Abstract
Selfish genes demonstrate transmission bias and invade sexual populations despite conferring no benefit to their hosts. While the molecular genetics and evolutionary dynamics of selfish genes are reasonably well characterized, their effects on hosts are not. Homing endonuclease genes (HEGs) are one well-studied family of selfish genes that are assumed to be benign. However, we show that carrying HEGs is costly for Saccharomyces cerevisiae, demonstrating that these genetic elements are not necessarily benign but maybe parasitic. We estimate a selective load of approximately 1–2% in ‘natural’ niches. The second aspect we examine is the ability of HEGs to affect hosts' sexual behaviour. As all selfish genes critically rely on sex for spread, then any selfish gene correlated with increased host sexuality will enjoy a transmission advantage. While classic parasites are known to manipulate host behaviour, we are not aware of any evidence showing a selfish gene is capable of affecting host promiscuity. The data presented here show a selfish element may increase the propensity of its eukaryote host to undergo sex and along with increased rates of non-Mendelian inheritance, this may counterbalance mitotic selective load and promote spread. Demonstration that selfish genes are correlated with increased promiscuity in eukaryotes connects with ideas suggesting that selfish genes promoted the evolution of sex initially.
Keywords: VDE, Saccharomyces cerevisiae, homing endonuclease gene, selfish gene, fitness
1. Introduction
Evolutionary conflicts often occur when natural selection operates in contrary directions at different levels of biological organization. For example, ‘selfish’ non-Mendelian genes bias their transmission through meiosis, become over-represented in the next generation [1,2], and can invade populations without conferring any fitness advantage to the individuals carrying them [1,3,4]. Homing endonuclease genes (HEGs) are a family of selfish genes that are phylogenetically widespread, have been well molecularly studied, and even harnessed to engineer populations [1,5–7]. HEGs achieve a transmission advantage over the rest of the genome by inducing a biased gene-conversion event known as ‘homing’ and are thus inherited by a disproportionate number of offspring. HEGs may very quickly spread in populations and this has been shown in experiments with microbes and insects [4,7]. One factor that will temper HEG spread is the breeding system of the host species [1,8]. As has been shown experimentally, HEGs (and all selfish genes) rely on outcrossed sex for spread [4], as here HEG heterozygotes are formed and thus homing may occur. One model suggests that HEGs may persist in the long term through periodic oscillations with pseudo-genes [9]. Another model suggests that longer term purifying selection for HEG function is maintained via recurrent horizontal transfer to, and subsequent invasion of, new species, which it appears that at least some HEGs are adapted for [10,11].
There are a number of fundamental evolutionary aspects of selfish genes that have been explored little. Here, we use VDE (also known as PI-SceI), which is a well-studied HEG that resides in the essential nuclear VMA1 gene [12] of a range of yeast species [11,13], to examine some of these aspects. VDE splices at the protein level, homes during meiosis [5,13,14] and has been shown to display super-Mendelian inheritance of between 75 and 90%, rather than the Mendelian 50%. VDE has been shown to invade sexually outcrossed populations [4,13] and is well adapted to recurrently invade different species [11]. VDE is found in Saccharomyces cerevisiae, among other species, and here we examine the effects VDE has on this host.
(a). The mitotic fitness effects of homing endonuclease genes
Owing to their small size and self-splicing properties, HEGs are thought to not confer a replication burden or affect the functions of host genes they are usually inserted within and are thus thought to be benign [1]. However, theoretically even detrimental HEGs may spread owing to their super-Mendelian ‘driving’ actions. Thus, there is a fitness landscape across which HEGs might exist, from commensal (benign) to parasitic. The limit on the extent of tolerable HEG parasitic load will be a function of the rate of non-Mendelian inheritance and host outcrossing rate (see box 6.1 in [1], which assumes HEG neutrality). While selection will primarily operate on the propensity to drive, in theory selection will also operate to decrease HEGs’ effects on host fitness if this cost is not correlated with the ability to drive. The only previous study to evaluate an HEG's fitness effect examined VDE in S. cerevisiae in artificial laboratory media and indirectly estimated it at less than 1% [4]. Fitness is patently environmentally dependent, and estimating VDE's fitness effect using only benign artificial media may not provide an accurate evaluation of the consequences of harbouring VDE in nature. Here, we directly evaluate VDE's effect on its host in ecologically relevant niches by measuring growth rates (r) and carrying capacities (K) among populations.
(b). Homing endonuclease genes potential to increase sexual promiscuity
It is well documented that certain parasites have evolved mechanisms to manipulate their hosts in ways that enhance their probabilities of transmission, and Leucochloridium spp. trematodes are a classic example with their manipulation of snail behaviour [15]. Might selfish genetic elements similarly modify hosts? All non-Mendelian elements critically rely on sex for spread. In obligate sexual species, selfish genes are assured of frequent exposure to meiosis, and thus transmission bias. However, in facultative sexual organisms the frequency of sex, and thus opportunities for spread, are uncertain. In theory, one expects selection to enhance a selfish gene's ability to increase their host's propensity to undergo sex, because this enhances transmission. Models analysing selfish elements show that the probability of population invasion is increased if an element elevates the level of sexuality of its bearers [16]. While this makes intuitive sense, we are not aware of any experimental studies examining the potential for selfish genes, HEG or otherwise, to directly influence their host's propensity to engage in sex. In yeasts, this could manifest by a manipulation of the shift from mitotic growth to sporulation, the process that comprises meiosis with the production of sexual haploid spores. The idea of selfish elements affecting sexuality links with broader theories proposing that sex initially evolved because of selfish-elements’ actions, which manipulated hosts to promote the fusion of independent genomes to ensure spread [17]. This idea is an attractive one, not least as there is a precedent for such actions in bacteria with plasmids that may promote conjugation [18].
