Abstract
Thyroid neoplasias represent among the fastest growing cancers. While surgery has become the treatment of choice for most thyroid tumors, many require chemotherapy. In this review, we examine the contributions of work in the fruit fly Drosophila towards Multiple Endocrine Neoplasia Type 2 (MEN2), a Ret-based disease to which recent Drosophila models have proven useful both for understanding disease mechanism as well as helping identify new generation therapeutics.
Keywords: Drosophila, Medullary Thyroid Carcinoma, thyroid, cancer
Introduction
Thyroid cancers including Medullary Thyroid Carcinoma (MTC) have proven difficult targets for chemotherapies. This difficulty is not unique to the thyroid: most solid tumors do not demonstrate long-lasting responses and eventually develop resistance to chemotherapeutic intervention. Where do the difficulties lie?
Some lie with the nature of cancer itself: cancers show a wide range of complexity and variability from individual to individual. In this regard, the genetic simplicity of, e.g., monogenic Ret-based tumors should help simplify the task. Solid tumors with a limited palate of mutations are rare, and tumor types such as medullary thyroid carcinoma present an especially genetically tractable problem. These tumors can also provide a useful platform to test new approaches for cancer therapeutics. Here, we review the use of Drosophila to explore some of these new approaches and discuss lessons that emerge from these efforts.
Drosophila and Cancer
Drosophila provides perhaps the most powerful genetic tools of any multicellular model. Using these tools, Drosophila biologists revolutionized a developmental biology field that was dominated by mammalian embryology approaches. Among their many contributions—recognized by a Nobel Prize in 1995—was emphasizing the importance of considering the details of local cell interactions. These interactions are lost when an experiment focuses on cell lines or whole tissues. The current state of the cancer field has some similarities, with a wealth of knowledge that is still working to establish useful and unifying themes. Time will tell if Drosophila can make a similarly important contribution in extracting key cancer-related principles from amidst this large body of knowledge. To date, the Drosophila community’s impact on cancer has mainly been from work that is indirectly related to cancer: epithelial patterning, signal transduction, etc. [1].
An important example is the Drosophila field’s establishment of the concept of “compensatory proliferation”. During normal development, tissues regulate their overall size through a number of pathways including the “hippo/ste20” pathway worked out primarily in Drosophila (reviewed in [2]). Forcing proliferation of a cell leads to compensatory death of neighboring cells to maintain precise tissue size (the fly needs perfectly sized wings to achieve efficient flight) [3]. Conversely, dying cells secrete Wnt and BMP signals to induce proliferation of their local neighbors, leading to the well-studied phenomenon termed “compensatory proliferation”. Artificially triggering apoptosis signals while simultaneously blocking caspase activity leads to extended growth signaling by these “undead” cells and often massive overgrowth [4,5]. These experiments recapitulate important aspects of an emerging tumor: high levels of caspase activity but low levels of apoptosis. Recently, the mammalian field has begun to consider this phenomenon as well (e.g., [6]).
A small but increasing number of laboratories are bringing fly’s remarkable tools to bear specifically on fundamental questions of cancer biology. In essence two approaches have been taken. In the first approach, Drosophila has been used to explore synergy between pairs of cancer-related genes. Pairing Ras and the cell polarity protein Scribble leads to overgrowth and invasion of transformed cells into neighboring compartments. EGFR plus PTEN-knockdown, when targeted to the developing fly nervous system, establishes an interesting fly glioma model with several aspects reflective of its human disease counterpart [7]. And pairing Ras plus Src—directly or by knocking down the Src inhibitor Csk—reflects a combination found in a host of human tumors including breast, lung, and pancreatic [7–9]. These efforts have allowed us to explore issues of emergent signaling properties that reflect the complexity of most solid tumors including thyroid.
