Abstract
The evolution of plants exhibiting different sexes, or dioecy, is correlated with a number of ecological and life-history traits such as woody growth form and animal-dispersed seeds, but the underlying causes of these associations are unclear. Previous work in seed plants has suggested that the evolution of fleshy cones or seeds may favour dioecy. In this study, we use a well-sampled molecular phylogeny of conifers to show that although dioecy and fleshiness strongly co-occur at the species level, this relationship has not resulted from numerous separate origins of this trait combination or from differential rates of diversification. Instead, we suggest that two character combinations—the ancestral dry-monoecious condition and the derived fleshy-dioecious condition—have persisted in conifers longer than other combinations over evolutionary time. The persistence of these trait combinations appears to reflect differences in the rate of successful transition into and out of these character states over time, as well as the geographical restriction of species with rare combinations and their consequent vulnerability to extinction. In general, we argue that such persistence explanations should be considered alongside ‘key innovation’ hypotheses in explaining the phylogenetic distribution of traits.
Keywords: seed dispersal biology, dioecy, gymnosperm, cone
1. Introduction
Seed plants exhibit a wide array of breeding systems, ranging from species with individual plants that produce both seeds and pollen to those that produce only one or the other [1,2]. The causes and consequences of this diversity have been the subject of a large body of research, particularly in regards to the evolution of solely unisexual individuals, a condition known as dioecy in plants [3–5]. Although originally viewed simply as a mechanism to prevent inbreeding, it has long been appreciated that dioecy is correlated with a variety of ecological or life-history traits, such as tropical distribution, woody growth habit and abiotic pollination [6–9]. Coupled with the relative rarity of dioecy (around 6% of flowering plant species [8]), these associations suggest that the repeated evolution of separate seed- and pollen-bearing plants (more than 100 times within flowering plants [10]) may be a response to specific environmental or ecological circumstances. Analyses of these correlations, however, have been confounded by the difficulty of establishing the phylogenetic sequence of acquisition of the relevant traits [2,11].
Seed dispersal biology represents another important trait that is associated with breeding system [6,7,12], but whose interpretation has been controversial [11]. Givnish [7] noted a strong correlation at the species, genus and family levels between dioecy and the presence of fleshy or succulent tissues in the seeds or cones of non-flowering seed plants (‘gymnosperms’), which attract animal seed dispersers [13,14]. Givnish then developed a conceptual model of female and male fitness as a function of reproductive effort, illustrating how individuals devoting more resources to the production of fleshy cones or seeds would disproportionately gain in fitness by attracting more animals to disperse their seeds [7, also see 12]. Such circumstances could create an opportunity for unisexual individuals to invade ancestrally mixed populations. More recent modelling has also shown that successful dispersal of a high proportion of propagules through animal dispersal can favour the evolution of stable dioecious populations [4,15,16].
Donoghue [11], however, questioned the correlation that stimulated Givnish's initial evolutionary model. He suggested that the large number of fleshy, dioecious species might instead result from higher diversification rates in just a few clades with these traits, and thus their correlation may not reflect a direct ecological interaction between them. Although later studies have suggested that dioecy may be linked with diversification, the relationship is not generally straightforward. For example, dioecious clades are less diverse overall than flowering plants with other breeding systems [17], but those that possess multiple traits traditionally correlated with dioecy, particularly fleshy seeds or fruits, appear to be more diverse than their sister clades [18].
Recent advances in phylogenetics, especially in terms of building and analysing large trees, allow us to explore these interactions within a much more detailed phylogenetic context. In this study, we use a large, well-sampled molecular phylogeny of conifers [19] to test the correlation between dioecy and fleshy seeds or cones. Conifers have several potential advantages for this type of study. They are an old and diverse group, with approximately 630 extant species and a fossil record stretching back 300 million years [20], but many aspects of their biology are less variable than those of flowering plants, making correlations easier to interpret. For example, conifers are all wind-pollinated trees or shrubs, and their reproductive organs are borne on separate pollen-producing or seed-producing cones [21]. Conifers do, however, vary in their breeding system from species where individuals produce both types of cone, a condition termed monoecy, to those that are dioecious, with individuals bearing only one type. In this relatively simple system, we show that the abundance of dioecious species with fleshy cones results from neither increased net diversification rates nor numerous independent origins of this combination of traits. Rather, our analyses favour an explanation based on the long-term persistence of both the fleshy-dioecious and dry-monoecious character state combinations.
