Abstract
Vertebrates respond to unpredictable noxious environmental stimuli by increasing secretion of glucocorticoids (CORT). Although this hormonal stress response is adaptive, high levels of CORT may induce significant costs if stressful situations are frequent. Thus, alternative coping mechanisms that help buffer individuals against environmental stressors may be selected for when the costs of CORT levels are elevated. By allowing individuals to identify, anticipate and cope with the stressful circumstances, cognition may enable stress-specific behavioural coping. Although there is evidence that behavioural responses allow animals to cope with stressful situations, it is unclear whether or not cognition reduces investment in the neuroendocrine stress response. Here, we report that in birds, species with larger brains relative to their body size show lower baseline and peak CORT levels than species with smaller brains. This relationship is consistent across life-history stages, and cannot be accounted for by differences in life history and geographical latitude. Because a large brain is a major feature of birds that base their lifetime in learning new things, our results support the hypothesis that enhanced cognition represents a general alternative to the neuroendocrine stress response.
Keywords: stress response, brain size, cognition, innovation, phylogenetic comparative analysis
1. Introduction
Organisms are constantly challenged by their environment. Most of these challenges may be perceived as stressful and how organisms cope with such stress can have important fitness consequences [1]. One important mechanism by which vertebrates respond to stressful stimuli is the neuroendocrine stress response, which is a highly conserved reaction for two reasons. First, its physiological background, the activation of the hypothalamus–pituitary–adrenal axis, is common in all vertebrates; and second, a large variety of uncontrollable noxious stimuli (commonly referred to as stressors) trigger the same general response [2,3]. In reaction to stressors, the secretion of glucocorticoid (CORT) hormones can increase within 2–3 min, usually reaching peak concentrations within 15–60 min [4,5]. Such stress-induced increase in CORT concentrations shows heritable variation [6,7] and it is thought to be adaptive because it redirects behaviour and physiology towards immediate survival functions (inducing an ‘emergency life-history stage (LHS)’; [4,8–11]). However, chronic elevation of CORT levels owing to frequent exposure to stressors may result in substantial costs in terms of survival and reproduction, including an impairment of immune function and a reduction of metabolic efficiency [3,8], although this view is not free of criticisms [12].
Despite qualitative similarities in the neuroendocrine stress response, the magnitude of the stress response dramatically varies among different taxa [13–16], implying that animals may react to similar challenges in diverse ways. For instance, among bird species (where variation of the neuroendocrine stress response is best documented), there is approximately 12-fold variation in the maximum stress hormone levels within a single LHS, and the baseline hormone concentrations show even higher variation, exceeding an 80-fold difference (see the electronic supplementary material, appendix A). This huge and seemingly adaptive interspecific variation in circulating stress hormone levels suggests that the benefits and costs associated with coping mechanisms differ among avian taxa. Extrinsic factors may be accounted for part of this variation. For example, it has long been known that unpredictable stressors provoke strong, persistent CORT responses in rats [17]. However, it is increasingly becoming clear that the optimal stress response also depends on the existence of alternative mechanisms that allow buffering individuals against stress without any need to increase hormone levels, for example by avoiding the stressor [3].
To efficiently cope with a stressor requires the capacity to identify, anticipate and behaviourally cope with the stressful circumstance [18]. Cognition, defined as the neuronal processes concerned with the acquisition, retention and use of information [19], may be one of the alternative mechanisms that enable stress-specific behavioural coping. There is ample evidence that changes in behaviour are an important mechanism through which animals deal with stressful situations, including predation risk, food shortages and human disturbances (reviewed in [20,21]). For example, elk (Cervus elaphus) reduces the stress caused by wolf presence by increasing the proportion of time spent vigilant and by moving into the protective cover of wooded areas [22]. Interestingly, the response to wolf presence is better explained by changes in behavioural patterns than by changes in CORT concentrations [22]. The role of behaviour in coping with stress is expected to be particularly important in animals whose fitness heavily depends on learning new things throughout their lifetime (cognitive life style, hereafter). Baboons (Papio anubis), for example, rarely experience stressors associated with famine or predators, but instead may suffer from social stressors. Baboons are able to handle such stress by cultivating friendships [23]. Although there is evidence to support the cognitive buffer hypothesis [24–26], suggesting that behavioural modifications can buffer individuals from stressful situations, whether cognition serves as a general alternative to activation of the neuroendocrine stress responses is presently unknown. We ask here whether species characterized by a cognitive life style tend to invest less in a neuroendocrine stress system using a comprehensive phylogenetic-based comparative analysis in birds.
Characterizing the cognitive life style on large comparative datasets covering a broad spectrum of species is extremely difficult [27,28], yet the size of the whole brain relative to body size (relative brain size, hereafter) can serve as an indirect approximation. Although finer morphological measures to quantify the performance of the brain in cognitive tasks have been proposed (e.g. association areas of the brain, and density of neurons and glia), these are difficult to obtain and hence are only available for a few species [27]. However, there exists a tight relationship between neuron numbers, the size of the association areas and brain size after removing the common effects of body size (reviewed in [27]). More importantly, the validity of relative brain size as a surrogate of cognitive life style is supported in birds by experimental and comparative evidence that large brains are associated with a higher propensity for learning [29–33] and better performance in dealing with novel or altered environments [34–36].
