Abstract
Objective:
The purpose of our study is to correlate renal echogenicity with serum creatinine in order to determine the significance of renal echogenicity when it comes to identifying the progression of chronic kidney disease (CKD) and for the sonographic grading of CKD.
Materials and Methods:
Sixty patients above 30 years of age who had been diagnosed with CKD according to the guidelines of the National Kidney Foundation were included in the study. Patients on kidney replacement therapy or with fatty liver findings on ultrasonography were excluded. Ultrasounds of kidneys were performed by two radiologists who were blind to the patients’ serum creatinine levels. Renal cortical echogenicity was compared with serum creatinine. Statistical analysis was performed using one-way ANOVA followed by Scheffe's test. The relationship between serum creatinine and sonographic features was assessed by correlation coefficient analysis. A P value less than 0.05 was considered statistically significant.
Results:
Mean serum creatinine was 2.80 mg/dl for Grade 1 (range: 0.9-9.2 mg/dl), 3.69 mg/dl for Grade 2 (range: 1.2-10.3 mg/dl), 3.86 mg/dl for Grade 3 (range: 1.1-6.5 mg/dl), and 7.90 mg/dl for Grade 4 (range: 3.1-11.4 mg/dl). The grades being determined by cortical echogenicity on imaging A statistically significant, positive correlation was observed between serum creatinine and grading based on cortical echogenicity (P = 0.004).
Conclusion:
Renal echogenicity and its grading correlates better with serum creatinine in CKD than other sonographic parameters such as longitudinal size, parenchymal thickness, and cortical thickness. Hence, renal echogenicity is a better parameter than serum creatinine for estimating renal function in CKD, and has the added advantage of irreversibility.
Keywords: Chronic kidney disease, renal echogenicity, serum creatinine

INTRODUCTION
Chronic kidney disease (CKD) is one of the common causes of renal failure. It involves a progressive loss over the course of months in the structure and function of the kidneys, with or without a decreased glomerular filtration rate (GFR). CKD can be diagnosed by its pathological abnormalities, changes in the levels of kidney function markers in the blood or urine, or by imaging investigations.[1] Ultrasound is the ideal imaging modality in CKD because of its non-invasiveness, and because it provides easy accessibility and visualization of the kidneys. Ultrasonography is the first, and, in most cases, the only imaging investigation required in the work-up of chronic renal failure. Observation of a small kidney with a thin, echogenic cortex or parenchyma indicates irreversible damage.[2,3] The best screening modality to evaluate renal insufficiency in patients is sonography.[4] As ultrasonographic findings like echogenicity, longitudinal length, parenchymal, and cortical thickness represent irreversible changes, ultrasonography is a better imaging modality when it comes to ascertaining the progression of the disease.[2,3]
The serum creatinine level is an endogenous serum marker that is commonly used to estimate GFR, and accordingly, the stage of CKD.[5] The aim of our study is to correlate renal echogenicity with serum creatinine levels and to investigate the significance of renal echogenicity in identifying the progression of CKD, as well as use sonographic imaging in grading CKD.
MATERIALS AND METHODS
The ethical committee of our institute approved this prospective study. The duration of study was one year, from May 2011 to April 2012. Sixty patients above 30 years of age who had been diagnosed with CKD according to the guidelines of the National Kidney Foundation were selected.[1] Patients on kidney replacement therapy (hemodialysis, peritoneal dialysis, and renal transplantation), as well as those with fatty liver and other liver diseases diagnosed on ultrasonography, were excluded. Detailed information from patients regarding age, sex, duration of diabetes mellitus if diabetic, duration of hypertension if hypertensive, other causes of chronic renal failure, and treatment history was collected.
Using a standard B mode grayscale ultrasound (Voluson GE PRO 730), ultrasound of the kidneys and liver were performed by two radiologists with respective experience of 8 and 5 years using curved array transducers of 2.5t-4 MHz. Speckle reduction imaging (SRI) and low tissue harmonic imaging were applied to visualize the liver and kidney echogenicity. A manual method of adjusting the gain and time gain compensation (TGC) was used so that inter-observer bias could be reduced. The radiologists were blind to patients’ serum creatinine values, and all patients were reviewed by both radiologists.