A second aspect of fly cancer models has been the ability to examine the relationship between a tumor and its environment. Altered metabolism is a central tumor driver and our laboratory is currently using flies to explore the relationship between diet, metabolism, and cancer. Flies have also demonstrated the importance of local cell-cell interactions between transformed cells and their epithelial neighbors [10,11] as well as the hematopoietic system [12]. All of these aspects are key for regulating tumorigenesis and will inform us on the factors that determine the nature of thyroid tumor progression. With regard to therapeutics, processes that reflect environmental signaling such as “compensatory proliferation” will require phenotype-based drug screening in the context of the whole animal, an approach we discuss below. These key interactions are not readily modeled in cell culture or in vitro.
Drosophila does not have a thyroid and interpretations of fly data have to be done with care. Nevertheless, their powerful tools have proven useful as a first step towards understanding mechanism and therapeutics in a whole animal setting. We next review Drosophila’s contributions specifically to thyroid cancer.
Fly model of thyroid cancer
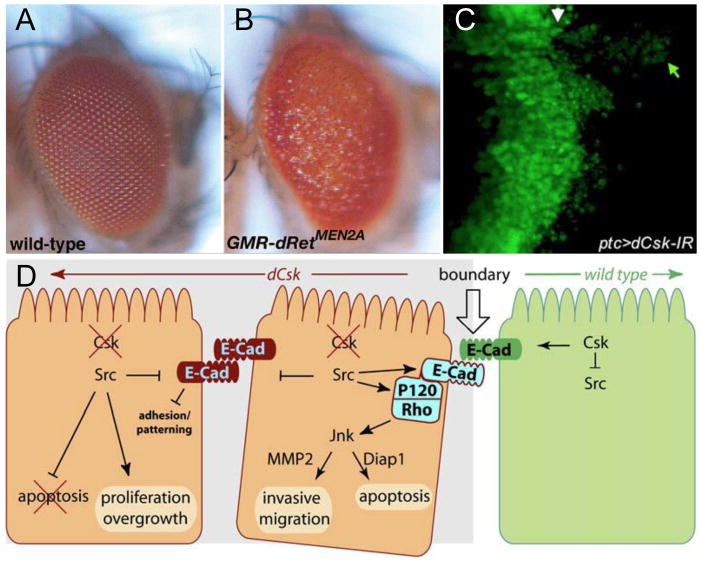
Cancer is fundamentally a genetic defect and flies offer the ability to explore the genetics of the disease in a thorough, unbiased fashion. Multiple Endocrine Neoplasia type 2 (MEN2) offered our laboratory an opportunity to model a monogenic tumor. As flies do not have a thyroid we targeted oncogenic Ret—a receptor tyrosine kinase (RTK) that mediates cell signaling—to the developing eye, one of the best characterized epithelia in terms of signal transduction [13]. To model MEN2 we fused oncogenic Ret (RetMEN2) to an eye specific promoter. The result was a ‘rough eye’ phenotype (Figure 1B) due to overproliferation, switches in cell fate, and compensatory apoptosis [13]. Although not a perfect model of thyroid cancer it proved a useful one.
Figure 1. Oncogenic Ret directs tumorigenesis in Drosophila.
(A) A wild type Drosophila adult eye. (B) Targeting expression of the oncogenic Ret isoform dRetMEN2B to the eye led to overgrowth and other defects, visible as a “rough eye” adult phenotype. (C) Knockdown of dCsk in a strip in the developing wing epithelium (marked by green fluorescent protein) led to activation of Src and migration of transformed cells (green arrow) away from the original site (white arrow). (D) Some of the molecules identified in Drosophila screens that mediate oncogenic dRetMEN2 migration. Neighboring normal cells (green) may signal to their transformed neighbors (orange) to promote migration. A,B are from [13], C,D are from [10].
Genetic modifiers of Ret-based tumors
We used the ease of scoring the eye phenotype to test nearly 3,000 genes in an unbiased screen for ‘genetic modifiers’ of the RetMEN2-based rough eye. We found 140 genetic modifiers: 93 genes yielded a stronger rough eye phenotype (“enhancers”) when their genomic complement was reduced; 47 yielded a weaker rough eye phenotype (“suppressors”). These genetic modifiers defined multiple signaling pathways including Ras, Src, Jnk, etc. as well as chromatin remodeling proteins and other cancer-related loci [13]. This provided a broad view of the factors that mediate RetMEN2-based transformation.
