary hypotheses
All methods are described in detail in the electronic supplementary material. We use multiple methods to address two primary questions. First, do speciation, extinction and dispersal rates vary between temperate and tropical regions? Second, is variation in those rates related to ecological factors such as climate, ecosystem energy or geographical area? We address these questions using new methods on a large-scale tree (tree-based analyses; see below). Numerous other factors may underlie higher tropical richness, either by affecting diversification rates or allowing more species to accumulate locally and regionally over time [5]. These include the length of time that clades have occupied regions (time-for-speciation; [13,17,18]), geographical area [32,33], rates of climatic-niche evolution [10] and diversity-dependent diversification [34,35]. We addressed these factors in two sets of analyses described below, one focused on clades (families) and the other on a set of 12 global ecoregions representing centres of amphibian diversity and endemism: tropical South America, Nearctic, Afro-tropical, western Palaearctic, eastern Palaearctic, Madagascar, Oceania, southeast Asia, south Asia, tropical middle America, temperate South America and the West Indies (see the electronic supplementary material, for detailed definitions).
(b). Phylogeny and distributional data
We first generated a time-calibrated tree containing 2871 species (from [36]). We then used the 2008 International Union for Conservation of Nature Global Amphibian Assessment (http://www.iucnredlist.org/initiatives/amphibians) to obtain range maps available for 6117 species (approx. 90% of known, extant species; [29]). We then classified all 6576 species covered in our taxonomic database as occurring in one or more of the 12 global ecoregions listed above, each having distinct amphibian faunas (i.e. many endemic species and genera) and broadly similar to those from recent analyses [37]. We then extracted the latitudinal and longitudinal extents, ranges and mid-points from the species-range maps. In theory, latitudinal diversity gradients might arise from random range-shuffling (the mid-domain effect; [38]) but simulation analyses [39] rejected this model (figure 1b; see the electronic supplementary material) and indicate that the tropics are diverse and temperate regions are depauperate relative to null models.
(c). Climatic data
Using the range maps, we calculated mean values for 21 environmental variables for 6117 species, including most species (2794) in the phylogeny (figure 1c). For estimates of local environmental conditions and species’ climatic niche, we used the WorldClim dataset [40]. This dataset contains 19 variables describing variation in monthly, quarterly and yearly measures of temperature and precipitation. The dataset is based on weather station data (and spatial interpolation between stations) from 1950 to 2000. The variables were projected globally at a spatial resolution of 2.5 min (approx. 5 km2). We also used mean annual temperature (BIO1) separately as a metric of solar energy [41].
As metrics of productive energy, we used remotely-sensed measurements of net primary productivity (NPP) from the NASA MODIS 17 dataset (http://www.ntsg.umt.edu/modis/) projected at 2.5 min resolution [42] and actual evapotranspiration (AET) projected at 30 min resolution [43]. These metrics measure the interaction between energy, temperature and water balance in local environments, and are strongly correlated with global amphibian diversity [44]. The use of these factors as measures of solar energy (BIO1) and productive energy (AET and NPP) is well established, particularly with regard to species–energy relationships [41]. However, we recognize that their correlations with richness may reflect factors besides energy.
These 21 variables (19 BIOCLIM variables, AET and NPP) were condensed into a single measure of realized climatic niche using phylogenetically corrected principal components analysis (PCA; [45]; see the electronic supplementary material). We used PC1 for analysing ecological correlates of rates of speciation, extinction and climatic-niche evolution. This axis explains 33% of the total variation, and emphasizes temperature and precipitation variables that are strongly correlated with latitude such as annual mean temperature and precipitation seasonality variables (see the electronic supplementary material, tables S1 and S2), which are known to be correlated with global amphibian diversity [44]. We acknowledge that climatic niches estimated from range maps and a single PC axis can have errors and will not reflect all relevant aspects of the climatic niche, but the significant correlations with climate that we find suggest that relevant climatic variation is not completely obscured by error.
(d). Tree-based analyses
We hypothesized that speciation, extinction and dispersal vary significantly between temperate and tropical lineages, and tested this phylogenetically using the GeoSSE algorithm [27]. Importantly, this method incorporates species not sampled in the phylogeny and their biogeographic locations. We estimated the sampling proportion of species in tropical, temperate and both regions from the 6576 species (approx. 94% of described amphibians) in our taxonomic database, based on their occurrence in the 12 ecoregions defined above (see the electronic supplementary material). The 2871-species phylogeny contains 75% of species that occur in both temperate and tropical areas (84 of 112), 39% of tropical species (2257 of 5802) and 80% of temperate species (530 of 662). We tested a series of 10 models that allowed speciation, extinction and dispersal rates to vary between these two climatic zones (see the electronic supplementary material, table S3).
We then tested the hypothesis that differences in speciation and extinction rates are influenced by climatic variables using the QuaSSE algorithm [28]. We fit models in which both speciation and extinction rates were invariant with respect to climatic niche (PC1), and in which these rates varied independently as sigmoidal or hump-shaped functions of PC1 (electronic supplementary material, table S4). We use PC1 rather than latitude, as the biological mechanisms influencing differential diversification rates at different latitudes are hypothesized to be related to climatic differences associated with those latitudes, rather than the latitudinal position of the species per se. The QuaSSE algorithm also incorporates missing species (sampling fraction 0.42 = 2794/6576) but does not incorporate information on the traits or biogeographic location of the missing species (but see the electronic supplementary material and Discussion for why this should not overturn our results).