Length of the kidney was measured pole to pole. Parenchymal thickness was measured from the renal hilum to the maximum convex border of the lateral renal margin. Cortical thickness was measured in the sagittal plane over a medullary pyramid, perpendicular to the capsule. When there was inter-observer variation, consensus was sought. Renal longitudinal size, parenchymal thickness, cortical thickness, cortical echogenicity, and corticomedullary differentiation were evaluated. In every case, the mean values of the right and left renal longitudinal size, parenchymal thickness, and cortical thickness were calculated. Renal cortical echogenicity was compared and graded with the echogenicity of the liver and renal medulla, where:
Grade 0: Normal echogenicity less than that of the liver, with maintained corticomedullary definition [Figure 1]
Figure 1.
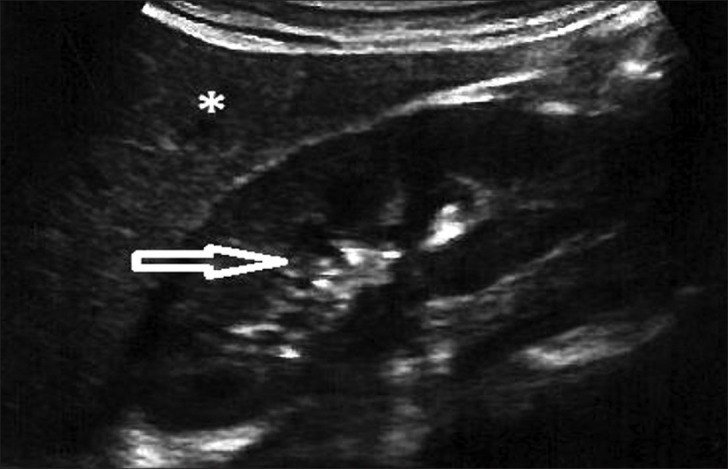
Ultrasound of abdomen (longitudinal section) shows renal cortical echogenicity Grade 0: Normal, echogenicity less than liver (star), with maintained cortico-medullary definition (arrow) of right kidney.
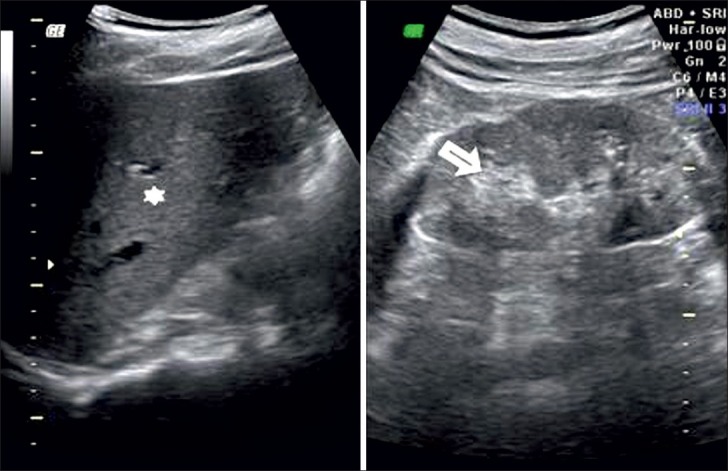
Grade 1: Echogenicity the same as that of the liver, with maintained corticomedullary definition [Figure 2]
Figure 2.
Ultrasound of abdomen (longitudinal section) shows renal cortical echogenicity Grade 1: Echogenicity same as the liver (star), with maintained cortico-medullary definition (arrow) of right kidney.