Candidate susceptibility loci
One important use of this functional database of RetMEN2 mediators was the ability to identify candidate ‘modifier loci’ in human tumors. Working with Paul Goodfellow, we matched our list of Drosophila RetMEN2 genetic enhancers with a PCR-based analysis of copy number variation in MEN2 patients’ tumor samples [13]. The result was the identification of two loci that are statistically linked to increased risk of secondary pheochromocytomas, the signaling kinase TNIK and the chromatin remodeling protein CHD3. This use of Drosophila to identify candidate biomarkers and susceptibility loci represents an interesting potential use of Drosophila for cancer genomic studies.
Src directs invasion
C-terminal Src-kinase (Csk), a negative regulator of Src activity, was identified in the RetMEN2 genetic modifier screen. Our studies showed that loss of Csk in a discrete patch of cells adjacent to wild-type cells resulted in cell invasive behavior (Figure 1C). Using genetics, we identified a pathway that mediates Csk/Src-dependent migration that includes E-Cadherin, Rho1, Jnk, and matrix metalloproteases (Figure 1D). Surprisingly, while discrete loss of Csk led to Src-dependent cell invasion, loss of Csk throughout the whole tissue resulted in overgrowth and proliferation but not invasion. This indicated that interactions between normal and transformed cells are instrumental in directing invasion, the first step towards metastasis. It also implied that cells at the periphery of human tumors may be potentially more invasive than more internal transformed cells [10].
Sin3a regulates a genetic module of invasion
Sin3a, a HDAC-associated co-factor, was also identified in this Ret screen. Recently, our genetic, ChIP-seq, and molecular analysis in flies have shown that Sin3a is a suppressor of invasion downstream of Ret (and other RTK’s). By virtue of being a global regulator Sin3a controls a ‘module of genes’ involved in invasion, making it an ideal point of deregulation for tumor progression to occur. We further showed that human Sin3 mRNA’s were consistently altered in a number of human tumor types, and that knockdown of human Sin3A increased invasive behavior of human lung cancer cells. Previous studies in human cell lines and analysis of genetic nulls in mice had failed to uncover this aspect of Sin3a function. Careful titration of Sin3a knockdown in our fly studies uncovered a novel role for Sin3A proteins in regulating tumor progression in humans [14]. These studies once again demonstrate the value of whole animal studies as well as the tractability of the fly genetic system.
Drosophila and Ret-based tumors: validating Caprelsa
Although Drosophila has proven a useful tool for exploring the mechanisms by which oncogenic RetMEN2 can direct metastatic tumors, our long-term goal was to help identify and validate useful therapeutics. To this end, we used our RetMEN2 flies to screen candidate drugs as well as compound libraries that included FDA approved drugs, natural compounds, etc. Flies were fed with multiple doses of each compound, then scored for drugs that suppressed the rough eye phenotype while permitting the fly to survive to adulthood. This was a stringent screen: most drugs effective against oncogenic Ret also inhibited additional targets and were likely to kill the developing fly, which required precisely regulated signaling for proper development.
Carlomagno, Santoro, and colleagues demonstrated significant activity of ZD6474/Vandetanib/Caprelsa against oncogenic Ret and against a human MEN2A cell line [15]. Caprelsa was originally developed as a VEGFR inhibitor, and this work suggested it may act on MTCs through multiple targets. Working with Sam Wells and AstraZeneca, we demonstrated that Caprelsa could suppress the rough eye phenotype while permitting fly viability when fed orally [16]. Completing Phase III trials in 2010, Caprelsa became the first FDA-approved chemotherapy for Ret-based thyroid tumors in April 2011 [17]. Caprelsa is an exciting new option for advanced MTC patients. It also validated the potential utility of flies as a predictive assay for candidate lead compounds.
