Abstract
Iron overload is associated with significant morbidity and mortality yet is easily treated. The objective of this study was to create a tool that could be easily adapted to clinical practice that indicates the likelihood of a patient having undetected iron overload. We used the National Health and Nutrition Examination Survey (NHANES) 1999–2002 for US adults aged 20 years and older to build a model (unweighted n=8,779). We chose potential variables for inclusion that could be gathered by self-report or measured without laboratory data and were suggested by past literature on hemochromatosis and iron overload. We computed logistic regressions to create the scores by initially evaluating the variables’ relationship with elevated ferritin and elevated transferrin saturation and then using odds ratios to correspond to scores. The resulting score on the IRon Overload ScreeNing Tool (IRON) was then validated with data on 13,844 adults in the NHANES III, 1988-94. Predictors in the final tool were age, gender, previous diagnoses of liver condition, osteoporosis or thyroid disease. The IRON score yielded an area under the curve (AUC) in the NHANES 1999-02 of 0.720 and an AUC of 0.685 in the NHANES III validation sample. The IRON score is a tool to assist in identification of patients with iron overload that has several qualities that make it attractive for use in clinical practice with an undifferentiated patient population including brevity, easily collected information and predictive ability comparable to other tools that help in directing screening.
Introduction
Hereditary hemochromatosis (HH), once considered a rare disease, is now recognized as one of the most common autosomal recessive disorders, occurring in approximately five persons per 1,000 in populations of northern European descent. Approximately one million people in the United States are at risk for development of iron overload, attributable primarily to HH; in addition one in 10 non-Hispanic Whites is a carrier [1,2]. This inborn error of iron metabolism is characterized by excessive dietary iron absorption, which in a subset of patients leads to progressive iron accumulation and reaching toxic levels by middle life [3,4]. The excess iron is deposited in multiple organs, causing oxidative tissue damage and substantial morbidity. The disease progresses silently and often is not discovered until irreversible damage has occurred. However, these disease manifestations can be prevented by therapeutic phlebotomy to remove excess iron [5]. Thus, there is a strong rationale for early detection of this treatable condition to achieve an optimal outcome.
Most patients with HH are homozygous for a mutation in the hemochromatosis (HFE) gene at position 282 (C282Y) of the HFE protein [6]. A second common mutation results in substitution of aspartic acid for histidine at position 63 (H63D); its relationship to iron overload is less clear, although compound heterozygosity with C282Y (C282Y/H63D) has been associated with iron overload in some patients.
Universal screening is not currently recommended by the American College of Physicians or the U.S. Preventive Services Task Force, although there is value of early identification [7,8]. With no universally accepted screening recommendations for hemochromatosis or iron overload, detection in an undifferentiated patient population in primary care is difficult and may lead to detection late in the course of illness, missing the opportunity for the use of appropriate therapy that can prevent clinical manifestations and organ dysfunction [9,10].
Although the identification of the HFE gene and the common C282Y and H63D mutations related to hemochromatosis suggests potential screening based on these mutations, large population-based screening studies have shown that disease penetrance in HFE-related hereditary hemochromatosis is lower than previously believed, making universal population-based screening for iron overload with genetic markers unattractive [11]. In addition, although detection of HH in presymptomatic stages is desirable, it is also important to identify affected persons who have begun to develop early disease manifestations, and this may have a higher yield than population-based screening.
One of the problems of identifying patients with HH in clinical practice is that the disease manifestations are similar to those found in other common disorders. However, once identified, many of the complications can be reversed by phlebotomy therapy [12]. Consequently, targeted screening or case finding based on clinical features associated with iron overload is a useful strategy.
One way to identify iron overload may be to use a multi-variable risk score. Many conditions, like iron overload, have multiple risk factors that might increase the clinician’s suspicion and need for screening or targeted case finding. A risk score simplifies clinical decision making by taking many risk factors for an outcome and combining them into a single predictive measure. It is not practical to evaluate mentally the relative influence of more than two or three risk factors at a time, so a risk score provides an automated way to take into account the relationships among risk factors and is a valuable way to target resources for case finding of individuals with ongoing disease [13,14]. Current guidelines for a number of conditions, such as osteoporosis and diabetes, recommend screening or treatment based on the use of risk scores [15,16]. However, no multivariate risk score exists to guide targeted screening for iron overload. Our goal was to create a tool that indicates the likelihood of undetected iron overload in a general population and that could be easily adapted to clinical practice.