Here, we construct diploid S. cerevisiae populations that are free of VDE (−/−), heterozygous for VDE (−/+) and homozygous for VDE (+/+) but are genetically identical at all other loci and initiated experimental populations.
2. Material and methods
(a). Experimental organisms
The four haploid strains described in Goddard et al. [4], DH89 (MATα ho ura3 VDE−), DH90 (MATa ho ura3 VDE−), DH91 (MATα ho ura3 VDE+) and DH95 (MATa ho ura3 VDE+) were mated appropriately to generate VDE-homozygotes (VDE+/+), VDE-heterozygotes (VDE+/−) and VDE-free homozygotes (VDE−/−). The construction of these three diploid strains means that they are isogenic at all other loci except VDE. Diploidy and VDE status were confirmed via PCR.
(b). Experimental media
Sporulation media (SM) comprised 1% potassium acetate (w/v), 0.1% yeast extract (w/v) and 0.05% glucose (w/v) with 5 mg l−1 uracil, and routine growth was conducted in artificial laboratory YPD media (10 g l−1 yeast extract, 20 g l−1 peptone and 20 g l−1 glucose). We measured fitness in four niches from which S. cerevisiae has been consistently isolated, and thus comprise at least some of its common niches of residence [19–22]. Vitis vinifera var Sauvignon blanc grape juice (pH 3.06) deriving from Marlborough, New Zealand was sterilized with 500 µl l−1 of dimethyl dicarbonate (DMDC). Vineyard soil from Mate's Vineyard in west Auckland was air-dried at 70°C for 10 days. Pouches of soil were soaked for 36 h to produce a 50 g l−1 soil solution (pH 4.63) and this was filter sterilized at 0.20 µm. Vitis vinifera var Chardonnay vine bark samples were collected from Mate's Vineyard, and Quercus robur (oak) bark samples were collected from the University of Auckland's city campus, and air-dried at 60°C for 4 days. A 50 g l−1 bark solutions (pH 5.81 and 4.41 for vine and oak, respectively) were filter sterilized at 0.20 µm. As the strains are uracil auxotrophs, 20 mg l−1 of uracil was added to each medium prior to experimentation.
(c). Fitness measurements
Seven replicates of VDE−/−, VDE +/− and VDE+/+ genotypes were inoculated into 50 ml of each experimental niche, except YPD, which was conducted in triplicate. One replicate of the VDE +/− for vine bark and one replicate of the VDE−/− for oak bark were lost leaving n = 6 for these genotypes in these niches, and thus 91 observations in total. Flasks (250 ml) were continuously shaken and the optical density (OD) at 600 nm was recorded every hour in triplicate and the mean was taken, with necessary dilutions if the OD > 1, until at least three stationary phase points were recorded. Carrying capacity (K) was determined as the maximum OD value, and exponential growth rate per hour (r) was taken as ln(ODf–ODi)/t, where ODi and ODf are the initial and final ODs across a period of t hours of exponential phase population expansion, which was determined empirically by evaluating the best linear fit of ln transformed data using a sliding window approach for each replicate. The difference in rates of exponential increase per hour, is the difference in relative Malthusian fitness (m) and the corresponding percent Darwinian fitness (w), was calculated as w = 100[exp(m)−1]. One-way and two-way ANOVAs were conducted in JMPv.10 (SAS) and R [23].
(d). Sporulation assay
Six populations of VDE+/+, VDE+/− and VDE−/− were propagated in YPD until densities reached approximately 1 × 107 cells ml−1, after which cells were harvested, washed with distilled water and re-suspended in 30 ml SM in 200 ml flasks. Populations were allowed to sporulate for 30 days. The percentage of sporulated cells from all replicates was measured daily in triplicate by visualization via light microscopy and counting 300 individuals and classifying them as sporulated or unsporulated (ambiguously sporulated cells were not counted). The sporulation proportion data were arcsine transformed and fit with the cubic smoothing spline algorithm in the grofit package [24] within R, and the maximum sporulation rate (slope) of each spline fit was determined with gcFitModel in the same package. We also conducted breakpoint regression analyses with the ‘segmented’ package in R [25]. ANCOVAs were conducted in R. We drew a distinction between sporulation rate, which we define as the maximum increase in the fraction sporulated over time and sporulation efficiency, which we define as the final proportion of the population sporulated. These are analogous to r and K. Lastly, the total sporulation data were evaluated and the area under each genotype's sporulation curve was measured using the trapz function of the R package ‘pracma’.
(e). VDE invasion model
We model a mitotic phase where VDE+/+ and VDE+/− genotypes have m- and mh-decreased Malthusian population expansion factors respectively, compared with VDE−/− and where the nature of dominance is determined by h. The resulting relative proportions of genotypes then undergo sporulation (meiosis), with the VDE−/− and VDE+/− genotypes doing so with decreased probability c and cb, respectively, where b models the nature of dominance. If x = VDE+/+, y = VDE+/− and z = VDE−/−, then the relative genotype frequencies after time hours of mitotic selection followed by c altered rates of sporulation (promiscuity) are
Substitution of x′, y′ and z′ here for x, y and z into Equations 3.1 to 3.3 described in Goddard et al. [4] allows one to calculate resulting genotype frequencies also accounting for HEG super-Mendelian inheritance during meiosis (d), and the proportion of spores that germinate and mate with a spore from a different tetrad (outcross, t) or mate with a spore from the same tetrad (inbreed). The change in VDE frequency accounting for all the above parameters may be calculated using the equations found in the electronic supplementary material and their recursion allow VDE dynamics to be evaluated across a number of mitotic : meiotic cycles (generations). A Mathematica file implementing these equations and their recursion is available as the electronic supplementary material.
