SUMMARY
Pre-invasive breast carcinoma cells that proliferate and accumulate within the non-vascular, closed intraductal niche are under severe hypoxic and metabolic stress. Understanding the survival mechanisms used by these cells has revealed therapeutic strategies for killing pre-invasive neoplasms. We have found that autophagy (“self eating”) is a major survival strategy used by pre-invasive carcinoma and breast cancer stem-like cells. Based on this finding, we have opened a clinical trial that is exploring neoadjuvant oral chloroquine anti-autophagy therapy for DCIS. We envision that antiautophagy therapy can be administered in combination with other treatments such as those which elevate intracellular calcium, to create a state of intolerable stress for pre-invasive neoplastic cells, and thereby stop breast cancer before it starts.
Keywords: autophagy, breast, calcium, chemoprevention, chloroquine, DCIS, microenvironment, PMCA2, pre-invasive, vitamin D
FUTURE PERSPECTIVE
We speculate that In the next 10 years a new type of oral breast cancer prevention therapy that kills or suppresses pre invasive lesions will be available for use in high risk patients, and eventually in all women in the post reproductive years. It will be a low toxicity therapy administered for a short time (one month), and this course of therapy may be repeated every 2–5 years. We are currently studying the first generation of this therapy, chloroquine, which disrupts autophagy, as a neoadjuvant therapy for DCIS and ADH (NCT01023477). The outcome of this trial should be known within the next 2 years. If we are successful in showing that chloroquine a) will reduce the radiologic size of the DCIS lesions, or b) reduce proliferation, and perhaps increase apoptosis, of the intraductal neoplastic cells, after the 30 day treatment course, then this will set the stage for wider confirmatory studies by others. The molecular basis of this new class of prevention will take advantage of growing scientific knowledge about the mechanisms used by pre-invasive carcinoma cells to survive and proliferate in the high stress microenvironment of the hypoxic and nutrient deprived breast intraductal niche. The biologic mechanism of this new form of therapy will be different than what has been envisioned for chemoprevention agents in the past. Instead of blocking early stages of the carcinogenic process by long term therapy, the new short term therapy will selectively kill or suppress already transformed genetically abnormal cells that have arisen within the breast duct as precursor lesions of invasive breast cancer.
In this perspective we show how autophagy appears to be an ideal target because pre-invasive carcinoma cells may have become addicted to this form of self-cannibalization to generate energy needed for proliferation under stress. We further envision that other therapies can be combined with anti-autophagy therapy, such as modulators of calcium efflux, so as to enhance the therapy effectiveness, and reduce the number of repeated treatments over a women’s lifetime.
Treatment of pre-invasive breast lesions as a new path to breast cancer prevention
Pre-invasive breast lesions proliferate within the confines of the breast duct and do not extend through the intact ductal basement membrane. One-third of all newly diagnosed breast cancer cases in the US are pre-invasive cancer [1]. Pre-invasive breast neoplasms are non-obligate precursors to invasive and metastatic cancer [1–5]. Thus a subpopulation of women with pre-invasive cancer will go on to develop invasive carcinoma. Compared to the general population, women harboring atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH) have a 3.5 to 5 fold increased risk of developing invasive cancer [1]. The increased relative risk is 7 to 9 fold for lobular carcinoma in situ (LCIS) and 4–12 fold for ductal carcinoma in situ (DCIS)[1]. Pre-invasive lesions with a higher degree of aggressive histologic features (e.g. a higher grade) have a greater risk of developing invasive cancer compared to patients with low grade lesions [2,6–8]. Lesion size, degree of nuclear atypia, and the presence of comedo necrosis (central luminal inflammation interspersed with apoptotic cells) are histopathological parameters identified as affecting the risk of recurrence within the heterogeneous spectrum of premalignant breast lesions [2,6,8]. Genetic, histopathologic and epidemiologic evidence supports that most, if not all, invasive ductal and lobular carcinomas were derived from a precursor pre-invasive lesion [2,9–14]. Consequently, an intervention therapy that directly kills all pre-invasive carcinoma lesions has the potential to eliminate the subset of precursor lesions that will go on to become invasive cancer.
Conventional chemoprevention embraces pharmacologic agents that block one or more early steps of carcinogenesis, initiation, and genetic progression, in cell culture or animal models. Nevertheless, translation of these findings to clinical trials suffers from a 5 to 20 year waiting period to yield the reduction of breast cancer incidence in the target population. In contrast to conventional chemoprevention, we propose that a short term therapy which kills premalignant breast lesions will prevent subsequent invasive cancer. Furthermore, such a therapy can be evaluated for safety and effectiveness as a neoadjuvant therapy for pre-invasive lesions in a time period of only a few years [11,15,16].
Requirements for a therapy that targets pre-invasive breast neoplasia
What are the requirements for a therapy that treats pre-invasive lesions? Firstly, the therapy must possess very low toxicity and be orally administered. These criteria are essential for targeting pre-invasive lesions because no justification exists for administering a toxic intravenous therapy to a woman who may harbor occult pre-invasive lesions but is otherwise healthy. Secondly, the therapy must be a short course, patient administered treatment. Long term therapies are disrupted in their effectiveness by patient non-compliance. Moreover, many therapies that show low toxicity under short term administration exhibit significant toxicity when administered for long time periods. If we imagine a therapy that attacks pre-invasive carcinoma cells, do we want that therapy to block invasion, or to directly kill the carcinoma cells? A large body of investigation has identified molecular mechanisms of invasion which constitute targets for anti-invasion therapies [17,18]. Unfortunately there are two problems with anti-invasion therapies. Firstly, they are expected to have serious side effects since invasion and migration is a normal part of wound healing and ongoing tissue remodeling. Secondly, such a therapy would only target the carcinoma cells that are in the act of invasion. Consequently, an anti-invasion therapy will have to be administered continuously for many years, since we do not know when a pre-invasive lesion will unleash its invasive potential. We conclude that the ideal therapy should be a short term therapy that directly kills the pre-invasive cells.
In this review we will show how a new understanding of pre-invasive carcinoma cells survival in the nutrient deprived, hypoxic, intraductal microenvironment has led to therapeutic strategies that fulfill our criteria for the optimal short term, oral therapy that will directly kill pre-invasive cells. We have found that autophagy (auto-self, phagy-eating) is a major survival strategy used by pre-invasive cells within the breast duct. Based on this finding, we are currently evaluating the safety and effectiveness of chloroquine, an oral, neoadjuvant anti-autophagy therapy for DCIS. We envision that anti-autophagy therapy can be administered in combination with other treatments such as those which elevate intracellular calcium in the carcinoma cells, to create a state of intolerable stress for pre-invasive cells, and thereby stop breast cancer before it starts.
Therapy that kills pre-invasive breast lesions is a short-cut to the identification of a short term intervention therapy that eradicates the precursors of breast cancer [11,15,16]. (Figure 1) A treatment that kills pre-invasive breast lesions follows the same rationale used for the prevention of a) colorectal cancer by colon polypectomy, and b) cervical cancer by ablating cervical dysplasia.
Figure 1.
Treating pre-invasive lesions is a means to prevent breast cancer. Pre-invasive carcinomas of the breast are non-obligate precursors to subsequent invasive and metastatic carcinoma. A short term therapy that kills pre-invasive carcinoma cells, administered to women who harbor pre-invasive lesions with truncate the emergence of invasive cancer.