Materials and Methods
Description of data sets and participants
Data used in this study were derived from the National Health and Nutrition Examination Survey (NHANES) 1999–2002 and the NHANES III (1988–1994). These surveys were nationally representative samples of the noninstitutionalized US population. Conducted in 1988–1994, the NHANES III included over 40,000 participants from 89 communities across the United States. In 1999, the survey became a continuous program with a changing focus on a variety of health and nutrition measurements to meet emerging needs. The NHANES 1999–2002 examined a nationally representative sample of about 5,000 persons each year. These persons were located in communities across the country, 15 of which were visited each year. To produce reliable statistics, NHANES over-samples persons 60 and older, African Americans, and Hispanics [17]. The NHANES includes physical exams, laboratory tests, and interviews with participating individuals. We used the NHANES 1999–2002 to develop our risk score and the NHANES III to validate it. The entire NHANES 1999–2002 sample ≥20 years old was used to develop the risk score, and NHANES III participants 20–74 years old were used to validate the risk score. Participants ≥75 years old were excluded from our NHANES III data set because an essential question about iron was not asked of these persons.
Identification of elevated iron measures
Our risk score is intended to predict cases with elevated iron measures, potentially indicative of iron overload in an undifferentiated patient population. Here, the term elevated iron measures refer to an individual having both elevated transferrin saturation and elevated serum ferritin. Transferrin saturation (%) was calculated as the ratio of serum iron concentration to total iron binding capacity (TIBC) multiplied by 100 in both data sets. Transferrin saturation ≥45%, serum ferritin >300 ng/ml in men, and ferritin >200 ng/ml in women were considered elevated [18].
Creation of the score in the development data set
Based on its proposed use as a way to determine whether further laboratory assessment is necessary, the developed risk score did not include any laboratory testing and instead focused on an individual’s history and symptoms. The value of the risk score is that it does not require laboratory tests to increase the suspicion among clinicians that the patient may have iron overload and require further screening. Following that logic, we examined a variety of candidate variables for inclusion in the risk score that can be assessed easily by a clinician. We chose the list of potential distinguishing predictors based on major disease entities related to hemochromatosis and iron overload (e.g., diabetes, CHF, liver problem), other signs/symptoms that have been identified from population based studies (e.g., arthritis), and other disease entities discussed in the literature (e.g., osteoporosis) [5,19,20]. The demographic predictor variables tested were as follows: age in three categories (20–44, 45–64, and ≥65 years old), gender, and race/ethnicity in four categories (non-Hispanic white, non-Hispanic black, Hispanic, and other race). The clinical variables tested were reports of physician diagnosis of arthritis, liver condition, osteoporosis/brittle bones, diabetes, congestive heart failure, and thyroid disease/problem. Patient reports of joint pain/aching/stiffness in the past year were also evaluated.
Because of the complex survey design in the NHANES 1999–2002, we used SUDAAN software (Research Triangle Park, NC) to perform logistic regression analyses with study design variables and weights, which made the results generalizable to the noninstitutionalized U.S. population. The modeling strategy followed was backward elimination. Variables not significant at the P < 0.05 level in the first logistic regression were removed and logistic regression was repeated to confirm that the slope parameters for variables remaining were still significantly different from zero. The same variables were identified using both for-ward selection and backward elimination strategies.
Creation of the iron overload risk score (IRON score) followed the methods used in the creation of the Charlson Comorbidity Index [21]. Odds ratios for elevated iron calculated during the second logistic regression were used to assess risk scores. Odds ratios less than 1.3 receive a score of 0. Significant odds ratios in the range of 1.30 to 1.49 receive a score of 1. Significant odds ratios in the range of 1.50 to 2.49 receive a score of 2. Significant odds ratios in the range of 2.50 to 3.49 receive a score of 3, etc. The total IRON score was the sum of the scores for the individual variables.