Other PC axes also explain considerable climatic variation (e.g. PC2: 20%; PC3: 16%; PC4: 11%; see the electronic supplementary material, table S1) but we did not include them in our QuaSSE analyses. Current implementations of QuaSSE (in diversitree [46]) do not allow for simultaneous analysis of multiple variables [28], making their inclusion problematic. However, like PC1, PC2–4 are also correlated with latitude (see the electronic supplementary material) and would presumably show similar patterns of rate differences between temperate and tropical climates.
Finally, we note that neither GeoSSE nor QuaSSE accounts for diversity dependence (see below), and GeoSSE does not model changes in rates over time. Nevertheless, both methods show strong relationships between diversification and occurrence in tropical regions, suggesting that they are not confounded by the effects of diversity dependence.
(e). Clade-based analyses
As species richness in clades can only be directly affected by clade age and diversification rates, we hypothesized that clade age and rates of speciation and extinction differ between temperate and tropical lineages and that these rates may be related to ecological variables and rates of climatic-niche evolution. For the 66 amphibian families (classification following [36]), we first calculated total richness (from [29]; see the electronic supplementary material) and latitudinal mid-point, environmental energy proxies (AET, NPP, BIO1) and geographical area, using the range maps for 6117 species. Although families are an arbitrary taxonomic unit, we focused on them given that relationships, species richness and composition of families are generally more well established than that for lower ranked taxa (e.g. genera), and most families are endemic to a single ecoregion (see the electronic supplementary material).
We calculated stem and crown ages for all families from the time-calibrated phylogeny. We first tested for a significant relationship between clade age (crown and stem) and diversity. A positive age–diversity relationship is expected under a birth–death process [47,48] but this has proved rare for stem-group ages in comparative datasets [49]. However, recent analytical models have shown that this pattern can also arise as a result of high extinction along the stem lineage [48], and few studies have compared both crown and stem ages to diversity. A positive crown–diversity relationship and flat or negative stem–diversity relationship may indicate higher rates of extinction, as entire subclades are pruned from the stem [25]. We tested for latitudinal gradients in crown and stem ages to determine whether this signature of extinction varied latitudinally.
We then used subtrees for each family to estimate the rate of climatic-niche evolution (using PC1, see the electronic supplementary material), and net diversification rate (r = speciation–extinction) and relative extinction fraction (ɛ = extinction/speciation; also known as turnover, with higher frequency of lineage replacement through time at larger values of ɛ [50]). The latter two estimates are based on a phylogenetic method (see the electronic supplementary material; [50]) that incorporates richness of all species in these families, including those not in the phylogeny [51]. We used multiple regression to link species richness and diversification rates to time, area and energy. We used linear combinations of variables, as interactions would be difficult to interpret, and would introduce many additional parameters. We accounted for phylogenetic relationships among clades using independent contrasts for all multiple-regression analyses using these estimated rates (see the electronic supplementary material).
These estimates of net diversification rate do not include estimates of changes in speciation and extinction over time, such as if clades started out with higher speciation rates which then declined over time owing to diversity dependence [34,35,52]. Preliminary analyses testing for diversity dependence against the null hypothesis of monotonic decay in rates over time ([35]; see the electronic supplementary material) found support for negative linear diversity dependence in speciation rate in 33 family-level clades (including all families with more than 50 species). Thus, it is possible that the ecological factors which are correlated with higher diversification rates (e.g. climate, energy) influence species richness by allowing more species to coexist locally in tropical clades (a long-standing hypothesis for high tropical diversity; [5]). By contrast, if fewer species can coexist locally in temperate regions and species richness of temperate clades is more saturated (i.e. clades are closer to their carrying capacities) at higher latitudes, this might provide a potential explanation for latitudinal gradients in both speciation rates and total diversity (even if the specific mechanisms relating local diversity, limited resources and speciation are unknown).
We used new models that explicitly account for incomplete species sampling in phylogenies [34] to estimate equilibrium species-richness (K′; total carrying capacity without extinction), as well as λ0 (initial speciation, before onset of diversity-dependent effects) and μ (extinction, assumed to be constant over time) for each of 60 family-level clades with more than 1 species. These estimates were then used to test the hypothesis that environmental conditions in tropical regions increased the carrying capacity for species richness. Specifically, we tested for relationships between area and energy (AET, NPP and BIO1) and K′ using multiple regression, as well as for latitudinal gradients in K′, λ0, μ and saturation (N/K′), using linear regression. We note, however, that interpreting these phylogenetic results in terms of carrying capacity for local or regional richness relies on numerous assumptions (see the electronic supplementary material for discussion) about ecological interactions among species that are not tested here [34,35,52–54].
(f). Ecoregion-based analyses
Finally, we hypothesized that differences in species richness among ecoregions might be related to time-for-speciation within ecoregions. Importantly, the time-for-speciation effect may drive patterns of species richness without variation in rates of speciation and extinction, and this ecoregion-based analysis is the only one here that can address this hypothesis. We first tested the relationship between the richness of the 12 global ecoregions and a set of ecological and evolutionary variables similar to those analysed above. Mean values for AET, NPP and BIO1 were calculated for each region as for species (see the electronic supplementary material; see above). We used multiple regression to link species richness to area and energy (AET, NPP and BIO1). We hypothesized that amphibians will show the classic species–area and species–energy relationships [32], though these variables must act through their effects on diversification rates, which we address above with the tree- and clade-based analyses.
We then estimated the timing of the earliest colonization of each region by extant amphibians using biogeographic reconstructions on the phylogeny (see the electronic supplementary material), and tested for a relationship between timing of colonization and species richness of regions using linear regression. We recognize that the total land area of ecoregions is not equivalent to the extent of habitable biomes within those ecoregions, which may have a stronger effect over time [12,55]. However, land area provides a standard metric for assessing the impact of habitable area.