Histopathology of pre-invasive breast lesions: microenvironment constraints for intraductal neoplastic cells
Intraductal lesions are classified based on their morphologic appearance. Lobular lesions consist of cuboidal cells characteristic of luminal cells of normal breast acini, whereas ductal lesions are comprised of moderate-large columnar cells similar to normal breast duct cells [1]. The pre-invasive stages of lobular breast cancer include atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS). ALH and LCIS are considered as non-obligate precursors of invasive lobular carcinoma (ILC). The corresponding lesions of the ductal subtype include flat epithelial atypia (FEA), atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS)[1]. FEA, ADH, and DCIS are considered the non-obligate precursors of invasive ductal carcinoma (IDC). The pathological distinction between ADH and DCIS is based upon the degree of architectural atypia and the size and the extent of epithelial proliferation [1,6,8]. DCIS is further sub-classified into low-, intermediate-, and high-grade categories using histomorphological parameters that include cytomorphological and architectural features of the breast gland such as tubule formation/differentiation, nuclear pleomorphism, and mitotic index (proliferation rate)[1]. Necrosis can be a prominent feature of low and high grade DCIS, and the degree of necrosis correlates with the probability of invasive cancer [1,11,19].
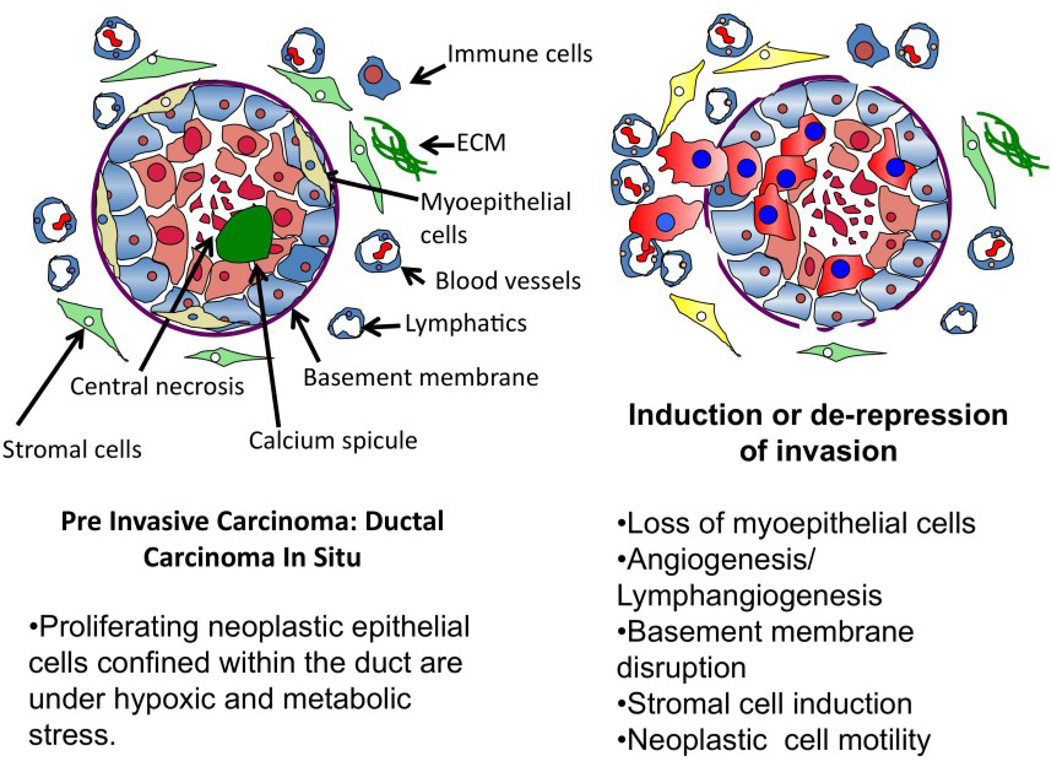
All breast pre-invasive lesions are a proliferation of neoplastic epithelial cells within the closed environment of the intraductal lumen or terminal duct lobular unit (TDLU). The intraductal space in which pre invasive lesions continue to proliferate does not appear to have blood vessels or lymphatics, and is normally surrounded by myoepithelial cells and an intact basement membrane [2,20]. The outside perimeter of the basement membrane interfaces with the connective tissue stroma, mesenchymal cells, immune cells, lymphatics and vasculature [1,2]. The pathologic hallmarks heralding the transition from in situ to invasive carcinoma are a) the extraductal extension of the neoplastic cells and b) an alteration in the cellular microecology of the lesion. By definition, pre-invasive neoplasms have not yet crossed the basement membrane boundary [1,21] to invade beyond their intraductal origin, and then metastasize. Microinvasion is recognized as a group of neoplastic cells that have traversed the duct basement membrane and have come into direct contact with the stroma where they can invade blood vessels, nerves and lymphatics. In addition to a disruption of the basement membrane, the cellular histomorphologic feature that distinguishes DCIS from infiltrating ductal carcinoma (IDC) is the disappearance of the organized myoepithelial layer [1,11,19,22,23] (Figure 2).
Figure 2.
Microenvironment of the pre-invasive breast cancer lesion. Pre-invasive neoplastic cells must adapt to proliferate in a high stress environment. Adaption to stress may promote genetic instability, carcinogenesis, and invasion. A. Proliferating neoplastic epithelial cells confined within the duct are under hypoxic and metabolic stress. B. Induction of invasion coincides with Loss of myoepithelial cells, angiogenesis/lymphangiogenesis, basement membrane disruption, stromal cell induction, and neoplastic cell motility.
Intraductal neoplastic cells proliferate in a high stress microenvironment
Pre-invasive neoplastic cells that proliferate and accumulate within the non-vascular intraductal space are under severe hypoxic and metabolic stress. Pre-invasive cells must adapt to hypoxic stress within the duct in order to survive and proliferate. The vascular density of tissues is homeostatically regulated in all tissues and all metazoan species to restrict the maximum distance between tissue cells and the nearest blood vessel. The radii of low and high grade DCIS lesions can be as great as 200 to 500 microns (15 to 60 cell diameters) [2]. This greatly exceeds the homeostatic limitations of blood vessel minimum density which has been measured to fall in the range of 25 to 50 microns [24,25]. Multicellular spheroids grown in culture provide a model for the oxygen diffusion limitations of the non-vascularized DCIS cell colony packed within the duct bounded by the basement membrane [11,12]. Spheriods grown in culture exhibit central necrosis when the radius of the spheroid exceeds a minimum distance required for oxygen to diffuse in from the surface of the spheroid. The width of the viable rim of multicellular tumor spheroids grown in spinner culture was reported to be 150 microns over a wide range of spheroid diameters from 400 to 1000 microns [26,27]. This distance is in the range of the average distance of vessels from the nearest area of necrosis studied in solid tumors [28,29]. Therefore typical intermediate or high grade pre-invasive lesions expand to a diameter greatly exceeding the limitations of oxygen diffusion. High grade pre-invasive lesions may contain a central zone of necrosis with surrounding a viable rim of 5 to 25 cell layers thick cells. The viable intraductal rim can exceed the minimum distance for adequate oxygenation, demonstrating that pre-invasive cells have adapted to survive and proliferate under hypoxic conditions. However, adaption to stress within the duct may contribute to the carcinogenic process [30–32].
