Highlights
-
•
Macrophages can internalise LDL through scavenger receptor-independent mechanisms.
-
•
Macropinocytosis has been shown to contribute significantly to foam cell formation.
-
•
Cytokines such as TGF-β, IL-33, IFN-γ and IL-17A can modulate macropinocytosis.
-
•
TGF-β mediated inhibition of macropinocytosis is a Smad-2/-3-independent process.
-
•
Macropinocytosis is a promising target for therapeutic intervention of atherosclerosis.
Abbreviations: AcLDL, acetylated LDL; Apo, apolipoprotein; BMDM, bone marrow-derived macrophage; CD-36, cluster of differentiation 36; DiI, 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyane perchlorate; THP-1, human acute monocytic leukemia cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HMDM, human monocyte-derived macrophages; IL, interleukin; IFN-γ, interferon-γ; LDL, low-density lipoproteins; LY, lucifer yellow; OxLDL, oxidized LDL; SR-A, scavenger receptor A; shRNA, short hairpin RNA; TGF-β, transforming growth factor-β
Keywords: Macropinocytosis, Cytokine, Foam cell, Atherosclerosis, Low density lipoprotein
Abstract
A key event during the formation of lipid-rich foam cells during the progression of atherosclerosis is the uptake of modified low-density lipoproteins (LDL) by macrophages in response to atherogenic mediators in the arterial intima. In addition to scavenger receptor-dependent uptake of LDL, macropinocytosis is known to facilitate the uptake of LDL through the constitutive and passive internalization of large quantities of extracellular solute. In this study we confirm the ability of macropinocytosis to facilitate the uptake of modified LDL by human macrophages and show its modulation by TGF-β, IFN-γ, IL-17A and IL-33. Furthermore we show that the TGF-β-mediated inhibition of macropinocytosis is a Smad-2/-3-independent process.
1. Introduction
Atherosclerosis is a chronic inflammatory disease of the vasculature that is governed by many risk factors including genetic predisposition and diet. The disease itself is characterized by the accumulation and retention of apolipoprotein B (apoB)-containing lipoproteins, such as low-density lipoprotein (LDL), within the arterial wall and the subsequent formation of foam cell-rich fibrotic plaques that, upon rupture, result in myocardial infarction and stroke [1]. The formation of lipid laden foam cells in the arterial intima, as a result of modified LDL uptake by macrophages, represents a critical step during the progression of atherosclerosis. The uptake of modified forms of LDL is understood to take place through a receptor-dependent mechanism that is propagated by the increased expression of key genes, such as scavenger receptor-A (SR-A) and CD36, by invading macrophages [1,2]. Furthermore, this phenotypic change is known to be tightly regulated by a multitude of both novel and classical cytokines implicated in the control of atherogenesis, such as interleukin (IL)-33, interferon-γ (IFN-γ), and transforming growth factor-β (TGF-β) [3–5], and currently represents the most accepted and well documented paradigm of modified LDL uptake by macrophages.
In addition to this “classical” mode of lipid uptake, it has been shown that scavenger receptor-independent processes, such as macropinocytosis, may contribute significantly to the uptake of LDL by macrophages [6] and therefore drive the process of foam cell formation. Macropinocytosis is a form of fluid-phase endocytosis where solute uptake is directly proportional to the volume of liquid internalized and the solute concentration. The process itself involves the actin-dependent ruffling of the plasma membrane and the subsequent fusion of the membrane to itself in order to form intracellular fluid filled vacuoles 0.5–5 μM in diameter [7,8]. This process has been shown to contribute to the uptake of oxidized (Ox-) LDL by mouse RAW264.7 macrophages [9], an observation that may account for the appearance of LDL sized nano-particles within macrophages present in atherosclerotic lesions of apoE deficient mice [10]. Furthermore, macropinocytosis has also been shown to contribute to the uptake of native and modified forms of LDL by human peripheral blood-derived macrophages [11], phorbol 12-myristate 13-acetate (PMA)-derived macrophages [6] and macrophage colony-stimulating factor (MCS-F)-derived macrophages in vitro [12,13]. In addition, it has also been shown that minimally oxidized LDL (mmLDL) can induce macropinocytosis in macrophages through a toll-like receptor (TLR) 4/spleen tyrosine kinase (SYK) dependent mechanism and therefore potentiate the uptake of both native and oxLDL thus contributing to the progression of atherosclerosis [14,15].
