Abstract
Objectives
Our aim was to determine the effect of metformin exposure on survival in patients with advanced pancreatic adenocarcinoma (PAC).
Methods
A retrospective cohort study was conducted using The Health Improvement Network (THIN), a primary care electronic medical record database from the United Kingdom (2003–2010). The study cohort included all subjects with a diagnostic code for incident PAC and a pre-existing diagnosis of type 2 diabetes mellitus (T2DM). Subjects were classified as exposed if they were prescribed metformin around the time of PAC diagnosis (between 6 months prior and 1 month after). A secondary analysis was performed only on exposed subjects without prior (i.e., 6 months before PAC diagnosis) exposure to metformin. The primary outcome was overall survival. The analysis was performed using univariate and multivariable Cox proportional-hazards models.
Results
The study included 516 subjects with pre-existing T2DM and PAC, 247 of which were exposed to metformin. In univariate and multivariable analysis, there was no difference in survival between those exposed and those unexposed to metformin in the primary analysis (HR = 1.11 [0.89, 1.38], p = 0.367) or the secondary analysis (HR = 1.09 [0.80, 1.47], p = 0.585).
Conclusions
Metformin use is not associated with improved survival in subjects with advanced PAC.
Keywords: pancreatic cancer, metformin, prognosis, pharmacoepidemiology, diabetes mellitus
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States1. Despite new chemotherapeutic agents, improved surgical technique, and growing experience in the last two decades, mortality has remained unchanged, and may even be climbing2. Currently, pancreatic adenocarcinoma (PAC) can only be cured through surgical resection for local disease3. However, local disease only occurs in 5–10% of patients at diagnosis3. Treatment for locally advanced and metastatic cancer involves chemotherapy and is palliative in nature.
There are two predominantly used initial chemotherapeutic regimens: 1) gemcitabine as monotherapy or in combination with other agents, namely capecitabine and 2) a combination chemotherapy known as FOLFIRINOX (5-fluorouracil, leucovorin, oxaliplatin, irinotecan). Both regimens have been shown to have modest mortality benefit ranging from two to six months 4–8 but have significant limitations. For example, FOLFIRINOX can only be used in those without cholestasis or decreased functional status8. Furthermore, treatment is associated with high morbidity 4,7,8, relatively expensive, and requires the placement of invasive indwelling intravenous access.
Metformin is a medication in the biguanide class used as an oral hypoglycemic agent in the treatment of type 2 diabetes mellitus (T2DM).9 The mechanism of action to lower blood glucose is not entirely understood, but metformin achieves its effects by decreasing hepatic glucose production and increasing insulin sensitivity. Therapy is cheap, safe, and well tolerated. Metformin has been used in non-diabetic conditions such as polycystic ovary syndrome and non-alcoholic steatohepatitis.9 In vitro studies suggest that metformin may inhibit the growth of PAC cells, through a mechanism related to its effects on lowering insulin and IGF-1 levels. These two hormones may stimulate cancer cell growth through their interaction with G protein-coupled receptors that promote mitogenic signaling.10–13 Metformin may also have direct inhibitory effects by activating the AMPK protein, a serine/threonine kinase usually activated in adenosine monophosphate rich states and hypoxia.14–16 AMPK in turn phosphorylates and inactivates proteins in the mTOR pathway, a regulatory pathway that inhibits cell proliferation, polarity, and division. 17–21
Recent studies have supported these findings on a population level, suggesting that metformin may reduce the incidence of PAC. Li et al published a hospital based case control study, which suggested that metformin use at any time was associated with a significantly lower odds of developing PAC compared to those never exposed.22 Currie et al reported in a retrospective cohort study of a United Kingdom (UK) primary care database, The Health Improvement Network (THIN), that amongst subjects with T2DM, those on metformin monotherapy carried the lowest hazards ratio for the development of all cancers, including PAC.23 A similar study performed in a Taiwanese cohort showed a significant hazards ratio of 0.15 for the development of PAC in metformin users compared to non-metformin users.24 To date, there has been only one study of metformin’s effects on survival in PAC by Sadeghi et al, which showed a survival benefit for metformin with an overall hazards ratio of 0.64, though that benefit was only significant in non-metastatic patients.25.