Survival and adaption of premalignant cells within the stressful, hypoxic and nutrient deprived intraductal microenvironment may promote genetic instability and the selection of neoplastic cells with invasive potential. Metabolic acidosis [33,34] and hypoxic stress within the tumor microenvironment induces mutagenesis and genetic instability [19,30–32]. Adaption to survival under stress within the intraductal microenvironment can override normal cellular stress responses, leading to continued growth of genetically damaged cells.
In hypoxic conditions, DNA binding transcription factors form complexes with hypoxia inducible factors (HIFs) directing most of the adaptation. Hypoxia inducible factor mediates the adaptive response to maintain oxygen homeostasis [28,32]. Under normal oxygen levels prolyl hydroxylases (PHD1–3) use oxygen as a substrate to modify proline residues on the oxygen dependent subunit HIF-1α. Hydroxylated HIFα is recognized by the von Hippel Lindau tumor suppressor (VHL) and thereby targeted for proteasomal degradation [35]. Consequently, normal oxygen levels are associated with degradation of HIFα, and conversely, HIFα levels are increased by hypoxia. HIF mediated adaptation to hypoxia can inhibit p53 mediated death of DNA damaged cells [32]. Hypoxic stress, independent of HIF, is associated with decreased rate of DNA damage repair, and the up-regulation of invasion and metastatic potential [36–38]. Consequently pre-invasive cells that adapt to living in a state of hypoxia can proliferate in the presence of genetic mutations that drive tumor progression and induce or de-repress invasion [38,39]. This stressful intraductal DCIS microenvironment is a “training ground” for malignant cells [11,12]. (Figure 2)
Living in the harsh intraductal microenvironment requires cells to perform two major functions [11]: a) suppress pathways designed to normally eliminate damaged cells, and b) find other ways to harness energy. Genotoxic, metabolic, hypoxic, and oxidative stress engage stress-response programs in normal cells. If DNA damage is significant, then general classes of pathways are activated to eliminate propagation of damaged cells by suppressing the replication rate (senescence) or increasing the death (apoptosis) rate of damaged cells. Premalignant and malignant cells have been proposed to down regulate suppressor pathways or up-regulate prosurvival pathways [6,34]. Nevertheless, even if a cell can resist programmed cell death or senescence it will not survive in a hypoxic nutrient deprived environment unless it can find alternative sources of energy for cellular functions. Pre-invasive cells can exhibit a high rate of proliferation as indicated by Ki-67 staining, thus they are avoiding senescence. Alternative pathways for obtaining energy include autophagy, anaerobic respiration, or increasing the efficiency of aerobic respiration [6,34,40]. Understanding how proliferating pre-invasive cells circumvent stress-induced death, or cell cycle arrest, and find alternative sources of energy provides a new functional approach to create intervention strategies for premalignant breast lesions.
Pre-invasive cells use autophagy to find new sources of energy under stress
Understanding cell processes, which promote survival of malignant progenitor cell in DCIS, provides strategies for killing DCIS cells, by removing their ability to survive in the stressful intraductal space. We have found that autophagy is a major mechanism used by DCIS cells to survive in the high stress environment of the intraductal space [11,12]. We examined the hypothesis that fresh human DCIS lesions contain pre-existing carcinoma precursor cells with breast cancer stem-like cell properties, and possess invasive properties [12]. Our model system for ex vivo organoid culture of fresh human DCIS lesions, induced the emergence of neoplastic epithelial cells exhibiting the following characteristics: a) spontaneous generation of hundreds of spheroids (mammospheres) and duct-like 3-D structures in culture within 2–4 weeks, from both low and high grade lesions, b) tumorigenicity in NOD SCID mice, c) cytogenetically abnormal (copy number loss or gain in chromosomes including 1, 5, 6, 8, 13, 17) compared to the normal karyotype of the non-neoplastic cells in the source patient’s breast tissue, d) in vitro migration and invasion of autologous breast stroma, and e) up-regulation of signal pathways linked to, and components of, cellular autophagy and prosurvival [12].
Multiple markers of autophagy were present in the patient’s original DCIS lesion and the mouse xenograft [12]. Treatment with a lysosomotropic inhibitor (chloroquine phosphate) of autophagy completely suppressed the generation of DCIS spheroids/3-D structures, suppressed ex vivo invasion of autologous stroma, induced apoptosis, suppressed autophagy associated proteins including Atg5, AKT/PI3 Kinase, and mTOR, eliminated cytogenetically abnormal cells from the organ culture, and completely prevented tumor xenograft growth. Therefore, these malignant precursor cells must utilize cellular autophagy for survival [12]. Recently it has been confirmed that cultured lines of breast cancer stem cells require autophagy for survival [41]. Thus breast cancer stem like cells first arise within pre-invasive lesions prior to the overt manifestation of invasion. Since these cells survive by the use of autophagy, this pathway constitutes an exciting therapeutic target.
What is autophagy? Autophagy is known to be a main determinant of cell fate in response to metabolic stress [40,42–45]. Autophagy (literally “self-eating”) denotes any cellular pathway involving the delivery of cytoplasmic material to the lysosome for enzymatic digestion. There are at least three types of autophagy, chaperone-mediated autophagy, microautophagy, and macroautophagy. Chaperone-mediated autophagy is a mechanism that allows the direct lysosomal import of proteins which contain a particular pentapeptide motif [46]. In contrast, both microautophagy and macroautophagy involve dynamic membrane rearrangements which snare or engulf the cytoplasmic target for loading into the lysosome. In microautophagy, cytoplasmic material is directly engulfed at the surface of the lysosome by septation, protrusion or invagination of the limiting membrane.
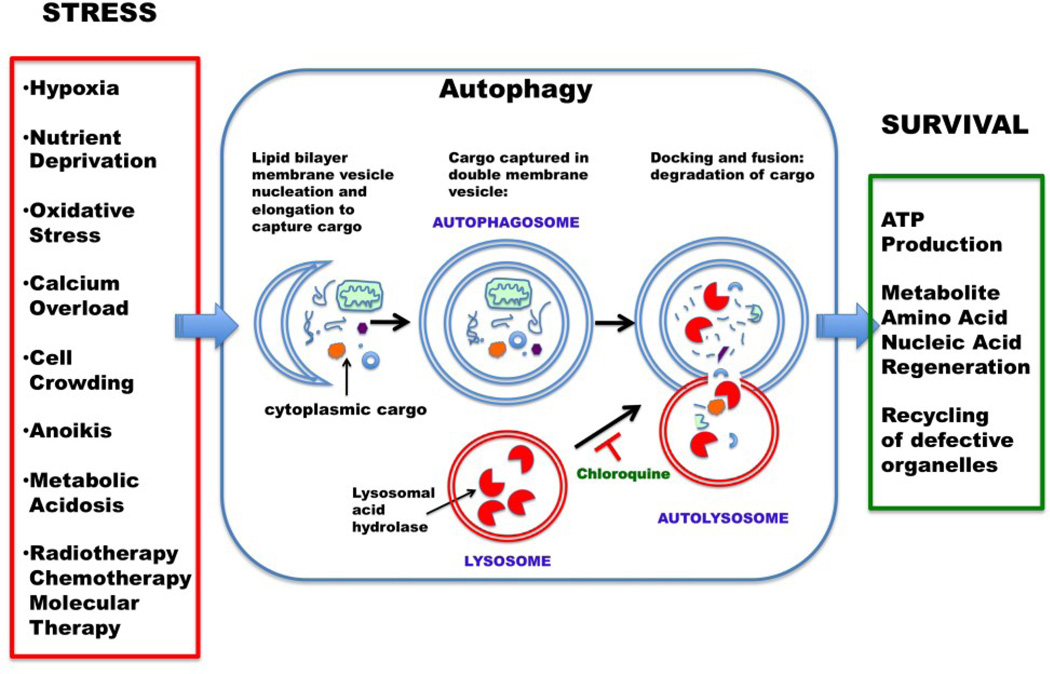
Macroautophagy involves the sequestration of cytoplasmic contents into a separate double-membrane cytosolic vesicle referred to as an autophagosome, (Figure 3) that subsequently fuses with a late endosome or lysosome to form an autophagolysosome (or autolysome). Inside the autophagolysosome, the lysosomal hydrolases degrade the sequestered material. The degrade products then become available to the cell for recycling. Macroautophagy (usually just termed “autophagy”) is the major regulated cellular pathway for degrading long-lived proteins and the only known pathway for degrading cytoplasmic organelles [46].