Despite its clear implication during the process of foam cell formation, the effect of novel and established foam cell regulators, such as IL-33, IL-17A, IFN-γ and TGF-β, on macropinocytosis remains unexamined. Therefore, the objective of this study was to confirm the role of macropinocytosis on modified LDL uptake in human macrophages and examine the effect of key cytokines known to regulate atherosclerotic progression on this process.
2. Materials and methods
2.1. Reagents and cell culture
All chemicals were purchased from Sigma–Aldrich (Poole, UK) unless otherwise stated. Recombinant human TGF-β, IL-33, IL-17A and IFN-γ were supplied by Peprotech (London, UK). Acetylated LDL (AcLDL) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethyllindocarbocyane perchlorate (Dil)-labeled AcLDL (DiI-AcLDL) were purchased from Intracel (Frederick, Maryland, USA).
Human monocyte-derived macrophages (HMDM) were differentiated from monocytes isolated from buffy coats supplied by the Welsh Blood service using Ficoll-Hypaque purification described elsewhere [3]. Ethical approval and informed consent for each donor was granted by the Welsh Blood Service for the use of human blood samples. Human acute monocytic leukemia cells (THP-1) and HMDM were maintained in complete RPMI-1640 supplemented with 10% (v/v) heat-inactivated FCS, penicillin (100 U/ml), streptomycin (100 μg/ml) and l-glutamine (2 mmol/L) (all Invitrogen, Paisley, UK), at 37 °C in a humidified atmosphere containing 5% (v/v) CO2. THP-1 monocytes were differentiated into macrophages using 160 nM PMA for 24 h and this ensured high expression levels of scavenger receptors and other genes implicated in the control of macrophage foam cell formation [16]. In all experiments cells were incubated for 24 h with either 30 ng/ml of TGF-β, 10 ng/ml of IL-33, 100 ng/ml of IL17A or 1000 U/ml of IFN-γ. THP-1 macrophages were pre-incubated with cytochalasin-D for 1 h prior to the addition of AcLDL, DiI-AcLDL or lucifer yellow (LY) dipotassium salt and cytokines. Cytokines and cytochalasin-D were reconstituted in PBS/0.1% bovine serum albumin (BSA) or DMSO respectively that were subsequently used as a vehicle controls.
2.2. DiI-AcLDL and lucifer yellow uptake assays
Cells were incubated for 24 h with DiI-AcLDL (10 μg/ml) or LY (100 μg/ml) in RPMI-1640 containing 0.2% (v/v) fatty-acid free BSA (Sigma–Aldrich) at 37 °C. The concentration of LY reflects that commonly used in the literature [17]. DiI-AcLDL and LY uptake were analyzed by flow cytometry on a FACS Canto (BD Biosciences, Oxford, UK) flow cytometer with at least 10,000 events acquired for each sample. DiI-AcLDL and LY uptake is represented as a percentage with the vehicle-treated control indicated as 100%.
2.3. Real-time quantitative PCR
RNA extraction, reverse transcription and real-time quantitative PCR analysis was performed as described elsewhere [3]. Oligonucleotides specific for 60S ribosomal protein L13a (RPL13A) (forward 5′-CCTGGAGGAGAAGAGGAAAGAGA-3′, reverse 5′-TTGAGGACCTCTGTGTATTTGTCAA-3′), CD36 (forward 5′-GAGAACTGTTATGGGGCTAT-3′, reverse 5′-TTCAACTGGAGAGGCAAAGG-3′) [18] and SR-A (forward 5′-CCAGGGACATGGAATGCAA-3′, reverse 5′-CCAGTGGGACCTCGATCTCC-3′) [18] were purchased from Sigma Aldrich (Poole, UK). Fold changes in expression were calculated using 2−(ΔCt1 − ΔCt2), where ΔCt represents the difference between the threshold cycle (CT) for each target gene and RPL13A mRNA transcript levels. Melting curve analysis was performed on each primer set to confirm amplification of a single product and all amplicons were sequenced to ensure reaction specificity (data not shown).