Because species vary in relative brain size [32], the importance of cognition in dealing with stress is also expected to vary across taxa. This may result in a trade-off that defines a continuum of the stress response, ranging from species that primarily rely on plastic behavioural responses to species that mostly rely on organism-wide hormonal responses. If so, we predict that the magnitude of the hormonal stress response should be negatively correlated with cognitive capacity as reflected by the species’ brain size. To test this prediction, we compiled a global database documenting 330 CORT level values of 119 avian species from temperate and tropical regions and tested the association between brain size and CORT levels with a recent Bayesian modelling technique [37,38] that allows for an analysis of intraspecific variation while correcting for problems associated with phylogenetic and regional non-independence among data. Given the correlative nature of our analyses, we were careful to control for key factors that could obscure or confound any brain–CORT association (for a similar approach, see [39,40]).
2. Methods
(a). Glucocorticoid levels
We compiled a database from the primary literature published before May 2010 on circulating CORT concentrations. We searched for articles on the ISI Web of Knowledge database using the keyword ‘corticosterone’ and filtered out studies on non-avian taxa. Based on the original articles, we categorized the LHSs in which CORT was measured into migration, wintering, pre-breeding and parental phase of breeding. We excluded those data where the description of the studies did not allow for unambiguously classifying the LHS. We also excluded studies from the moult stage, because often it was not clearly reported whether or not the birds were actually moulting during the post-breeding moulting period and also because during moult the CORT production is heavily downregulated [13]; therefore, in many species, the measured values were close to or below the detection limits of the assays. Migration included both spring and autumn migration (the electronic supplementary material, appendix A contains which type of migration was used for a given species). Wintering was defined as all non-breeding periods except for the post-breeding moult period. Pre-breeding was defined as LHSs from territory establishment through the onset of incubation, and therefore included samples collected during the periods of courtship, copulation, nest building, egg formation and egg-laying. The parental phase of breeding included both incubation and chick-rearing.
From the published records, we extracted two hormone levels (in ng ml−1): (i) baseline CORT, i.e. sampled immediately upon capture within 3 min [41] or more if the study validated that CORT had not begun to increase during that time interval (range 0–7 min; mean + s.d. = 3.08 + 0.92, n = 648). This measure is an approximation for the seasonal baseline level of CORT that the animals should maintain to cope with the predictable demands of the current LHS (allostatic load, ‘state B’ sensu [42]); and (ii) peak CORT, i.e. the highest stress-induced level among all measurements following capture and restraint for 5–70 min (mean + s.d. = 35.33 + 22.18 min, n = 444). This measure corresponds to the acutely elevated levels of CORT triggered by unpredictable perturbations that cannot be prepared for and may shift the animals into the ‘emergency LHS’ (allostatic overload, ‘state C’ sensu [42]). Whenever the given study allowed, we collected CORT data separately for the sexes, otherwise we collected CORT data combined for the sexes. Although sex-specific analyses were not carried out, the reason for collecting sex-specific values was to control for potential bias in sampling in the original studies. For both baseline and peak CORT in each sex, we calculated the weighted mean (by sample size) of the different estimates, and then we used the mean value of the sexes to generate one baseline and one peak value per species. We excluded CORT data measured in individuals that were subjected to experimental manipulations (e.g. hormone implantation, brood size manipulation) or extreme conditions (e.g. severe storms) that are expected or known to alter their CORT levels. All reported CORT values are total levels (i.e. free CORT plus CORT bound to plasma binding proteins; free CORT levels were available only for 22 species). In total, we used CORT and brain data for 119 bird species from 189 studies of CORT (see the electronic supplementary material, appendix A).
(b). Brain size
Brains vary in whole size, size of their parts, density of neurons and glia, as well as density of neurotransmitter receptors, and each of these features has been suggested to reflect the performance of the brain in cognitive tasks [27]. We focused on size of the whole brain mainly for reasons of data availability. The analysis of whole-brain size is justified for three main empirical reasons (reviewed by [27] and see also references therein). First, neuron density and relevant brain component volumes (e.g. the Pallium areas of the avian brain associated with innovation and learning) are tightly correlated with whole-brain volumes. Second, several measures of behavioural flexibility, including innovation and learning, correlate with brain volume in birds and mammals. Finally, large-brained birds and mammals tend to exhibit higher ability to survive novel ecological challenges, as suggested by their higher success when introduced in novel environments or when dealing with environmental alterations. Thus, although we agree with Healy & Rowe [43] on the importance of examining brain structure and components when appropriate data become available, our assumption that a large brain may facilitate behavioural responses to stressful situations is well supported by empirical evidence.