Figure 3.
Macroautophagy (Autophagy) is a controlled cell process of self-cannibalization that can be used to generate energy, and survive, in the face of stress. Autophagosomes ensnare cytoplasmic organelles and proteins within a double membrane vesicle which fuses with a lysosome containing proteinases that digest the cargo, thereby releasing degradation products for recycling or energy production. Chloroquine interferes with autophagy by altering the lysosome pH which blocks the fusion of the autophagosome with the lysosome thereby suppressing the digestion of the cargo.
Autophagy in essence is a controlled process of self-cannibalization [47,48]. The output of the lysosomal digestion are inputs to cellular metabolism, generating energy to build new proteins and membranes. During cell starvation, autophagy provides an internal source of nutrients for energy generation and, thus, survival [45,49]. Pre invasive neoplastic cells can multiply within the nutrient and oxygen deprived intraductal space because they exploit autophagy to survive [11,12]. Invasive carcinoma cells also exploit autophagy to survive in the face of chemotherapy or molecular targeted therapy [11,50–53].
Autophagy is triggered through diverse sources of stress impinging on pre-invasive breast cancer. The first trigger is hypoxia and nutrient stress. Proliferating ductal epithelial cells accumulating within the breast duct do not have access to the vasculature outside the duct. For this reason, high grade DCIS is associated with central necrosis, and the accumulation of lipofuschin and calcium. The activation of autophagy may divert the hypoxic DCIS cells away from apoptosis. Autophagy may also be integral to the removal of dead and dying intraductal DCIS cells, since the intraductal space is nonvascular and dead cells are not removed by immune phagocytes which are excluded from the special closed duct niche [54].
The second trigger of autophagy is anoikis [55], the activation of apoptotic cell death for cells that have been separated from their normal adhesion substratum. Autophagy has been shown to be a key regulator of survival for cells deprived of an anchoring substratum, and may play an important role for cell survival in any anchorage independent state. Normal glandular epithelial cells require attachment to, or association with, the basement membrane extracellular matrix (ECM) for continued survival. During ductal hyperplasia and dysplasia epithelial cells exist at a substantial distance away from association with the peripheral basement membrane. Moreover, autophagy may assist invading carcinoma cells as they migrate into the stroma in the absence of a basement membrane anchor. A third trigger of autophagy is matrix degradation. High grade DCIS, microinvasion, and overt carcinoma invasion is associated with interruptions, remodeling, and enzymatic breakdown of the basement membrane and the stromal ECM [1,21]. Autophagy may facilitate cell movement through areas of degraded matrix by the phagocytic processing of matrix breakdown fragments. A fourth trigger for autophagy is calcium. Microcalcifications are mammographic indicators of high grade DCIS, and calcium phosphate precipitates are potent inducers of autophagy [56].
Autophagy, a new a target for eliminating pre-invasive carcinoma cells
Chloroquine (CQ) is an orally administered small molecule inhibitor that blocks the autophagy pathway [57–60] fulfills the criteria set forth above for the optimal therapy that targets pre-invasive cancer. Some authors have proposed that anti-cancer therapies should stimulate autophagy (instead of inhibiting autophagy) so that the tumor cell will eat itself to death [61]. The current data shows the opposite: autophagy is a survival mechanism co-opted by pre-invasive cells and breast cancer stem cells [41,51]. There is strong pre clinical and clinical data justifying the use of chloroquine as an anti-cancer agent. The safety profile of CQ has been well established for long term prophylaxis, and acute therapy of malaria worldwide [58]. CQ has been shown to suppress N-methyl- N-nitrosurea induced mouse breast carcinogenesis [62], enhances the effectiveness of tyrosine kinase inhibitor treatment of primary CML stem cells [50], and has been proposed as a potential means to enhance the effectiveness of tamoxifen in vitro for tamoxifen resistant breast carcinoma cells by blocking autophagy dependent cell survival [52,63]. CQ has been proposed as a therapy for Myc induced lymphomagenesis because CQ induces lysosomal stress that causes a p53 dependent cell death that does not require caspase mediated apoptosis [64]. Beyond breast cancer treatment, CQ therapy has also been shown to reduce lymphoma progression [64,65], suppress melanoma invasion [66,67] and arrest pancreatic cancer in animal models [68,69], and has been employed in clinical trials of glioblastoma [70]. Potential additional primary and secondary benefits of chloroquine, beyond direct killing of pre-invasive cells are a) the suppression of carcinogen induced transformation or progression of breast epithelium, and b) the enhancement of tamoxifen sensitivity in ER+ breast carcinoma. Finally, CQ, in contrast to a large molecule such as a therapeutic antibody, is a small molecule that can rapidly penetrate the basement membrane of the pre-invasive lesions to gain immediate access to the neoplastic cells [58,71].
PINC Trial (Preventing Invasive breast Neoplasia with Chloroquine)
Our ongoing clinical study (clinicaltrials.gov identifier NCT01023477), is examining the safety and effectiveness of chloroquine phosphate (Aralen) administration for a one month period to patients with low, intermediate, or high grade DCIS (Figure 4). This trial is based on our pre-clinical studies [12] in cell culture and animal models which document that a) spheroid forming neoplastic cells can be harvested and propagated from human DCIS lesions, b) the spheroid forming DCIS cells have stem-like properties and generate invasive tumors in mouse xenografts, thus they can be classified as carcinoma precursors, c) the propagated DCIS stem-like cells up regulate autophagy, and autophagy is up regulated in the patient’s DCIS lesions by IHC, and d) Chloroquine kills the DCIS spheroid forming cells in culture and Chloroquine blocks DCIS cell generated tumor growth in xenografts. Chloroquine is known to rapidly enter the lysosomal compartment of cells [58,71]. In our pre-clinical studies Chloroquine therapy induced death and apoptosis in the DCIS cells in less than a week [12].
Figure 4.
Graphic representation of the PINC (Preventing Invasive Neoplasia with Chloroquine) trial design. Chloroquine neoadjuvant therapy is being assessed for all grades of DCIS, in both ER negative and ER positive breast DCIS lesions. The clinical trial endpoints are a) shrinkage by MRI, b) pathologic regression, c) reduction of lesion proliferation index, d) progenitor cell reduction, and e) elimination of genetically abnormal cells.