2.4. Adenoviral infection of THP-1
Adenovirus encoding shRNA against Smad-2, Smad-3 and GAPDH were prepared as previously described [19]. THP-1 monocytes were infected with RAd-GAPDH shRNA or RAd-Smad-2 shRNA or RAd-Smad-3 shRNA at a multiplicity of infection (MOI) of 100 for each virus in 0.5 ml RPMI-1640 medium for 2.5 h at 37 °C (rocking) prior to addition of 1 ml of RPMI-1640 medium (including 160 nM PMA to induce differentiation into macrophages) and incubation for a further 72 h. An MOI of 100 was sufficient to infect >98% THP-1 cells as measured by flow cytometry following infection with a GFP-expressing recombinant adenovirus (data not shown).
2.5. Statistical analysis
All data are presented as mean [±standard deviation (SD)] on the assigned number of independent experiments or, in experiments involving HMDM, experiments performed using samples from different donors. For single comparisons, values for p were calculated using the Student’s t-test. For multiple comparisons, values of p were calculated using Welch’s robust test of equality of means followed by Games–Howell post hoc analysis. Values of p were considered significant below 0.05.
3. Results
3.1. Cytochalasin-D inhibits the uptake of LY and AcLDL in human macrophages
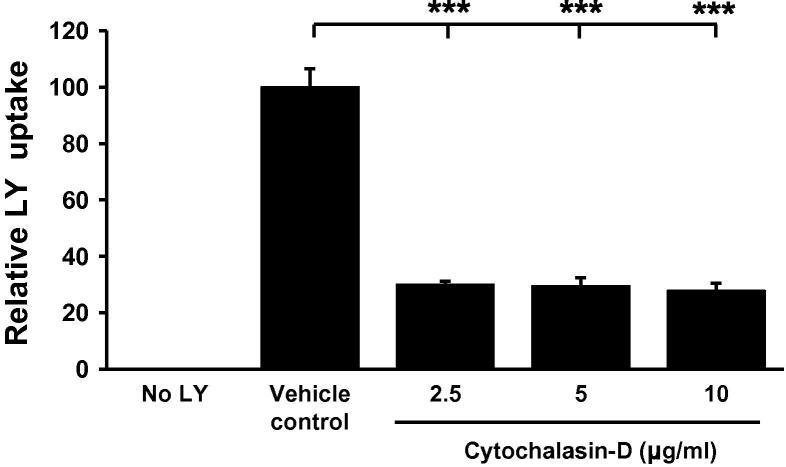
The PMA differentiated THP-1 model is commonly used to delineate human macrophage cellular functions associated with atherosclerosis due to their conserved responses with primary HMDM and in vivo data [3,4,20]. This cellular system was therefore employed to examine the potential contribution of macropinocytosis during foam cell formation. In light of this we first examined the effect of the established macropinocytosis (not micropinocytosis [11]) inhibitor, cytochalasin-D, on the uptake of LY, a fluorescent dye commonly used to monitor macropinocytosis [7,21], by monocyte derived THP-1 macrophages. As shown in Fig. 1, cytochalasin-D, a cell permeable myotoxin that inhibits macropinocytosis through the de-polymerization of actin filaments and tubulin microtubules [11,12], attenuates the uptake of LY by approximately 70% (at 2.5 μg/ml) when compared to the vehicle control treated cells.
Fig. 1.
Cytochalasin-D inhibits LY uptake in THP-1 macrophages. LY uptake was measured in THP-1 macrophages in response to 24 h incubation with varying concentrations of cytochalasin-D (n = 3). DMSO was used as a vehicle control. Data represents the mean ± SD. Statistical analysis was performed using Welch’s test of equality of means with Games–Howell post hoc analysis, ***P < 0.001.