3. Results
(a). VDE affects host fitness
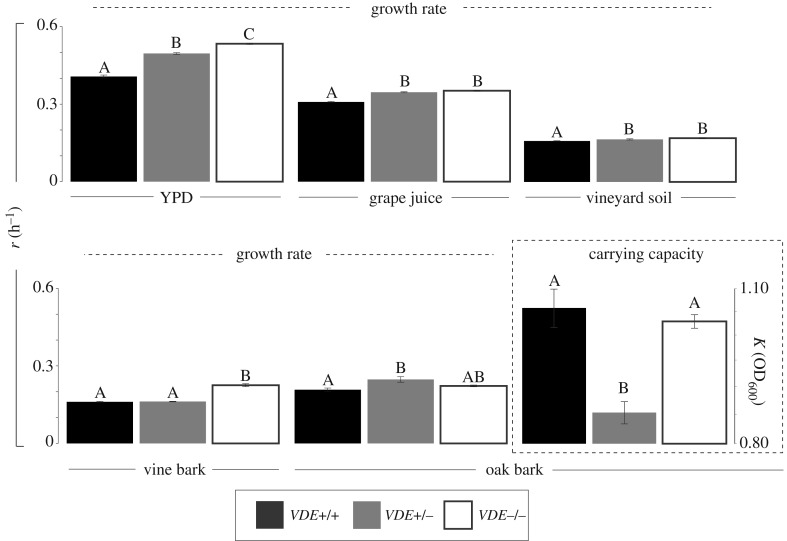
We evaluated the effects of carrying VDE in these niches by conducting a two-way ANOVA to determine whether VDE affects r overall, and if so whether VDE status differentially affects r between niches. The ANOVA reveals that VDE status significantly affects r generally (main effect F2,76 = 151.75, p < 0.0001), but the extent of fitness differences between genotypes with differing VDE status is contingent on niche (interaction F8,76 = 27.92, p < 0.0001). These niches differ greatly in their concentrations of major and minor nutrients, and unsurprisingly, niche significantly affects growth rate (main effect F4,76 = 1737.06, p < 0.0001).
We conducted one-way ANOVAs to determine the nature of the interaction between niche and VDE status, and these revealed a significant effect of VDE status on rates of exponential increase in each niche independently (YPD (recall n = 3), F2,6 = 247.02, p < 0.0001; grape juice, F2,18 = 133.88, p < 0.0001; vineyard soil, F2,18 = 11.08, p = 0.0007; vine bark (one replicate lost), F2,17 = 99.31, p < 0.0001; and oak bark (one replicate lost), F2,17 = 7.00, p = 0.0061). Tukey's HSD post hoc comparisons revealed populations homozygous for VDE (+/+) had among the lowest growth rates across all niches (p < 0.01; figure 1). In four of the five niches, VDE-free populations (−/−) had among the greatest growth rates. Thus, VDE+/− populations had intermediate fitness in four niches, and overall the empirical degree of dominance (h) of VDE's mitotic fitness load in these niches equals 0.46. Together this suggests that VDE's effect on fitness is largely dose dependent, i.e. that its effect on fitness is additive or semidominant. VDE displayed a different fitness effect in oak bark, where heterozygotes had the greatest fitness. Compared with VDE-free populations, the mean Malthusian and corresponding Darwinian fitness difference for individuals carrying either one or two copies of VDE in each niche is shown in table 1. This shows the greatest negative effect of VDE on fitness is in YPD (the only artificial niche) and the least effect in oak bark (note the Tukey's HSD test shows VDE+/− and −/− not to differ at p < 0.01). Among the ‘natural niches’ examined, VDE confers a fitness load of 1.5% (m = 0.015) on average.
Figure 1.
The mean (±s.e., n = 6–7 for natural niches and n = 3 for YPD) growth rate (r) and carrying capacities (K) of VDE+/+, VDE+/− and VDE−/− populations in various niches. The exponential growth rate per hour for all populations in all niches is shown. Only the carrying capacity in oak bark is displayed, as carrying capacity in all other niches did not significantly differ between genotypes. ANOVAs revealed significant differential effects of VDE status between niches (F8,76 = 27.924,p < 10–16), and letters above the bars show within-niche tests for differences between populations with differing VDE status using a post hoc Tukey's HSD test at p < 0.01. Letters connecting populations with differing VDE status in the same niche do not significantly differ.
Table 1.
The difference in mean Malthusian (m) and Darwinian (w) fitness (both per hour) of populations homozygous (VDE+/+) and heterozygous (VDE+/−) compared with populations that were free of VDE (−/−). The mean fitness effect of VDE was calculated simply by the sum of fitness for +/+ and +/− divided by three (there are three copies of VDE). An asterisk indicates a significant difference in fitness from the VDE−/− genotype in each niche as revealed by Tukey's HSD test.
| niche |
VDE+/+ |
VDE+/− |
mean fitness per copy of VDE |
|||
|---|---|---|---|---|---|---|
| m | w (%) | M | w (%) | m | w (%) | |
| YPD | −0.1269 | −13.53* | −0.0376 | −3.83* | −0.05483 | −5.64 |
| grape juice | −0.0435 | −4.45* | −0.0058 | −0.58 | −0.01643 | −1.66 |
| soil | −0.0110 | −1.10* | −0.0047 | −0.47 | −0.00522 | −0.52 |
| vine bark | −0.0643 | −6.64* | −0.0631 | −6.51* | −0.04245 | −4.34 |
| oak bark | −0.0148 | −1.49 | +0.0250 | +2.47 | +0.0034 | +0.34 |
| grand mean | −0.0521 | −5.44 | −0.0172 | −0.0179 | −0.02310 | −2.34 |
| ±s.e. | ±0.0189 | ±0.0202 | ±0.0135 | ±0.0138 | ±0.009 | ±1.02 |
*p < 0.05.