Drosophila and Ret-based tumors: rational polypharmacology
While Caprelsa provides an exciting new option for MTC patients it is not the end of the story. A significant proportion progressed or showed early resistance [18]. Toxicity issues that are commonly observed with tyrosine kinase inhibitors (TKIs) were also reported for Caprelsa, limiting dose and potentially length of treatment for many patients. Further, resistance has been identified in some patients and this will likely be a growing problem over time.
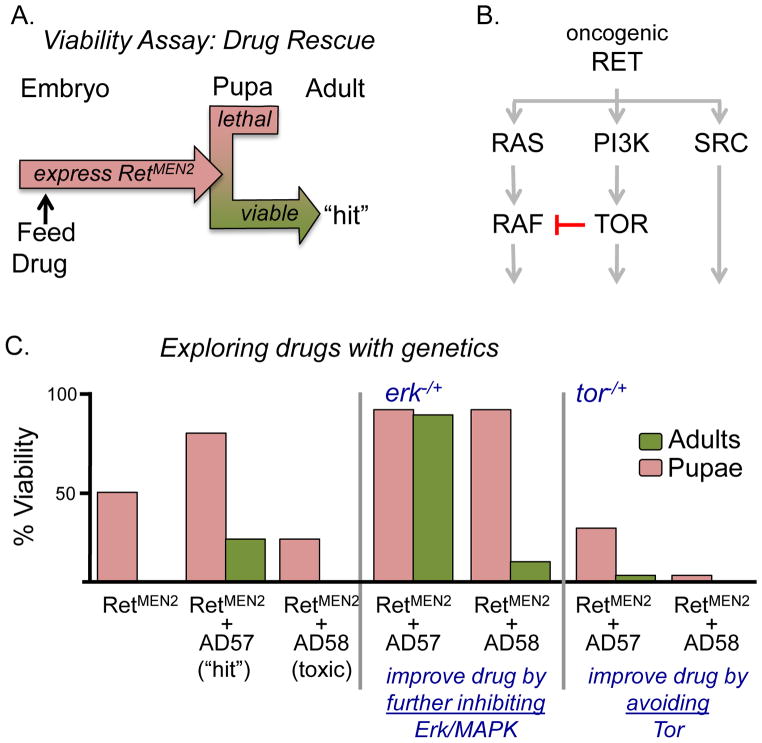
With this in mind our laboratory established a collaboration [19] with Kevan Shokat and Arvin Dar, medicinal chemists who specialize in developing TKIs. We reasoned that drugs that depended on targeting Ret alone would invariably run the risk of high toxicity (due to the importance of Ret for homeostasis as well as unintended off target effects) and resistance. We screened a chemical library developed in the Shokat laboratory designed to target multiple kinases [20]; by contrast to the concept of “off-target effects”, our goal was to develop a useful method for developing drugs with rational, stepwise “multi-target effects”. In place of screening eye phenotypes, we developed a model in which oncogenic RetMEN2 killed the fly; candidate hits would result in live animals in a whole animal assay that could be quantitated (Figure 2A; [19]). We identified one significant hit, the DFG-out Type II inhibitor compound AD57 [21]. AD57 showed better efficacy than Caprelsa, the Ret-targeting inhibitor DL06, or any other compound we had tried. We next worked to improve it.
Figure 2. Combining genetic and drug screens.
(A) Viability assay approach for drug screening. Oncogenic Ret expression (ptc>dRetMEN2B) in developing flies results in death during larval and pupal stages. Addition of drugs that inhibit Ret pathway activity can rescue them to adult stages. (B) At least three pathways mediate dRetMEN2B transformation. Schematic leaves out many pathway components to focus on key targets. Red indicates crosstalk inhibition on Ras pathway signaling by TOR. (C) Quantitating viablity assay. Compared to controls, AD57 rescued a significant number of dRetMEN2B embryos to adulthood whereas the close analog AD58 proved toxic. Fly genetics identified mechanisms for improving AD57: genetically reducing the Ras downstream effector Erk resulted in better efficacy of AD57 and AD58 on dRetMEN2B flies; reducing Tor made the drugs more toxic. From [19].