We obtained published data on whole-brain size based on previously published papers [44–47]. We used actual brain mass, where available, but we also included cranial endocast measures converted to mass by multiplying the reported value by the density of fresh brain tissue (1.036 g ml−1) [34]. Previous studies have shown that both metrics are strongly correlated and that combining them in a similar analysis does not alter the conclusions [32,48,49]. When information on brain size was available from different sources, we used mean values or the value based on larger sample sizes. Information on brain mass was available for 97 of the 119 species that we considered. For the remaining species, brain size was estimated by using the average brain mass of the closest taxonomic level (genus, which predicts 91% of the variance at the species level; [34]). Restricting our analyses only to those species where brain mass data was available did not change our results qualitatively (see the electronic supplementary material, table S17).
To remove the allometric effect on brain size (larger birds have larger brains), we calculated the residuals from a linear regression between log-transformed brain mass and log-transformed body mass (residual brain mass henceforth). This model produced a highly significant relationship between the two variables (r2 = 0.94, p < 0.0001) and visual inspection revealed no sign of heteroscedasticity or any pattern in the residuals, indicating a good fit [50]. Thus, a positive residual brain mass indicates that the brain is larger than expected by body size, whereas a negative residual brain mass indicates that the brain is smaller than expected by body size.
(c). Confounding variables
To control for the effects of potentially confounding variables, we collected data on aspects of interspecific life-history variation that have been shown to be related to CORT levels. First, according to Bókony et al. [14], we calculated the ‘brood value’ for each species, i.e. the number of offspring in the current reproduction relative to the number of offspring that can be produced during the lifetime of an average individual of the species. This variable has a negative effect on avian stress response during the parental phase of breeding [14]. Brood value was calculated as log10(clutch size/(clutch size × number of broods per year × average reproductive lifespan)).
Second, we gathered data on body mass for two reasons. On the one hand, larger birds have lower baseline CORT levels, indicating a lower overall metabolism and/or that large mass may serve as a buffer against some stressors [14,15]. On the other hand, body mass may also influence the magnitude of the stress response (i.e. peak CORT), because body mass is strongly related to pace of life which may affect CORT across various LHSs [51]. We calculated the average of male and female body mass where sex-specific body mass data were available, and log-transformed body mass before the analyses.
Third, CORT levels may vary latitudinally [14,52,53]. Therefore, we collected data on the latitude of each study site, then we calculated the mean latitude weighted by the sample size for each species and for each LHS. We used the absolute values of latitude in the analyses. The complete dataset and data sources are given in the electronic supplementary material, appendix A.
(d). Modelling approach
We investigated the evolutionary relationships between CORT levels and brain size with Bayesian phylogenetic mixed-effects models based on Markov chain Monte Carlo (MCMC) estimations, as implemented in the R package ‘MCMCglmm’ v. 2.17 [37,38,54]. MCMCglmm allows the simultaneous analysis of multiple response variables (baseline and peak CORT, in our case) while controlling for the effect of shared ancestry and repeated measures of the response (repeated CORT measures within species, in our case). Shared ancestry was taken into account by specifying a phylogenetic variance–covariance matrix based on a phylogenetic supertree (see the electronic supplementary material, appendix B; [55] assuming gradual branch lengths, i.e. proportional to the number of nodes (Nee's method; [56]). Parameter estimates are based on the posterior distribution with 95% credible intervals (lower and upper CI).
Analyses were run in three stages (details in the electronic supplementary material, pp. 2–10). In the first stage, we estimated the repeatability of baseline and peak CORT levels within species [57]. This is essential, because if CORT levels are not repeatable, then they cannot be used in the interspecific comparative analyses. Repeatability was estimated separately for baseline and peak CORT as the intraclass correlation coefficient obtained by dividing species variance by total variance while taking into account LHS and phylogeny.
In the second stage, we investigated whether baseline and peak CORT show correlated phenotypic evolution or evolve independently as two traits, and we also tested the effect of phylogeny in these analyses. This allowed us to define the best structure of random effects for subsequent analyses. We tested four structures (see the electronic supplementary material, table S1): (i) no phylogenetic effects (i.e. species as independent data points) yet evolutionary correlation between baseline and peak CORT levels; (ii) no phylogenetic effects and no evolutionary correlation between CORT levels; (iii) phylogenetic effects (i.e. unstructured phylogenetic variance–covariance matrix allowing the estimation of variance of each trait due to evolutionary history) and a covariance between baseline and peak CORT levels attributable to shared ancestry between species [39]; and (iv) phylogenetic effects yet covariance between baseline and peak CORT levels set to zero (i.e. assuming independent evolution of the traits). We compared these models using the information-theoretic approach [58]. Specifically, we used the deviance information criterion (DIC) [37], which is the Bayesian equivalent of the more commonly used Akaike information criterion.
In the last stage, we used the best structure of random factors to model baseline and peak CORT levels (multi-response variables) as a function of residual brain mass and all confounding variables (explanatory variables). We again investigated the relative importance of the explanatory variables using the information-theoretic approach [58]. We built a priori candidate models explaining the interspecific variation in CORT levels (table 1), and ranked the models based on their DIC differences (ΔDIC). The simplest model merely describes the differences of CORT values between baseline and peak levels and across LHSs. All the rest of the models also control for the potentially confounding effects of brood value, body mass and latitude, differing only in the interaction terms included. The terms ‘level × brain’ and ‘LHS × brain’ express that the relationship between CORT and brain size differs between baseline and peak levels and across LHSs, respectively, whereas the term ‘level × LHS × brain’ tests whether baseline and peak CORT is differently related to brain size in different LHSs (i.e. different slopes for baseline and CORT in every LHS). We report the parameter estimates (posterior means) and corresponding 95% CI values for the fully parametrized model, parameter estimates of all other models are reported in the electronic supplementary material, tables S2–S8.