Our objective was to determine the effects of metformin exposure on survival in patients with advanced PAC. Based on all available epidemiologic and in vitro studies, we hypothesized that metformin exposure would be associated with a longer median survival after the diagnosis of PAC.
Materials and Methods
We conducted a retrospective cohort study to determine the effect of metformin exposure on survival in subjects with advanced pancreatic adenocarcinoma.
Data Source
This study was conducted in THIN, which is primary care electronic medical record database well-suited for pharmacoepidemiologic research.26,27 The database contains data on more than 7.5 million patients of acceptable research quality from 495 general practitioner offices in the UK. Patients included in THIN are similar in age, sex, and geographic characteristics to the general UK population.27 The data are collected from routine charting records from participating general practitioners through a software package named Vision. The records are then collected, deidentified, and aggregated at regular intervals. The database contains information on all past and current medical diagnoses, both acute and chronic (coded using British Read codes), and prescribed medications (coded using British National Formulary codes). Laboratory values, social habits, radiologic studies, and some physical examination elements are also electronically captured. The diagnoses of T2DM and PAC, as well as information on death in THIN have all been previously validated.28–30
Study Cohort
The inclusion criteria were a diagnosis of pancreatic adenocarcinoma with a prior diagnosis of T2DM. The exclusion criteria were a diagnosis of PAC other than adenocarcinoma, discontinuation of metformin prior to the diagnosis of PAC, renal failure, a history of pancreatic cancer resection, and age < 40 at the time of incident PAC diagnosis.
Due to the nature of the THIN database, subjects were only defined as having pancreatic adenocarcinoma if their first appropriate diagnostic code occurred after the latter of two dates 1) Vision date – the date that the primary care practice first instituted the Vision software package and 2) six months after registration date – the date that the patient was registered with a particular practice. Only diagnoses entered after Vision software implementation are likely to represent actively recorded medical diseases rather than the retroactive documentation of pre-existing conditions. The six month window after registration allows for the “purge” of diagnoses upon registration. Restricting diagnoses after these two dates allows for the identification of incident disease, rather than prevalent disease.31 Only subjects with adenocarcinoma of the pancreas were included for this study to avoid a mixed effect from differing cancer types and biologies.
The rationale for using subjects only with T2DM is that they are the population that is at risk for being exposed to metformin, i.e., those without T2DM would generally not be eligible to be prescribed metformin. Similarly, only those with a diagnosis of T2DM prior to their PAC diagnosis were included because those who developed T2DM after their diagnosis of PAC would not have been eligible for metformin at the time of PAC diagnosis. We included patients whose first recorded diagnosis of T2DM occurred up to one month after their PAC diagnosis to ensure that primary care physicians had been given the opportunity to enter the diagnosis of T2DM into the medical record system.
All subjects not at-risk for exposure to metformin were excluded. Specifically, those subjects in which metformin was prescribed prior to, but not after, the one month post-diagnosis cutoff time were excluded on the basis that they may not be eligible for metformin use at the time of PAC diagnosis due to intolerance, adverse reaction, allergy, etc. Renal failure is a contraindication for metformin use.9 Those with glomerular filtration rates (GFR) less than 30 ml/min were excluded. This was determined using the last creatinine prior to PAC diagnosis to calculate a glomerular filtration rate using the Modification of Diet in Renal Disease (MDRD) formula.32
A small fraction of patients with a history of PAC resection were excluded as these patients may have early stage disease. This was done to make the study population as homogenous as possible containing subjects only with higher stage disease, i.e., locally advanced or metastatic PAC. This was also done in an attempt to minimize potential lead time bias from incidentally discovered cancer. As exploratory analyses, we conducted secondary analyses where we 1) included those who underwent resection in the study cohort and 2) repeat the primary analysis after restricting the study cohort to those who underwent resection only.