2. Material and methods
This study uses our previously published time-calibrated conifer phylogeny that sampled 489 species, or approximately 80% of living conifer diversity [19]. Based on published literature, we scored each species in the dataset for two characters—seed/cone type (dry, fleshy) and breeding system (monoecy, dioecy)—or for a single combined character state (dry-monoecy, fleshy-monoecy, dry-dioecy and fleshy-dioecy). In this study, ‘dry’ refers to species whose seed cones are either woody or heavily sclerified at maturity (i.e. at the time of seed dispersal), whereas ‘fleshy’ propagules are succulent, pulpy or otherwise indehiscent at maturity, reflecting their role in biotic seed dispersal. Although we have been unable to sample all extant conifer species, the relative frequency of the various character states is similar to that of conifers as a whole (see electronic supplementary material for further details).
We first examined the minimum number of character state transitions for individual and combined character states inferred under parsimony. We next used maximum-likelihood methods to reconstruct character evolution, using two broad classes of models that differed in whether transitions between states occurred at equal rates or whether they were allowed to vary. Within these categories, we fit models where cone type and breeding system evolve independently and those where they evolve as combined character states. For the combined character states, we tested five separate models: one in which all transitions between states can occur, one in which all transitions occur except simultaneous shifts in two traits (e.g. dry-monoecy to fleshy-dioecy) and three different sequences in which the fleshy-dioecious state specifically evolves through a fleshy-monoecious intermediate state. These three sequences differed in the degree of irreversibility once a lineage transitioned away from the dry-monoecious character state (see electronic supplementary material).
We further used these likelihood models to quantify the amount of time spent in each character state through stochastic character mapping, a simulation-based method that samples character histories from a posterior distribution conditioned on the transition rate estimates from the best-fit model. We generated a sample of 1000 likely discrete character histories using phytools [22], and for each tree, we summed the time spent in each state along all tree edges. All other character reconstruction analyses were carried out using the phangorn [23] and corHMM [24] packages written for R [25].
The relative abundance of any particular character state could be the product of asymmetric transitions rates, as implied by the models discussed above. Differences in diversification, however, could equally influence the distribution of character states; for example, monoecious clades with dry seed cones might be common because they have higher diversification rates. Furthermore, both these processes could work together to generate observed character frequencies. We therefore used the binary state speciation and extinction model (BiSSE; [26]), a method that obtains joint estimates of speciation, extinction and transition rates for the two binary characters (dry versus fleshy; monoecy versus dioecy). We then used MuSSE, the multistate implementation of BiSSE [27], to analyse the combined character states. A large, well-sampled tree is ideal for these methods, because they can lack sufficient statistical power in trees containing fewer than several hundred species [28,29].
MuSSE analyses used the same set of five combined character models as in the likelihood analyses described above, but in this case, each combined character state has associated speciation and extinction rates. We analysed BiSSE and MuSSE models in a Bayesian framework, using Markov chain Monte Carlo methods to obtain posterior distributions for the parameters estimated under each model. In all cases, the Markov chain was run for 10 000 steps, with the first 2500, steps removed as burn-in, resulting in 7500 samples comprising the marginal posterior distribution for each parameter in any given model. In order to select among the different models tested, we computed the deviance information criterion (DIC) for each model, which is a Bayesian analogue of Akaike's information criterion (AIC; see the electronic supplementary material). We focus here on net diversification rate (speciation rate–extinction rate), because studies have suggested that BiSSE and MuSSE models recover this parameter with greater confidence than specific speciation or extinction rates [30,31]. All analyses described above were carried out in the R package diversitree [27].
Finally, we tested for shifts in diversification rate independently of whether or not they were associated with character state changes, using the step-wise AIC framework proposed by Alfaro et al. [32] and implemented in the R package turbo-MEDUSA. This likelihood-based method measures the fit of shifts in net diversification rate, added in a stepwise manner, until the addition of new parameters exhausts the information contained within the tree.