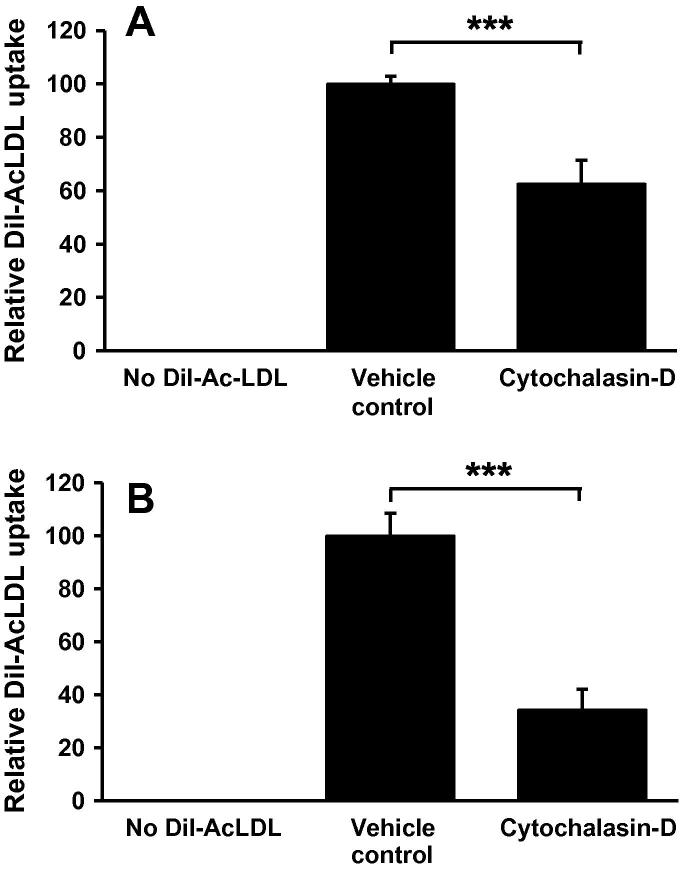
Next, in order to further substantiate the existing evidence suggesting a role for macropinocytosis in foam cell formation [7,12], we examined the effect of cytochalasin-D (at the concentration defined above) on the uptake of DiI-AcLDL, a form of LDL used extensively during in vitro foam cell formation assays due to its substantial intracellular accumulation [22]. As shown in Fig. 2A, cytochalasin-D significantly inhibited the uptake of AcLDL by THP-1 macrophages by approximately 37%, an observation that was also conserved in HMDM (Fig. 2B). This response was confirmed by an observed reduction in the intracellular cholesterol content of cytochalasin-D treated THP-1 cells and HMDM (see Supplementary Fig. 1).
Fig. 2.
Cytochalasin-D inhibits AcLDL uptake in human macrophages. Dil-AcLDL uptake was measured in (A) THP-1 macrophages or (B) HMDM in response to 24 h incubation with cytochalasin-D (2.5 μg/ml, n = 3). DMSO was used as a vehicle control in all experiments. Data represents the mean ± SD. Statistical analysis was performed using the Student’s t-test, ***P < 0.001.
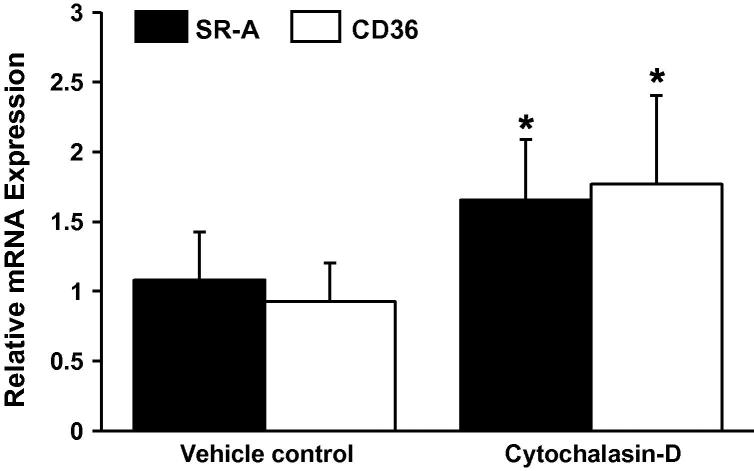
The uptake of AcLDL is principally regulated by cell surface receptors that are known to facilitate the internalization of AcLDL, as well as other forms of modified-LDL, during foam cell formation [1]. Numerous studies have used cytochalasin-D in order to specifically examine the impact of macropinocytosis, and not receptor-mediated endocytosis, on LDL uptake [9,11,12]. Nevertheless, in order to confirm the specificity of the inhibitor for macropinocytosis we next examined the expression of CD36 and SR-A mRNA in response to cytochalasin-D treatment. Both CD36 and SR-A are involved in receptor-mediated uptake of modified LDL. As shown in Fig. 3, both CD36 and SR-A mRNA expression were significantly increased, and not decreased, in response to cytochalasin-D when compared to the vehicle control.