Subjects less than the age of 40 were excluded to avoid including patients with genetic or family syndromes for PAC whose tumor biology may act independently or differently from those with sporadic PAC.
Exposure
Metformin exposure was defined based on the temporal pattern of metformin use in relation to the time of initial diagnosis (see table 1). The follow-up time of each patient was divided into three times periods by two points in time: 6 months pre- and 1 month post-cancer diagnosis. These define a pre-diagnosis (prior to 6 months pre-diagnosis), peri-diagnosis (between 6 months pre- and 1 month post-diagnosis), and post-diagnosis (after 1 month post-diagnosis) exposure periods. Subjects prescribed metformin at any time in the peri-diagnosis period were defined as exposed. This window was felt to be sufficiently large to identify subjects that were being prescribed metformin. Subjects not exposed during that time period were considered unexposed, regardless of peri- and post-diagnosis exposure. We categorized subjects solely on their exposure status around the time of PAC diagnosis, or an “initial treatment carried forward” for several reasons. The “initial treatment carried forward” is analogous to “intention to treat” in a clinical trial since the exposure status is determined during an enrollment period (equivalent to the peri-diagnosis period in the current study) and patients are considered to remain in the same exposure group for the duration of follow-up even if they switch to the alternative exposure. This approach will specifically address the more clinically relevant question as to whether putting patients on metformin at the time of PAC diagnosis will influence survival. The alternative was an “as treated” approach which categorizes exposure as time-varying and allows subjects to switch between the different treatments accruing follow-up time for both exposure groups at different times. However, the results of as treated analysis are much less informative for the clinical decision of whether or not to put a patient just diagnosed with PAC on metformin to improve survival. In addition, change in exposure status during follow-up may be associated with the outcome of interest in our study, as those discontinued from metformin may be discontinued due to worsening end-of-life disease. On the other hand, those who were started on metformin well into their disease course may be so secondary to better survival. Therefore, the “initial treatment carried forward” approach may also help minimize these potential confounding effects.
Table 1.
Patterns of metformin use. The three times periods that define metformin exposure include the period prior to 6 months before the diagnosis of pancreatic cancer (pre-diagnosis), the time between 6 months before diagnosis and 1 month after diagnosis (peri-diagnosis), and the time after 1 month after diagnosis (post-diagnosis). The *’d exposure statuses are those that will be excluded in the secondary analysis.
| Pre-diagnosis | Peri-diagnosis | Post-diagnosis | Exposure status |
|---|---|---|---|
| + | + | + | Exposed* |
| + | + | – | Excluded |
| + | – | – | Excluded |
| – | + | + | Exposed |
| – | – | + | Unexposed |
| + | – | + | Unexposed* |
| – | + | – | Exposed |
| – | – | – | Unexposed |
A secondary exposure definition was also used; subjects prescribed metformin during the peri-diagnosis period were considered exposed but were excluded if prescribed metformin during the pre-diagnosis period. This secondary exposure group was used to define “new starters” of metformin to avoid any potential confounding associated with past exposure to metformin. A sensitivity analysis was performed by extending the peri-diagnosis period to include only 3 months prior to, and up to 12 months prior to PAC diagnosis.
Outcome
The primary outcome was overall survival. Follow-up time for exposed and unexposed groups began 1 month after the diagnosis of PAC. This lag would account for any short delays in initiation of therapy in the exposed group after the diagnosis of PAC. The time to death was defined as the time between 1 month after diagnosis and death. The date of death was recorded as the exact date of death in the THIN database. For those alive, those who withdrew from the database/primary care practice were censored at the date of last follow-up.
