Abstract
The differential diagnosis for a cardiac mass includes primary and metastatic neoplasms. While primary cardiac tumors are rare, metastatic disease to the heart is a common finding in cancer patients. Several “tumor-like” processes can mimic a true cardiac neoplasm with accurate diagnosis critical at guiding appropriate management. We present a pictorial essay of the most common benign cardiac masses and “mass-like” lesions with an emphasis on magnetic resonance imaging features.
Keywords: Benign neoplasms, cardiac, cardiac masses, magnetic resonance, magnetic resonance imaging

INTRODUCTION
Primary cardiac neoplasms are rare and usually benign, with a reported frequency of 0.02%.[1,2] Metastatic disease to the heart is common, occurring in 10% of cancer patients.[3] Transthoracic ultrasound has long been the primary modality for identifying and evaluating cardiac masses. The technology is noninvasive, cheap, widely available, and allows for real-time imaging for mass mobility. Evaluation may be limited due to patient body-habitus, a poor sonographic window, or operator inexperience. Contrast resolution on ultrasound is also relatively poor. Magnetic resonance imaging (MRI) combines noninvasive multiplanar imaging and the ability to acquire functional information with excellent contrast resolution. A regular heart rate and electrocardiographic gating are crucial for acquiring diagnostic images.
Although there is overlap of the MRI characteristics of several cardiac masses, MRI can be diagnostic in several settings and provide useful information in others. Lipomas, fibromas, and hemangiomas as well as thrombus or lipomatous hypertrophy may be confidently diagnosed with MRI. Heterogeneous signal, enhancement, necrosis, extra-cardiac spread, or pericardial effusion may be identified, with the absence of these findings suggesting benignity.[4]
Electrocardiogram (ECG)-gated T1-weighted spin-echo (SE) images are initially acquired in the axial plane for the evaluation of suspected cardiac masses. Sagittal or coronal plane sequences may also be obtained for further delineation of tumor extent. T2-weighted SE images help enhance the contrast between tumor and myocardium and aid in identifying possible cystic or necrotic features. Gadopentetic acid (Gd-DTPA) is administered to improve contrast between tumor and myocardium on T1-weighted images. Fat-saturation techniques are effective for characterizing lipomas. Steady-state free precession (SSFP) sequences display the blood pool with high signal intensity (white blood imaging). Cine MR SSFP images provide valuable information about the movement of masses relative to other cardiovascular structures.[5]
BENIGN MASSES
Myxoma
Approximately 50% of primary cardiac neoplasms are myxomas, with 75% of these originating from the left atrium.[2,6] On cine imaging, (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm30.gif Fig 1a) a narrow stalk that attaches to the inter-atrial septum or prolapse of the myxoma across the atrioventricular valve may be observed [Figure 1a]. Myxomas are often isointense to myocardium on SSFP/T1-weighted and hypointense on T2-weighted imaging [Figure 1b] and [1c]. Variable heterogeneous enhancement may be seen after contrast administration [Figure 1d]. On transthoracic echocardiogram (TTE), myxomas appear lobular and heterogeneously echogenic with a characteristic stalk and relative mobility [ e]. In patients with a cardiac myxoma, a careful history and physical assessment is advised as myxomas of the heart, skin, or breast can be seen in patients with spotty skin pigmentation, schwannomas, and endocrine disorders, including Cushing syndrome.[7] This autosomal dominant condition was originally described, nearly 3 decades ago, as being most often due to a mutation in the tumor suppressor gene PRKAR1A that encodes the protein kinase A regulatory subunit.[8]
Figure 1.