Receiver Operating Characteristic (ROC) curves were computed using MedCalc software and the iron overload risk scores for the NHANES 1999–2002 data set. This analysis used unweighted data, as there was no provision for weighting of data in MedCalc Area Under the Curve (AUC) was calculated for the ROC curve. The higher the area under the curve, the more useful the tool is for screening. An AUC of 1.0 would indicate a perfect relationship and an AUC of 0.50 indicates there is no relationship. We also computed weighted ROC curves using a SAS based procedure. However, this strategy does not allow for computation of sensitivity and specificity values for particular iron-overload risk scores and does not produce ROC curve graphs [22]. The AUCs calculated were virtually the same using weighted (0.712) and unweighted data (0.720) with the NHANES 1999–2002 data.
Testing of the risk score in other samples
We chose to use the NHANES III data set for validating the risk score. The NHANES III was conducted between 1988 and 1994. Persons 20–74 years old were included in our study because the question about liver condition was not asked of persons 75 years old or older. With the NHANES III, we used age (20–44, 45–64, and 65–74 years old), gender, liver disorder caused pain, osteoporosis, and thyroid disease for calculation of the risk score as these were the variables that were significant after the first logistic regression. One other difference between the NHANES 1999–2002 questions used to develop the score and the NHANES III questions used to validate the score was a slight difference in the wording of the question asking of self report of physician diagnosed liver problems. In the NHANES 1999–2002, the variable was worded as “liver condition,” whereas in the NHANES III, the variable was worded as“liver disorder caused pain.” This would suggest that the responses to the NHANES 1999–2002 could include diagnoses resulting from asymptomatic testing.
IRON scores were computed for the NHANES III sample. These risk scores were then evaluated using ROC curves and AUCs calculated using MedCalc software. These analyses were done using unweighted data. The AUCs calculated were virtually the same using weighted data in SAS (0.687) and unweighted data in MedCalc (0.685).