Statistical Analysis
To compare exposure and un-exposed group, Pearson’s chi-squared test was used for categorical and Student’s t-test was used for continuous variables. The primary analysis was performed using univariate and multivariable Cox proportional-hazards models. Non-proportionality was assessed using scaled Schoenfeld residual score testing. All p-values were two-sided, and p-values < 0.05 were considered significant.
We examined the following potential confounding variables that are associated with the exposure, and possibly, the outcome: age at diagnosis, gender, duration of T2DM, last HbA1c prior to PAC diagnosis, BMI calculated from the last height and weight prior to the diagnosis of PAC, history of pancreatitis, and smoking status at time of diagnosis. We also measured Charlson comorbidity index, which was validated using Read codes and included this as a possible confounding variable as it is a predictor of mortality.33 All subjects were assigned two points for PAC and one point for T2DM. Race information is not available in THIN. We also measured exposure status to other antidiabetic medications such as sulfonylureas, thiazolidinediones, and insulin using the same criteria as was used to define exposure status for metformin. This was measured in an effort to control for confounding by indication as these medications may be used to replace metformin and indicate worsening T2DM and PAC status. Average daily dose of metformin was calculated for all exposed subjects.
Whether or not a variable was included in the final Cox regression model depended on the extent to which it affected the hazards ratio. In order to determine which variables to include in the multivariable models, we determined if the unadjusted hazards ratio differed from the hazards ratio adjusted for each potential confounder by more than 10% of the unadjusted hazards ratio. Those variables that create such a difference between unadjusted and adjusted odds ratios were be included in the model; this approach has been shown to be superior to other methods of selecting confounders in case-control studies.34
Similar methods were used to assess the relationship with the secondary definition of exposure.
Records were complete for all 516 subjects in the study for all variables except for HbA1c (135 missing, 26% overall, 33% in unexposed), BMI (37 missing, 7.2% overall, 9.3% in unexposed), GFR (42 missing, 8.1% overall, 11.1% in unexposed). Multiple imputation was performed to impute missing HbA1c data given its degree of missingness and potential to be a confounder in the final analysis. Values were imputed using the Gaussian normal imputation method using the following variables: the presence of diabetic complications, duration of T2DM, age, sex, pancreatitis, smoking status, and use of insulin, thiazolidinediones, or sulfonylureas.
Prior data indicate that the median survival time of high stage PAC is about 3 months. With 247 exposed subjects and 269 unexposed subjects, we can detect a difference in true median survival times of about 20 days with 80% power. The Type I error was assumed to be 0.05.
Data extraction and statistical analyses were performed using STATA v.12.
Results
The initial data extraction was performed to include all individuals with any diagnostic code of a pancreatic malignancy. This yielded 6684 subjects. After applying the inclusion and exclusion criteria, there were 729 final subjects for analysis, of which 516 survived past 30 days, the first day of follow-up (see figure 1).
Figure 1.

Inclusion/exclusion criteria, flow diagram.
Subject characteristics are summarized in table 2. The cohort was 51% male. The mean (±sd) age was 72.5±10 years, and the mean BMI was 26.8±5.4 kg/m2. Sixty-six percent had a history of smoking, and 42% were smokers at the time of their diagnosis. Complications from diabetes (retinopathy, nephropathy, amputation, etc.), was present in 8.5%, and the mean duration of T2DM diagnosis was 1680±2430 days. Average HbA1c before PAC diagnosis was 8.1±2.2%. Average Charlson index was 4.1±1.8. The average MDRD GFR was 74.2±22.2 mL/min. 47.8% were exposed to metformin according to the primary exposure definition, while 22% were exposed according to the secondary exposure definition. The proportion of subjects ever exposed to acarbose, gliptins, GLP-agonists, insulin, meglitinides, metformin, sulfonylureas, or thiazolidinediones were 2.0%, 0.7%, 0.7%, 31.7%, 1.2%, 50.8%, 56.1%, and 8.9%, respectively.
Table 2.