Patients with DCIS who are ER negative (expected to be approximately one half of the high grade DCIS cases) or who are ER positive, are both eligible for our neoadjuvant trial. Patients, regardless of histologic grade, are randomized to receive chloroquine at one of two doses: 500 mg/week, or 250 mg/week, for four weeks. MRI studies are performed on each patient at enrollment and just before surgical therapy one month after treatment. At the conclusion of the one month treatment period, all patients receive standard of care surgical therapy: mastectomy or lumpectomy depending on the size and confluence of the primary DCIS lesion. Outcome measures, post versus pre therapy, are a) reduction in DCIS lesion volume by MRI b) pathologic regression, c) the reduction or elimination of genetically abnormal tumorigenic DCIS stem like cells, d) and the suppression of cellular proliferation, induction of apoptosis, or disruption of autophagy, as measured by changes in proteomic markers in the post treatment versus the pre-treatment specimen.
The design of the PINC trial has several positive features. The trial takes advantage of the standard of care waiting period between the initial pathologic diagnosis of DCIS and the subsequent surgical therapy. During this waiting period CQ is administered orally. A further positive aspect is that the patient is used as her own control, because the effectiveness of the therapy will be based on a comparison of the pre versus post treatment specimen. In the neoadjuvant trial as designed we have the opportunity to evaluate the DCIS lesions radiologically before therapy, at the time of diagnostic biopsy, and then again, after therapy, prior to surgical standard of care excision of the neoplasm [Figure 4]. Radiologic measurement of efficacy is based on standard RECIST criteria. Formal molecular measures of pathologic efficacy in the pre versus post treatment lesion include a) a reduction in proliferative index by IHC using PCNA or KI67, b) an increase in apoptosis index by IHC using cPARP, and c) a disruption of autophagy by IHC using LC3B staining of autophagosomes.
A final important aspect of this trial is that it can be modified to test other potential therapies, alone or in combination with CQ. Thus the PINC trial can be a short cut for the identification of mono or combination therapies that can be documented to kill or suppress DCIS lesions following one month of oral therapy.
We imagine a future in which a limited course of low toxicity therapy is administered to suppress or eradicate pre-malignant pre-invasive breast lesions, in high-risk patients, even if they are undetectable by standard imaging. Eventually this type of therapy could be extended to the general population. While CQ is a therapeutic strategy being currently evaluated, additional anti-autophagy therapies may become available in the future. Moreover, as we learn more about the survival strategies used by breast carcinoma cells, we can envision new therapies that can be used alone, or in combination with ant-autophagy treatments. An exciting opportunity for combination therapy exists based on recent findings made by one of the co-authors (JW) concerning the importance of calcium export in breast epithelial function and breast cancer cell survival. Calcium metabolism may have a special relevance to pre-invasive breast cancer progression. Pathologists and surgeons have long questioned whether intraductal calcifications, the common radiologic diagnostic feature are a cause, or consequence, of breast cancer [2].
Intraductal microcalcifications record long standing hypoxic stress
Ninety percent of DCIS mammographic diagnoses are based on the presence of microcalcifications [72,73]. While microcalcifications of all types are associated with a broad spectrum of breast lesions, and have a 30–40% overall specificity for malignancy [2], the radiologic location, shape, size, and density of the microcalcification can be highly specific DCIS [74]. Fine linear, occasionally branching, microcalcifications, and pleomorphic small (<0.5mm) calcifications, are typically found within the ductal tree, and are associated with necrotic areas of high grade and intermediate grade DCIS. In contrast, microcalcifications restricted to the lobules, and not the ductal tree, are almost always associated with benign disease such as microcystic adenosis. The chemical composition of most DCIS associated microcalcifications is a subtype of calcium phosphate, hydroxyapatite, which is easily detectable by conventional light microscopy. Upon histopathologic examination, microcalcifications are hematoxyphilic (blue) deposits present within the necrotic center of the DCIS lesion duct, and are often surrounded by a viable rim of DCIS neoplastic cells. The individual calcifications often appear concentrically layered, giving the impression that calcium deposition is accreting over time [75]. Suspicious microcalcifications have been associated with the later stages of fat necrosis [2], and this further supports the concept that calcium phosphate deposition follows the accumulation of necrotic cellular material. As such, microcalcifications provide an important clue about the age of intermediate and high grade DCIS lesions. Since hypoxia induced necrosis precedes calcium deposition, and calcium deposition occurs over time, we can conclude that most intermediate and high grade DCIS lesions are subjected to hypoxic stress for a long period of time prior to diagnosis. Thus, microcalcifications can be a signature of ongoing hypoxic stress and conditions favoring genetic instability [76]. While it is unclear if microcalcifications are related to the pathogenesis of DCIS, they may contribute to the persistence of the DCIS lesions. Insoluble calcium induces autophagy, and may contribute to the local oxidative or metabolic stress within the duct [56].
Proper calcium management by breast epithelium may be a critical determinant of cell survival
An important source of cellular stress in breast epithelium is the intracellular accumulation of calcium ions which are toxic to the cell [77,78]. Export of calcium by breast epithelium, through specialized calcium export channels, is an additional strategy for pre-invasive (and invasive) cancer cells to survive under hypoxic and metabolic stress [79,80].
Alterations in intracellular calcium (Ca+2) serve as important signals that modulate many cellular processes [77,78]. In order to regulate Ca+2, each cell has a calcium (Ca2+) signaling “toolkit” consisting of various ion pumps, transporters, and binding proteins that interact to coordinate cell- and stimulus-specific changes in Ca+2 [77,80]. One of the tools in this kit is the PMCA family, which transport Ca2+ out of the cell in response to ATP hydrolysis [81,82]. There are 4 PMCA genes, of which PMCA1 and 4 are expressed ubiquitously. MECs transport large amounts of Ca2+ against a gradient from the systemic circulation into milk, and the cells must manage the large transcellular Ca2+ flux to avoid disruptions in Ca2+ signaling, Ca2+ toxicity and apoptosis [80]. A specific splice variant of the PMCA2 gene, PMCA2wb, which traffics to the apical membrane, is greatly upregulated in the breast at the onset of lactation, and the loss of PMCA2 reduces milk Ca2+ by 60–70% [80,83,84]. Ca2+ stimulates its own transport into milk, a process mediated by the calcium-sensing receptor (CaSR), which activates the enzymatic activity of PMCA2. Thus PMCA2 is responsible for transporting Ca2+ from MECs into milk at baseline and in response to activation of the CaSR. PMCA2 regulates apoptosis. During pregnancy, MECs proliferate rapidly to supply the large numbers of secretory alveoli necessary for milk production. At the end of lactation, most new MECs die in a two-step process of coordinated apoptosis [85]. Distension of alveoli by retained milk after weaning alters the shape of the epithelial cells, which, in turn, causes rapid downregulation of PMCA2 levels [79]. As a result, there is a decrease in Ca2+ export, a sustained increase in Ca+2, the activation of calpain and the onset of apoptosis. Apoptosis results from intracellular Ca2+ toxicity caused by the reduced ability of PMCA2-null cells to transport Ca2+ out of the cell when Ca2+ uptake is increased in preparation for milk production. Therefore, in addition to its importance for directional Ca2+ transport, PMCA2 also protects against cell death in response to the large influx of Ca2+ into MECs during lactation.