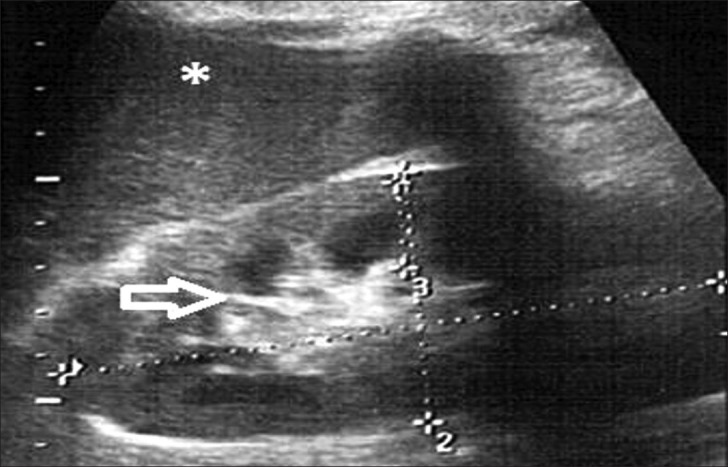
Grade 2: Echogenicity greater than that of the liver, with maintained corticomedullary definition [Figure 3]
Figure 3.
Ultrasound of abdomen (longitudinal section) shows renal cortical echogenicity Grade 2: Echogenicity more than the liver (star), with maintained cortico-medullary definition (arrow) of left kidney.
Grade 3: Echogenicity greater than that of the liver, with poorly maintained corticomedullary definition [Figure 4]
Figure 4.
Ultrasound of abdomen (longitudinal section) shows renal cortical echogenicity Grade 3: Echogenicity more than the liver (star), with poorly maintained cortico-medullary definition (arrow) of right kidney.
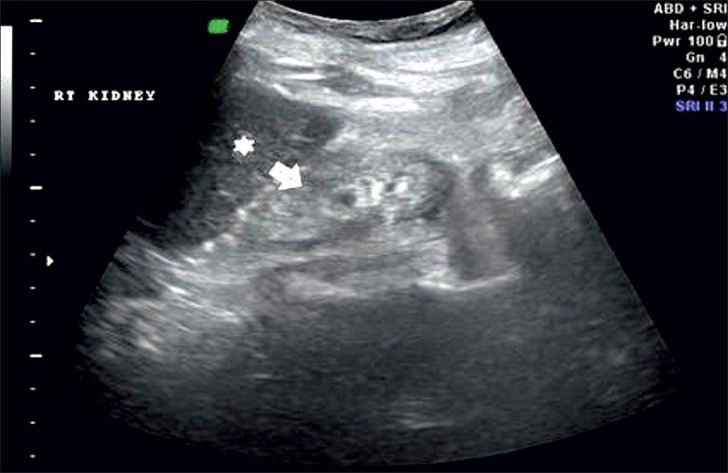
Grade 4: Echogenicity greater than that of the liver, with a loss of corticomedullary definition [Figure 5].
Figure 5.
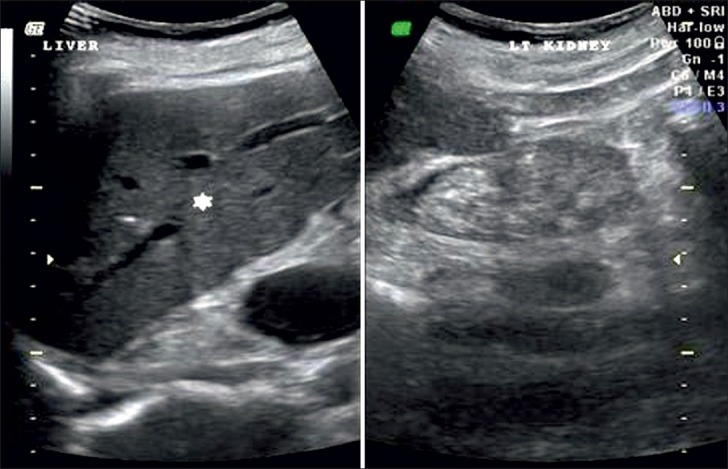
Ultrasound of abdomen (longitudinal section) shows renal cortical echogenicity Grade 4: Echogenicity more than the liver (star), with loss of cortico-medullary definition (arrow) of left kidney.