3. Results
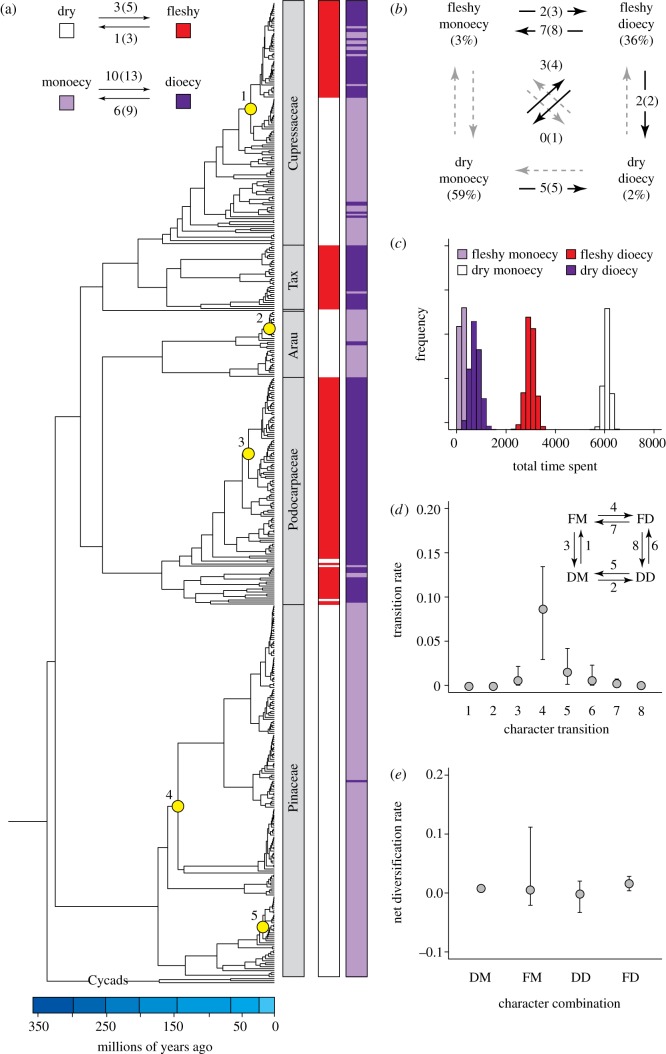
Parsimony reconstructions indicate that dry cones are plesiomorphic in conifers, with shifts to fleshy cones occurring between three and five times, and shifts back to dry cones occurring between one and three times (depending on how ambiguous nodes are optimized; figure 1a). Breeding system appears to be more labile, with transitions to dioecy occurring between 10 and 13 times, and shifts back to the ancestral monoecious condition occurring between six and nine times (figure 1a). Two combinations of individual character states—dry-monoecy and fleshy-dioecy—are found most frequently in extant species, although alternative combinations (fleshy-monoecy, dry-dioecy) occur sporadically (figure 1a). Similar patterns were recovered in the analyses of the combined character states, which infer transitions between most of the character states (figure 1b).
Figure 1.
(a) Phylogenetic distribution of cone type (dry, fleshy) and breeding system (monoecy, dioecy) in extant conifers. The number of transitions between individual cone and breeding system states are counts based on parsimony reconstructions, with the difference between the minimum and maximum number due to ambiguous nodes given in parentheses. Numbered yellow circles indicate nodes that are associated with an increase in diversification rate based on the MEDUSA analysis (1, Juniperus + Cupressus sensu lato clade; 2, New Caledonian Araucaria clade; 3, Podocarpus clade; 4, Pinus + Piceae + Cathaya clade; 5, clade of Abies not including A. bracteata and A. mariesii). (b) Minimum number of observed transitions among the combined character states as reconstructed using parsimony, with the maximum number provided in the parentheses. (c) Persistence of the four combined character states based on stochastic character mapping. Distributions for each state represent different persistence times calculated for 1000 separate character maps. Frequency is an arbitrary measure scaled to the median of each distribution. (d) Transition rates among combined character states based on the best-fit MuSSE model. A schematic of this model is shown in the upper right hand corner of the panel, with numbers identifying the various character state transitions (DM, dry-monoecy; FM, fleshy-monoecy; DD, dry-dioecy; FD, fleshy-dioecy). (e) Net diversification rate (speciation rate–extinction rate) for each combination of character states based on the best-fit MuSSE model. Error bars in (d,e) represent 95% credibility intervals calculated from the posterior distribution of parameter estimates (see Material and methods).