Results
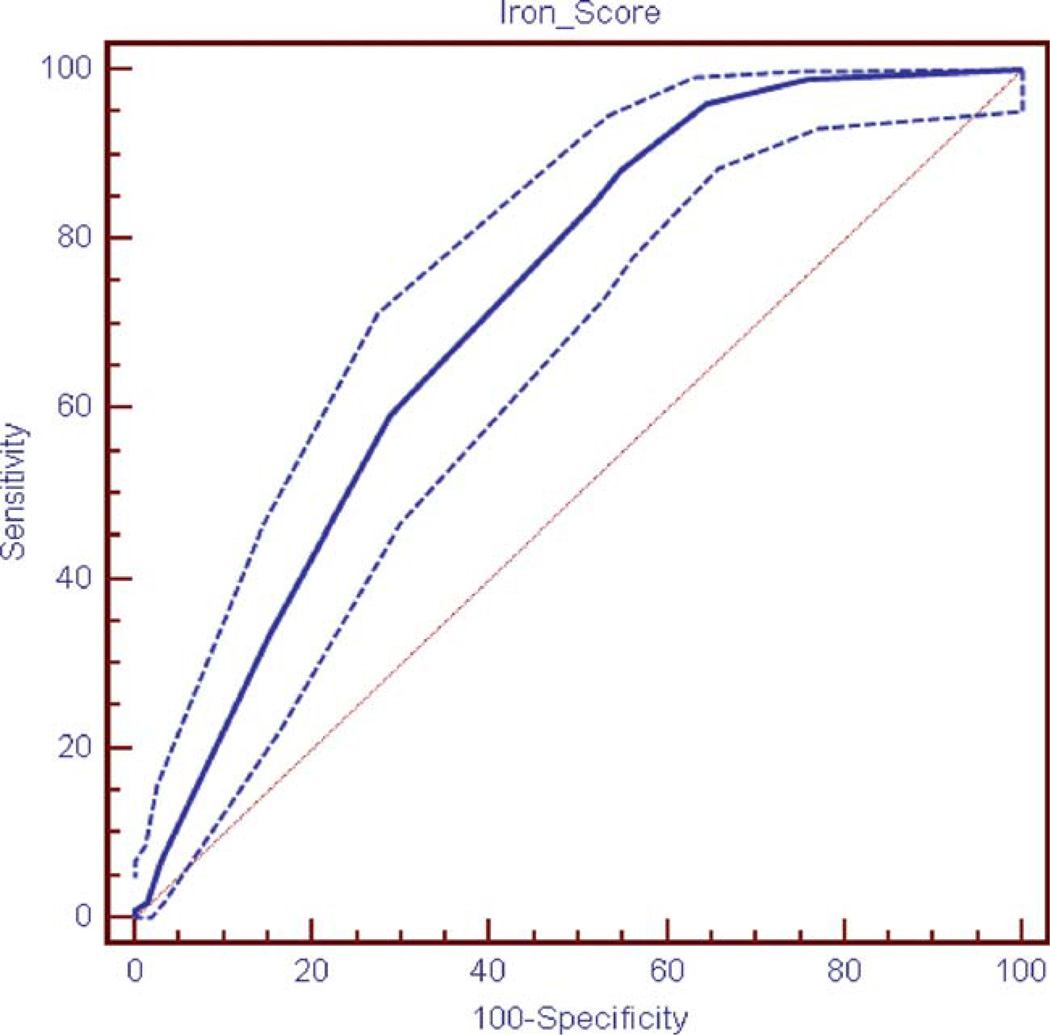
There were 8,779 individuals in the NHANES 1999–2002 sample, representing 186,389,488 persons. Demographics of these individuals are shown in Table I. There were 101 unweighted cases of individuals with elevated transferrin saturation and elevated ferritin in the NHANES 1999–2002 accounting for 1% of the adult population or 1,928,202 individuals. Results of the initial logistic regression are presented in Table II and results of the final logistic regression and corresponding IRON scores are shown in Table III. The IRON score included five variables with a higher score on the IRON score indicating a greater risk that the patient currently has iron overload. The variables are age (20–44 = 0; 45–64 = 2; ≥65 = 3), gender (women = 0; men = 6), physician diagnosis of liver condition (no = 0; yes = 3), physician diagnosis of osteoporosis (no = 0; yes = 3), and physician diagnosis of thyroid disease/problem (no = 0; yes = 3). The individuals’ total scores ranged from 0 to 18. The AUC for the NHANES 1999–2002 ROC curve was 0.720 (Fig. 1). Sensitivity and specificity of the ROC curves are presented in Table IV.
TABLE I.
Demographics of the NHANES 1999–2002
| NHANES 1999–2002 | ||
|---|---|---|
| Total | Individuals with elevated iron measures |
|
| Unweighted N | 8,779 | 101 |
| Population estimate | 186,389,488 | 1,928,202 |
| Age (year) (%) | ||
| 20–44 | 51.2 | 26.6 |
| 45–64 | 32.3 | 42.3 |
| ≥65 | 16.5 | 31.2 |
| Gender (%) | ||
| Male | 48.1 | 78.7 |
| Female | 51.9 | 21.3 |
| Race/Ethnicity (%) | ||
| Non-Hispanic White | 72.3 | 69.9 |
| Non-Hispanic Black | 10.3 | 7.8 |
| Hispanic | 13.8 | 17.8 |
| Other race | 3.5 | 4.5 |
| Arthritis (%) | ||
| Yes | 21.4 | 31.1 |
| No | 78.6 | 68.9 |
| Liver condition (%) | ||
| Yes | 3.0 | 11.1 |
| No | 97.0 | 88.9 |
| Joint pain (%) | ||
| Yes | 44.2 | 50.6 |
| No | 55.8 | 49.4 |
| Osteoporosis (%) | ||
| Yes | 4.4 | 9.7 |
| No | 95.6 | 90.3 |
| Diabetes (%) | ||
| Yes | 6.4 | 12.3 |
| No | 93.6 | 87.7 |
| Congestive heart failure (%) | ||
| Yes | 2.1 | 1.8 |
| No | 97.9 | 98.2 |
| Thyroid disease (%) | ||
| Yes | 7.5 | 15.9 |
| No | 92.5 | 84.1 |
TABLE II.