Blood samples were collected from the selected patients. In vitro estimation of serum creatinine was conducted using a modified kinetic Jaffe reaction.[6,7] Statistical analysis was calculated using one way ANOVA followed by Scheffe's test. The relationship between serum creatinine and sonographic parameters were assessed by correlation coefficient analysis. P values less than 0.05 were considered statistically significant.
RESULTS
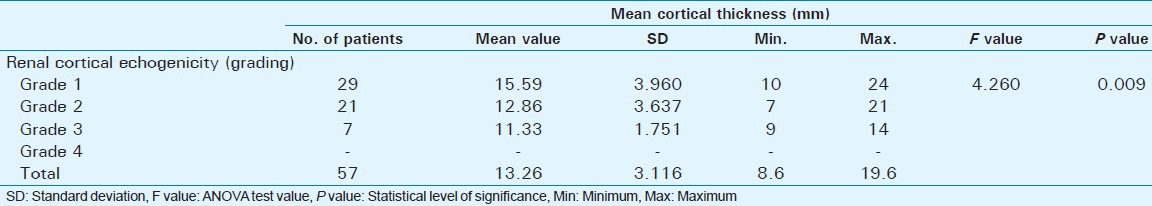
Out of 60 selected patients, 42 were male and 18 were female. Twenty-nine patients(48.3%) had sonological Grade 1 CKD, 21(35%) had Grade 2 CKD, 7(11.7%) had Grade 3 CKD, and 3(5%) had Grade 4 CKD (Figure 6). The mean serum creatinine was 2.8 mg/dl for Grade 1 (range: 0.9-9.2 mg/dl), 3.69 mg/dl for Grade 2 (range: 1.2-10.3 mg/dl), 3.86 mg/dl for Grade 3 (range: 1.1-6.5 mg/dl), and 7.9 mg/dl for Grade 4 (range: 3.1-11.4 mg/dl) [Table 1]. The mean longitudinal size was 101.38 mm for Grade 1 (range: 76-124 mm), 91.43 mm for Grade 2 (range: 63-115 mm), 89.43 mm for Grade 3 (range: 60-111 mm), and 78 mm for Grade 4 (range: 67-91 mm) [Table 2]. The mean parenchymal thickness was 47.38 mm for Grade 1 (range: 37-61 mm), 41.14 mm for Grade 2 (range: 30-61 mm), 40 mm for Grade 3 (range: 21-50 mm), and 37.33 mm for Grade 4 (range: 31-44 mm) [Table 3]. The mean cortical thickness was 15.59 mm for Grade 1 (range: 10-24 mm), 12.86 mm for Grade 2 (range: 7-21 mm), and 11.33 mm for Grade 3 (range: 9-14 mm) [Table 4]. By definition, Grade 4 involves more echogenicity than the liver, with a loss of corticomedullary definition. Once corticomedullary definition is lost, cortical thickness cannot be measured; hence, [Table 4] includes 57 cases.
Figure 6.
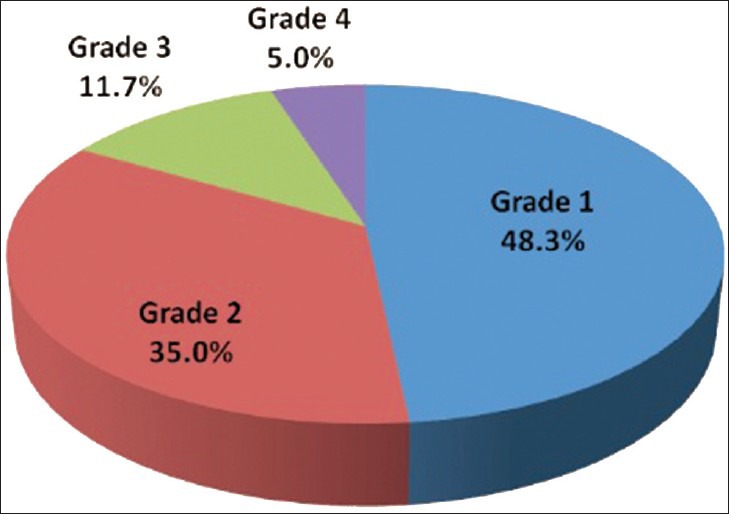
Pie chart displays 29 patients had sonological Grade 1 CKD, 21 patients had sonological Grade 2 CKD, 7 patients had sonological Grade 3 CKD, and 3 patients Grade 4 CKD.