3. Results
(a). Tree-based analyses
The time-calibrated tree is available in the electronic supplementary material, file S1, and the estimated topology and dates are congruent with several recent analyses [30,56,57]. The best-fit GeoSSE model (see the electronic supplementary material, table S3) estimates a speciation rate for tropical lineages that is 1.5 times higher than that of temperate lineages (0.0565 lineages × Myr−1 versus 0.0379 lineages × Myr−1), and 3.5 times higher than that of the relatively few species (approx. 85) occurring in both regions (0.0161). Extinction rates are also relatively high in temperate zones (0.00823 lineages × Myr−1), roughly 22% of speciation rates. Remarkably, compared with high temperate extinction, estimated extinction rates for the tropics are not merely lower, but are close to zero (0.0000103 lineages × Myr−1). Net diversification rates (speciation–extinction; r) in the tropics are thus 1.9 times higher than those in temperate regions (0.0565 lineages × Myr−1 versus 0.0297 lineages × Myr−1), whereas relative extinction fractions (extinction/speciation; ɛ) are approximately 0 in the tropics and 0.22 in temperate areas. Finally, estimated dispersal rates from tropical to temperate regions are five times lower than temperate-to-tropical rates (0.00082 lineages × Myr−1 versus 0.0041 lineages × Myr−1), despite the higher richness in tropical regions (strongly suggesting ecological constraints on dispersal in this direction).
The best-fit QuaSSE model (see the electronic supplementary material, table S4) indicates that both speciation and extinction rates exhibit a sigmoidal response to the multivariate climatic-niche estimate from PC1, (figure 1d; see the electronic supplementary material). This yields minimum and maximum speciation rates of 0.0491 lineages × Myr−1 and 0.0577, respectively, and minimum and maximum extinction rates of 0.0023 and 0.0213, respectively. Climatic niche (PC1) exhibits a significantly positive latitudinal gradient (Pearson's correlation rP = 0.77; p < 0.00001; figure 1e). Thus, high speciation rates and low extinction rates correspond to low PC1 values, which occur near the equator (figure 1e and table 1). This results in a latitudinal gradient in net diversification rates (0.028 lineages × Myr−1 to 0.055) and relative extinction fractions (0.435–0.039) from high to low latitudes, respectively (figure 1e).
Table 1.
Correlations between latitude, and variables relating to species richness and diversification. (Data are means or parameter estimates for 66 family-level clades regressed against the latitudinal mid-point of the family, analysed using Pearson's correlation (rP), with significant results (p-value) (see the electronic supplementary material for discussion of multiple comparisons).)
| latitude versus: | rP | p-value |
|---|---|---|
| species richness (ln) | −0.45 | 0.00014 |
| stem age (Ma) | 0.47 | 0.00008 |
| crown age (Ma) | −0.27 | 0.03277 |
| carrying capacity (K′) | −0.61 | <0.00001 |
| saturation (N/K′) | 0.36 | 0.00555 |
| r (lineages × Myr−1) | −0.42 | 0.00058 |
| ɛ (extinction/speciation) | 0.31 | 0.01412 |
| area (km2) | −0.40 | 0.00102 |
| NPP (g[C] × m2 × y−1) | −0.54 | <0.00001 |
(b). Clade-based analyses
We find that species richness of families show a strong negative relationship with latitude (table 1 and figure 2a,b). Using multiple regression, family richness shows positive relationships with area and NPP (overall R2 = 0.58; see the electronic supplementary material, table S5, for model coefficients). However, these factors can only influence species richness by influencing speciation and extinction over time. Most variation in richness among families is explained by positive relationships with net diversification rate and crown-group age (unlike many recent studies [49,58]) and a negative relationship with stem-group age (overall R2 = 0.79; see the electronic supplementary material, table S6; figure 2a). Further, these ages tend to vary based on the overall latitudinal position of clades (table 1 and figure 2b), with older stem-group ages and younger crown-group ages at higher latitudes.
Figure 2.
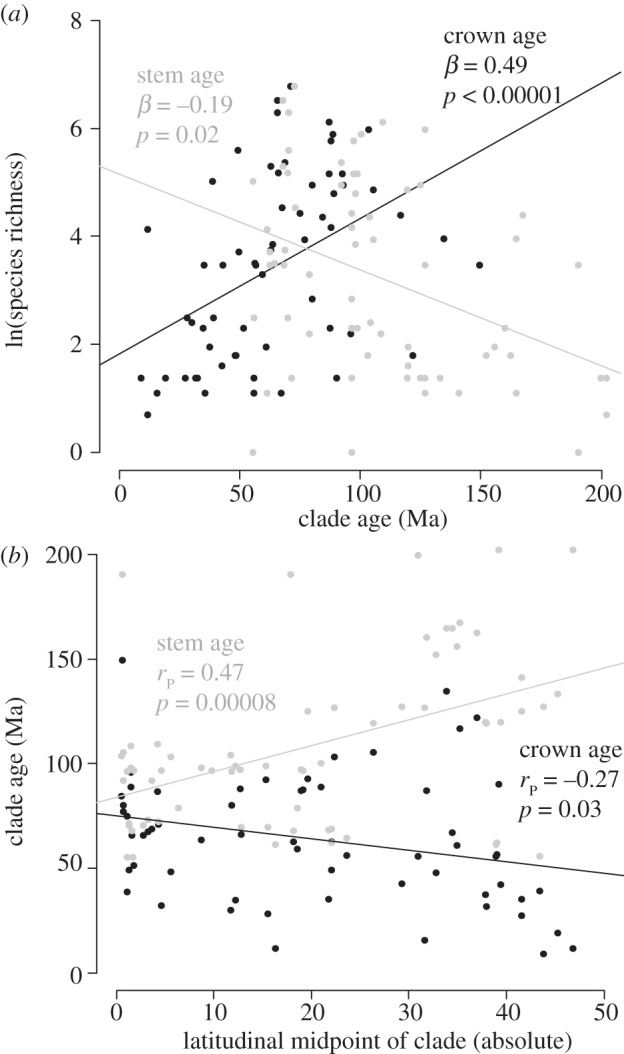
Relationships between (a) the stem-group and crown-group ages of clades (Ma, millions of years ago) and their species richness (multiple regression controlling for diversification rates; see the electronic supplementary material, table S6), and (b) the latitudinal mid-point of clade distributions and their stem- and crown-group ages (correlation; table 1). Grey circles correspond to stem-group ages and black circles correspond to crown-group ages. The clades are the 66 families of extant amphibians (sensu [36]).