Our previous genetic screens indicated that Ras/Raf, Src, and PI3K/Tor were primary mediator pathways of oncogenic RetMEN2 (Figure 2B; [13]). Accordingly, in vitro human kinase panel analysis of AD57 indicated it directly inhibited these pathways’ rate-limiting kinases Raf, Src, and mTor as well as Ret. To determine whether these targets were hit optimally, we reduced the genetic copy number of Raf, Src, or Tor pathway components in the presence of AD57. This proved informative. For example, erk(−/+) plus AD57 improved animal survival. We concluded that Ras pathway activity is a key target and that an improved second generation drug would need to further reduce Ras pathway signaling (Figure 2C).
Conversely, tor(−/+) plus AD57 increased toxicity and decreased efficacy: heterozygous animals were otherwise fine but died more readily in the presence of drug (Figure 2C). That is, Tor represents an “anti-target” and an improved drug should avoid it. But how? Fly data proved useful on this point as well. First, genetic and biochemical analysis indicated that reducing tor—alone or in the presence of AD57—led to activation of Ras pathway signaling though a recently described feedback loop in which Tor inhibits Ras pathway activity (Figure 2B; [22,23]). Second, the close chemical analog AD58 inhibited Tor but not Raf and proved highly toxic to the flies; we could show this was due to increased Ras pathway signaling throughout the fly [19]. We concluded that unbalanced Tor/Raf inhibition led to overall animal toxicity.
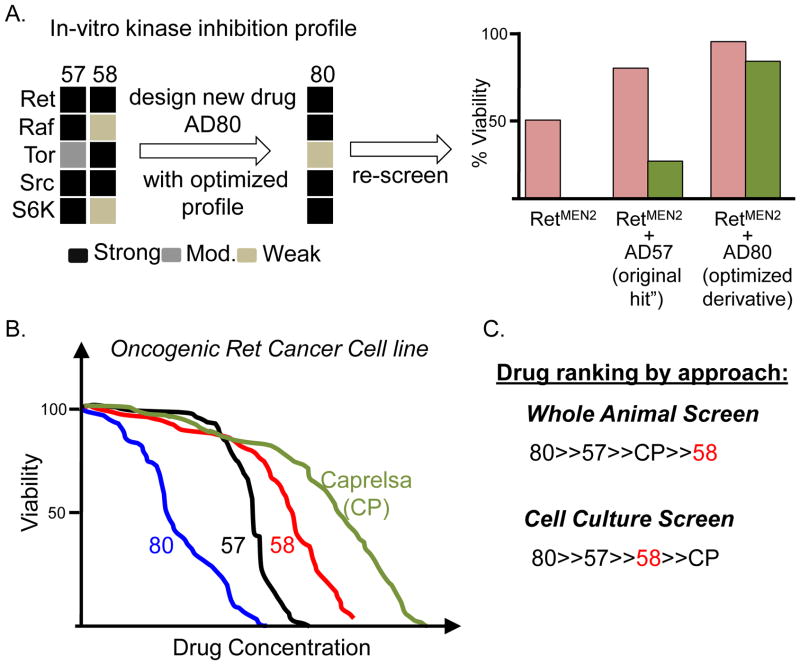
The toxic compound AD58 differed from AD57 only in that it lacked a trifluro-methyl group on the terminal phenyl ring. From this and from chemical modeling, the Shokat laboratory deduced that adding additional residues to the terminal ring—essentially moving away from AD58’s structure—would further reduce activity against Tor. Adding chloride or fluoride groups yielded derivative compounds AD80 and AD81. As hoped, these compounds maintained suppression of Ret, Raf, and Src but had reduced activity against Tor. They retained activity against the downstream PI3K pathway effector S6K, allowing them to suppress the pathway downstream of the problematic Tor-mediated feedback loop (Figure 3A). The result was compounds with strongly improved efficacy against Drosophila RetMEN2 transformation with strongly reduced animal toxicity (Figure 3A).
Figure 3. Developing an improved AD57 derivative.