Table 1.
Comparison of serum creatinine with renal cortical echogenicity (Grading determined by ultrasound features)
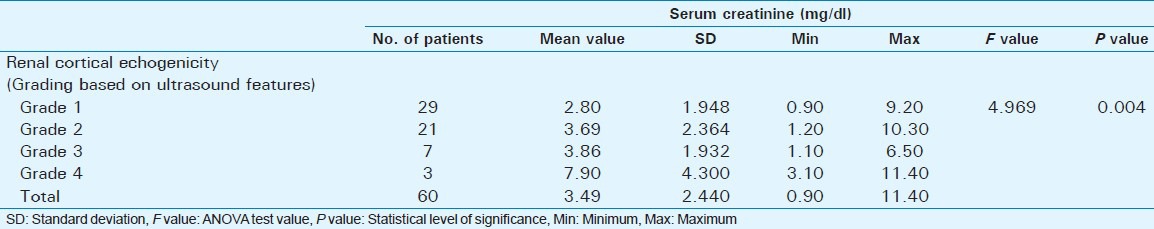
Table 2.
Comparison of mean Longitudinal size with renal cortical echogenicity (Grading determ ined by Ultrasound features)
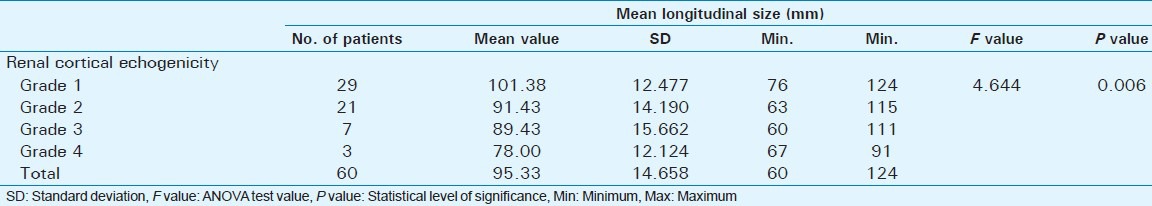
Table 3.
Comparison of mean parenchymal thickness with renal cortical echogenicity (Grading determined by ultrasound features)
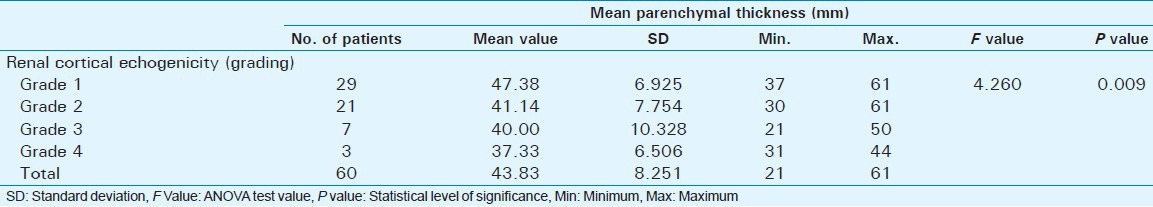
Table 4.