Fig. 3.
Cytochalasin-D inhibits LDL uptake through a scavenger receptor-independent mechanism. The relative CD36 and SR-A mRNA expression was determined in THP-1 macrophages incubated with Cytochalasin-D (2.5 μg/ml, n = 3). Gene-specific mRNA expression levels were calculated using the comparative Ct method and normalized to RPL13A levels with vehicle treated cells given an arbitrary value of 1. DMSO was used as a vehicle control. Data represents the mean ± SD. Statistical analysis was performed using the Student’s t-test, *P < 0.05.
3.2. Macropinocytosis contributes to the cytokine-mediated regulation of foam cell formation
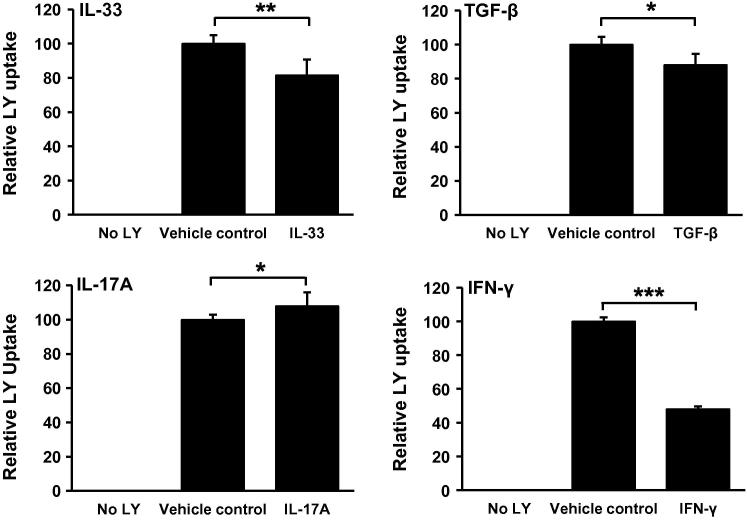
A plethora of research focusing on atherogenic cytokines, such as IFN-γ, IL-33, IL-17A and TGF-β, has highlighted their ability to modulate macrophage foam cell formation through scavenger receptor-dependent mechanisms [3–5] although their effect on macropinocytosis remains undetermined. To this end, we examined the effect of these cytokines on the uptake of LY by THP-1 macrophages. As shown in Fig. 4, IFN-γ, IL-33 and TGF-β significantly inhibited LY uptake when compared to the vehicle treated control cells. Conversely, IL-17A significantly increased LY uptake when compared to the vehicle treated control cells. The concentrations of cytokines used in these experiments reflect pre-defined optimal levels for LY uptake (Supplementary Fig. 2) or, in the case of TGF-β, a clinically relevant concentration that has been previously shown to reduce modified LDL uptake in our system [5,19].
Fig. 4.
Differential regulation of LY uptake in THP-1 macrophages by cytokines. LY uptake was measured in THP-1 macrophages in response to 24 h incubation with IL-33 (10 ng/ml, n = 5), TGF-β (30 ng/ml, n = 4), IL-17A (100 ng/ml, n = 3) or IFN-γ (1000 U/ml, n = 4). PBS/0.1% BSA was used as a vehicle control in all experiments. Data represents the mean ± SD. Statistical analysis was performed using the Student’s t-test, *P < 0.05; **P < 0.01; ***P < 0.001.