Initial Logistic Regression for Predictors of Elevated Iron Levels in the NHANES 1999–2002 Data Set
| Odds ratio | 95% CI | |
|---|---|---|
| Age (year) | ||
| 20–44 | 1.00 | – |
| 45–64 | 2.32 | 1.21–4.48 |
| ≥ 65 | 3.51 | 1.62–7.58 |
| Gender | ||
| Male | 5.94 | 3.37–10.44 |
| Female | 1.00 | – |
| Race/Ethnicity | ||
| Non-Hispanic White | 1.00 | – |
| Non-Hispanic Black | 1.10 | 0.57–2.13 |
| Hispanic | 1.79 | 0.96–3.35 |
| Other Race | 1.91 | 0.51–7.22 |
| Arthritis | ||
| Yes | 1.06 | 0.59–1.91 |
| No | 1.00 | – |
| Liver condition | ||
| Yes | 3.09 | 1.18–8.08 |
| No | 1.00 | – |
| Joint pain | ||
| Yes | 1.05 | 0.63–1.74 |
| No | 1.00 | – |
| Osteoporosis | ||
| Yes | 2.66 | 1.15–6.16 |
| No | 1.00 | – |
| Diabetes | ||
| Yes | 1.21 | 0.47–3.13 |
| No | 1.00 | – |
| Congestive heart failure | ||
| Yes | 0.40 | 0.12–1.33 |
| No | 1.00 | – |
| Thyroid disease | ||
| Yes | 2.67 | 1.03–6.95 |
| No | 1.00 | – |
TABLE III.
Final Logistic Regression for Predictors of Elevated Iron Levels in the NHANES 1999–2002 Data Set
| Odds ratio | 95% CI | IRON score | |
|---|---|---|---|
| Age (year) | |||
| 20–44 | 1.00 | – | 0 |
| 45–64 | 2.24 | 1.18–4.26 | 2 |
| ≥65 | 3.21 | 1.63–6.31 | 3 |
| Gender | |||
| Male | 5.77 | 3.22–10.36 | 6 |
| Female | 1.00 | – | 0 |
| Liver condition | |||
| Yes | 3.05 | 1.17–7.93 | 3 |
| No | 1.00 | – | 0 |
| Osteoporosis | |||
| Yes | 2.68 | 1.17–6.17 | 3 |
| No | 1.00 | – | 0 |
| Thyroid disease | |||
| Yes | 2.66 | 1.03–6.85 | 3 |
| No | 1.00 | – | 0 |
Figure 1.
Receiver Operating Characteristic (ROC) curve for the IRON score in the NHANES 1999–2002 (with 95% confidence limits shown). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE IV.
Sensitivity and Specificity for the IRON Score Receiver Operating Characteristic (ROC) Curves in the NHANES 1999–2002 and NHANES III
| NHANES 1999–2002 | NHANES III | |||
|---|---|---|---|---|
| Criterion | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) |
| >0 | 99 | 24 | 94 | 30 |
| >2 | 96 | 36 | 86 | 43 |
| >3 | 88 | 45 | 80 | 50 |
| >5 | 84 | 48 | 78 | 52 |
| >6 | 59 | 71 | 41 | 79 |
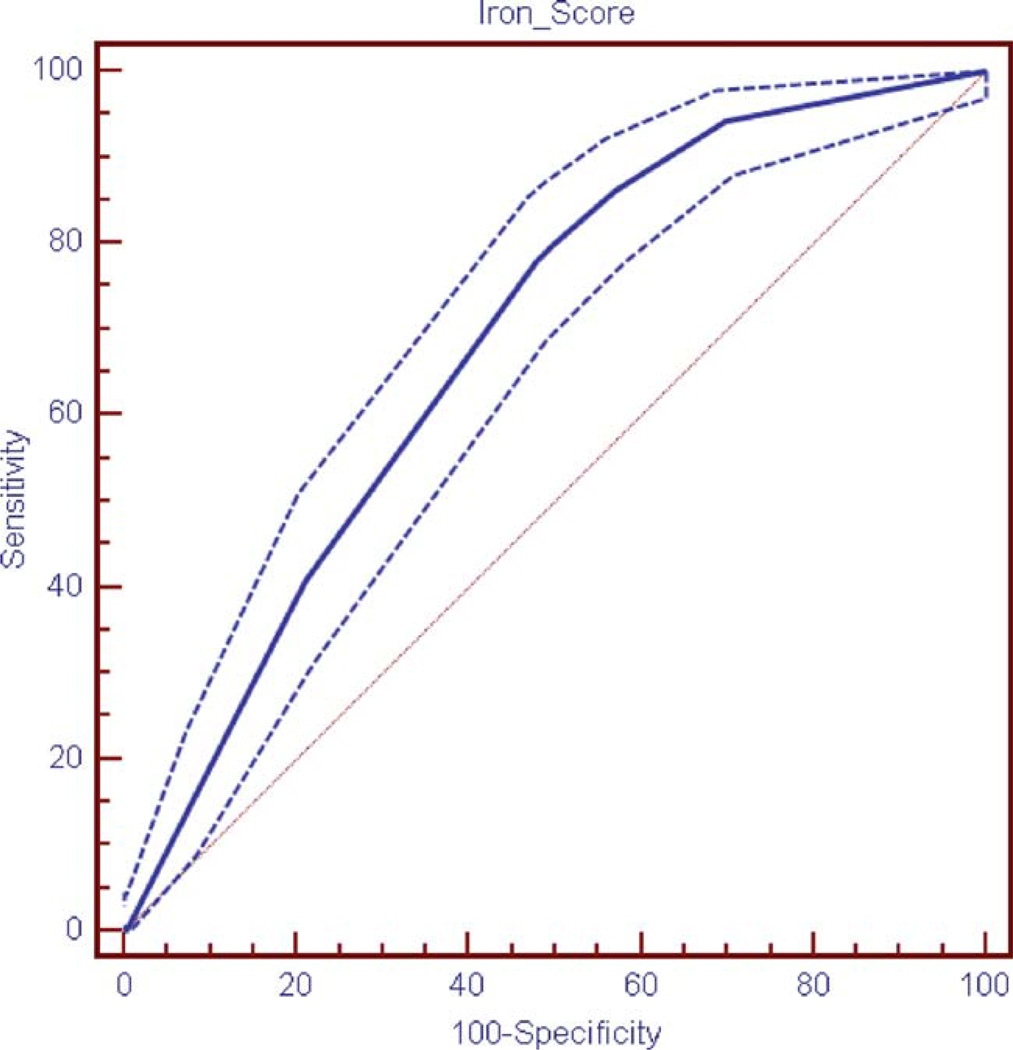
Based on the results from NHANES 1999–2002, IRON scores were also computed for NHANES III. The NHANES III included 13,844 unweighted cases in the sample, of which 159 had both elevated transferrin saturation and elevated ferritin representing 1% of the adult population or 1,575,404 individuals. Demographics of these individuals are shown in Table I. The AUC for the NHANES III ROC curve was 0.685 (Fig. 2).
Figure 2.
Receiver Operating Characteristic (ROC) curve for the IRON score in the NHANES III (with 95% confidence limits shown). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