Calcium regulation in breast epithelium: a new target for therapies that kill pre-invasive carcinoma
Intracellular Ca2+ transients regulate cellular processes important for cancer, including proliferation, adhesion, migration and apoptosis [86,87]. Ca2+ entry across the plasma membrane activates proliferation. This results both from direct effects of Ca2+ and calmodulin on cell cycle regulators and from the activation and amplification of the Ras/Raf/MAPK pathway [88–90]. While calcium influx is tied to cell proliferation, there is a tight upper limit on the intracellular calcium concentration. Excess accumulation of Ca2+ by cancer cells induces cell death. Thus, cancer cells must reprogram intracellular Ca2+ dynamics to allow for the proliferative effects of Ca2+ entry, while preventing apoptosis from calcium overload [86,87]. Calcium export through PMCA channels, therefore, can be a major mechanism to prevent apoptosis when pre invasive cells are proliferating in a high stress microenvironment.
1α,25-Dihydroxyvitamin D3 (1,25D3) is the activated form of vitamin D3 and a major regulator of calcium homeostasis. We hypothesize that it can be used, as a therapeutic strategy to overload pre-invasive cells with Ca+2 and overwhelm the PMCA2 efflux survival mechanism, thereby promoting apoptosis. During lactation, and in cancer cells, vitamin D increases the uptake of calcium into cells through a combination of voltage dependent and independent channels [91–93]. However, a significant distinction is that in normal cells, vitamin D induces a transient rise in intracellular calcium levels, while in breast cancer cell lines, it induces a sustained increase in intracellular calcium and induces cell-cycle arrest in malignant breast cells, but not normal mammary epithelium in culture [93,94]. Therefore there is strong rationale for a future strategy to kill DCIS cells by stimulating intracellular calcium uptake with Vitamin D treatment while also inhibiting autophagy with chloroquine and/or inhibiting PMCA2 function. These combined maneuvers should induce intracellular calcium crisis specifically in DCIS lesions and convert autophagic [95,96] related and calcium export survival pathways into apoptotic death pathways. If our hypothesis is correct, these experiments will provide the rationale for a neoadjuvant clinical trial of combination high dose Vitamin D and chloroquine for DCIS.
EXECUTIVE SUMMARY.
Treatment of pre-invasive breast lesions are a new path to breast cancer prevention
Pre-invasive breast neoplasms are non-obligate precursors to invasive and metastatic cancer.
Pre-invasive breast carcinoma cells that proliferate and accumulate within the non-vascular closed intraductal niche are under severe hypoxic and metabolic stress. Understanding the survival mechanisms used by these cells has revealed therapeutic strategies for killing pre-invasive neoplasms before they can invade out of the duct.
Short term treatment of pre-invasive breast lesions are a new path to breast cancer prevention.
Requirements for a therapy that targets pre-invasive breast neoplasia
The ideal treatment agent is an orally administered small molecule with very low toxicity.
We have found that autophagy is a major survival strategy used by pre-invasive cells, and breast cancer stem like cells. Our findings indicate that breast cancer stem like cells that exist within invasive carcinoma first arise within pre invasive lesions prior to the overt manifestation of invasion. Since these cells survive by the use of autophagy, this pathway constitutes an exciting therapeutic target.
Chloroquine (CQ) is an orally administered small molecule inhibitor that blocks the autophagy pathway. The safety profile of CQ has been well established for long term prophylaxis, and acute therapy of malaria worldwide.
Histophatology of pre-invasive breast lesions: microenvironment constraints for intraductual neoplastic cells
All breast pre-invasive lesions are a proliferation of neoplastic epithelial cells within the closed environment of the intraductal lumen or terminal lobular unit.
DCIS can be distinguished from infiltrating ductal carcinoma (IDC) by the disappearance of the organized myoepithelial layer.
Intraductual neoplastic cells proliferate in a high stress microenvironment
Pre-invasive neoplastic cells that proliferate and accumulate within the non-vascular intraductal space are under sever hypoxic and metabolic stress.
Adaption to survival under stress within the intraductal microenvironment can override normal cellular stress responses, leading to continued growth of genetically altered cells.
Living in the harsh intraductal microenvironment requires cells to perform two major functions: a) suppress pathways designed to normally eliminate damaged cells, and b) find alternate cellular methods of harnessing energy.
Pre-invasive cells use autophagy to find new sources of energy under stress
Autophagy (literally “self-eating”) is a controlled process of self-cannibalization. Cells capture and consume their own cytoplasm and organelles. The output of the lysosomal digestion is energy, providing ATP for cellular metabolism.
During cell starvation, autophagy provides an internal source of nutrients for energy generation and, thus, survival. Pre-invasive neoplastic cells can multiply within the nutrient and oxygen deprived intraductal space because they exploit autophagy to survive.
Invasive carcinoma cells also exploit autophagy to survive in the face of cytotoxic stress caused by chemotherapy or molecular targeted therapy.
Autophagy, a new target for eliminating pre-invasive carcinoma cells
Chloroquine is an orally administered small molecule that blocks the autophagy pathway. Chloroquine can rapidly penetrate the basement membrane of the pre-invasive lesions.
The safety profile of chloroquine has been well established worldwide for long-term prophylaxis and acute therapy of malaria.
Chloroquine provides a potential therapy for estrogen receptor (ER) negative breast DCIS lesions.
PINC Trial (Preventing Invasive breast Neoplasia with Chloroquine)
Based on our finding that autophagy is a target for pre-invasive cancer, we are currently evaluating the safety and effectiveness of chloroquine, an anti-autophagy oral neoadjuvant therapy for DCIS in the PINC trial (Preventing Invasive Neoplasia with Chloroquine).
As we learn more about the survival strategies used by breast carcinoma cells, we can envision future therapies that can be used alone, or in combination with, ant-autophagy treatments.
Therapy that kills pre-invasive breast lesions is a short-cut to the identification of a short term intervention therapy that eradicates the precursors of breast cancer. A treatment that kills pre-invasive breast lesions follows the same rationale used for the prevention of a) colorectal cancer by colon polypectomy, and b) cervical cancer by ablating cervical dysplasia.
Intraductual microcalcifications record long standing hypoxic stress
Intraductal calcium spicules are radiologic hallmarks of pre invasive breast cancer. An exciting opportunity for combination therapy exists based on recent findings concerning the importance of calcium export in breast epithelial function and breast cancer cell survival.
We propose a future strategy to kill pre-invasive cells by stimulating intracellular calcium uptake with Vitamin D treatment while also inhibiting autophagy with chloroquine and/or inhibiting breast epithelial calcium export function. These combined maneuvers should induce intracellular calcium crisis specifically in DCIS lesions and convert autophagic and calcium export survival pathways into apoptotic death pathways.
We imagine a future in which a limited course of low toxicity therapy is administered to suppress or eradicate premalignant pre invasive breast lesions, in high-risk patients, even if they are undetectable by standard imaging.
Acknowlegements
The work described herein was supported by grants to LAL from the Department of Defense Breast Cancer Research Program [U.S. Army Medical Research Acquisition Activity], and the Susan G. Komen Foundation.