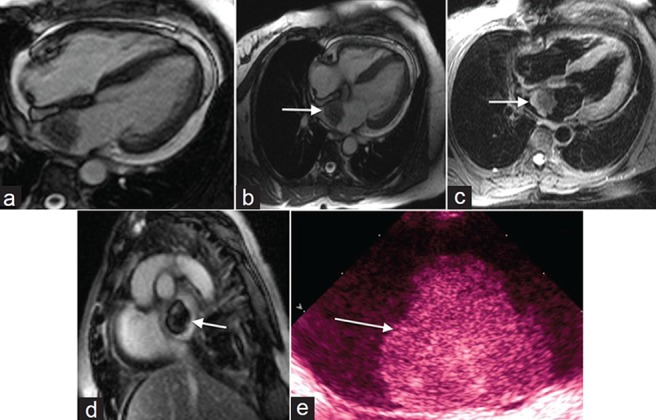
60-year-old female with shortness of breath diagnosed with myxoma. (a) Steady-state free precession (SSFP) cine imaging demonstrates a mobile mass in the left atrium originating from the interatrial septum suggestive of myxoma. (b) SSFP imaging shows the mass is isointense to myocardium and (c) on T2- weighted imaging the mass is hypointense. (d) Post contrast enhancement shows heterogeneous signal and (e) gray-scale transthoracic echocardiogram TTE demonstrates a lobular, heterogeneously echogenic left atrial mass shows a myoxma (white arrows).
Papillary fibroelastoma
Papillary fibroelastomas are the most common primary cardiac tumor to originate from a cardiac valve, and the second most common tumor overall.[2] There is a slight predilection for fibroelastomas to occur on the aortic and mitral valve. While left-sided tumors may present with symptoms related to systemic embolization, right-sided tumors are usually asymptomatic.[9] Papillary fibroelastomas are typically seen on TTE as a small mobile mass attached to a cardiac valve with a characteristic “shimmer” artifact at the interface of the mass with adjacent blood [Figure 2a].[10] Visualizing papillary fibroelastomas on MR has traditionally been limited due to the typically small size at detection. If visualized on MRI cine SSFP imaging, the mass would typically be mobile and attached to a cardiac valve by a short stalk with adjacent turbulent blood flow [Figure 2b]. The signal characteristics of papillary fibroelastomas are not well-reported. A case at our institution presented as a hypointense mass on SSFP and post-contrast T1-weighted imaging was conspicuous due to bright blood signal on these sequences which outlined the mass [Figure 2c and 2d]. The mass was not well-visualized on T2-weighted images [Figure 2e].
Figure 2.
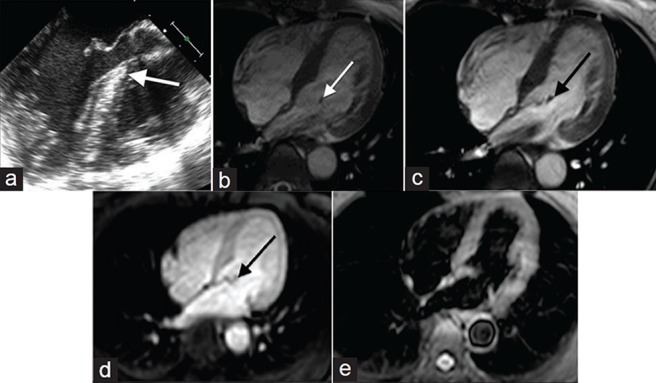
50-year-old female with a history of chest pressure diagnosed with papillary fibroelastoma. (a) Gray-scale TTE and (b-e) MR imaging of the heart: (a) TTE shows a small mobile mass attached to a cardiac valve with “shimmer” artifact (white arrow) compatible with a papillary fibroelastoma, (c) SSFP cine shows the mass was attached to the mitral valve by a short stalk and mobile with turbulent blood flow, (c) On SSFP the mass was hypointense and (d) post contrast T1-weighted image shows the mass was also hypointense (black arrow) and (e) T2-weighted imaging reveals the mass is inconspicuous.
Lipoma
Cardiac lipomas are rare benign primary cardiac neoplasms of mature adipose tissue that may originate from the epicardium or endocardium. When cardiac lipomas originate from the endocardium, decreased mobility and a broad base of attachment on cine imaging (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm32.gif Fig 3a) may help differentiate a lipoma from a myxoma [Figure 3a]. Lipomas are characteristically hyperintense on both T1- and T2-weighted images without enhancement after contrast administration [Figure 3b-d]. Lipomas are echogenic, lobular, and homogeneously echogenic on TTE [Figure 3e].
Figure 3.