(A) In vitro human kinase inhibition panel indicated that AD58’s ratio of Tor:Raf inhibition is high; genetics predicted this would be toxic (Figure 2C). AD57 is more balanced but (Figure 2C) can be improved. Chemical analog AD80, modelled to avoid inhibiting Tor, retained strong inhibition of Ret, Src, S6K, and Raf. AD80 proved to be better than AD57 in the fly viability assay. (B) Fly viability assay was predictive of results in human cell culture: increased whole animal viability correlated with decreased growth of cell culture lines. (C) AD58’s whole animal toxicity was only apparent in a whole animal setting. From [19].
In human TT and MZ-CRC-1 cell lines—derived from MEN2A and MEN2B patients, respectively—AD80 reduced proliferation approximately 150-fold more effectively than Caprelsa (Figure 3B). It was also significantly improved compared to Caprelsa in TT-based mouse xenograft studies. These mouse studies further demonstrate the promise of stepwise, rational polypharmacology developed in a whole animal assay.
These studies show the value of carrying out drug screening in the context of a whole animal. For example, the advantages of targeting multiple pathways beyond simply RetMEN2 are most apparent in a whole animal setting; indeed we cannot exclude the possibility that some of AD80’s utility lies in targeting tissues other than the tumor itself. In another example, cell culture-based screens rank AD58 as better than Caprelsa, despite its high animal toxicity (Figure 3C). Screening directly for bottom-line efficacy and minimal toxicity can identify novel chemical spaces for useful therapeutic indices.
Future of Drosophila and Thyroid Drug Discovery
These are promising times for the development of therapeutics targeting the most intractable thyroid tumors. Just a few years ago there were relatively few options available for treatment. New Ret and Raf inhibitors show promise in clinical trials and Caprelsa represents the first FDA-approved therapeutic specifically for Ret-based tumors. However, current drugs including Caprelsa are not the final word on thyroid tumor therapeutics. As discussed in this review, rational polypharmacology approaches have great promise but, importantly, the approach remains currently unproven. Advancing genomics will identify additional targets, and Drosophila is well positioned to rapidly assess the utility of these targets and to explore how they contribute to tumorigenesis and drug sensitivity. Drosophila is most useful when closely paired with mammalian models. Perhaps our current greatest unmet need is improved mammalian models that are rapid, robust, and predictive.
Conclusions
A reasonable question would be whether Drosophila is required to identify these compounds. As emphasized in this review, our approach to rational polypharmacology can only be readily done in flies. Considering some alternatives:
Cell line and in vitro screening
Cell lines and in vitro approaches are best at identifying drugs that hit single targets. Screening with, for example, a luciferase readout is a powerful tool for screening large drug libraries. But the track record of these approaches has been mixed for cancer. They fail to capture the complexity of the cell-cell interactions described above. Indeed, we wonder whether many drugs that succeed in the marketplace attack both the tumor and also targets outside the tumor, elsewhere in the tissue or the body. This complexity is missed. By starting with metastatic cells, xenograft models also fail to model many of these interactions.
Mammalian model systems
Whole animal screening has many advantages in its ability to capture whole body complexity. Phenotypic screening has lost popularity in recent decades but it remains a powerful method of identifying new drugs and we anticipate a resurgence in its use. However, screening in mouse or rat is not practical beyond a small number of drugs; certainly the ability to screen with robotics is not available. Mouse is also limited in the number of genes that can be tested in the presence of drugs, limiting the ability to predict secondary targets. Mice are more typically used in secondary screens, where their similarity to humans can be leveraged.
Other simple organisms
The zebrafish D. rerio and the worm C. elegans have powerful genetic tools available. However, fish have yet to be used extensively for drug screening of solid tumors due to a long life-cycle and the difficulty and cost of testing large numbers of drugs in adult fish. Worms have powerful genetic tools at a level similar to Drosophila, and diseases such as metabolic dysfunction has been usefully modeled. They are also accessible to whole animal drug screening. However, the simple nature of their epithelia has made solid tumor modeling uncommon in the field. In both cases, as technology changes these simple models may be used increasingly to model solid tumors including thyroid.