Diversification rates for families are positively linked to area and NPP (R2 = 0.55; see the electronic supplementary material, tables S7 and S10). A previous study suggested that increased rates of climatic-niche evolution may drive increased diversification rates in salamanders [10], but this is not significant across amphibians (see the electronic supplementary material, table S7). Relative extinction fraction shows a positive latitudinal gradient (table 1) and a negative relationship with area and NPP (R2 = 0.16; see the electronic supplementary material, tables S8 and S11). There is no correlation between clade age and either net diversification rate (rP = 0.043; p = 0.74) or relative extinction fraction (rP = 0.10; p = 0.43), suggesting that these estimates are not artefactually biased [58]. While relative extinction fraction is not significant in the best-fit model for explaining species richness (see above), there is a negative correlation between r and ɛ (rP = −0.54; p < 0.00001), indicating that variation in net diversification rates among clades is related to variation in both extinction (which varies latitudinally and with area and NPP; table 1; see the electronic supplementary material, tables S8 and S11) and speciation rates. Thus, family-level clades at lower latitudes cover larger areas with greater NPP, conditions associated with increased diversification (see the electronic supplementary material, tables S7 and S10) and reduced turnover (table 1).
Within the family-level clades, we also find some support for temporal decreases in speciation rate under a model of linear diversity dependence [35]. Using phylogeny-based models accounting for incomplete sampling [34], we estimated carrying capacities (K′), saturation (N/K′), initial speciation (λ0) and constant extinction (μ) for 60 families with more than 1 species. Carrying capacity is positively related to area and NPP (R2 = 0.64; electronic supplementary material, tables S9 and S12) and exhibits a negative latitudinal gradient (table 1 and figure 3). Saturation also shows a positive latitudinal gradient (table 1) but not initial speciation or extinction (see the electronic supplementary material). Summing estimates of carrying capacity across families (see the electronic supplementary material, table S14) suggests a global capacity of approximately 10 700 species for the 60 clades tested or approximately 70% saturation at current diversity levels (approx. 7000 species; see the electronic supplementary material for discussion of assumptions).
Figure 3.
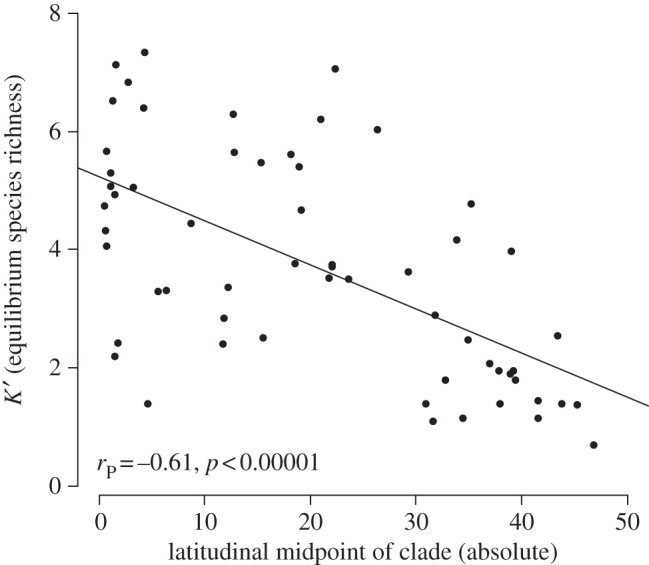
Latitudinal variation in equilibrium species-richness (K′), regressed against the absolute value of the latitudinal mid-point of the geographical range of 66 family-level clades (table 1).
(c). Ecoregion-based analyses
Using multiple regression, ecoregions show strong species–area and species–energy (AET) relationships (see the electronic supplementary material, tables S13 and S15). However, AET and area must influence richness by impacting diversification rates or time of colonization (dispersal). Timing of colonization is not significantly related to species richness (rP = 0.12, p = 0.72). Given the non-significant results relating species richness to time and the significant results related to diversification rates, the species–area and species–energy relationships must be caused by biogeographic differences in speciation and extinction rates (see above). While there is no significant effect of time-for-speciation on diversity in ecoregions, variation in speciation and extinction rates may be related to changes in the extent of suitable habitat over time [7,55] in those ecoregions (see Discussion).
4. Discussion
We demonstrate here, to our knowledge, for the first time that high tropical amphibian diversity is explained by both high speciation and low extinction in the tropics, and low speciation and high extinction in temperate regions. The tree-based analyses show that differences between temperate and tropical extinction are proportionally much larger than the differences in speciation (figure 1d). We find that early colonization and higher extinction has yielded many ancient, species-poor clades in temperate regions, whereas relatively young clades have rapidly accumulated high diversity in tropical regions [25]. For example, tropical South America was first colonized by extant amphibian lineages approximately 96 Ma and has more than 2300 species, whereas the temperate Nearctic was colonized approximately 200 Ma and has only 300 species (see the electronic supplementary material, table S15). Furthermore, we find that the diversity gradient is apparently maintained by limited dispersal out of the tropics into temperate regions, whereas colonization of the tropics by temperate lineages is proportionally much higher (see also [7,14]). The QuaSSE and clade-based analyses show that these differences are influenced by ecological variables including area, energy and climate (table 1 and figure 1d). Importantly, this variation is not recent: the ancient age of the temperate stem lineages compared with their recent crown ages (figure 2b and table 1; [25]) indicates impacts of temperate extinction over tens to hundreds of millions of years (see [48]).