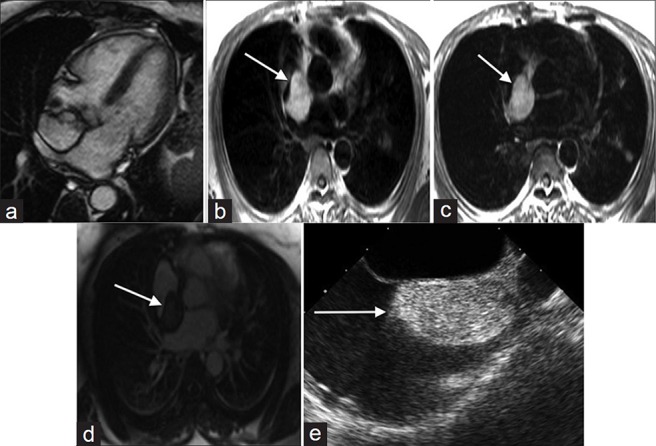
79-year old female with shortness of breath diagnosed with cardiac lipoma. (a) SSFP cine shows a non-mobile circumscribed mass with a broad base of attachment to the inter-atrial septum compatible with a lipoma. (b) T1-weighted, (c) T2- weighted, and (d) without enhancement images show the mass is hyperintense (white arrows) and (e) TTE shows the mass is echogenic, lobular, and homogeneously echogenic (white arrow).
Fibroma
Fibromas are rare benign fibrous tumors that primarily occur within the interventricular septum or left ventricular free wall of children.[2] On cine imaging (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm33.gif Fig 4a), fibromas appear as a non-contractile mass that often narrows the ventricular cavity [Figure 4a]. The fibrous nature of the tumor produces hypointense signal on SSFP and T2-weighted imaging [Figure 4b] and [4c]. While little or no tumoral enhancement is often reported, enhancement patterns are variable and include heterogeneous, homogeneous, and peripheral enhancement.[11]
Figure 4.
16-year old asymptomatic female with a history of atrial septal defect repair as an infant diagnosed with fibroma. (a) SSFP cine of the heart shows a non-mobile mass originating from the right ventricular wall. (b) SSFP and (c) T2-weighted images show that the fibrous nature of the tumor produces hypointense signal (white arrows), compatible with a fibroma.
Hemangioma
Hemangiomas account for approximately 5% of cardiac tumors and may occur in either the endocardium, myocardium, or epicardium.[12] On MR cine SSFP imaging (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm34.gif Fig 5a) a cardiac hemangioma may appear as a non-mobile, non-contractile mass without predilection for a given cardiac chamber [Figure 5a]. On T1-weighted imaging, hemangiomas are isotense secondary to slow flow with hyperintense signal on T2-weighted imaging [Figure 5b] and [5c]. Strong heterogeneous to homogeneous enhancement is observed after contrast administration [Figure 5d].
Figure 5.
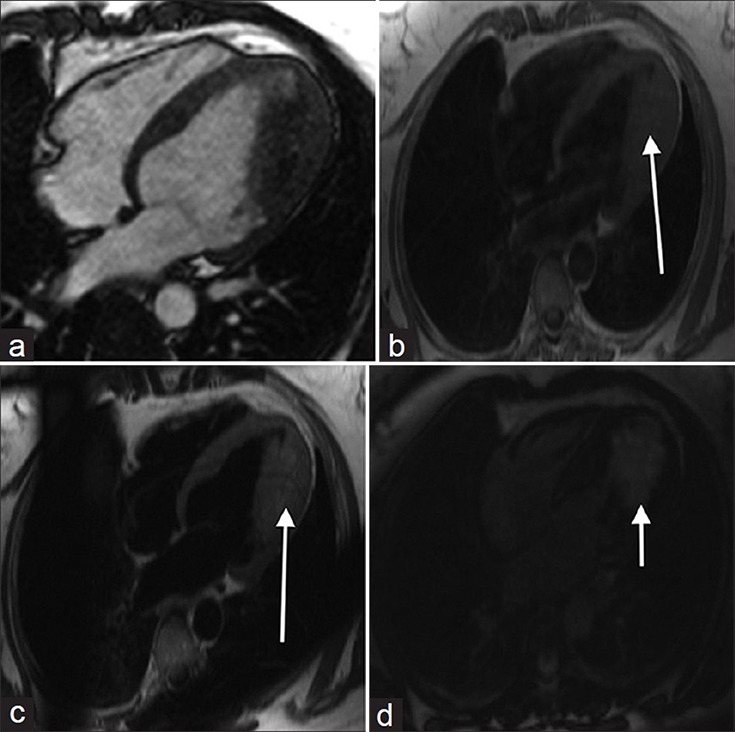
49-year old female with a 20-year history of episodic supraventricular tachycardia and Wolff-Parkinson-White syndrome later diagnosed on pathology investigations with hemangioma. (a) MR cine SSFP imaging shows a non-mobile, non-contractile mass in the left ventricle. (b) T1-weighted image shows the mass is isointense, (c) T2-weighted image reveals the mass is hyperintense, and (d) After contrast administration, the mass demonstrates strong enhancement (white arrows).