Maximum-likelihood methods reconstructed a somewhat different pattern of character evolution because the best-supported model was a combined character state model in which simultaneous trait shifts between states did not occur (see electronic supplemental materials, table S2). Intermediate character states were therefore intercalated between observed multistate transitions, such as fleshy-monoecy between dry-monoecy and fleshy-dioecy. Models with cone type and breeding system evolving independently were not strongly supported (see electronic supplemental material, table S2). Instead, the probability of evolving monoecy or dioecy appears to be dependent on whether cones are fleshy or dry [33]. Stochastic character maps based on the best-supported likelihood model suggest that conifers have spent much more total time in the dry-monoecious and fleshy-dioecious states than in the other states (figure 1c).
Transition rates between character states inferred from the best-fit likelihood model (see electronic supplemental material, table S3) were broadly consistent with those estimated from the best-fit MuSSE model. We show here only results from the latter, because this model also assesses diversification. Transition rates among the combined character states were generally low (figure 1d), but transitions away from the uncommon character states (3–6 in figure 1d) were higher on average than transitions away from the common states, particularly the shift from fleshy-monoecy to fleshy-dioecy. MuSSE results also suggest that net diversification rates do not differ significantly among the character combinations (figure 1e), although precise values for fleshy-monoecy and dry-dioecy are difficult to estimate due to their rarity. It is worth noting, however, that a MuSSE model in which net diversification rate was constrained to be the same for all of the combined character states received almost as much support as the best-fit model (see electronic supplemental material, table S5). BiSSE results likewise suggested that neither cone type nor breeding system on their own were associated with higher net diversification rates (see electronic supplementary material, table S4).
4. Discussion
Two potential explanations for the prevalence of dioecious species with fleshy propagules in conifers are that this combination of traits either arose many times independently or that these traits individually or jointly increase net diversification. Our results suggest that the distribution of character states in the conifers does not result from either of these processes. Transitions among character states occur relatively infrequently, whether reconstructed using parsimony or maximum likelihood, and the distribution of traits suggests that dioecy has evolved less than 10 times within fleshy lineages (figure 1a,b). In terms of diversification, neither dioecy nor fleshiness is associated with higher net diversification rates, and the fleshy-dioecious combination likewise does not show elevated net diversification (figure 1a,e). It is also noteworthy that dioecy in conifers is not associated with lower species richness or with significantly lower net diversification rates, in contrast to results from some studies of angiosperms [17,34].
An alternative explanation for the prevalence of fleshy-dioecious species, as well as of monoecious species with dry cones, is that these character combinations are simply the most stable reproductive strategies and they therefore appear in the largest number of conifer species. This interpretation is consistent with previous modelling studies suggesting that monoecy is an effective reproductive strategy for sessile organisms such as plants [4,7], but that efficient long-distance dispersal of seeds can favour dioecy as it overcomes the deleterious ‘seed-shadow effect’ in which offspring establish beneath parent plants [15,16,34]. In the model presented by Givnish for gymnosperms, selection should specifically operate against a mutant monoecious plant that appears within a fleshy-dioecious population as well as against strictly male or female mutants in a dry-monoecious population [7] (T. Givnish 2013, personal communication). Overall, these models suggest that the prevalence of the dry-monoecious and fleshy-dioecious states in conifers reflects the general advantage of these two reproductive strategies, whereas other combinations are either selected against or rapidly transition to alternative states.
Our results support some of the major predictions of these models. For example, we infer that species accumulate over time in the dry-monoecious and fleshy-dioecious character states, because transition rates away from them are low (figure 1c,d). Additionally, the elevated transition rate between the fleshy-monoecious and fleshy-dioecious states is consistent with fleshiness rapidly promoting the evolution of dioecy, as predicted by the Givnish model [7, see also 13]. Other aspects of conifer reproductive biology also suggest that the fleshy-dioecious combination may represent a stable strategy. For instance, the major fleshy-dioecious lineages (Juniperus, Podocarpaceae and Taxaceae) have highly reduced cones that often contain only a single seed, which is consistent with theoretical predictions on the allocation of resources that would maintain stable dioecy [15].