Our analyses also reject other explanations for the latitudinal diversity gradient in amphibians (e.g. mid-domain effect, climatic-niche rates and time-for-speciation). Climatic-niche rates were previously shown to affect diversification rates in plethodontid salamanders [10], but this pattern may not be widespread across amphibians or the lack of a strong effect here may be caused by our use of a single PC axis to describe climatic niches both across and within tropical and temperate regions. Some recent studies have found a strong time-for-speciation effect on regional richness and no evidence of latitudinal variation in diversification rates [13,16,17,22]. However, the amphibian groups (Hylidae and Ranoidea) showing this pattern are relatively young and may have invaded temperate regions too recently to be impacted by higher temperate extinction. Many amphibian families are endemic to either temperate or tropical ecoregions (i.e. do not show high dispersal), and many ancient amphibian clades are temperate [25].
The lack of a time-for-speciation effect across ecoregions seems to be at least partially explained by higher rates of temperate extinction yielding old, depauperate clades at high latitudes. We find that older crown-group clades do accumulate more species as expected, that temperate regions have been colonized longer, and temperate stem-group ages are older (table 1 and figure 2a). However, higher temperate extinction may slow the accumulation of diversity as lineages (including entire subclades) are pruned at higher rates from stem branches, and temperate crown groups are thus younger and less diverse [25]. By contrast, low tropical extinction may allow more lineages to accumulate over time, yielding older crown-group ages in the tropics (table 1 and figure 2b). A stem–crown age imbalance owing to extinction might also provide a generalized explanation for the widespread disconnection between stem-clade age and diversity [48,49,58].
Our analyses of rates of speciation and extinction revealed a particularly surprising result: that the estimated extinction rate for tropical amphibians is very low, with some estimates close to zero. We know of no other studies that have found a similar pattern of very low tropical extinction, contrasted with high extinction in temperate regions. There has been debate whether extinction can be accurately estimated from molecular phylogenies [59], but tree-based estimators such as BiSSE and QuaSSE have yielded accurate results in simulations, given sufficient sampling of species [28,60]. The robustness of our extinction estimates are thus supported by three lines of evidence: (i) simulations show that GeoSSE and QuaSSE can accurately estimate rate parameters including extinction [27,28], even on much smaller phylogenies (200–500 species), (ii) the relationships between climate and estimated extinction rates (figure 1d) indicate that rate differences are non-random (i.e. they are related to ecological factors), and thus unlikely to be purely artefactual, and (iii) similar estimates are obtained by diverse methods (GeoSSE, QuaSSE and clade-based) including rates estimated across the tree and separately for each family (see the electronic supplementary material).
An additional concern for rate estimation may be the relative imbalance in sampling of temperate versus tropical species in our tree (approx. 80% versus approx. 40%). Both QuaSSE and GeoSSE explicitly account for missing species [27,28], with GeoSSE also incorporating their geographical distributions. However, no studies have specifically addressed how GeoSSE and QuaSSE are impacted by non-randomly missing species. Nevertheless, the concordance of these results with each other and the clade-based analyses suggests that the core conclusions (higher tropical speciation and higher temperate extinction) reflect real signal present in the data. Simulations [31] have shown that the BiSSE analytical framework used by GeoSSE and QuaSSE is robust with up to 50–60% missing taxa, similar to our phylogeny (approx. 60%). Furthermore, the clade-based results show no relationship between the proportion of taxa sampled in a clade and estimated rates (see the electronic supplementary material), indicating that our speciation and extinction estimates are not skewed by missing species. Most importantly, if our estimates were biased by missing species, they should be biased towards underestimating tropical speciation or overestimating tropical extinction, given the higher proportion of missing tropical species. Thus, adding missing species should only reinforce the patterns of high tropical speciation and low tropical extinction (or at least relative extinction fraction) that form our main conclusions. Similarly, there are hundreds of undescribed species not included here. But as most are presumably tropical, adding them should only strengthen our results.
We acknowledge that our surprising finding of extremely low tropical extinction should be further tested in other groups and with other methods and other data (e.g. paleontological). Unfortunately, the fossil record of amphibians is relatively sparse, although there seems to be many extinct fossil taxa known from present-day temperate regions compared with relatively few from tropical regions [61], as might be expected from our phylogenetic results. Our results do not predict a complete lack of fossil amphibians in the tropics, but we expect a higher proportion of temperate fossils relative to present temperate species richness. Groups with a more complete record may offer a more robust system to test for differences in temperate and tropical extinction using fossil data [62].
Given our overall results, a major challenge for future studies is now to understand how ecological variables (particularly, temperature and energy) might increase tropical speciation and temperate extinction. This is probably a complex mixture of processes across space and time [3–5,7,32,62]. One potential explanation is that tropical climates foster higher rates of molecular evolution, leading to increased speciation rates [63]. Additionally, relative extinction fractions are higher at higher latitudes (table 1), indicating greater turnover of lineages (extinction with replacement; [51]) through time in temperate clades [19], which may be caused by more rapidly changing ecological conditions in temperate zones driving higher extinction.