Drosophila
At the moment, Drosophila sits a unique position as providing a genetically tractable animal with complex epithelia that reflect our own structures and which is accessible to whole animal screening. Most oncogenes and tumor suppressors have clear fly orthologs. The majority of cancer relevant drugs we have fed to flies have shown significant activity, mirroring recent studies that have validated flies as a useful model to test compounds [24]. We anticipate that the Drosophila field will continue to find ways to use this remarkable insect in new and innovative approaches to cancer therapy.
Acknowledgments
T. K. D. and R. L. C. were supported by NIH grants R01 CA109730 and R01 CA084309, and American Cancer Society Grant 120616-RSGM-11-018-01-CDD. T. K. D. was also supported by American Cancer Society Grant 120886-PFM-11-137-01-DDC.
Footnotes
Conflict of Interests
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudrapatna VA, et al. Drosophila cancer models. Dev Dyn. 2012;241 (1):107–118. doi: 10.1002/dvdy.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumaneng K, et al. Organ Size Control by Hippo and TOR Pathways. Curr Biol. 2012;22 (9):R368–379. doi: 10.1016/j.cub.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Cova C, et al. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117 (1):107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 4•.Huh JR, et al. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14(14):1262–1266. doi: 10.1016/j.cub.2004.06.015. An excellent example of how studies in Drosophila epithelial maturation speak directly to issues of oncogenesis. [DOI] [PubMed] [Google Scholar]
- 5.Ryoo HD, et al. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7 (4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Huang Q, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17 (7):860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read RD, et al. A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 2009;5 (2):e1000374. doi: 10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302(5648):1227–1231. doi: 10.1126/science.1088474. A landmark paper demonstrating how Drosophila can be used to explore issues of cancer metastasis. [DOI] [PubMed] [Google Scholar]
- 9.Vidal M, et al. Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 2007;67 (21):10278–10285. doi: 10.1158/0008-5472.CAN-07-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Vidal M, et al. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10(1):33–44. doi: 10.1016/j.devcel.2005.11.007. Demonstration of the importance of whole animal cancer modeling: local interactions between tumor and normal epithelial cells promote migration of tumor cells at the border. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, et al. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463 (7280):545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordero JB, et al. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18 (6):999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Read RD, et al. A Drosophila model of multiple endocrine neoplasia type 2. Genetics. 2005;171(3):1057–1081. doi: 10.1534/genetics.104.038018. The first Drosophila thyroid tumor model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das TK, et al. Sin3a Acts through a Multi-Gene Module to Regulate Invasion in Drosophila and Human Tumors. Oncogene. 2012 doi: 10.1038/onc.2012.326. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Carlomagno F, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62(24):7284–7290. A key paper demonstrating the utility of Vandetanib/Caprelsa in inhibiting MTC. [PubMed] [Google Scholar]
- 16••.Vidal M, et al. ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res. 2005;65(9):3538–3541. doi: 10.1158/0008-5472.CAN-04-4561. Validating the utility of Vandetanib/Caprelsa in a whole animal transgenic model (flies) [DOI] [PubMed] [Google Scholar]
- 17.Wells SA, Jr, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28 (5):767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Wells SA, Jr, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. Landmark study finalizing the establishment of Vandetanib/Caprelsa for MTC patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Dar AC, et al. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature. 2012;486(7401):80–84. doi: 10.1038/nature11127. Demonstrating how fly models of thyroid cancer can be used to develop novel, polypharmacology-based drugs in a stepwise, rational manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apsel B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4 (11):691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dar AC, et al. Small molecule recognition of c-Src via the Imatinib-binding conformation. Chem Biol. 2008;15 (10):1015–1022. doi: 10.1016/j.chembiol.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gedaly R, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30 (12):4951–4958. [PMC free article] [PubMed] [Google Scholar]
- 23.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118 (9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das T, Cagan R. Drosophila as a novel therapeutic discovery tool for thyroid cancer. Thyroid. 2010;20 (7):689–695. doi: 10.1089/thy.2010.1637. [DOI] [PubMed] [Google Scholar]