Comparison of mean cortical thickness with renal cortical echogenicity (Grading based on ultrasound features)
DISCUSSION
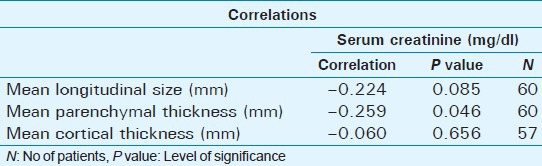
A statistically significant positive correlation was observed between serum creatinine and cortical echogenicity grading (P = 0.004). There was also a statistically significant positive correlation between mean longitudinal size and renal echogenicity (P = 0.006), parenchymal thickness, and renal echogenicity (P = 0.009), and cortical thickness and renal echogenicity (P = 0.008). A statistically significant negative correlation was observed between mean longitudinal size and serum creatinine (P = 0.085); a statistically significant negative correlation was observed between mean parenchymal thickness and serum creatinine (P = 0.046); and a statistically significant negative correlation was observed between mean cortical thickness and serum creatinine (P = 0.656) [Table 5].
Table 5.
Statistical correlation between serum creatinine and mean longitudinal size, mean parenchymal thickness, and mean cortical thickness
Our study showed statistically significant positive correlations between serum creatinine and renal echogenicity grading (P = 0.004) [Table 1] from Grade 1 to Grade 4 CKD. A study by Moghazi et al., showed that renal echogenicity has the strongest correlation with histologic parameters (glomerular sclerosis, tubular atrophy, interstitial fibrosis, and interstitial inflammation).[8] Research by Päivänsalo et al., showed that a highly echogenic cortex was the most common abnormality; this was slightly more frequent in tubulointerstitial disease (75%) than in glomerular disease (61%).[9] In a previous study, Hricak et al., showed a statistically significant positive correlation between cortical echogenicity and the severity of global sclerosis, focal tubular atrophy, the number of hyaline casts per glomerulus, and focal leukocytic infiltration.[10] Our results contradict those of Platt et al., who found that renal echogenicity equal to the echogenicity of the liver is not a good indicator of disease.[11] Using speckle reduction imaging (SRI) and low tissue harmonic imaging technology, normal renal echogenicity is less than that of liver in the normal population and shows better difference in echogenicity between the liver and renal cortex. This has also been described by Rosenfield and Siegel.[12]
A statistically significant positive correlation was seen between renal echogenicity grading and mean longitudinal size (P = 0.006) [Table 2]. Renal length has traditionally been considered a surrogate marker of renal function because renal length decreases with decreasing renal function.[13] When repeating renal measurements, estimation of renal length should be preferred to renal volume.[14] A study by Miletić et al., revealed that relative renal length (calculated using the kidney length to body height ratio) better represents kidney size than absolute renal length (measurements of longitudinal renal diameter) because it eliminates sex and height differences.[15]
There was a statistically significant positive correlation observed between renal echogenicity grading and parenchymal thickness (P = 0.009) [Table 3]. As the echogenicity increased, there was a decrease in the mean parenchymal thickness. A study by Moghazi et al., showed that parenchymal thickness, but not cortical thickness, correlated with tubular atrophy.[8]
There was a statistically significant positive correlation between renal echogenicity grading and cortical thickness (P = 0.008) [Table 4]. As the echogenicity increased, there was a decrease in mean cortical thickness. Study done by Beland et al., showed that cortical thickness measured on ultrasound appears to be more closely related to GFR than renal length.[16]
Our study group included few patients with Grade 3 and Grade 4 CKD. This may have occurred because, as our institution is a tertiary referral center, most cases were treated with renal replacement therapies like hemodialysis, peritoneal dialysis, and renal transplantation due to complications associated with CKD. As serum creatinine increases, there is increased renal cortical echogenicity. Since changes in renal echogenicity are irreversible, a sonological grading of CKD can be carried out, allowing the severity of CKD to be assessed.
The P value of renal echogenicity (P = 0.004) was statistically more significant than the P values for mean longitudinal size (P = 0.006), mean parenchymal thickness (P = 0.009), and mean cortical thickness (P = 0.008).