Indeed, a major factor that may influence diversity within regions is the variable extent of habitable areas within ecoregions over time [12,55,64]. In our analyses, we refer to the contemporary extent of clades (clade-based analyses) and the current geographical area of ecoregions (ecoregion-based analyses), but we recognize that the geographical range of clades and the habitable area of ecoregions have shifted through time. A recent analysis of major biomes (e.g. tropical forests, deserts) showed that time-integrated area and productivity plus temperature (i.e. greater available area, productivity and temperature over time) provided the best-fit model for contemporary amphibian diversity [55]. Our results suggest that the effect of these factors is to promote speciation and reduce extinction. The changing extent of habitats through time may drive higher temperate extinction, as these changes are much greater at higher latitudes owing to glacial cycles [64]. Our results are also potentially consistent with the idea that the reduced area of temperate zones in the past may have limited temperate diversification [7].
Along these lines, latitudinal variation in resource availability may impact both speciation and extinction rates, particularly in temperate areas, if ecological factors limit the number of species that can co-occur locally. Our results from diversity-dependent models are consistent with the long-standing idea that an increased quantity or diversity of resources in tropical regions may allow co-occurrence of more species in the tropics [5]. Specifically, there is a significant latitudinal gradient in both estimated carrying capacity and saturation of clades (figure 3), suggesting that temperate clades have lower total carrying capacities, and are generally closer to these limits than tropical clades. These gradients suggest a greater and faster slowdown in speciation rate over time in temperate clades than in tropical clades (table 1). These may then lead to higher rates of tropical speciation (and accumulation of more tropical diversity over time), even though λ0 (initial speciation rate) does not significantly vary with latitude. Lower carrying capacities for temperate clades might also help explain limited dispersal out of the tropics, if there have been fewer open niches available in temperate regions over time [65].
There are also some problems with these interpretations based on estimated saturation and carrying capacity. For example, these analyses do not actually incorporate data on local co-occurrence or resource abundance, nor address which resources are limited for temperate amphibians (if any), nor identify how these resources actually influence speciation (especially given evidence for frequent allopatric speciation in amphibians; [66–68]). Some amphibian analyses also show similar local richness between some mesic lowland tropical sites and temperate sites, and greater apparent diversity dependence in more species-rich regions [22]. There may also be issues of power for estimating carrying capacity for small clades, from which there is relatively little phylogenetic data for parameter estimation. Thus, the latitudinal gradient in carrying capacity and saturation may simply reflect the gradient in clade richness (table 1). Additional simulations are needed, and this pattern should be investigated in other groups.
Further, the lack of a latitudinal gradient in extinction rates under diversity dependence is inconsistent with some of our other results (GeoSSE, QuaSSE and clade-based). One possible explanation is that the diversity-dependent model used here enforces constant, rather than diversity-dependent extinction rates (i.e. only diversity-dependent speciation is included). If limited resources in temperate regions prevent co-occurrence of many species, this might also result in diversity dependent increases in temperate extinction over time. Furthermore, diversity-dependent estimates within families may not reflect extinctions above the family-level in older temperate groups, as suggested by the conflicting patterns of diversity over time in stem versus crown groups (see above). These models also do not account for the co-occurrence of multiple clades or temporal variation in carrying capacities within lineages. In summary, the diversity-dependent analyses provide a potential explanation that links environmental factors to the observed patterns in the processes that change richness (i.e. speciation, extinction), but future studies will be needed to fully resolve these mechanisms.
5. Conclusion
Here, we use large-scale phylogenetic analyses to show that the global latitudinal diversity gradient in a major species-rich group (amphibians) is driven by increased tropical speciation and increased temperate extinction and maintained by limited dispersal from tropical to temperate regions. These differences in speciation and extinction rates are strongly linked to ecological factors, including area, climate and ecosystem energy. Our study is, to our knowledge, the first to consider these mechanistic explanations simultaneously using a large-scale phylogeny. Nevertheless, many previous studies are concordant with parts of these results, especially those showing higher tropical diversification rates in groups such as plants [21], insects [23], marine invertebrates [62] and birds [8,9,11], and those showing limited tropical-to-temperate dispersal [13–14]. A major challenge for future studies will be to determine the precise mechanisms by which ecological variables differentially affect speciation, extinction and dispersal rates in temperate and tropical regions. Finally, we estimate strikingly low rates of past extinction for tropical amphibians. These low rates are in stark contrast to current patterns, with human impacts leading to high extinction in the tropics [69].
Acknowledgements
We thank I. Cuthill, M. Cardillo, G. Thomas, S. Smith, D. Rabosky, E. Goldberg, R. FitzJohn, A. Phillimore, F. Burbrink, J. Lombardo and T. Guiher for comments and assistance with this study. The dated phylogeny, ecoregion occurrences and PC1 scores (climatic-niche estimates) for the 2871 species analysed are provided as electronic supplementary material.
Funding statement
This research was supported by US N.S.F. Bioinformatics Postdoctoral grant no. DBI-0905765 to R.A.P.