Table 1.
Comparison of candidate models of CORT levels based on deviance information criterion (DIC). (Baseline and peak CORT are simultaneously modelled in a multi-response Bayesian phylogenetic mixed model. Variables included as predictors in the models are level (i.e. baseline versus peak CORT), life-history stage (LHS), log body mass (mass), absolute distance from equator (latitude), brood value (BV) and residual brain mass (brain).)
| model no. | fixed effects in the model | DIC | ΔDIC |
|---|---|---|---|
| 1 | level + LHS + BV + mass + latitude + brain + brain × level + brain × LHS | 1940.36 | 0.00 |
| 2 | level + LHS + BV + mass + latitude + brain + brain × level + brain × LHS + brain × level × LHS | 1940.54 | 0.18 |
| 3 | level + LHS + BV + mass + latitude + brain + brain × LHS | 1940.91 | 0.54 |
| 4 | level + LHS | 1948.68 | 8.32 |
| 5 | level + LHS + BV + mass + latitude | 1950.16 | 9.80 |
| 6 | level + LHS + BV + mass + latitude + brain + brain × level | 1951.87 | 11.50 |
| 7 | level + LHS + BV + mass + latitude + brain | 1952.73 | 12.37 |
3. Results
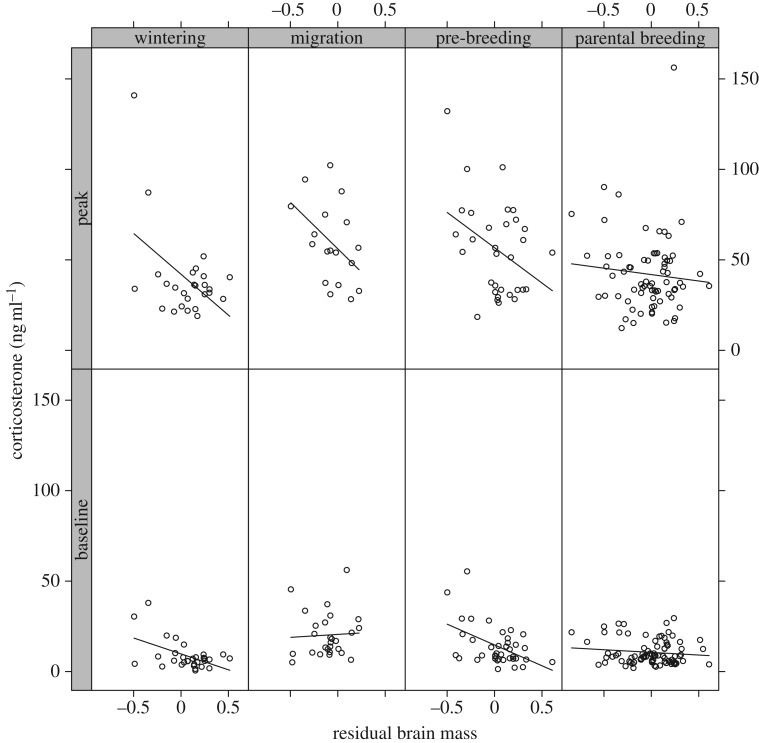
CORT levels exhibited substantial variation among LHSs (figure 1). The baseline and peak CORT measures were consistent within species, showing repeatabilities of 0.60 (CI: 0.47–0.76) and 0.49 (CI: 0.32–0.65), respectively. Thus, CORT measures can be considered species’ traits and are thus valid for interspecific comparative analyses.
Figure 1.
Relationship between residual brain size and levels of baseline and peak corticosterone (for statistics, see table 2). Residual brain mass was calculated as residuals from a linear regression between log body mass (independent variable) and log brain mass (response variable). Lines are linear regression fits to the raw data in each life-history stage.
Models assuming correlated evolution of baseline and peak CORT received substantially more support than models assuming independent evolution of the two traits, both when species were treated as independent data points (ΔDIC = 21.13) and when evolutionary history was taken into account (ΔDIC = 2.42). From these correlated evolution models, the best support was for the model where species were treated as independent evolutionary units, indicating only a moderate phylogenetic effect on the CORT levels (phylogenetic heritability was 0.39). We consequently used models with correlated evolution and species treated as independent data points in the next analyses (for models with phylogeny, see electronic supplementary material, tables S9–S16).
As expected from our hypothesis, CORT levels were consistently lower in species with larger residual brain mass (figure 1). Indeed, our top three models in the model selection approach received almost equal support, and they all included residual brain mass, and an interaction between LHS and residual brain mass, whereas models excluding this interaction or brain size altogether received considerably less support (table 1). Our top three models differ only in the slope parameters describing how peak CORT levels vary with residual brain size.