Baseline characteristics of the patients.
| Variable | Unexposed (269) | Exposed (247) | p-value |
|---|---|---|---|
| Gender (Male) | 136 (51%) | 140 (57%) | p = 0.164 |
| Diabetic Complication | 14 (5%) | 30 (12%) | p = 0.005 |
| Pancreatitis | 30 (11%) | 7 (3%) | p < 0.001 |
| Ever Smoker | 183 (68%) | 162 (66%) | p = 0.556 |
| Current Smoker | 110 (41%) | 113 (46%) | p = 0.266 |
| Acarbose Use | 4 (1%) | 7 (3%) | p = 0.290 |
| Gliptin Use | 1 (0%) | 3 (1%) | p = 0.275 |
| GLP Use | 0 (0%) | 2 (1%) | p = 0.139 |
| Insulin Use | 85 (32%) | 105 (43%) | p = 0.010 |
| Meglitinide Use | 2 (1%) | 6 (2%) | p = 0.122 |
| Sulfonylurea Use | 111 (41%) | 186 (75%) | p < 0.001 |
| TZD | 6 (2%) | 44 (18%) | p < 0.001 |
| Duration of DM(±sd) (days) | 1225 (159) | 1920 (137) | p < 0.001 |
| Age(±sd) (yr) | 72.0 (0.64) | 71.5 (0.60) | p = 0.529 |
| Charlson Score(±sd) | 4.15 (0.11) | 4.23 (0.12) | p = 0.597 |
| HbA1c(±sd) (%) | 7.66 (0.16) | 8.63 (0.15) | p < 0.001 |
| BMI(±sd) (kg/m2) | 25.8 (0.31) | 27.5 (0.34) | p < 0.001 |
| GFR(±sd) (mL/min) | 72.8 (1.34) | 76.7 (1.53) | p = 0.055 |
Those who were exposed were significantly more likely to have complications of T2DM, use insulin, sulfonylureas, and thiazolidinediones, have longer duration of T2DM, higher HbA1c, increased BMI, and have less pancreatitis (p < 0.05). With regards to duration of T2DM, 36% (CI 31%–41%) of unexposed subjects had recent onset T2DM (< 180 days), compared to 18% (CI 14%–22%) of exposed subjects.
In the Cox proportional hazards model, 447 deaths were observed with 118,305 patient-days of follow-up. The earliest time of failure was at day 0. The last observed failure was at time 4339. The median survivals in the exposed and unexposed groups were 99 (range 2 to 2642) days and 114 (range 1 to 4339) days, respectively. A Kaplan Meier curve can be seen in figure 2. The test of non-proportionality revealed a test score that was not significant, and therefore the assumption of proportionality was not violated.
Figure 2.
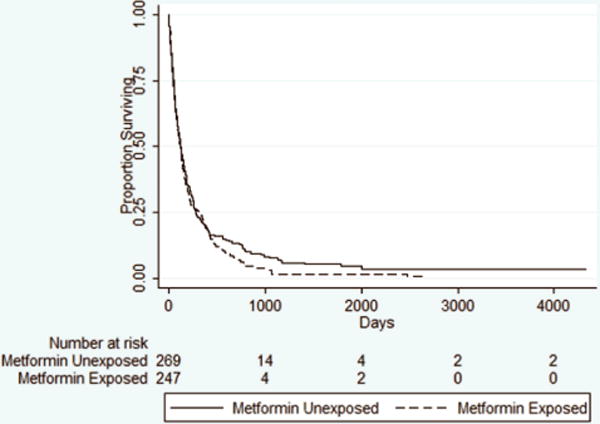
Kaplan-Meier Curves for Survival in the As-Treated Analysis.