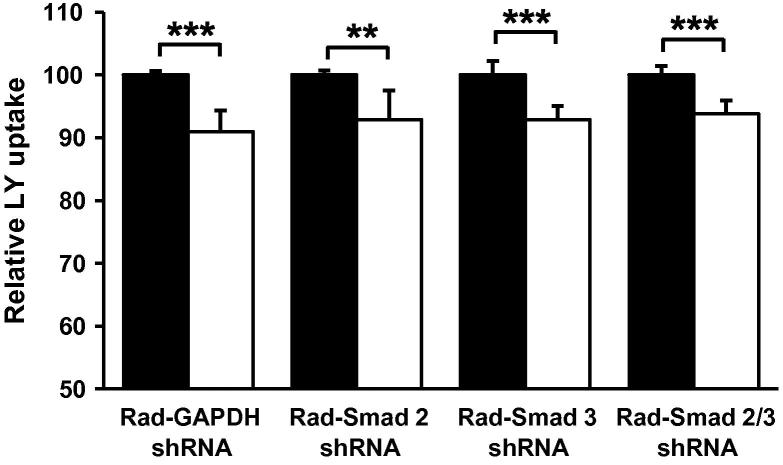
The signaling pathways underlying the cytokine-mediated regulation of receptor-dependent foam cell formation are well-defined [3–5,23]. For example, a recent study by our group has shown that the Smad signal transduction pathway is responsible for the TGF-β-mediated inhibition of AcLDL uptake by THP-1 macrophages [19]. Therefore, in order to determine if a similar pathway plays a role in the TGF-β-mediated inhibition of macropinocytosis, we examined the effect of TGF-β on LY uptake in our previously validated adenovirus-mediated Smad deficient in vitro macrophage model [19]. As shown in Fig. 5, adenoviral administration of shRNA’s specific for Smad-2 (RAd-Smad-2 shRNA), Smad-3 (RAd-Smad-3 shRNA) or both (RAd-Smad-2/3 shRNA) failed to abolish the statistically significant reduction in LY uptake observed between untreated and TGF-β treated GAPDH shRNA (RAd-GAPDH shRNA) infected cells.
Fig. 5.
TGF-β inhibits LY uptake by THP-1 macrophages through a Smad-independent mechanism. LY uptake was measured in THP-1 macrophages following infection with RAd-GAPDH shRNA or RAd-Smad-2 shRNA or RAd-Smad-3 shRNA or RAd-Smad-2 shRNA and RAd-Smad-3 shRNA together in the presence (empty bars) or absence (filled bars) of 24 h TGF-β (30 ng/ml) stimulation (n = 4). PBS/0.1% BSA was used as a vehicle control. Data represents the mean ± SD. Statistical analysis was performed using the Student’s t-test, **P < 0.01; ***P < 0.001.
4. Discussion
Complete understanding of the mechanisms by which LDL is internalized by macrophages during inflammation is fundamental to the progression of atherosclerotic research. To date, receptor-mediated endocytosis represents the best-understood mechanism of LDL uptake: however, it has emerged that macropinocytosis can significantly contribute to the process of foam cell formation [6,9,11,12]. In this report we confirm that modified LDL can indeed be internalised by human macrophages through a potential receptor-independent mechanism as significant reductions in AcLDL internalisation and intracellular cholesterol content were observed in response to cytochalasin-D treatment in both THP-1 macrophages and HMDM (Fig. 2 and Supplementary Fig. 1). Cytochalasins have been routinely used to specifically inhibit macropinocytosis in many studies and its inhibition of lipoprotein uptake has now been confirmed in various monocyte-derived macrophage lineages [9,11,12]. Despite this, we could not eliminate the possibility that our observed changes in AcLDL uptake were the result of inhibition of receptor-mediated LDL uptake by cytochalasin-D. Subsequently, we were able to show that the transcript levels of two well-established markers of receptor-mediated modified LDL uptake, namely SR-A and CD36 [3,24], are not decreased by cytochalasin-D treatment. Considering that previous work by our group has consistently shown a direct correlation between transcripts levels and protein expression of CD36 and SR-A in our system [3,19], these data suggest that macropinocytosis is likely to be the mechanism responsible for our observed changes in AcLDL uptake and intracellular cholesterol content. Interestingly, the increased expression of SR-A and CD36 mRNA observed in our study may indicate the existence of a compensatory mechanism employed by the macrophage to maintain modified LDL uptake and cholesterol levels in the absence of the macropinocytosis pathway. Similar compensatory mechanisms can be seen in other systems such as during the regulation of calcium transport by plasma membrane calcium ATPase 1b in the intestine of Calbindin-D9k knockout mice and the increased expression of β1-Adrenoreceptors to modulate murine gastrointestinal tissue relaxation in the absence of the β3-Adrenoreceptor [25,26].