It can be difficult to diagnosis patients with iron overload when they are in the early stages of the condition because they typically have nonspecific symptoms such as chronic fatigue, abdominal pain, or joint pain which may be attributed to other causes. The diagnosis may not be suspected even in patients with more severe manifestations such as liver disease, cardiomyopathy, or diabetes mellitus because these conditions are common in the general population. The risk assessment tool that we developed to help physicians identify patients who might have hereditary hemochromatosis may provide an effective strategy for promoting early detection of iron overload and prevention of morbidity and mortality associated with this condition [23].
The IRON score can be used as an assessment tool for screening in clinical and population settings or as a prescreening tool to help identify potential patients that need to have further laboratory testing to assess their iron measures. This is the first risk score created to assist in identifying patients at risk for current iron overload without a physical examination or laboratory assessment. It consists of a minimal amount of self reported information that could be collected through either a quick personal history or an electronic health record.
The IRON score has a predictive utility and AUC values (AUC = 0.720 for development data set; 0.685 for validation data set) similar to tools that help to target screening for other chronic conditions, such as diabetes and osteoporosis [14,24,25]. Three previously published screening tools are the TAG-IT (AUC = 0.740 for development data set; 0.744 for validation data set) which can be used to identify persons likely to have impaired fasting glucose; the FRAX tool (validated in 11 clinical trials with AUC = 0.57–0.77; mean = 0.66) used to identify women with at risk for fracture; and the ARIC CHD Risk Calculator (AUC = 0.721 for women with diabetes; 0.672 for men with diabetes) for assessing cardiovascular risk in persons having diabetes [14,16,24,25]. The use of risk score tools was recommended in the recent scientific statement by the American Diabetes Association, American Heart Association and American College of Cardiology Foundation (ADA/AHA/ACCF) on aspirin for primary prevention of cardiovascular events in people with diabetes [16]. This approach is consistent with the current recommendations for screening and preventive treatment for a variety of common conditions by the United States Preventive Services Task Force (USPSTF) and other organizations.