In the univariate unadjusted Cox proportional hazards model, metformin exposure was associated with a trend towards an increased risk of death (HR = 1.13 [0.94, 1.36], p = 0.197). (See table 3.) The variables that fit the criteria of confounder included duration of T2DM, presence of diabetic complications, history of pancreatitis, Charlson index, BMI, GFR, smoking at the time of diagnosis, history of insulin use, history of sulfonylurea use, and history of thiazolidinedione use. Given that the variable HbA1c had a high degree of missingness (25% of subjects did not have a HbA1c), multiple imputation was performed using the Gaussian normal imputation method. Using the imputed values, HbA1c also fit the criteria for confounding. Age and sex were also included in the final model as these were felt to be important covariates regardless of statistical significance. The adjusted hazards ratio for metformin exposure was 1.11 [0.89, 1.38], p = 0.367.
Table 3.
Univariate and Multivariate Analysis.
| Primary Analysis | # of Subjects | Crude HR | Adjusted HR* |
|---|---|---|---|
| Metformin Unexposed | 269 | 1 | 1 |
| Metformin Exposed | 247 | 1.13 [0.94, 1.36], p = 0.197 | 1.11 [0.89, 1.38], p = 0.367 |
| Secondary Analysis | Crude HR | Adjusted HR* | |
| Metformin Unexposed | 269 | 1 | 1 |
| Metformin Exposed | 101 | 1.16 [0.88, 1.52], p = 0.290 | 1.09 [0.80, 1.47], p = 0.585 |
adjusted for age, sex, duration of diabetes, presence of diabetic complications, history of pancreatitis, Charlson index, BMI, GFR, smoking at the time of diagnosis, history of insulin use, history of sulfonylurea use, history of thiazolidinedione use, and HbA1c
The same analysis was performed with the secondary definition of exposure, that is, only newly exposed subjects were considered exposed and previously exposed subjects were excluded. There were a total of 460 subjects with 101 exposed. In that analysis, history of pancreatitis, sex, diabetic complications, ever history of smoking, sulfonylurea use, TZD use, duration of T2DM, Charlson score, and HbA1c were all associated with the exposure. Diabetes with complications, pancreatitis, insulin use, and age were associated with the outcome. In univariate analysis, metformin exposure was associated with a trend towards increased mortality HR 1.16 [0.88, 1.51], p = 0.290. Adjusting for the same confounders mentioned previously, the adjusted HR was 1.09 [0.80, 1.47], p = 0.585. Stratified analysis was performed for all variables. None of the variables were found to be effect modifiers in the primary or secondary analyses.
In the sensitivity analysis, none of the previously described measures of effect were significantly changed by adjusting the time definition of the peri-diagnosis period. Additionally, analysis stratified by average metformin dose quartile revealed no significant change in the prognosis with increasing metformin dose.
When we included those patients who underwent resection, the primary results are essentially unchanged (HR 1.04 [0.83, 1.31]). Furthermore, an analysis restricted among those patients who underwent resection did not demonstrate a beneficial effect of metformin (HR 1.32 [0.68, 2.58]).
Discussion
The main finding of this study is that despite experimental evidence suggesting otherwise, there is no survival benefit to metformin exposure in subjects with advanced PAC. Even when including only subjects that were “new starters” of metformin to reduce the bias from previous metformin exposure, there was still no survival benefit. This effect was completely independent of all other measured confounders, including HbA1c, presence of diabetic complications, duration of T2DM, comorbidity index, and other medication usage.
These results are in concordance with the recent study by Sadeghi et al which showed that metformin imparted a survival benefit in PAC in non-metastatic subjects but not amongst those with metastatic disease. In our exploratory analysis restricted among those who underwent resection and thus potentially with early stage cancer, we did not observe a survival advantage among metformin users. However, given the wide confidence interval, this analysis could not exclude a potential modest protective effect of metformin in this patient population.
The main limitations of this study are those inherent to the study design, mainly bias and confounding. Given the retrospective nature of the data, much of this data is dependent upon physician entry into the computerized medical record system and patient adherence to the prescribed medication. There is some reassurance provided by the fact that many of the main diagnoses, namely cohort-, exposure-, and outcome-defining variables have been validated in previous studies, minimizing the possibility of misclassification bias. A window for exposure classification was also utilized to allow for prescription and diagnosis entry which should also minimize misclassification bias. Further, prescription data is particularly robust in THIN as all prescriptions are issued through the general practitioner.