It is now becoming apparent that macropinocytosis is not a completely constitutive process and is subject to modulation by various mediators of atherosclerosis [14]. For example, recent studies by our own group have demonstrated its inhibition by the athero-protective long chain n-3 polyunsaturated fatty acids [27] such as eicosapentaenoic acid and docosahexaenoic acid which serves to further define the contribution of macropinocytosis during foam cell formation. Similarly, our current study shows that IL-33 and IL-17A, two cytokines whose roles in atherogenesis have been recently identified [3,28], are able to modulate LY uptake by THP-1 macrophages (Fig. 4) in a manner consistent with their previously defined atherogenic properties. IL-33, an anti-atherogenic cytokine known to reduce foam cell formation in human macrophages [3], significantly reduces the amount of LY internalised by human macrophages while IL-17A, mainly a pro-athergenic cytokine that has been shown in some studies to promote the progression of lesion development in apoE knockout mice [28], is able to induce LY uptake. Furthermore, our study also shows that IFN-γ can significantly reduce the uptake of LY by THP-1 macrophages (Fig. 4) suggesting an anti-atherogenic role for IFN-γ during foam cell formation. In light of both pro- and anti-atherogenic roles of IFN-γ, at least in vitro [29], it seems plausible to suggest that the magnitude of IFN-γ induced receptor-dependent LDL uptake seen in various studies [29] is sufficient to mask its inhibitory effect on macropinocytosis resulting in a net foam cell stimulatory effect. However, it is possible that inhibition of macropinocytosis represents another anti-atherogenic action of IFN-γ in vitro.
Finally, our study shows that the anti-atherogenic cytokine, TGF-β, can also inhibit the uptake of LY by THP-1 macrophages (Fig. 4), which is consistent with a plethora of existing evidence demonstrating the athero-protective properties of this cytokine [30]. Interestingly, as seen in our previously established virally mediated Smad-deficient system (Fig. 5), this process was found to occur independently of the Smad signal transduction pathway, unlike the Smad-dependent TGF-β-mediated inhibition of receptor-dependent AcLDL uptake [19], suggesting that macropinocytosis and receptor-mediated endocytosis may be regulated by distinct signaling pathways. Although the mechanisms underlying the TGF-β-mediated inhibition of macropinocytosis remains un-elucidated, TGF-β is known to signal through many other signalling molecules such as c-Jun N-terminal kinase, p38 kinase and casein kinase 2 [30], which may therefore represent promising avenues for future macropinocytotic research.
4.1. Conclusion
In summary, we have confirmed that macropinocytosis plays a significant role during the process of foam cell formation by facilitating the internalisation of AcLDL in human macrophages. Furthermore we demonstrate, for the first time, that the process of macropinocytosis is modulated by key cytokines implicated in atherosclerosis such as IFN-γ, TGF-β, IL-33 and IL-17A in a manner mostly consistent with their ability to promote or hinder foam cell formation and suggest that TGF-β inhibits macropinocytosis through a Smad-independent pathway. Together, these data clearly demonstrate the importance of macropinocytosis during foam cell formation and highlight it as a potential target for therapeutic intervention.
Acknowledgements
This work was supported by the British Heart Foundation (PG/08/073/25520).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cyto.2013.05.016.
Appendix A. Supplementary material
Supplementary material contains Figs. 1 and 2.