Considering the possible morbidity and mortality from unidentified iron overload, this tool has significant potential for improving care by allowing clinicians to identify affected individuals before the onset of significant clinical manifestations without requiring the resources that would be used if universal genetic or laboratory screening was recommended. Another benefit is that that instead of calculating a future risk, it calculates risk of currently having elevated iron measures which may indicate iron overload. Finally, this tool has potential application as a Web-based screening instrument to improve awareness and population health [26,27]. Further studies to evaluate the cost effectiveness of the IRON score to identify patients with current iron overload are needed.
Although the IRON score has potential utility in a clinical setting, we should note limitations in its design. First, this tool was developed from cross-sectional data and examines only the likelihood of currently having elevated iron measures. This score does not predict future risk for developing iron overload or disease sequelae of iron overload. However, for its suggested purpose of guiding targeted screening, identifying those currently having elevated iron measures is most appropriate. However, there is not currently universal agreement on those levels [18,28]. Second, due to the data that were available, different questions had to be used in the developmental data set and validation data sets to represent risk score components, specifically when identifying liver issues. NHANES 1999–2002 asked about liver conditions and NHANES III asked about liver disorder causing pain. This difference limits the predictive ability found in the validation data set, as the more inclusive liver condition question would be expected to perform better than one asking about pain due to a liver condition, which will miss individuals who are pain free but have liver disease. However, we purposefully did not attempt to use laboratory findings to identify liver disease because we wanted the score to include easily available data. Third, neither the NHANES III nor the NHANES 1999–2002 included a question regarding whether a doctor had ever told the respondent that he or she had hemochromatosis. Consequently, we could not exclude individuals with diagnosed hemochromatosis from the population. It is likely, however, that most individuals who were successfully managing their disease would not still meet our criteria of elevated iron measures.
In conclusion, the IRON score is a tool to assist in identification of patients with iron overload that has several qualities that make it attractive for use in clinical practice with an undifferentiated patient population including brevity, easily collected information, and predictive ability comparable to other tools that help in directing screening. Future research may focus on evaluating the utility of the IRON score tool in practice.
Acknowledgments
As a cooperative agreement authors Mary M. Hulihan and Althea M. Grant from the Centers of Disease Control and from the R01 grant authors Christine E. McLaren and Gordon D. McLaren, MD helped in all aspects of this study.
Contract grant sponsor: Centers for Disease Control and Prevention; Contract grant number: MM1144 (Cooperative Agreement).
Contract grant sponsor: National Heart, Lung, and Blood Institute; Contract grant number: R01 HL083328.
Footnotes
Conflict of interest: Nothing to report
References
- 1.Steinberg KK, Cogswell ME, Chang JC, et al. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. JAMA. 2001;285:2216–2222. doi: 10.1001/jama.285.17.2216. [DOI] [PubMed] [Google Scholar]
- 2.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 3.Fowler C. Hereditary hemochromatosis: Pathophysiology, diagnosis, and management. Crit Care Nurs Clin North Am. 2008;20:191–201. doi: 10.1016/j.ccell.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.McLaren CE, Barton JC, Eckfeldt JH, et al. Heritability of serum iron measures in the hemochromatosis and iron overload screening (HEIRS) family study. Am J Hematol. 2010;85:101–105. doi: 10.1002/ajh.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaren GD, McLaren CE, Adams PC, et al. Hemochromatosis and Iron Overload Screen (HEIRS) Study Research Investigators. Clinical manifestations of hemochromatosis in HFE C282Y homozygotes identified by screening. Can J Gastroenterol. 2008;22:923–930. doi: 10.1155/2008/907356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 7.Qaseem A, Aranson M, Fitterman N, et al. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for hereditary hemochromatosis: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517–521. doi: 10.7326/0003-4819-143-7-200510040-00010. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock EP, Garlitz BA, Harris EL, et al. Screening for hereditary hemochromatosis: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2006;145:209–223. doi: 10.7326/0003-4819-145-3-200608010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Waalen J, Felitti VJ, Beutler E. Screening for hemochromatosis by measuring ferritin levels: A more effective approach. Blood. 2008;111:3373–3376. doi: 10.1182/blood-2007-07-102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friexenet N, Moreno-Rosel MS, Barcelo MJ, et al. Detection of hereditary hemochromatosis and biochemical iron overload in primary care: A multicenter case finding study in Spain. Am J Hematol. 2010;85:294–296. doi: 10.1002/ajh.21634. [DOI] [PubMed] [Google Scholar]
- 11.Phatak PD, Bonkovsky HL, Kowdley KV. Hereditary hemochromatosis: Time for targeted screening. Ann Intern Med. 2008;149:270–272. doi: 10.7326/0003-4819-149-4-200808190-00009. [DOI] [PubMed] [Google Scholar]
- 12.Witte DL, Crosby WH, Edwards CQ, et al. Practice guideline development task force of the College of American Pathologists. Hereditary hemochromatosis. Clin Chim Acta. 1996;245:139–200. doi: 10.1016/0009-8981(95)06212-2. [DOI] [PubMed] [Google Scholar]
- 13.Grover SA, Lowensteyn I, Esrey KL, et al. Do doctors accurately assess coronary risk in their patients? Preliminary results of the coronary health assessment study. BMJ. 1995;310:975–978. doi: 10.1136/bmj.310.6985.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koopman RJ, Mainous AG, III, Everett CJ, Carter RE. Tool to assess likelihood of fasting glucose impairment (TAG-IT) Ann Fam Med. 2008;6:555–561. doi: 10.1370/afm.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. [Accessed on January 21, 2011];Screening for Osteoporosis. 2011 at http://www.uspreventiveservicestaskforce.org/uspstf/uspsoste.htm.
- 16.Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395–1402. doi: 10.2337/dc10-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) [Accessed on January 20, 2010];National Health and Nutrition Examination Survey. 2011 http://www.cdc.gov/nchs/nhanes.htm.
- 18.Iron Disorders Institute. [Accessed on December 30, 2010];Iron Tests. 2009 at http://www.irondisorders.org/iron-tests/.
- 19.Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 20.Tsay J, Yang Z, Ross FP, et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood. 2010;116:2582–2589. doi: 10.1182/blood-2009-12-260083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Izrael D, Battaglia AA, Hoaglin DC, Battaglia MP. Use of the ROC curve and the bootstrap in comparing weighted logistic regression models, Paper 248–27; Proceedings of the Twenty-Seventh Annual SAS Users Group International Conference; April 14–17, 2002; Orlando, Florida: SAS Institute Inc. [Google Scholar]
- 23.Cogswell ME, McDonnell SM, Khoury MJ, et al. Iron overload, public health, and genetics: Evaluating the evidence for hemochromatosis screening. Ann Intern Med. 1998;129:971–979. doi: 10.7326/0003-4819-129-11_part_2-199812011-00008. [DOI] [PubMed] [Google Scholar]
- 24.Folsom AR, Chambless LE, Duncan BB, et al. The Atherosclerosis Risk in Communities Study Investigators. Prediction of coronary heart disease in middle-aged adults with diabetes. Diabetes Care. 2003;26:2777–2784. doi: 10.2337/diacare.26.10.2777. [DOI] [PubMed] [Google Scholar]
- 25.Kanis JA on behalf of the World Health Organization Scientific Group. Technical Report. UK: University of Sheffield: World Health Organization Collaborating Centre for Metabolic Bone Diseases; 2007. Assessment of osteoporosis at the primary health-care level. 2007, Printed by the University of Sheffield. [Google Scholar]
- 26.Grover SA, Lowensteyn I, Joseph L, et al. Patient knowledge of coronary risk profile improves the effectiveness of dyslipidemia therapy: The CHECK-UP study: A randomized controlled trial. Arch Intern Med. 2007;167:2296–2303. doi: 10.1001/archinte.167.21.2296. [DOI] [PubMed] [Google Scholar]
- 27.Koopman RJ, Mainous AG., III Evaluating multivariate risk scores for clinical decision making. Fam Med. 2008;40:412–416. [PubMed] [Google Scholar]
- 28.Center of Disease Control. [Accessed on April 13, 2011];Hemochromatosis (Iron Storage Disease): Diagnosis. 2010 Jun; at http://www.cdc.gov/ncbddd/hemochromatosis/diagnosis.html.