In other ways, however, our results conflict with expectations based on previous models. Character combinations such as fleshy-monoecy and dry-dioecy, which are not expected to be successful strategies, have arisen repeatedly in conifers. In fact, the minimum number of times that these combinations have originated is roughly similar to that of the more common character states. In particular, we note that fleshy-monoecy has evolved from the fleshy-dioecious condition at least seven times, and dry-dioecy has evolved from the dry-monoecious condition at least five times (figure 1b). These results suggest that all combinations of traits can and do evolve under certain circumstances, but that only dry-monoecy and fleshy-dioecy tend to persist. Although selection operating within populations may contribute to the stability and widespread distribution of the common character states, it appears that this mechanism alone does not provide an adequate explanation for the origin and distribution of all the various trait combinations in conifers, including the appearance of dioecy in many clades.
Differential extinction among clades may represent an additional factor shaping the observed distribution of character states, both in terms of their actual occurrence and in terms of our ability to reconstruct their evolution. Simulation studies have shown that extinction can decrease the statistical power of BiSSE or MuSSE models; and in particular, high extinction in clades with uncommon characters can also inflate transition rates to more common states [28]. Although direct fossil evidence of breeding system is extremely rare in the conifers, extinction does not appear to be specifically associated with uncommon reproductive strategies. In the Northern Hemisphere, for example, a number of extant genera underwent significant range contraction, and possibly species extinction, during the Neogene (e.g. Cathaya, Pseudolarix, Taiwania [35,36]), but these groups appear to have been monoecious with dry cones. The current distribution of the dry-dioecy trait combination, on the other hand, is more likely to have been directly influenced by extinction. This combination has nearly always evolved in the Southern Hemisphere (at least six separate times in three unrelated lineages), particularly clades that live in temperate environments [37–39]. Regardless of the specific factors that may have favoured the origin of this trait combination, the limited areal extent of these environments, coupled with the significant contraction during the Pleistocene of wet temperate forests where most of these clades live [40,41], has likely contributed to the rarity of the dry-dioecy combination in extant conifers. As the extant dry-dioecious species are diverse in reproductive biology, producing wind, water and occasionally animal-dispersed seeds [42], their rarity is unlikely to reflect extinction related to the possession of specific reproductive traits.
Much more work is required on the specific ecological conditions that select for the various breeding systems and cone types in conifers. In this regard, the large and actively diversifying clade Juniperus may be a promising study system because it exhibits a spectrum of breeding systems from pure monoecy to pure dioecy, even though dioecious species with fleshy cones broadly characterize the clade [43]. We stress, however, that population-level evolutionary explanations need to be combined with a consideration of macroevolutionary dynamics such as the biogeography-vulnerability hypothesis outlined above. Moreover, we need to expand the set of possible mechanisms beyond simple differences in diversification rate to also consider evolutionary persistence in time and space as a legitimate explanation for phylogenetic patterns. Although such persistence explanations are somewhat subtle, and lack the immediate appeal of ‘key innovation’ hypotheses, they may nevertheless be crucial in accounting for the phylogenetic distribution of many biological features of interest.
Acknowledgements
We thank Tim Brodribb, Aljos Farjon, Patrick Knopf and Robert Mill for information on breeding systems in conifers. We thank Brian O'Meara for methodological advice, and we are especially grateful to Tom Givnish for his comments on an earlier draft, which both clarified his outlook and improved our interpretations. Two anonymous reviewers provided helpful suggestions.