The second parameter we measured was carrying capacity (K). A two-way ANOVA again reveals a significant effect of niche (F4,76 = 4724.51, p < 0.0001), but there is no effect of VDE status generally (F2,76 = 2.83, p = 0.0654) and only a very weak interaction between niche and VDE status (F8,76 = 2.12, p = 0.0441). One-way ANOVAs examining the effect of VDE status within each niche independently revealed oak bark was the only niche which displayed a differential effect of VDE status on carrying capacity (ANOVA F2,17 = 15.11, p = 0.0002; figure 1). Here, VDE-heterozygotes had a significantly lower carrying capacity than both VDE+/+ and VDE−/− (Tukey's HSD p < 0.0016).
(b). VDE increases sporulation rates
Figure 2 shows that there was a marked escalation in the percentage of sporulated cells in the VDE+/+ populations compared with the VDE+/− and VDE−/− from about the fifth day. An ANCOVA of sporulation rates, calculated using the spline method, revealed rates differed significantly across the three genotypes (F2,15 = 10.24, p = 0.0016). Tukey's HSD post hoc comparisons indicate that the sporulation rate of populations carrying two copies of VDE were significantly greater than populations carrying just one or no copies (VDE+/+ = 3.33±0.24 versus VDE+/− = 2.22±0.12, p = 0.0061 and VDE−/− = 2.08 ± 0.21, p = 0.0024). However, sporulation rates did not significantly differ between VDE+/− and VDE−/− populations (p = 0.8899), but we note a weak trend for VDE+/− to be intermediate. We also analysed sporulation rates with a segmented regression analysis, using day 10 as a breakpoint as revealed by breakpoint analyses. Again, an ANCOVA revealed significant differences across genotypes (F2,15 = 19.78, p = 0.0006) and Tukey's post hoc comparisons confirmed that VDE+/+ had a significantly greater sporulation rate than that of both VDE+/− (p = 0.0004) and VDE−/− (p = 0.0001) populations, but the rates of VDE+/− and VDE−/− did not significantly differ (p = 0.754). Lastly, the total data were evaluated, and a one-way ANOVA examining the area under each genotype's sporulation curve showed a significant effect of VDE status on sporulation (F2,15 = 10.31, p = 0.0015). A Tukey's HSD post hoc analysis again revealed that VDE+/+ had an overall sporulation behaviour that significantly differed from that of VDE+/− (p = 0.006) and VDE−/− (p = 0.0024), and the latter two genotypes show no significant difference (p = 0.911). We found no significant difference in overall sporulation efficiencies among all three genotypes when we analysed the sporulation percentages on day 30 at the end of the experiment (ANOVA F2,15 = 0.23, p = 0.797). On average, VDE+/+ genotypes sporulated faster than VDE+/− and VDE−/− genotypes and showed a 38% increased likelihood of successfully completing meiosis after 14 days.
Figure 2.
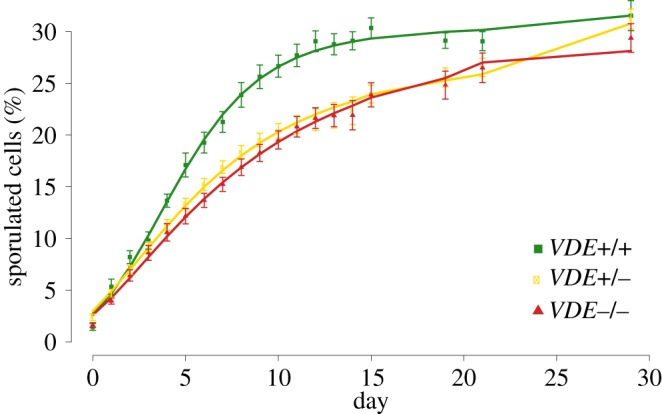
The change in percentage sporulated cells over 30 days for populations with differential VDE status. Each point represents the mean (±s.e., n = 7), and the line is the best fit for each using the spline method with cubic smoothing. (Online version in colour.)
(c). Modelling VDE dynamics
The above data reveal VDE may affect host fitness in both mitotic and meiotic phases of yeast's life cycle. The proportion of time spent in mitosis will clearly affect VDE's spread because selection against VDE occurs here. VDE's mitotic costs may be countered by its ability to affect non-Mendelian inheritance during meiosis: the extent of over-representation in the next generation ameliorates the actions of selection in the current generation. Here, we define generation as one period of mitotic division followed by meiosis. Along with inbreeding and the skewed speed with which different genotypes successfully complete meiosis, the nature of dominance for both VDE's selective load and increased promiscuity will also modulate VDE dynamics to a degree. Thus, together there are at least seven interdependent parameters that will determine if and how rapidly VDE may spread: the extent of non-Mendelian inheritance/meiotic drive (d); mitotic cost (m) and the nature of dominance (h); increased promiscuity (c) and the nature of dominance (b); the extent to which the population is outcrossed (t); and the ratio of mitosis : meiosis (time).