Our best model predicts that baseline CORT levels decrease with increasing residual brain mass, but this effect is more pronounced in the wintering and pre-breeding stages than during parenting and disappears during migration (see the electronic supplementary material, figure S1 and table 2). Peak CORT levels vary with residual brain mass more steeply than baseline levels in each LHS, but these differences in slope remain the same in different LHSs (see the electronic supplementary material, table S2 and figure S1). Our second best model is fully parametrized, where both baseline and peak CORT levels vary with residual brain mass differently in each LHS (three-way interaction), predicting different slopes for baseline and peak levels as a function of residual brain mass (table 2 and electronic supplementary material, figure S2). Our third model predicts different CORT levels for different LHSs, and an effect of residual brain mass that also varies between LHSs (brain : LHS interaction in the electronic supplementary material, table S4 and figure S3). However, for any given LHS, this model predicts identical slopes for peak and baseline levels.
Table 2.
Parameter estimates for the fully parametrized Bayesian phylogenetic mixed-effects model (model no. 2 from the candidate model set in table 1). (Dependent variable was CORT levels (both baseline and peak). The intercept was removed for easier interpretation, therefore baseline and peak CORT levels show parameter estimates for the reference factor level (wintering), and other parameters give parameters according to the model specification. LHS refers to life-history stage. Posterior means with 95% lower and upper credibility intervals (CI) and Bayesian p-values are reported.)
| parameter estimates | posterior mean | lower CI | upper CI | p-value |
|---|---|---|---|---|
| baseline CORT (wintering) | 17.17 | 6.58 | 27.91 | 0.002 |
| peak CORT (wintering) | 51.32 | 40.13 | 62.71 | <0.001 |
| LHS migrationa | 9.15 | 4.14 | 13.95 | <0.001 |
| LHS pre-breedinga | 3.41 | −0.52 | 7.31 | 0.089 |
| LHS parental breedinga | 0.39 | −3.24 | 3.65 | 0.797 |
| brood value | 3.74 | −3.42 | 10.36 | 0.279 |
| body mass (log) | −0.78 | −1.76 | 0.14 | 0.108 |
| latitude (absolute value) | 0.03 | −0.09 | 0.15 | 0.598 |
| residual brain massb | −21.35 | −34.78 | −6.42 | 0.004 |
| peak CORT × residual brain mass | −43.10 | −77.71 | −9.19 | 0.012 |
| LHS migration × residual brain massc | 27.49 | 8.13 | 45.66 | 0.007 |
| LHS pre-breeding × residual brain massc | 1.30 | −16.27 | 18.87 | 0.878 |
| LHS parental breeding × residual brain massc | 19.85 | 5.88 | 35.36 | 0.012 |
| peak CORT × LHS migration × residual brain massd | 26.90 | −27.31 | 84.13 | 0.354 |
| peak CORT × LHS pre-breeding × residual brain massd | 33.18 | −10.18 | 77.17 | 0.134 |
| peak CORT × LHS parental breeding × residual brain massd | 37.96 | 1.39 | 73.57 | 0.042 |
aContrast with baseline CORT in the wintering LHS.
bFor baseline levels in the wintering LHS.
cContrast with the residual brain mass effect on baseline CORT in the wintering LHS.
dContrast with the residual brain mass effect on peak CORT in the wintering LHS.
Potential confounding effects (body mass, latitude and brood value) have little, if any, effect on CORT levels overall (see the electronic supplementary material, tables S2–S8).
4. Discussion
Comparing the stress hormone levels and brain sizes of 119 avian species (including 24 species with genus-level estimates of brain size), our study has revealed a negative relationship between CORT concentrations and residual brain mass that varied across LHSs. Both baseline and peak CORT levels showed a decreasing slope with increasing residual brain mass during the wintering and pre-breeding phases and, to a lesser extent, also in the parental breeding phase. For the migratory phase, peak but not baseline CORT exhibited the same negative relationship with brain size. Thus, our prediction that birds with larger brains have lower circulating levels of stress hormones is supported in the majority of the comparisons.
Our analyses show that baseline and peak CORT levels evolve as correlated traits with low phylogenetic inertia. Given that hormone levels are able to change rapidly as a function of the environment, it is reasonable that we do not find a strong phylogenetic constraint on these traits [14]. However, our analyses cannot contribute to discern whether the effect of brain size on the evolution of CORT levels happens primarily through natural selection acting on baseline levels (with correlated responses on peak levels), peak levels (with correlated responses on baseline levels) or other factors not considered in the study that affect both baseline and peak levels. We also acknowledge that CORT levels are just one part of a very complex physiological system that includes other hormones, binding proteins, two types of cellular receptors (mineralocorticoid and glucocorticoid receptors) and at least one type of membrane-integrated receptor and several target genes [59]. Although all these regulators of CORT action may also differ across species [60] and vary depending on physiological states and environmental conditions [61], we found relatively high repeatabilities for both baseline and peak CORT levels, corroborating our earlier results [14]. This supports the notion that hormone levels coevolve with downstream endocrine components; therefore, plasma hormone levels may be considered as a relatively easily measurable trait of the phenotypic variation in the underlying endocrine machinery (reviewed by Williams [62] and see also Bókony et al. [14]).