CONCLUSIONS
Renal echogenicity and its grading correlates better with serum creatinine in CKD than other sonographic parameters like longitudinal size (P = 0.085), parenchymal thickness (P = 0.046), and cortical thickness (P = 0.656). As serum creatinine is an indicator of kidney function, renal echogenicity is a better parameter to estimate renal function with the added advantage of irreversibility when compared to serum creatinine, which improves with kidney replacement therapy like -hemodialysis, peritoneal dialysis, and renal transplantation in chronic kidney disease.[17]
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/28/114809
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.National Kidney Foundation. Bailie GR, Uhlig K, Levey AS. Clinical practice guidelines in nephrology. Evaluation, classification, and stratification of chronic kidney disease. Am J Kidney Dis. 2002;39(suppl 2):S1–266. [PubMed] [Google Scholar]
- 2.O’Neill WC. Chronic renal failure. In: O’Neill WC, editor. Atlas of renal ultrasonography. Philadelphia: W.B. Saunders Company; 2001. pp. 41–3. [Google Scholar]
- 3.O’Neill WC. Sonographic evaluation of renal failure. Am J Kidney Dis. 2000;35:1021–38. doi: 10.1016/s0272-6386(00)70036-9. [DOI] [PubMed] [Google Scholar]
- 4.Khati NJ, Hill MC, Kimmel PL. The role of ultra-sound in renal insufficiency: The essentials. Ultrasound Q. 2005;21:227–44. doi: 10.1097/01.wnq.0000186666.61037.f6. [DOI] [PubMed] [Google Scholar]
- 5.Tietz NW. Philadelphia: W.B. Saunders Co; 1994. Textbook of clinical chemistry; p. 1531. [Google Scholar]
- 6.Larsen K. Creatinine assay by a reaction/kinetic approach. Clin Chem Acta. 1972;41:209–17. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb RH, Weinberg EP, Rubens DJ, Monk RD, Grossman EB. Renal sonography: Can it be used more selectively in the setting of an elevated serum creatinine level? Am J Kidney Dis. 1997;29:362–7. doi: 10.1016/s0272-6386(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 8.Moghazi S, Jones E, Schroepple J, Arya K, McClellan W, Hennigar RA, et al. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005;67:1515–20. doi: 10.1111/j.1523-1755.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 9.Päivänsalo M, Huttunen K, Suramo I. Ultrasonographic findings in renal parenchymal diseases. Scand J Urol Nephrol. 1985;19:119–23. doi: 10.3109/00365598509180238. [DOI] [PubMed] [Google Scholar]
- 10.Hricak H, Cruz C, Romanski R, Uniewski MH, Levin NW, Madrazo BL, et al. Renal parenchymal disease: Sonographic-histologic correlation. Radiology. 1982;144:141–7. doi: 10.1148/radiology.144.1.7089245. [DOI] [PubMed] [Google Scholar]
- 11.Platt JF, Rubin JM, Bowerman RA, Marn CS. The inability to detect kidney disease on the basis of echogenicity. AJR Am J Roentgenol. 1988;151:317–9. doi: 10.2214/ajr.151.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfield AT, Siegel NJ. Renal Parenchymal disease: Histopathologic-sonographic correlation. AJR. 1981;137:793–8. doi: 10.2214/ajr.137.4.793. [DOI] [PubMed] [Google Scholar]
- 13.American College of Radiology Website. ACR practice guideline for the performance of an ultra- sound examination of the abdomen and/or retro-peritoneum (in collaboration with the American Institute of Ultrasound in Medicine AIUM) 2007. [Last accessed on 2010 Apr 14]. Available from: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/us/us_abdomen_retro.aspx . Revised.
- 14.Emamian SA, Nielsen MB, Pedersen JF. Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. A comparison of linear measurements and volumetric estimates. Acta Radiol. 1995;36:399–401. [PubMed] [Google Scholar]
- 15.Miletić D, Fuckar Z, Sustić A, Mozetic V, Stimac D, Zauhar G. Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound. 1998;26:185–9. doi: 10.1002/(sici)1097-0096(199805)26:4<185::aid-jcu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Beland MD, Walle NL, Machan JT, Cronan JJ. Renal cortical thickness measured at ultrasound: Is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am J Roentgenol. 2010;195:W146–9. doi: 10.2214/AJR.09.4104. [DOI] [PubMed] [Google Scholar]
- 17.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]