Many differential combinations of these parameter values may result in equilibrium/loss/spread of VDE. Thus, to make progress into analysing the effect of certain parameters on VDE dynamics, we used empirically derived estimates for parameters where available, including those revealed here. Unless otherwise stated we used average literature-derived values for VDE's rate of drive (d = 0.81) [4,13], and the average rate of outcrossing in S. cerevisiae populations (t = 0.18) [22,26,27]. We use the Malthusian fitness cost, and its dominance, empirically determined here from comparisons of fitness in ‘natural niches’ (m = 0.015 and h = 0.46). Using the equations supplied in the electronic supplementary material, we analysed the ability of a VDE allele to invade a yeast population by introducing a VDE+/+ migrant into VDE−/− population at a frequency of 0.1%.
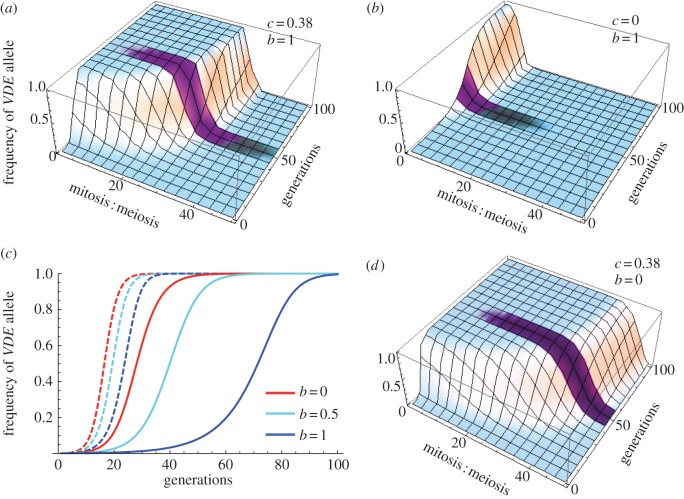
The first question we addressed is whether the effect on promiscuity we observed may speed VDE's invasion as hypothesized. Time in mitosis will also critically affect VDE's spread and interplay with any effect of increased promiscuity, and so we simultaneously assessed the effect of increasing promiscuity and the mitotic : meiotic ratio. Comparing figure 3a and b shows the increased rate of invasion that the observed increased promiscuity confers to VDE. It is clear that VDE may invade more rapidly and across a larger mitosis : meiosis ratio when the promiscuity of VDE-homozygotes is increased. In the absence of altered rates of promiscuity, invasion is not possible above mitotic : meiotic ratios of approximately 10 : 1. However, the observed rate of increased promiscuity (c = 0.38) allows invasion to occur up to a ratio of approximately 40 : 1. Examining a parameter space where a lack of promiscuity does not prevent VDE invasion (mitosis : meiosis of 10 : 1), then the observed elevated rates of promiscuity in VDE-homozygotes allow VDE to invade populations 20 times more rapidly (in 30 compared to 600 generations when VDE has no effect on promiscuity). This analysis supports verbal arguments and suggests that selection will operate on VDE variants that effect greater host promiscuity. That increased promiscuity was observed only in VDE-homozygotes is puzzling: conceptually selection should operate to increase promiscuity in VDE-heterozygotes as well. If the trait is modelled as dominant, then at the 10 : 1 mitosis : meiosis ratio used above, VDE may spread in just 25 generations, as opposed to 30 when recessive. The difference in invasion times between the altered promiscuity trait being dominant and recessive is 10-fold less than the difference in invasion times between the presence and absence of the altered promiscuity trait (approx. 600 generations). This suggests selection may relatively weakly act on the nature of dominance compared with the altered promiscuity trait itself, at least under these parameter values. To further evaluate the effect of dominance, we took cross sections spanning the parameter space where only increased promiscuity allows invasion (figure 3a,b). Across this space, figure 3c clearly shows that VDE spreads most rapidly when the increased promiscuity trait is dominant (b = 0), and conversion of the increased promiscuity trait to dominance generally extends the mitotic : meiotic parameter space over which VDE invades, but this is to a relatively limited extent (figure 3d). Under conditions that result in protracted invasion times (greater and dominant VDE mitotic costs, lower levels of non-Mendelian inheritance and greater levels of inbreeding) then the dominance of the increased promiscuity trait will have a relatively greater effect on invasion times.
Figure 3.
Modelled dynamics of VDE invasion from rare across 100 ‘mitotic–meiotic generations’, with empirically determined rates of drive (d = 0.81), mitotic selective load and levels of dominance (m = 0.015 and h = 0.46), and inbreeding (t = 0.18). (a) Where the observed increase in promiscuity (c = 0.38) is effected for VDE+/+ only (it is recessive, b = 1), across a ratio of time in mitotic : meiotic phases from 1 : 1 to 50 : 1. (b) Where there is no effect of VDE on host promiscuity (c = 0) across a ratio of time in mitotic : meiotic phases from 1 : 1 to 50 : 1. VDE may invade more rapidly and across a larger mitosis : meiosis ratio when increased promiscuity. (c) The effect of dominance of the increased promiscuity trait on VDE spread across parameter space where only increased promiscuity allows VDE to invade: mitotic : meiotic of 15 : 1 (dashed lines) and 30 : 1 (solid lines). Dominance of the promiscuity trait increases speed of VDE spread. (d) Where the observed increase in promiscuity (c = 0.38) is effected for VDE+/+ and VDE+/− equally (it is dominant, b = 0), across a ratio of time in mitotic : meiotic phases from 1 : 1 to 50 : 1. (Online version in colour.)