References
- 1.Barrett SCH. 2002. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–283 (doi:10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- 2.Barrett SCH. 2010. Understanding plant reproductive diversity. Phil. Trans. R. Soc. B 365, 99–109 (doi:10.1098/rstb.2009.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geber MA, Dawson TE, Delph FL. (eds) 1999. Gender and sexual dimorphism in plants. New York, NY: Springer [Google Scholar]
- 4.Wilson WG, Harder LD. 2003. Reproductive uncertainty and the relative competitiveness of simultaneous hermaphroditism versus dioecy. Am. Nat. 162, 220–241 (doi:10.1086/376584) [DOI] [PubMed] [Google Scholar]
- 5.Ashman T-L. 2006. The evolution of separate sexes: a focus on the ecological context. In Ecology and evolution of flowers (eds Harder LD, Barrett SCH.), pp. 204–222 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Bawa KS. 1980. Evolution of dioecy in flowering plants. Annu. Rev. Ecol. Syst. 11, 15–39 (doi:10.1146/annurev.es.11.110180.000311) [Google Scholar]
- 7.Givnish TJ. 1980. Ecological constraints on the evolution of breeding systems in seed plants: dioecy and dispersal in gymnosperms. Evolution 34, 959–972 (doi:10.2307/2408001) [DOI] [PubMed] [Google Scholar]
- 8.Renner SS, Ricklefts RE. 1995. Dioecy and its correlates in the flowering plants. Am. J. Bot. 82, 596–606 (doi:10.2307/2445418) [Google Scholar]
- 9.Vamosi JC, Otto SP, Barrett SCH. 2003. Phylogenetic analysis of the ecological correlates of dioecy in angiosperms. J. Evol. Biol. 16, 1006–1018 (doi:10.1046/j.1420-9101.2003.00559.x) [DOI] [PubMed] [Google Scholar]
- 10.Charlesworth D. 2002. Plant sex determination and sex chromosomes. Heredity 88, 94–101 (doi:10.1038/sj.hdy.6800016) [DOI] [PubMed] [Google Scholar]
- 11.Donoghue MJ. 1989. Phylogenies and the analysis of evolutionary sequences, with examples from seed plants. Evolution 43, 1137–1156 (doi:10.2307/2409353) [DOI] [PubMed] [Google Scholar]
- 12.Bawa KS. 1982. Outcrossing and the incidence of dioecism in island floras. Am. Nat. 119, 866–871 (doi:10.1086/283960) [Google Scholar]
- 13.Geldenhuys CJ. 1993. Reproductive biology and population structures of Podocarpus falcatus and P. latifolius in southern Cape forests. Bot. J. Linn. Soc. 112, 59–74 (doi:10.1111/j.1095-8339.1993.tb00308.x) [Google Scholar]
- 14.Willson MF, Sabag C, Figueroa J, Armesto JJ. 1996. Frugivory and seed dispersal of Podocarpus nubigena in Chiloe, Chile. Rev. Chil. Hist. Nat. 69, 343–349 [Google Scholar]
- 15.Vamosi JC, Zhang Y, Wilson WG. 2007. Animal dispersal dynamics promoting dioecy over hermaphroditism. Am. Nat. 170, 485–491 (doi:10.1086/519856) [DOI] [PubMed] [Google Scholar]
- 16.Barot S, Gignoux J. 2004. How do sessile dioecious species cope with their males? Theor. Popul. Biol. 66, 163–173 (doi:10.1016/j.tpb.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 17.Heilbuth JC. 2000. Lower species richness in dioecious clades. Am. Nat. 156, 221–241 (doi:10.1086/303389) [DOI] [PubMed] [Google Scholar]
- 18.Vamosi JC, Vamosi SM. 2004. The role of diversification in causing the correlates of dioecy. Evolution 58, 723–731 (doi:10.1111/j.0014-3820.2004.tb00405.x) [DOI] [PubMed] [Google Scholar]
- 19.Leslie AB, Beaulieu JM, Rai HS, Crane PR, Donoghue MJ, Mathews S. 2012. Hemisphere-scale differences in conifer evolutionary dynamics. Proc. Natl Acad. Sci. USA 109, 16 217–16 221 (doi:10.1073/pnas.1213621109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor TN, Taylor EL, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, 2nd edn Burlington, MA: Academic Press [Google Scholar]
- 21.Owens JN, Takaso T, Runions CJ. 1998. Pollination in conifers. Trends Plant Sci. 3, 479–485 (doi:10.1016/S1360-1385(98)01337-5) [Google Scholar]