The negative relationship between CORT concentrations and residual brain mass was especially marked for peak CORT levels. Such CORT levels can be interpreted as acute hormonal responses induced by stressful situations, and hence the lower peak CORT levels of large-brained birds may reflect that these species have evolved compensatory mechanisms to minimize the use of their neuroendocrine stress response, such as enhanced cognitive capacities for coping with stressful situations. Interestingly, a less steep but still negative relationship was exhibited by baseline CORT levels in the wintering and pre-breeding stages, which may be explained by the role of baseline CORT in preparing the organism to perform better under expected future stressful situations [13]. According to the cognitive buffer hypothesis (reviewed by Sol [26]), this preparative effect should be less crucial for species with larger brains, more capable of dealing with ecological challenges through learning and other behavioural coping mechanisms.
The relationship between brain size and CORT levels is unlikely to be a by-product of their common relationship with the fast–slow continuum of life-history variation. This is because while species with a large brain relative to body mass tend to be at the ‘slow’ extreme of the continuum [63], these ‘slow’ species also tend to have higher CORT levels than short-lived ‘fast’ species [15]. If life history was the cause linking CORT and brain size, then we would thus have found a positive relationship between them, but we have found just the opposite. In addition, in our analyses, we have controlled for several potential confounding variables that capture various aspects of the species’ life history, notably body mass. Previous work by Hau et al. [15] showed that both baseline and maximum CORT levels varied inversely with body mass, although, in our study, the effects of body mass were non-significant. Given that a large body mass is associated with a slow pace of life [64], its negative relationship with CORT levels or the absence of such effects are contradictory to what we would expect if big brains and high CORT were a common outcome of having a ‘slow’ pace of life. In a similar vein, the absence of a negative relationship between CORT and body mass also renders the possibility unlikely that the negative CORT–brain association is simply driven by allometric effects in which selection acts on body mass rather than on brain size. Our results are thus consistent with the view that within long-lived species, only those with enlarged brains have been able to reduce CORT levels.
Admittedly, the negative effect of brain size on CORT levels was reduced in certain LHSs, notably during the reproductive and migration stages. The reduction during the reproductive stage may be explained by the opposing influence of brood value. Although brood value exhibited no consistent effect on CORT levels over all four LHSs (this study), it has been found important during the reproductive stage, because species with limited future breeding opportunities (i.e. with high brood value) cannot afford high CORT levels that impair reproduction [14]. A high brood value is often associated with a fast pace of life, as is small brain size [63]. Therefore, although species with small brains usually rely on the general neuroendocrine stress response, according to our results, evolution should favour the mitigation of stress response in these species when they are caring for their offspring. Contrarily, species with large brains may be less dependent on the hormonal stress response for coping with challenges, whereas they need not dampen it specifically for the breeding season as their own survival tends to be more valuable for them than current reproduction.
It remains less clear, however, why the negative relationship between brain size and baseline CORT breaks down in the migratory stage. Migrating birds often circulate highly elevated baseline CORT levels, perhaps as result of the high energetic demands of migration [13], and it is possible that large-brained species are just as reliant on this physiological ‘tuning’ as are small-brained species. Furthermore, CORT levels during the spring and autumn migrations can differ [13], which we did not consider in our analyses owing to small sample sizes. Finally, the lack of correlation between brain size and baseline CORT in the migratory stage could simply reflect the reduced variation in residual brain size observed in migratory species [49]. Clearly, more detailed studies are required on this matter.
Our findings contribute to the debate over the importance of corticosterone in mediating life-history trade-offs through their actions on behavioural processes [15]. Compared with short-lived species, species that prioritize future over present reproduction by extending their lifespan tend to have lower CORT levels during the reproductive stage [14], perhaps reflecting their lower urgency to breed or a longer season suitable for breeding [15]. These species also tend to have higher CORT levels outside the breeding season that facilitates the ‘emergency LHS’ that enhances adult survival [15]. However, animals with a longer life are also more likely to be exposed to stressful situations during their life, and hence the fitness costs of the hormonal responses to stress may be higher in the long term. As our analyses suggest, the large brain that has evolved in some long-lived species may provide a compensatory mechanism for avoiding or anticipating the stressors. This mechanism is based on information acquisition and behaviourally flexible responses, and hence may be applied to a variety of stressors including those individuals have never encountered before. The possibility that this is a general compensatory mechanism of vertebrates is intriguing and we anticipate that investigating this question may be an important avenue for future research.
Acknowledgements
We thank Frances Bonier and Creagh Breuner for useful comments and discussions at an early stage of the project. We also thank Laura Schoenle Thomas, anonymous referees and an associate editor who provided useful comments and constructive criticism to earlier versions of this manuscript.
Funding statement
Á.Z.L. was supported by the Hungarian Research Fund (OTKA PD76862), a Bolyai Fellowship from the Hungarian Academy of Sciences, and during the preparation of the manuscript by the National Science Fund (IOS-1145625) and an Eötvös State Grant from the Hungarian Scholarship Board (MÖB); V.B. by the Hungarian Research Fund (OTKA K84132) and an Eötvös State grant from the Hungarian Scholarship Board (MÖB); D.S. is supported by research funds from the Spanish Government (CGL2010-1838 and ‘Montes. CSD2008-00040); and F.A. is supported by the seventh research programme of the European Community FP7/2007–2013 (Marie–Curie Fellowship, no. 237034).