VDE's capacity to invade populations will also be affected by the host population's outcrossing rate (t), VDE's rate of drive (d) and mitotic selection coefficient (m). If the mitotic : meiotic ratio is set to 30 : 1 and all other parameters are as empirically determined, it would take 100 generations for VDE to invade (to go from a 0.1 to 99.9% frequency). In a completely outcrossed population (t = 1), this time is reduced to just 30 generations. A benign VDE (m = 0) takes 20 generations to invade an outcrossed population and this extends to only 25 generations in an inbred population (t = 0.18). A striking effect of increased promiscuity is shown through the interaction with the extent of inbreeding. Our model only allows for a limited time in the meiotic phase and the differing rate of sporulation we observe translates into an over-representation of VDE+/+ at the end of this limited time. If VDE has no effect on promiscuity, then it may not invade a completely inbred population regardless of the other parameter values. Complete inbreeding will prevent invasion because heterozygotes are never formed, and thus drive cannot occur. However, effecting over-representation by increased rates of sex means that VDE may invade a completely inbred population: this occurs not only because of its ability to drive, but also because of over-representation of VDE+/+ genotypes. If time in meiosis is not a limiting factor, then this effect will not be realized. Any degree of outcrossing in a population will mean over-representation by both meiotic drive and increased promiscuity, and these will act in concert to promote VDE invasion as increased promiscuity will also increase the proportions of heterozygotes. This is pertinent to populations that are naturally highly inbred, such as certain plants and yeasts.
4. Discussion
These experiments were conducted with genotypes that only differed in VDE copy number, and thus the differences in exponential growth and sporulation rates between populations seen here can be attributed to VDE. The fitness cost for each copy of the selfish 2 µm plasmid has previously been estimated at 0.17% for S. cerevisiae in artificial laboratory media [28]. However, we are not aware of any previous direct estimates for the fitness effects of a selfish gene in natural niches. In addition, as far as we are aware, this is the first report to show that a selfish gene may affect their carrier's promiscuity. Overall HEGs affect both mitotic and meiotic phases of S. cerevisiae's life cycle and this shows that selfish genes may have more subtle and far-reaching effects than previously appreciated.
During mitosis, these data show that on average VDE has a negative effect on the fitness of the organism within which it resides, and thus HEGs may be classed as parasitic. Thus, the assumption that HEGs are generally benign is not a valid one. The extent of this parasitic load appears both copy number and niche dependent. While there are exceptions, in general the greater the copy number the greater the load, and the load is close to semidominant. On average, each copy of VDE appears to suppress growth rate by approximately an additional 1.5% but this ranges from 4% to neutrality among the natural niches we examined. Given the rates of inbreeding displayed in natural S. cerevisiae populations, the parasitic load estimated for VDE delays the time for successful population invasion by about one quarter compared with a benign form when mitotic : meiotic ratios are approximately 30 : 1.
These data show that VDE fully meets the requirements to be labelled a ‘selfish gene’ as these genetic elements are parasitic but persist. The reason VDE confers a parasitic load is unknown. This might possibly be an economic burden, but this is unlikely as VDE is only 1 kb of the 13×103 kb in the yeast genome. During vegetative growth, VDE's endonuclease is expressed but homing is halted by its exclusion from the nucleus via host-encoded karyopherins [29,30]. A possibility for mitotic load is owing to unspecific chromosome breaks made by VDE leaking into the nucleus and the repair burden this might confer to the cell. Lastly, in the longer term selective loads for HEGs may mean the more rapid degeneration and loss of HEGs once fixed, which may play a part in accelerating the speed of the evolutionary cycle that HEGs are thought to follow [10].
The extent to which VDE's selective load is realized will critically depend upon the time the host population spends in mitosis. Analyses here, using the empirically determined values for inbreeding and rates of drive, suggest that transmission advantage during meiosis cannot counterbalance the selective load accumulated in mitosis with ratios greater than about 30 h of mitotic growth to one meiotic event. This seems a low ratio. Given the rates of population expansion determined here in the fruit niche, 30 h of exponential growth at 28°C would allow about 20 mitotic generations. We know little, if nothing, of the ratio of mitosis to meiosis in natural S. cerevisiae populations. An estimate for the ratio of outcrossed meiotic event to mitotic divisions of 50 000 : 1 has been advanced based on inferences from DNA sequences of just three strains [31]. Previous work estimates about one fifth of meiotic events are outcrossed for S. cerevisiae [22,26,27], which results in an extremely tentative mitotic : meiotic ratio estimate of approximately 10000 : 1. This is around 1000-fold less than the mitotic : meiotic ratios across which we infer that VDE may spread.
These data show VDE increases the sporulation speed of homozygotes by around 40% but has no effect on sporulation efficiencies. Under certain conditions, this increased promiscuity allows VDE to invade populations 20 times time more rapidly compared with when there is no effect on promiscuity. Even if VDE-homozygotes only have increased rates of sex, when VDE is rare this relatively increases the proportion of VDE alleles, and in the fraction of the population that outcrosses these thus go on to form a relatively greater proportion of heterozygotes. Increased heterozygote formation allows increased opportunities for homing, and thus population invasion. While it has been shown for classic parasites, as far as we are aware, this is the first demonstration of the presence of a selfish element correlating with an increased promiscuity trait in the host. The mechanism behind this altered rate of sex is unclear. The sporulation pathway is complex [32] and any aspect could potentially be affected by VDE. The difference in rates of sporulation seen here are greatest in the early stages of meiosis, which is where the sporulation timing variability between cells seems to lie [32]. While there is a trend for VDE-heterozygotes to have greater sporulation rates than VDE-free populations (figure 2), this difference is not significant. One question of interest is why sporulation rates are not manipulated in VDE-heterozygotes? It is clearly a recessive trait, and modelling suggests that selection may be acting relatively weakly on the nature of dominance of this trait. The extra chiasmata event that VDE homing represents during meiosis adds just one extra recombination event to the approximately 260 that occur ordinarily during meiosis [33] and is thus unlikely to confer a significant burden. In general, the mechanisms behind VDE's altered promiscuity (and load) are unknown but it is clear that many genes have pleiotropic effects [34]. Parameters other than the rate of sporulation might be affected by VDE, and it would be of interest to evaluate VDE's effect on tetrad disruption, spore germination and subsequent mating efficiencies as these parameters are also involved in VDE spread.