- 22.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (doi:10.1111/j.2041-210X.2011.00169.x) [Google Scholar]
- 23.Schliep KP. 2010. Phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 (doi:10.1093/bioinformatics/btq706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaulieu JM, O'Meara BC, Donoghue MJ. 2013. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst. Biol. 62, 725–737 (doi:10.1093/sysbio/syt034) [DOI] [PubMed] [Google Scholar]
- 25.R Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 26.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710 (doi:10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
- 27.Fitzjohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 6, 1084–1092 (doi:10.1111/j.2041-210X.2012.00234.x) [Google Scholar]
- 28.Davis MP, Midford PE, Maddison W. 2013. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evol. Biol. 13, 38 (doi:10.1186/1471-2148-13-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzjohn RG. 2010. Quantitative traits and diversification. Syst. Biol. 59, 619–633 (doi:10.1093/sysbio/syq053) [DOI] [PubMed] [Google Scholar]
- 30.Rabosky DL. 2010. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824 (doi:10.1111/j.1558-5646.2009.00926.x) [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu JM, Donoghue MJ. 2013. Fruit evolution and diversification in Campanulid angiosperms. Evolution. (doi:10.1111/evo12180) [DOI] [PubMed] [Google Scholar]
- 32.Alfaro ME, Santini F, Brock C, Alamillo H, Dornburg A, Rabosky DL, Carnevale G, Harmon LJ. 2009. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414 (doi:10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 (doi:10.1098/rspb.1994.0006) [Google Scholar]
- 34.Heilbuth JC, Ilves KL, Otto SP. 2001. The consequences of dioecy for seed dispersal: modeling the seed-shadow handicap. Evolution 55, 880–888 (doi:10.1111/j.0014-3820.2001.tb00605.x) [DOI] [PubMed] [Google Scholar]
- 35.LePage BA, Basinger JF. 1995. Evolutionary history of the genus Pseudolarix Gordon (Pinaceae). Int. J. Plant Sci. 156, 910–950 (doi:10.1086/297313) [Google Scholar]
- 36.Chou Y-W, Thomas PI, Ge X-J, LePage BA, Wang C-N. 2011. Refugia and phylogeography of Taiwania in East Asia. J. Biogeogr. 38, 1992–2005 (doi:10.1111/j.1365-2699.2011.02537.x) [Google Scholar]
- 37.Shapcott A, Brown MJ, Kirkpatrick JB, Reid JB. 1995. Stand structure, reproductive activity and sex expression in Huon Pine (Lagarostrobus franklinii (Hook L.) Quinn). J. Biogeogr. 22, 1035–1045 (doi:10.2307/2845833) [Google Scholar]
- 38.Grosfeld J, Barthélémy D. 2001. Dioecy in Fitzroya cupressoides (Molina) IM. Johnst. and Pilgerodendron uviferum (D. Don) Florin (Cupressaceae). C. R. Acad. Sci. Paris 324, 245–250 (doi:10.1016/S0764-4469(00)01289-0) [DOI] [PubMed] [Google Scholar]
- 39.Sanguinetti J, Kitzberger T. 2008. Patterns and mechanisms of masting in the large-seeded southern hemisphere conifer Araucaria araucana. Austral. Ecol. 33, 78–87 (doi:10.1111/j.1442-9993.2007.01792.x) [Google Scholar]
- 40.Kershaw AP, Martin HA, McEwen Mason JRC. 1994. The Neogene: a period of transition. In History of the Australian vegetation (ed. Hill RS.), pp. 299–327 Cambridge, UK: Cambridge University Press [Google Scholar]
- 41.Kale Sniderman JM. 2011. Early Pleistocene vegetation change in upland south-eastern Australia. J. Biogeogr. 38, 1456–1470 (doi:10.1111/j.1365-2699.2011.02518.x) [Google Scholar]
- 42.Enright NJ, Hill RS. 1995. Ecology of the southern conifers. Washington DC: Smithsonian Institution Press [Google Scholar]
- 43.Farjon A. 2005. A monograph of Cupressaceae and Sciadopitys. Richmond, UK: Royal Botanic Gardens, Kew [Google Scholar]