References
- 1.Hoffmann AA, Hercus MJ. 2000. Environmental stress as an evolutionary force. BioScience 50, 217–226 (doi:10.1641/0006-3568(2000)050[0217:ESAAEF]2.3.CO;2) [Google Scholar]
- 2.Selye H. 1973. The evolution of the stress concept. Am. Sci. 61, 692–699 [PubMed] [Google Scholar]
- 3.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends. Ecol. Evol. 19, 249–255 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 4.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone-behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 (doi:10.1093/icb/38.1.191) [Google Scholar]
- 5.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 6.Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. 2006. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J. Evol. Biol. 19, 343–352 (doi:10.1111/j.1420-9101.2005.01034.x) [DOI] [PubMed] [Google Scholar]
- 7.Miller DA, Vleck CM, Otis DL. 2009. Individual variation in baseline and stress-induced corticosterone and prolactin levels predicts parental effort by nesting mourning doves. Horm. Behav. 56, 457–464 (doi:10.1016/j.yhbeh.2009.08.001) [DOI] [PubMed] [Google Scholar]
- 8.Wingfield JC, Sapolsky R. 2003. Reproduction and resistance to stress: when and how? J. Neuroendocrinol. 15, 711–724 (doi:10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 9.Breuner CW, Patterson SH, Hahn TP. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 10.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 11.Wingfield JC. 2013. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct. Ecol. 27, 37–44 (doi:10.1111/1365-2435.12039) [Google Scholar]
- 12.Boonstra R. 2013. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct. Ecol. 27, 11–23 (doi:10.1111/1365-2435.12008) [Google Scholar]
- 13.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 14.Bókony V, Lendvai ÁZ, Liker A, Angelier F, Wingfield JC, Chastel O. 2009. Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598 (doi:10.1086/597610) [DOI] [PubMed] [Google Scholar]
- 15.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212 (doi:10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelier F, Ballentine B, Holberton RL, Marra PP, Greenberg R. 2011. What drives variation in the corticosterone stress response between subspecies? A common garden experiment of swamp sparrows (Melospiza georgiana). J. Evol. Biol. 24, 1274–1283 (doi:10.1111/j.1420-9101.2011.02260.x) [DOI] [PubMed] [Google Scholar]
- 17.Weiss JM. 1970. Somatic effects of predictable and unpredictable shock. Psychosom. Med. 32, 397–408 [DOI] [PubMed] [Google Scholar]
- 18.Badyaev AV. 2005. Role of stress in evolution: from individual adaptability to evolutionary adaptation. In Variation: a central concept in biology (eds Hallgrímsson B, Hall BK.), pp. 277–302 Burlington, MA: Academic Press [Google Scholar]
- 19.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Syst. 35, 347–374 (doi:10.1146/annurev.ecolsys.35.112202.130152) [Google Scholar]
- 20.Phillips BL, Suarez AV. 2012. The role of behavioural variation in the invasion of new areas. In Behavioural responses to a changing world: mechanisms and consequences, pp. 190–200 Oxford, UK: Oxford University Press [Google Scholar]
- 21.Sol D, Lapiedra O, Gonzalez-Lagos G. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112 (doi:10.1016/j.anbehav.2013.01.023) [Google Scholar]
- 22.Creel S, Winnie JA, Christianson D. 2009. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proc. Natl. Acad. Sci. USA 106, 12 388–12 393 (doi:10.1073/pnas.0902235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapolsky RM. 2004. Why zebras don't get ulcers, 3rd edn New York, NY: Holt Paperbacks. [Google Scholar]
- 24.Allman J, McLaughlin T, Hakeem A. 1993. Brain weight and life-span in primate species. Proc. Natl Aacd. Sci. USA 90, 118–122 (doi:10.1073/pnas.90.1.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deaner RO, Barton RA, Svan Schaik CP. 2003. Primate brains and life histories: renewing the connection. In Primate life histories and socioecology (eds Kappeler PM, Pereira ME.), pp. 233–265 Chicago, IL: University of Chicago Press. [Google Scholar]
- 26.Sol D. 2009. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130–133 (doi:10.1098/rspb.2005.3250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre L, Sol D. 2008. Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 72, 135–144 (doi:10.1159/000151473) [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre L. 2011. Taxonomic counts of cognition in the wild. Biol. Lett. 7, 631–633 (doi:10.1098/rsbl.2010.0556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. 1997. Feeding innovations and forebrain size in birds. Anim. Behav. 53, 549–560 (doi:10.1006/anbe.1996.0330) [Google Scholar]
- 30.Lefebvre L, Nicolakakis N, Boire D. 2002. Tools and brains in birds. Behaviour 139, 939–973 (doi:10.2307/4535964) [Google Scholar]
- 31.Webster SJ, Lefebvre L. 2001. Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim. Behav. 62, 23–32 (doi:10.1006/anbe.2000.1725) [Google Scholar]
- 32.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010 (doi:10.1016/j.anbehav.2009.06.033) [Google Scholar]