The strain of S. cerevisiae used here derived from a ‘wild-type’ background (Y55) that has not been extensively cultured in the laboratory. However, the extent to which these findings translate to other S. cerevisiae genetic backgrounds and species remains to be tested. In addition, the emulations of natural niches used here will likely little reflect the range of nutrients available across various grape juices, barks and soils, but the purpose of this experiment was to evaluate the relative effect of VDE in a range of niches and not the effect of these niches on fitness per se.
It is clear how VDE benefits from enhanced sporulation rates but this altered rate of sporulation may also affect the carrier cell's fitness. Yeasts generally sporulate to more resistant structures when unfavourable conditions are encountered, and spores survive better than vegetative cells in the gut of Drosophila, which is a known vector [35]. The data reported here suggest a VDE carrier would enjoy increased survival under these conditions. Finally, while we now have a good handle on the reasons that sex is maintained [36–39], we have less evidence for why sex evolved [18]. Selfish elements may spread through sex, and some ideas suggest that selfish elements drove the initial evolution of sex [17]. Indeed, Maynard Smith & Szathmary [18] suggest a prerequisite for the evolution of modern day meiosis required a ‘promiscuous’ population. To date, the precedent for this idea was drawn from the prokaryotic kingdom with the effects of plasmids on conjugation [18]. These data provide the first precedent for this idea from the eukaryotic kingdom, which is where sex appears to have evolved.
Acknowledgements
Juice was kindly donated by Constellation NZ, we thank M. Brajkovich M.W. for access to soil and bark from Kumeu River vineyards. We thank R. Aggio for help with data analysis. We thank anonymous reviewers for comments on an earlier manuscript.
References
- 1.Burt A, Trivers R. 2006. Genes in conflict. Cambridge, MA: Belknap, Harvard [Google Scholar]
- 2.Werren JH. 2011. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc. Natl Acad. Sci. USA 108, 10 863–10 870 (doi:10.1073/pnas.1102343108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurst GD, Werren JH. 2001. The role of selfish genetic elements in eukaryotic evolution. Nat. Rev. Genet. 2, 597–606 (doi:10.1038/35084545) [DOI] [PubMed] [Google Scholar]
- 4.Goddard MR, Greig D, Burt A. 2001. Outcrossed sex allows a selfish gene to invade yeast populations. Proc. R. Soc. Lond. B 268, 2537–2542 (doi:10.1098/rspb.2001.1830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoddard BL. 2005. Homing endonuclease structure and function. Q. Rev. Biophys. 38, 49–95 (doi:10.1017/S0033583505004063) [DOI] [PubMed] [Google Scholar]
- 6.Burt A, Koufopanou V. 2004. Homing endonuclease genes: the rise and fall and rise again of a selfish element. Curr. Opin. Genet. Dev. 14, 609–615 (doi:10.1016/j.gde.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 7.Windbichler N, et al. 2011. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (doi:10.1038/nature09937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burt A, Trivers R. 1998. Selfish DNA and breeding system in flowering plants. Proc. R. Soc. Lond. B 265, 141–146 (doi:10.1098/rspb.1998.0275) [Google Scholar]
- 9.Yahara K, Fukuyo M, Sasaki A, Kobayashi I. 2009. Evolutionary maintenance of selfish homing endonuclease genes in the absence of horizontal transfer. Proc. Natl Acad. Sci. USA 106, 18 861–18 866 (doi:10.1073/pnas.0908404106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goddard MR, Burt A. 1999. Recurrent invasion and extinction of a selfish gene. Proc. Natl Acad. Sci. USA 96, 13 880–13 885 (doi:10.1073/pnas.96.24.13880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koufopanou V, Goddard MR, Burt A. 2002. Adaptation for horizontal transfer in a homing endonuclease. Mol. Biol. Evol. 19, 239–246 (doi:10.1093/oxfordjournals.molbev.a004077) [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Gimble FS, Quiocho FA. 1997. Crystal structure of PI-SceI, a homing endonuclease with protein splicing activity. Cell 89, 555–564 (doi:10.1016/S0092-8674(00)80237-8) [DOI] [PubMed] [Google Scholar]
- 13.Gimble FS, Thorner J. 1992. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature 357, 301–306 (doi:10.1038/357301a0) [DOI] [PubMed] [Google Scholar]
- 14.Chevalier BS, Stoddard BL. 2001. Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res. 29, 3757–3774 (doi:10.1093/nar/29.18.3757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Study Behav. 41, 151–186 (doi:10.1016/S0065-3454(10)41005-0) [Google Scholar]
- 16.Johnson LJ, Brookfield JFY. 2002. Evolutionary dynamics of a selfishly spreading gene that stimulates sexual reproduction in a partially sexual population. J. Evol. Biol. 15, 42–48 (doi:10.1046/j.1420-9101.2002.00376.x) [Google Scholar]