References
- 1.Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (doi:10.1146/annurev.ecolsys.34.012103.144032) [Google Scholar]
- 2.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (doi:10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 3.Pianka ER. 1966. Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 100, 33–46 (doi:10.1086/282398) [Google Scholar]
- 4.Rohde K. 1992. Latitudinal gradients in species-diversity: the search for the primary cause. Oikos 65, 514–527 (doi:10.2307/3545569) [Google Scholar]
- 5.Mittelbach GG, et al. 2007. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (doi:10.1111/j.1461-0248.2007.01020.x) [DOI] [PubMed] [Google Scholar]
- 6.Ricklefs RE. 1987. Community diversity: relative roles of local and regional processes. Science 235, 167–171 (doi:10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- 7.Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 8.Cardillo M, Orme CDL, Owens IPF. 2005. Testing for latitudinal bias in diversification rates: an example using New World birds. Ecology 86, 2278–2287 (doi:10.1890/05-0112) [Google Scholar]
- 9.Ricklefs RE. 2006. Global variation in the diversification rate of passerine birds. Ecology 87, 2468–2478 (doi:10.1890/0012-9658(2006)87[2468:GVITDR]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 10.Kozak KH, Wiens JJ. 2010. Accelerated rates of climatic-niche evolution underlie rapid species diversification. Ecol. Lett. 13, 1378–1389 (doi:10.1111/j.1461-0248.2010.01530.x) [DOI] [PubMed] [Google Scholar]
- 11.Martin PR, Tewksbury JJ. 2008. Latitudinal variation in subspecific diversification of birds. Evolution 62, 2775–2788 (doi:10.1111/j.1558-5646.2008.00489.x) [DOI] [PubMed] [Google Scholar]
- 12.Fine PVA, Ree RH. 2006. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 168, 796–804 (doi:10.1086/508635) [DOI] [PubMed] [Google Scholar]
- 13.Wiens JJ, Graham CH, Moen DS, Smith SA, Reeder TW. 2006. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596 (doi:10.1086/507882) [DOI] [PubMed] [Google Scholar]
- 14.Smith BT, Bryson RWJ, Houston DD, Klicka J. 2012. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecol. Lett. 15, 1318–1325 (doi:10.1111/j.1461-0248.2012.01855.x) [DOI] [PubMed] [Google Scholar]
- 15.Salisbury CL, Seddon N, Cooney CR, Tobias JA. 2012. The latitudinal gradient in dispersal constraints: ecological specialisation drives diversification in tropical birds. Ecol. Lett. 15, 847–855 (doi:10.1111/j.1461-0248.2012.01806.x) [DOI] [PubMed] [Google Scholar]
- 16.Jansson R, Rodríguez-Castañeda G, Harding LE. 2013. What can multiple phylogenies say about the latitudinal diversity gradient? A new look at the tropical conservatism, out-of-the-tropics and diversification rate hypotheses. Evolution 67, 1741–1755 (doi:10.1111/evo.12089) [DOI] [PubMed] [Google Scholar]
- 17.Wiens JJ, Sukumaran J, Pyron RA, Brown RM. 2009. Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution 63, 1217–1231 (doi:10.1111/j.1558-5646.2009.00610.x) [DOI] [PubMed] [Google Scholar]
- 18.Stevens RD. 2006. Historical processes enhance patterns of diversity along latitudinal gradients. Proc. R. Soc. B 273, 2283–2289 (doi:10.1098/rspb.2006.3596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir JT, Schluter D. 2007. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (doi:10.1126/science.1135590) [DOI] [PubMed] [Google Scholar]
- 20.Buckley LB, et al. 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138 (doi:10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansson R, Davies TJ. 2008. Global variation in diversification rates of flowering plants: energy vs climate change. Ecol. Lett. 11, 173–183 [DOI] [PubMed] [Google Scholar]
- 22.Wiens JJ, Pyron RA, Moen DS. 2011. Phylogenetic origins of local-scale diversity patterns and the causes of Amazonian megadiversity. Ecol. Lett. 14, 643–652 (doi:10.1111/j.1461-0248.2011.01625.x) [DOI] [PubMed] [Google Scholar]
- 23.Condamine FL, Sperling FAH, Wahlberg N, Rasplus JY, Kergoat GJ. 2012. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 15, 267–277 (doi:10.1111/j.1461-0248.2011.01737.x) [DOI] [PubMed] [Google Scholar]
- 24.Cardillo M. 1999. Latitude and rates of diversification in birds and butterflies. Proc. R. Soc. Lond. B 266, 1221–1225 (doi:10.1098/rspb.1999.0766) [Google Scholar]
- 25.Wiens JJ. 2007. Global patterns of diversification and species richness in amphibians. Am. Nat. 170, S86–S106 (doi:10.1086/519396) [DOI] [PubMed] [Google Scholar]
- 26.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448 (doi:10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 27.Goldberg EE, Lancaster LT, Ree RH. 2011. Phylogenetic inference of reciprocal effects between geographic range evolution and diversification. Syst. Biol. 60, 451–465 (doi:10.1093/sysbio/syr046) [DOI] [PubMed] [Google Scholar]
- 28.FitzJohn RG. 2010. Quantitative traits and diversification. Syst. Biol. 59, 619–633 (doi:10.1093/sysbio/syq053) [DOI] [PubMed] [Google Scholar]
- 29.AmphibiaWeb 2013. Information on amphibian biology and conservation. Berkeley, CA: AmphibiaWeb: See (http://amphibiaweb.org/). [Google Scholar]
- 30.Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F. 2007. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892 (doi:10.1073/pnas.0608378104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FitzJohn RG, Maddison WP, Otto SP. 2009. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Syst. Biol. 58, 595–611 (doi:10.1093/sysbio/syp067) [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig ML. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Losos JB, Schluter D. 2000. Analysis of an evolutionary species-area relationship. Nature 408, 847–850 (doi:10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- 34.Etienne RS, Haegeman B, Stadler T, Aze T, Pearson PN, Purvis A, Phillimore AB. 2012. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc. R. Soc. B 279, 1300–1309 (doi:10.1098/rspb.2011.1439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabosky DL, Lovette IJ. 2008. Density-dependent diversification in North American wood warblers. Proc. R. Soc. B 275, 2363–2371 (doi:10.1098/rspb.2008.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyron RA, Wiens JJ. 2011. A large-scale phylogeny of amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 61, 543–583 (doi:10.1016/j.ympev.2011.06.012) [DOI] [PubMed] [Google Scholar]