- 33.Mehlhorn J, Hunt GR, Gray RD, Rehkämper G, Güntürkün O. 2010. Tool-making new Caledonian crows have large associative brain areas. Brain Behav. Evol. 75, 63–70 (doi:10.1159/000295151) [DOI] [PubMed] [Google Scholar]
- 34.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465 (doi:10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shultz S, Bradbury RB, Evans KL, Gregory RD, Blackburn TM. 2005. Brain size and resource specialization predict long-term population trends in British birds. Proc. R. Soc. B 272, 2305–2311 (doi:10.1098/rspb.2005.3250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maklakov AA, Immler S, Gonzalez-Voyer A, Rönn J, Kolm N. 2011. Brains and the city: big-brained passerine birds succeed in urban environments. Biol. Lett. 7, 730–732 (doi:10.1098/rsbl.2011.0341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–2220808728 [Google Scholar]
- 38.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508 (doi:10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 39.Cornwallis CK, West SA, Davis KE, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 40.Horváthová T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–170 (doi:10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero LM, Reed JM. 2005. Collecting baseline corticosterone samples in the field: is under 3min good enough? Comp. Biochem. Physiol. A 140, 73–79 (doi:10.1016/j.cbpb.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 42.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149 (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 43.Healy SD, Rowe C. 2007. A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464 (doi:10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mlíkovský J. 1989. Brain size in birds: 1. Tinamiformes through Ciconiiformes. Vést. cs. Spolec Zool. 53, 200–213 [Google Scholar]
- 45.Garamszegi LZ, Møller AP, Erritzøe J. 2002. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. Lond. B 269, 961–967 (doi:10.1098/rspb.2002.1967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mlíkovský J. 1990. Brain size in birds: 4. Passeriformes. Acta Soc. Zool. Bohemoslov 54, 27–30 [Google Scholar]
- 47.Iwaniuk AN. 2003. The evolution of brain size and structure in birds. PhD Thesis, Monash University, Clayton, Australia [Google Scholar]
- 48.Iwaniuk AN, Nelson JE. 2002. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 80, 16–23 (doi:10.1139/z01-204) [Google Scholar]
- 49.Sol D, Garcia N, Iwaniuk A, Davis K, Meade A, Boyle WA, Székely T. 2010. Evolutionary divergence in brain size between migratory and resident birds. PLoS ONE 5, e9617 (doi:10.1371/journal.pone.0009617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, 1st edn New York, NY: Springer. [Google Scholar]
- 51.Breuner CW. 2011. Stress and reproduction in birds. In Hormones and reproduction of vertebrates (eds Norris DO, Lopez KH.), pp. 129–151 London, UK: Academic Press [Google Scholar]
- 52.Silverin BB, Arvidsson B, Wingfield JC. 1997. The adrenocortical responses to stress in breeding willow warblers Phylloscopus trochilus in Sweden: effects of latitude and gender. Funct. Ecol. 11, 376–384 (doi:10.1046/j.1365-2435.1997.00097.x) [Google Scholar]
- 53.Breuner CW, Orchinik M, Hahn TP, Meddle SL, Moore IT, Owen-Ashley NT, Sperry TS, Wingfield JC. 2003. Differential mechanisms for regulation of the stress response across latitudinal gradients. Am. J. Physiol. 285, R594–R600 (doi:10.1152/ajpregu.00748.2002) [DOI] [PubMed] [Google Scholar]
- 54.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 55.Davis KE. 2008. Reweaving the tapestry: a supertree of birds. PhD Thesis, University of Glasgow, Glasgow, UK: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maddison WP, Maddison DR.2006. Mesquite: a modular system for evolutionary analysis, version 1.12. See http://mesquiteproject.org .
- 57.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26 (doi:10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 58.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 59.Malisch JL, Breuner CW. 2010. Steroid-binding proteins and free steroids in birds. Mol. Cell Endocrinol. 316, 42–52 (doi:10.1016/j.mce.2009.09.019) [DOI] [PubMed] [Google Scholar]
- 60.Desantis LM, Delehanty B, Weir JT, Boonstra R. 2013. Mediating free glucocorticoid levels in the blood of vertebrates: are corticosteroid-binding proteins always necessary? Funct. Ecol. 27, 107–119 (doi:10.1111/1365-2435.12038) [Google Scholar]
- 61.Breuner C, Orchinik M. 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175, 99 (doi:10.1677/joe.0.1750099) [DOI] [PubMed] [Google Scholar]
- 62.Williams TD. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the golden mean’. Phil. Trans. R. Soc. B 363, 1687–1698 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sol D, Székely T, Liker A, Lefebvre L. 2007. Big-brained birds survive better in nature. Proc. R. Soc. B 274, 763–769 (doi:10.1098/rspb.2006.3765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sibly RM, Brown JH. 2007. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl Acad. Sci. USA 104, 17 707–17 712 (doi:10.1073/pnas.0707725104) [DOI] [PMC free article] [PubMed] [Google Scholar]