Staging information is also not available in the THIN database. However, as this study population reflects the general UK population, a vast majority of subjects will have presented in advanced stages of PAC. Further, nearly all subjects with local disease should be removed by excluding those subjects that underwent pancreatic resection. Unfortunately, distribution of advanced stages and, more importantly, their effect on survival in the context of metformin exposure is not available owing to the nature of the database.
Missing data was present in this data set. One completely missing variable was that of race, as it is not recorded in THIN. This would be of concern if blacks have worsened outcome and if their medication usage and glycemic control are similarly different. However, this effect is likely minimized due to the national health care delivery system in the UK. Further, the black population is only 2% of the UK population.
There was minor missingness in some variables as mentioned earlier. Complete case analysis was performed as there was less than 10% missingness. Missing data was more substantial in HbA1c, but after multiple imputation was employed, it was found to be of minimal effect when included in a multivariable analysis, suggesting that it was not an important confounder.
There was a significantly larger proportion of recent-onset T2DM among the unexposed subjects. This may be of concern if recent-onset T2DM has a significant impact on survival. Chu et al suggested that subjects with new-onset T2DM may have worse outcomes (HR 1.75 [1.10–2.78]) as well as those with longstanding T2DM (HR 1.30 [0.75–2.25]) when compared to non-diabetic controls, however, it is unclear how their prognoses compared to each other. 35 We attempted to address this issue by controlling for this possible confounder in our multivariate analysis.
In summary, we did not observe a clinically important survival benefit from metformin use in advanced PAC, suggesting that the survival benefit from metformin may indeed be isolated to subjects with early stage disease.
Acknowledgments
This study is funded by the NIH T32-DK007740 to A.H.; NIH Clinical and Translational Science Award UL1 RR024134 to A.H.. and K.H. This project was also supported by Grant Number 8UL1TR000003 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of NCATS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This was presented at the American College of Gastroenterology Meeting in Las Vegas, NV, 2012
There are no financial disclosures or conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2008. [Google Scholar]
- 3.Jimenez RE, Fernandez-del Castillo C. Pancreatic Cancer. 9. 2010. [Google Scholar]
- 4.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 5.Scheithauer W, Schull B, Ulrich-Pur H, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003;14(1):97–104. doi: 10.1093/annonc/mdg029. [DOI] [PubMed] [Google Scholar]
- 6.Hess V, Salzberg M, Borner M, et al. Combining capecitabine and gemcitabine in patients with advanced pancreatic carcinoma: a phase I/II trial. J Clin Oncol. 2003;21(1):66–68. doi: 10.1200/JCO.2003.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozengurt E. Early signals in the mitogenic response. Science. 1986;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- 11.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213(3):589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 12.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Sasajima J, Mizukami Y, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic cells. PLoS One. 2010;5(1):e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn BB, Alquier T, Carling D, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277(28):25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 17.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 18.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67(1):1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi N, Abbruzzese JL, Yeung SC, et al. Metformin Use Is Associated with Better Survival of Diabetic Patients with Pancreatic Cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 28.Haynes K, Forde KA, Schinnar R, et al. Cancer incidence in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2009;18(8):730–736. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
- 29.Hall GC. Validation of death and suicide recording on the THIN UK primary care database. Pharmacoepidemiol Drug Saf. 2009;18(2):120–131. doi: 10.1002/pds.1686. [DOI] [PubMed] [Google Scholar]
- 30.Mulnier HE, Seaman HE, Raleigh VS, et al. Mortality in people with type 2 diabetes in the UK. Diabet Med. 2006;23(5):516–521. doi: 10.1111/j.1464-5491.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Khan NF, Perera R, Harper S, et al. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11:1. doi: 10.1186/1471-2296-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 35.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17(2):502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]