Paraganglioma
Paragangliomas are rare tumors of chromaffin cells that can present with symptoms related to catecholamine release, most commonly hypertension. A cardiac location for paragangliomas are exceedingly rare, with the roof of the left atrium and the inter-atrial septum the most common locations, when they do occur.[13] Paragangliomas are hypo- to iso-intense on T1-weighted imaging and classically hyper-intense on T2-weighted imaging with avid contrast enhancement.
“MASS-LIKE” LESIONS
Thrombus
Intra-cardiac thombi are relatively common and may be seen in the setting of atrial fibrillation or after myocardial infarction or central line placement. Transthoracic or transesophageal ultrasound combined with clinical correlation is often sufficient to arrive at the correct diagnosis. In the rare case where differentiating between thrombus and true mass is difficult, cardiac MRI can provide valuable information.
Delayed enhancement using a segmented inversion recovery sequence [Figure 6a] increases the sensitivity for thrombus detection compared to MRI cine SSFP sequences.[14] On cine imaging (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm35.gif Fig 6b), a location in an area of wall motion abnormality or in the left atrial appendage is suggestive of thrombus [Figure 6b]. The morphological appearance is clinically relevant as the risk for embolism is significantly higher with mobile or protruding thrombi compared to flat thrombi.[15] The signal on T1- and T2-weighted images may be variable and heterogeneous. Increasing the inversion times to 400-600 ms can increase contrast resolution between thrombus and myocardium during inversion recovery sequences (http://www.clinicalimagingscience.org/articles/2013/3/1/images/JClinImagingSci_2013_3_1_34_117458_sm36.gif Fig 6c) [Figure 6c].
Figure 6.
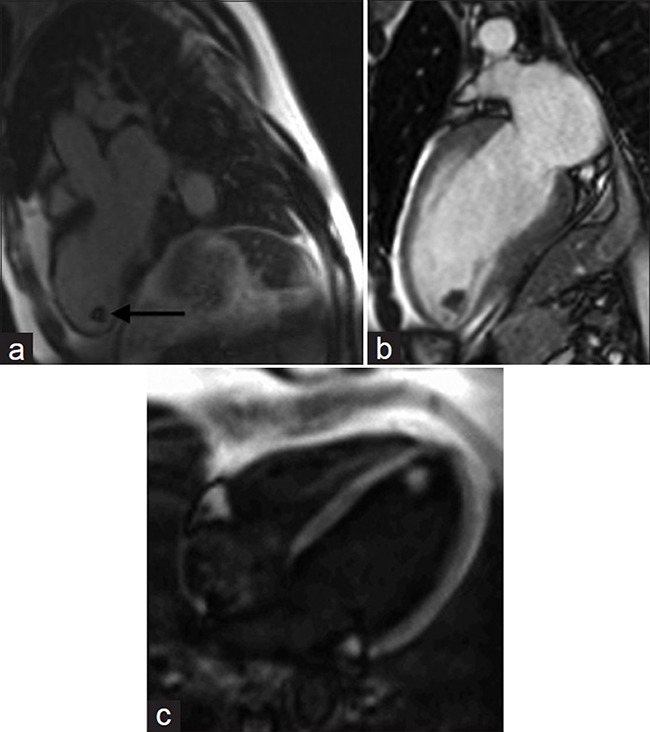
69-year-old male with chest pain diagnosed with left ventricular thrombus. (a-c) MR of the heart: (a) Delayed-enhancement inversion recovery sequence demonstrates a hypointense mass (black arrow) at the apex of the left ventricle. (b) SSFP cine imaging reveals hypokinesis of the left ventricular wall in this region, and (c) Cine of the left ventricle with different inversion times differentiates the mass from normal myocardium. Findings are diagnostic of left ventricular thrombus.