- 17.Hickey DA, Rose MR. 1988. The role of gene transfer in the evolution of sex. In The evolution of sex: an examination of current ideas (eds Michod RE, Levin BR.), pp. 161–193 Sunderland, MA: Sinauer [Google Scholar]
- 18.Maynard Smith JM, Szathmary E. 1997. The major transitions in evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 19.Sniegowski PD, Dombrowski PD, Fingerman E. 2002. Saccharomyces cerevisiae and Saccharomyces paradoxus coexist in a natural woodland site in North America and display diverent levels of reproductive isolation from European conspecics. FEMS Yeast Res. 1, 163–167 [DOI] [PubMed] [Google Scholar]
- 20.Aa E, Townsend JP, Adams RI, Nielsen KM, Taylor JW. 2006. Population structure and gene evolution in Saccharomyces cerevisiae. FEMS Yeast Res. 6, 702–715 (doi:10.1111/j.1567-1364.2006.00059.x) [DOI] [PubMed] [Google Scholar]
- 21.Liti G, et al. 2009. Population genomics of domestic and wild yeasts. Nature 458, 337–341 (doi:10.1038/nature07743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goddard MR, Anfang N, Tang R, Gardner RC, Jun C. 2010. A distinct population of Saccharomyces cerevisiae in New Zealand: evidence for local dispersal by insects and human-aided global dispersal in oak barrels. Environ. Microbiol. 12, 63–73 (doi:10.1111/j.1462-2920.2009.02035.x) [DOI] [PubMed] [Google Scholar]
- 23.R Core Development Team 2011. R: a language and environment for statistical computing; Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 24.Kahm M, Hasenbrink G, Lichtenberg-Frate H, Ludwig J, Kschischo M. 2010. grofit: fitting biological growth curves with R. J. Stat. Softw. 33, 1–2120808728 [Google Scholar]
- 25.Muggeo VMR. 2003. Estimating regression models with unknown break-points. Stat. Med. 22, 3055–3071 (doi:10.1002/sim.1545) [DOI] [PubMed] [Google Scholar]
- 26.Cubillos FA, Vásquez C, Faugeron S, Ganga A, Martínez C. 2009. Self-fertilization is the main sexual reproduction mechanism in native wine yeast populations. FEMS Microbiol. Ecol. 67, 162–170 (doi:10.1111/j.1574-6941.2008.00600.x) [DOI] [PubMed] [Google Scholar]
- 27.Gayevskiy V, Goddard MR. 2012. Geographic delineations of yeast communities and populations associated with vines and wines in New Zealand. ISME J. 6, 1281–1290 (doi:10.1038/ismej.2011.195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison E, Koufopanou V, Burt A, MacLean RC. 2012. The cost of copy number in a selfish genetic element: the 2-μm plasmid of Saccharomyces cerevisiae. J. Evol. Biol. 25, 2348–2356 (doi:10.1111/j.1420-9101.2012.02610.x) [DOI] [PubMed] [Google Scholar]
- 29.Nagai Y, Nogami S, Kumagai-Sano F, Ohya Y. 2003. Karyopherin-mediated nuclear import of the homing endonuclease VMA1-derived endonuclease is required for self-propagation of the coding region. Mol. Cell Biol. 23, 1726–1736 (doi:10.1128/MCB.23.5.1726-1736.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda T, Nogami S, Ohya Y. 2003. VDE-initiated intein homing in Saccharomyces cerevisiae proceeds in a meiotic recombination-like manner. Genes Cells 8, 587–602 (doi:10.1046/j.1365-2443.2003.00659.x) [DOI] [PubMed] [Google Scholar]
- 31.Ruderfer DM, Pratt SC, Seidel HS, Kruglyak L. 2006. Population genomic analysis of outcrossing and recombination in yeast. Nat. Genet. 38, 1077–1081 (doi:10.1038/ng1859) [DOI] [PubMed] [Google Scholar]
- 32.Neiman AM. 2011. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189, 737–765 (doi:10.1534/genetics.111.127126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roeder GS. 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11, 2600–2621 (doi:10.1101/gad.11.20.2600) [DOI] [PubMed] [Google Scholar]
- 34.Gavin AC, et al. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (doi:10.1038/415141a) [DOI] [PubMed] [Google Scholar]
- 35.Reuter M, Bell G, Greig D. 2007. Increased outbreeding in yeast in response to dispersal by an insect vector. Curr. Biol. 17, R81–R83 (doi:10.1016/j.cub.2006.11.059) [DOI] [PubMed] [Google Scholar]
- 36.Goddard MR, Godfray HCJ, Burt A. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434, 636–640 (doi:10.1038/nature03405) [DOI] [PubMed] [Google Scholar]
- 37.Colegrave N. 2002. Sex releases the speed limit on evolution. Nature 420, 664–666 (doi:10.1038/nature01191) [DOI] [PubMed] [Google Scholar]
- 38.Gray JC, Goddard MR. 2012. Gene-flow between niches facilitates local adaptation in sexual populations. Ecol. Lett. 15, 955–962 (doi:10.1111/j.1461-0248.2012.01814.x) [DOI] [PubMed] [Google Scholar]
- 39.Morran LT, Schmidt OG, Gelarden IA, Parrish RC, Lively CM. 2011. Running with the Red Queen: host–parasite coevolution selects for biparental sex. Science 333, 216–218 (doi:10.1126/science.1206360) [DOI] [PMC free article] [PubMed] [Google Scholar]