Abbreviations
- ADH
atypical ductal hyperplasia
- ALH
atypical lobular hyperplasia
- CaSR
calcium-sensing receptor
- CQ
chloroquine
- Ca+2
calcium
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- FEA
flat epithelial atypia
- HIF
hypoxia inducible factor
- IDC
infiltrating ductal carcinoma
- ILC
invasive lobular carcinoma
- LCIS
lobular carcinoma in situ
- MRI
magnetic resonance imaging
- PINC
Preventing Invasive Neoplasia with Chloroquine
- TDLU
terminal ductal lobular unit
- VHP
von Hippel-Lindau
REFERENCES
- 1. Sgroi DC. Preinvasive breast cancer. Annu Rev Pathol. 2010;5:193–221. doi: 10.1146/annurev.pathol.4.110807.092306. ** Comprehensive general review of pre invasive breast cancer pathology including an excellent discussion of genetic progression and the tumor microenvironment.
- 2. Boecker W. Preneoplasia of the Breast. Munich, Germany: Elsevier; 2006. * Excellent textbook of pre-invasive breast cancer pathology that includes a review of breast stem cell biology and breast morphogenesis.
- 3.Claus EB, Chu P, Howe CL, et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER-2/neu and hormone receptors. Exp Mol Pathol. 2001;70(3):303–316. doi: 10.1006/exmp.2001.2366. [DOI] [PubMed] [Google Scholar]
- 4.Hu M, Yao J, Carroll DK, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13(5):394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;49(4):751–758. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Berman HK, Gauthier ML, Tlsty TD. Premalignant breast neoplasia: a paradigm of interlesional and intralesional molecular heterogeneity and its biological and clinical ramifications. Cancer Prev Res (Phila Pa) 2010;3(5):579–587. doi: 10.1158/1940-6207.CAPR-10-0073. [DOI] [PubMed] [Google Scholar]
- 7.Betsill WL, Jr, Rosen PP, Lieberman PH, Robbins GF. Intraductal carcinoma. Long-term follow-up after treatment by biopsy alone. JAMA. 1978;239(18):1863–1867. doi: 10.1001/jama.239.18.1863. [DOI] [PubMed] [Google Scholar]
- 8.Lagios MD. Heterogeneity of duct carcinoma in situ (DCIS): relationship of grade and subtype analysis to local recurrence and risk of invasive transformation. Cancer Lett. 1995;90(1):97–102. doi: 10.1016/0304-3835(94)03683-a. [DOI] [PubMed] [Google Scholar]
- 9.Castro NP, Osorio CA, Torres C, et al. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 2008;10(5):R87. doi: 10.1186/bcr2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins LC, Tamimi RM, Baer HJ, et al. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer. 2005;103(9):1778–1784. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- 11. Espina V, Liotta LA. What is the malignant nature of human ductal carcinoma in situ? Nat Rev Cancer. 2010 doi: 10.1038/nrc2950. ** Initial proposal of the concept that anti-autophagy therapy is a strategy for killing pre-invasive breast cancer. Review of the high stress intraductal microenvironment, how this contributes to carcinogenesis, and why it is a target for therapy.
- 12. Espina V, Mariani BD, Gallagher RI, et al. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One. 2010;5(4):e10240. doi: 10.1371/journal.pone.0010240. ** Initial demonstration that tumorigenic cancer stem like cells can be propagated from preinvasive human breast cancer lesions. First demonstration for the role of autophagy in the survival of pre-invasive carcinoma.
- 13.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11(1):R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XJ, Salunga R, Tuggle JT, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100(10):5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelloff GJ, Sigman CC. Assessing intraepithelial neoplasia and drug safety in cancer-preventive drug development. Nat Rev Cancer. 2007;7(7):508–518. doi: 10.1038/nrc2154. * Recognition that pre-invasive neoplasms are an important target for therapeutic development and cancer prevention.
- 16. O'Shaughnessy JA, Kelloff GJ, Gordon GB, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8(2):314–346. * Early proposal that accelerated drug development should be focused on pre-invasive breast neoplasia.
- 17.Dickson RB, Johnson MD, Maemura M, Low J. Anti-invasion drugs. Breast Cancer Res Treat. 1996;38(1):121–132. doi: 10.1007/BF01803790. [DOI] [PubMed] [Google Scholar]
- 18.Seton-Rogers S. Metastasis: opposing forces in invasion. Nat Rev Cancer. 2011;11(9):624–625. doi: 10.1038/nrc3126. [DOI] [PubMed] [Google Scholar]
- 19.Bussolati G, Bongiovanni M, Cassoni P, Sapino A. Assessment of necrosis and hypoxia in ductal carcinoma in situ of the breast: basis for a new classification. Virchows Arch. 2000;437(4):360–364. doi: 10.1007/s004280000267. [DOI] [PubMed] [Google Scholar]
- 20.Tavassoli F. Intraductal Proliferative Lesions. In: Tavassoli F, Devilee P, editors. Tumors of the Breast and Female Genital Organs. Lyon: IARC-Press; 2003. pp. 63–73. [Google Scholar]
- 21.Liotta LA, Tryggvason K, Garbisa S, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 22.Mayr NA, Staples JJ, Robinson RA, Vanmetre JE, Hussey DH. Morphometric studies in intraductal breast carcinoma using computerized image analysis. Cancer. 1991;67(11):2805–2812. doi: 10.1002/1097-0142(19910601)67:11<2805::aid-cncr2820671116>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Pinder SE. Ductal carcinoma in situ (DCIS): pathological features, differential diagnosis, prognostic factors and specimen evaluation. Mod Pathol. 2010;23(Suppl 2):S8–S13. doi: 10.1038/modpathol.2010.40. [DOI] [PubMed] [Google Scholar]
- 24.Lyng H, Sundfor K, Trope C, Rofstad EK. Oxygen tension and vascular density in human cervix carcinoma. Br J Cancer. 1996;74(10):1559–1563. doi: 10.1038/bjc.1996.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 26.Boyer MJ, Barnard M, Hedley DW, Tannock IF. Regulation of intracellular pH in subpopulations of cells derived from spheroids and solid tumours. Br J Cancer. 1993;68(5):890–897. doi: 10.1038/bjc.1993.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannock IF, Kopelyan I. Influence of glucose concentration on growth and formation of necrosis in spheroids derived from a human bladder cancer cell line. Cancer Res. 1986;46(6):3105–3110. [PubMed] [Google Scholar]
- 28.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 29.Pirnia F, Pawlak M, Thallinger GG, et al. Novel functional profiling approach combining reverse phase protein microarrays and human 3-D ex vivo tissue cultures: expression of apoptosis-related proteins in human colon cancer. Proteomics. 2009;9(13):3535–3548. doi: 10.1002/pmic.200800159. [DOI] [PubMed] [Google Scholar]
- 30.Bindra RS, Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res. 2005;569(1–2):75–85. doi: 10.1016/j.mrfmmm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Li CY, Little JB, Hu K, et al. Persistent genetic instability in cancer cells induced by non-DNA-damaging stress exposures. Cancer Res. 2001;61(2):428–432. [PubMed] [Google Scholar]
- 32.Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465(7298):577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gatenby RA, Smallbone K, Maini PK, et al. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97(5):646–653. doi: 10.1038/sj.bjc.6603922. ** Immunohistochemical demonstration of hypoxia and metabolic acidosis in human pre-invasive carcinoma.
- 34. Gauthier ML, Berman HK, Miller C, et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12(5):479–491. doi: 10.1016/j.ccr.2007.10.017. ** Description of molecular changes associated with stress and proliferation in human DCIS.