References
- 1.McLaren J.E., Michael D.R., Ashlin T.G., Ramji D.P. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Michael D.R., Ashlin T.G., Buckley M.L., Ramji D.P. Macrophages, lipid metabolism and gene expression in atherogenesis: a therapeutic target of the future? Clin Lipidol. 2012;7:37–48. [Google Scholar]
- 3.McLaren J.E., Michael D.R., Salter R.C., Ashlin T.G., Calder C.J., Miller A.M. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–1229. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 4.Li N., McLaren J.E., Michael D.R., Clement M., Fielding C.A., Ramji D.P. ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 5.Irvine S.A., Foka P., Rogers S.A., Mead J.R., Ramji D.P. A critical role for the Sp1-binding sites in the transforming growth factor-beta-mediated inhibition of lipoprotein lipase gene expression in macrophages. Nucl Acids Res. 2005;33:1423–1434. doi: 10.1093/nar/gki280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruth H.S., Huang W., Ishii I., Zhang W.Y. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–34580. doi: 10.1074/jbc.M205059200. [DOI] [PubMed] [Google Scholar]
- 7.Jones N., Willingham M. Modified LDLs are internalized by macrophages in part via macropinocytosis. Anat Rec. 1999;255:57–68. doi: 10.1002/(SICI)1097-0185(19990501)255:1<57::AID-AR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Racoosin E., Swanson J. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao W., Li K., Liao K. Macropinocytosis contributes to the macrophage foam cell formation in RAW2647 cells. Acta Biochim Biophys Sin. 2009;41:773–780. doi: 10.1093/abbs/gmp066. [Shanghai] [DOI] [PubMed] [Google Scholar]
- 10.Buono C., Anzinger J.J., Amar M., Kruth H.S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruth H., Jones N., Huang W., Zhao B., Ishii I., Chang J. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B., Li Y., Buono C., Waldo S.W., Jones N.L., Mori M. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF) J Biol Chem. 2006;281:15757–15762. doi: 10.1074/jbc.M510714200. [DOI] [PubMed] [Google Scholar]
- 13.Anzinger J.J., Chang J., Xu Q., Buono C., Li Y., Leyva F.J. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler Thromb Vasc Biol. 2010;30:2022–2031. doi: 10.1161/ATVBAHA.110.210849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller Y.I., Choi S.H., Wiesner P., Bae Y.S. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol. 2012;167:990–999. doi: 10.1111/j.1476-5381.2012.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S.H., Harkewicz R., Lee J.H., Boullier A., Almazan F., Li A.C. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grewal T., Priceputu E., Davignon J., Bernier L. Identification of a gamma-interferon-responsive element in the promoter of the human macrophage scavenger receptor A gene. Arterioscler Thromb Vasc Biol. 2001;21:825–831. doi: 10.1161/01.atv.21.5.825. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg T.H., Newman A.S., Swanson J.A., Silverstein S.C. ATP4-permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 18.Draude G., Lorenz R. TGF-beta1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am J Physiol Heart Circ Physiol. 2000;278:H1042–H1048. doi: 10.1152/ajpheart.2000.278.4.H1042. [DOI] [PubMed] [Google Scholar]
- 19.Michael D.R., Salter R.C., Ramji D.P. TGF-β inhibits the uptake of modified low density lipoprotein by human macrophages through a Smad-dependent pathway: a dominant role for Smad-2. Biochim Biophys Acta. 2012;1822:1608–1616. doi: 10.1016/j.bbadis.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 21.Swanson J. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94(Pt 1):135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein J., Ho Y., Basu S., Brown M. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith E., Prasad K.M., Butcher M., Dobrian A., Kolls J.K., Ley K. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaren J.E., Calder C.J., McSharry B.P., Sexton K., Salter R.C., Singh N.N. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010;184:5827–5834. doi: 10.4049/jimmunol.0903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson D.S., Evans B.A., Summers R.J. Beta(1)-adrenoceptors compensate for beta(3)-adrenoceptors in ileum from beta(3)-adrenoceptor knock-out mice. Br J Pharmacol. 2001;132:433–442. doi: 10.1038/sj.bjp.0703828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G.S., Lee K.Y., Choi K.C., Ryu Y.H., Paik S.G., Oh G.T. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res. 2007;22:1968–1978. doi: 10.1359/jbmr.070801. [DOI] [PubMed] [Google Scholar]
- 27.McLaren J.E., Michael D.R., Guschina I.A., Harwood J.L., Ramji D.P. Eicosapentaenoic acid and docosahexaenoic acid regulate modified LDL uptake and macropinocytosis in human macrophages. Lipids. 2011;46:1053–1061. doi: 10.1007/s11745-011-3598-1. [DOI] [PubMed] [Google Scholar]
- 28.Erbel C., Chen L., Bea F., Wangler S., Celik S., Lasitschka F. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 29.McLaren J.E., Ramji D.P. Interferon gamma: a master regulator of atherosclerosis. Cytok Growth Factor Rev. 2009;20:125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Singh N.N., Ramji D.P. The role of transforming growth factor-beta in atherosclerosis. Cytok Growth Factor Rev. 2006;17:487–499. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material contains Figs. 1 and 2.