- 37.Holt B, et al. 2013. An update of Wallace's zoogeographic regions of the world. Science 339, 74–78 (doi:10.1126/science.1228282) [DOI] [PubMed] [Google Scholar]
- 38.Colwell RK, Rahbek C, Gotelli NJ. 2004. The mid-domain effect and species richness patterns: what have we learned so far? Am. Nat. 163, E1–E23 (doi:10.1086/382056) [DOI] [PubMed] [Google Scholar]
- 39.McCain CM. 2004. The mid-domain effect applied to elevational gradients: species richness of small mammals in Costa Rica. J. Biogeogr. 31, 19–31 (doi:10.1046/j.0305-0270.2003.00992.x) [Google Scholar]
- 40.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (doi:10.1002/joc.1276) [Google Scholar]
- 41.Evans KL, Warren PH, Gaston KJ. 2005. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 80, 1–25 (doi:10.1017/S1464793104006517) [DOI] [PubMed] [Google Scholar]
- 42.Phillips LB, Hansen AJ, Flather CH. 2008. Evaluating the species energy relationship with the newest measures of ecosystem energy: NDVI versus MODIS primary production. Remote Sens. Environ. 112, 3538–3549 (doi:10.1016/j.rse.2008.04.012) [Google Scholar]
- 43.Tateishi R, Ahn CH. 1996. Mapping evapotranspiration and water balance for global land surfaces. ISPRS J. Photogramm. 51, 209–215 (doi:10.1016/0924-2716(96)00015-9) [Google Scholar]
- 44.Buckley LB, Jetz W. 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B 274, 1167–1173 (doi:10.1098/rspb.2006.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 (doi:10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 46.FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3, 1084–1092 (doi:10.1111/j.2041-210X.2012.00234.x) [Google Scholar]
- 47.McPeek MA, Brown JM. 2007. Clade age and not diversification rate explains species richness among animal taxa. Am. Nat. 169, E97–E106 (doi:10.1086/512135) [DOI] [PubMed] [Google Scholar]
- 48.Pyron RA, Burbrink FT. 2012. Extinction, ecological opportunity, and the origins of global snake diversity. Evolution 66, 163–178 (doi:10.1111/j.1558-5646.2011.01437.x) [DOI] [PubMed] [Google Scholar]
- 49.Rabosky DL, Slater GJ, Alfaro ME. 2012. Clade age and species richness are decoupled across the eukaryotic tree of life. PLoS Biol. 10, e1001381 (doi:10.1371/journal.pbio.1001381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricklefs RE. 2007. Estimating diversification rates from phylogenetic information. Trends Ecol. Evol. 22, 601–610 (doi:10.1016/j.tree.2007.06.013) [DOI] [PubMed] [Google Scholar]
- 51.Nee S, May RM, Harvey PH. 1994. The reconstructed evolutionary process. Phil. Trans. R. Soc. Lond. B 344, 305–311 (doi:10.1098/rstb.1994.0068) [DOI] [PubMed] [Google Scholar]
- 52.Etienne RS, Haegeman B. 2012. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. Am. Nat. 180, E75–E89 (doi:10.1086/667574) [DOI] [PubMed] [Google Scholar]
- 53.Rabosky DL, Lovette IJ. 2009. Problems detecting density-dependent diversification on phylogenies: reply to Bokma. Proc. R. Soc. B 276, 995–997 (doi:10.1098/rspb.2008.1584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bokma F. 2009. Problems detecting density-dependent diversification on phylogenies. Proc. R. Soc. B 276, 993–994 (doi:10.1098/rspb.2008.1249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jetz W, Fine PVA. 2012. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 (doi:10.1371/journal.pbio.1001292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyron RA. 2011. Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Syst. Biol. 60, 466–481 (doi:10.1093/sysbio/syr047) [DOI] [PubMed] [Google Scholar]
- 57.Marjanovic D, Laurin M. 2007. Fossils, molecules, divergence times, and the origin of lissamphibians. Syst. Biol. 56, 369–388 (doi:10.1080/10635150701397635) [DOI] [PubMed] [Google Scholar]
- 58.Rabosky DL. 2009. Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol. Lett. 12, 735–743 (doi:10.1111/j.1461-0248.2009.01333.x) [DOI] [PubMed] [Google Scholar]
- 59.Rabosky DL. 2010. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824 (doi:10.1111/j.1558-5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]
- 60.Davis MP, Midford PE, Maddison WP. 2013. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evol. Biol. 13, 38 (doi:10.1186/1471-2148-13-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heatwole H, Carroll RL. (eds) 2000. Palaeontology: the evolutionary history of the Amphibia. Chipping Norton, Australia: Surrey Beatty Press [Google Scholar]
- 62.Jablonski D, Roy K, Valentine JW. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 (doi:10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 63.Wright S, Keeling J, Gillman L. 2006. The road from Santa Rosalia: a faster tempo of evolution in tropical climates. Proc. Natl Acad. Sci. USA 103, 7718–7722 (doi:10.1073/pnas.0510383103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120 (doi:10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiens JJ. 2011. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. 86, 75–96 (doi:10.1086/659883) [DOI] [PubMed] [Google Scholar]
- 66.Kozak KH, Wiens JJ. 2006. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 60, 2604–2621 [PubMed] [Google Scholar]
- 67.Hua X, Wiens JJ. 2010. Latitudinal variation in speciation mechanisms in frogs. Evolution 64, 429–443 (doi:10.1111/j.1558-5646.2009.00836.x) [DOI] [PubMed] [Google Scholar]
- 68.Cadena CD, et al. 2012. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R. Soc. B 279, 194–201 (doi:10.1098/rspb.2011.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 (doi:10.1126/science.1103538) [DOI] [PubMed] [Google Scholar]