- 35.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98(17):9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihaylova VT, Bindra RS, Yuan J, et al. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23(9):3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young SD, Hill RP. Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst. 1990;82(5):371–380. doi: 10.1093/jnci/82.5.371. [DOI] [PubMed] [Google Scholar]
- 38.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988;85(24):9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuvier C, Jang A, Hill RP. Exposure to hypoxia, glucose starvation and acidosis: effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin Exp Metastasis. 1997;15(1):19–25. doi: 10.1023/a:1018428105463. [DOI] [PubMed] [Google Scholar]
- 40.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong C, Bauvy C, Tonelli G, et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene. 2012 doi: 10.1038/onc.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo JY, Chen HY, Mathew R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. * General review of autophagy explaining how cancer cells utilize autophagy to survive under the stress of therapy.
- 44.Levine B, Ranganathan R. Autophagy: Snapshot of the network. Nature. 2010;466(7302):38–40. doi: 10.1038/466038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Zhu R, Wang L, et al. PIK3CA mutations mostly begin to develop in ductal carcinoma of the breast. Exp Mol Pathol. 2010;88(1):150–155. doi: 10.1016/j.yexmp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu X, Zou Z, Sun Q, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128(5):931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Bellodi C, Lidonnici MR, Hamilton A, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119(5):1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook KL, Shajahan AN, Warri A, et al. Glucose-Regulated Protein 78 Controls Cross-talk between Apoptosis and Autophagy to Determine Antiestrogen Responsiveness. Cancer Res. 2012;72(13):3337–3349. doi: 10.1158/0008-5472.CAN-12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5(3):400–403. doi: 10.4161/auto.5.3.7784. ** Initial experimental demonstration that autophagy is an important mechanism in development of tamoxifen resistance.
- 53. Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS One. 2009;4(7):e6251. doi: 10.1371/journal.pone.0006251. ** Initial experimental demonstration that autophagy is an important mechanism that facilitates resistance to anti-Her-2 therapy.
- 54.McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010;465(7301):1093–1096. doi: 10.1038/nature09127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19(3):797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gao W, Ding WX, Stolz DB, Yin XM. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4(6):754–761. doi: 10.4161/auto.6360. * Experimental evidence describing the role of calcium phosphate precipitates for initiating autophagy in cell lines.
- 57.Augustijns P, Geusens P, Verbeke N. Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol. 1992;42(4):429–433. doi: 10.1007/BF00280130. [DOI] [PubMed] [Google Scholar]
- 58.Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996;31(4):257–274. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 59.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009 doi: 10.1097/CAD.0b013e32832f4e50. [DOI] [PubMed] [Google Scholar]
- 60. Savarino A, Lucia MB, Giordano F, Cauda R. Risks and benefits of chloroquine use in anticancer strategies. Lancet Oncol. 2006;7(10):792–793. doi: 10.1016/S1470-2045(06)70875-0. * Excellent review of chloroquine as a re-purposed drug for anti-cancer therapy.
- 61. White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15(17):5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. ** Excellent review of autophagy and cancer highlighting how autophagy can positively and negatively influence cancer growth and response to therapy.
- 62.Loehberg CR, Thompson T, Kastan MB, et al. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Res. 2007;67(24):12026–12033. doi: 10.1158/0008-5472.CAN-07-3058. [DOI] [PubMed] [Google Scholar]
- 63.Samaddar JS, Gaddy VT, Duplantier J, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7(9):2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 64.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117(2):326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118(1):79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma XH, Piao S, Wang D, et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17(10):3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maddodi N, Huang W, Havighurst T, et al. Induction of autophagy and inhibition of melanoma growth in vitro and in vivo by hyperactivation of oncogenic BRAF. J Invest Dermatol. 2010;130(6):1657–1667. doi: 10.1038/jid.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirzoeva OK, Hann B, Hom YK, et al. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med (Berl) 2011;89(9):877–889. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 69.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briceno E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg Neurol. 2007;67(4):388–391. doi: 10.1016/j.surneu.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 71.van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a"binding site barrier". Cancer Res. 1991;51(18):4776–4784. [PubMed] [Google Scholar]
- 72.Evans A, Pinder S, Wilson R, et al. Ductal carcinoma in situ of the breast: correlation between mammographic and pathologic findings. AJR Am J Roentgenol. 1994;162(6):1307–1311. doi: 10.2214/ajr.162.6.8191988. [DOI] [PubMed] [Google Scholar]
- 73.Holland R, Hendriks JH, Vebeek AL, Mravunac M, Schuurmans Stekhoven JH. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990;335(8688):519–522. doi: 10.1016/0140-6736(90)90747-s. [DOI] [PubMed] [Google Scholar]
- 74.Hermann G, Keller RJ, Drossman S, et al. Mammographic pattern of microcalcifications in the preoperative diagnosis of comedo ductal carcinoma in situ: histopathologic correlation. Can Assoc Radiol J. 1999;50(4):235–240. [PubMed] [Google Scholar]
- 75.Foschini MP, Fornelli A, Peterse JL, Mignani S, Eusebi V. Microcalcifications in ductal carcinoma in situ of the breast: histochemical and immunohistochemical study. Hum Pathol. 1996;27(2):178–183. doi: 10.1016/s0046-8177(96)90372-x. [DOI] [PubMed] [Google Scholar]
- 76.Lev-Toaff AS, Feig SA, Saitas VL, Finkel GC, Schwartz GF. Stability of malignant breast microcalcifications. Radiology. 1994;192(1):153–156. doi: 10.1148/radiology.192.1.8208928. [DOI] [PubMed] [Google Scholar]
- 77. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. * Comprehensive review of calcium signaling and its role in cell biology.
- 78.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 79. VanHouten J, Sullivan C, Bazinet C, et al. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci U S A. 2010;107(25):11405–11410. doi: 10.1073/pnas.0911186107. ** Initial description of how the calcium export channels in breast cancer can contribute to the aggressiveness of breast cancer.
- 80.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148(12):5943–5954. doi: 10.1210/en.2007-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brini M. Plasma membrane Ca(2+)-ATPase: from a housekeeping function to a versatile signaling role. Pflugers Arch. 2009;457(3):657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- 82.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 83.Faddy HM, Smart CE, Xu R, et al. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008;369(3):977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinhardt TA, Lippolis JD. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem Biophys Res Commun. 2009;378(1):99–102. doi: 10.1016/j.bbrc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8(2):203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7(7):519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 87.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8(5):361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 88.Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39(2):101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Lipskaia L, Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96(1):55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Santella L, Ercolano E, Nusco GA. The cell cycle: a new entry in the field of Ca2+ signaling. Cell Mol Life Sci. 2005;62(21):2405–2413. doi: 10.1007/s00018-005-5083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bikle D, Adams J, Christakos S. VItamin D: production, metabolism, mechanism of action and clinical requirements. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: American Society for Bone and Mineral Research; 2008. pp. 141–149. [Google Scholar]
- 92.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1–2):80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011;347(1–2):55–60. doi: 10.1016/j.mce.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 94.Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys. 2011;523(1):107–114. doi: 10.1016/j.abb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gordon PB, Holen I, Fosse M, Rotnes JS, Seglen PO. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem. 1993;268(35):26107–26112. [PubMed] [Google Scholar]
- 96.Jia W, Pua HH, Li QJ, He YW. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J Immunol. 2010;186(3):1564–1574. doi: 10.4049/jimmunol.1001822. [DOI] [PMC free article] [PubMed] [Google Scholar]