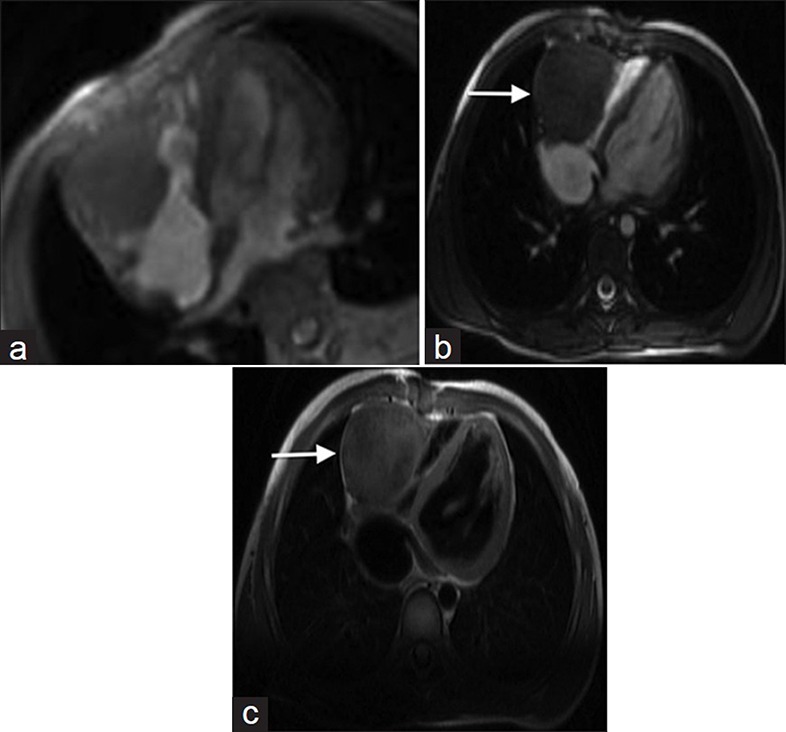
Lipomatous hypertrophy
Lipomatous hypertrophy of the intra-atrial septum is septal infiltration by non-encapsulated adipose, seen almost exclusively in the setting of obesity. Normally the inter-atrial septum is less than 1 cm in thickness, but thickening of the septum caudal to the fossa ovale to greater than 7 cm may be seen with lipomatous hypertrophy.[16] MRI cine SSFP sequence demonstrates non-encapsulated hyper-intense septal thickening [Figure 7a]. Lipomatous hypertrophy is hyper-intense on T1- and T2-weighted sequences and does not enhance after contrast administration [Figure 7b-d].
Figure 7.
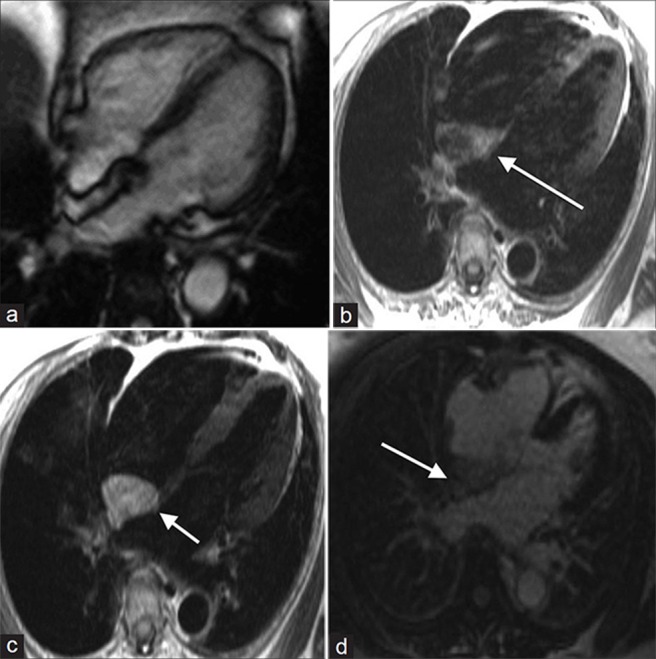
55-year-old male with dyspnea on exertion diagnosed with lipomatous hypertrophy. (a-d) MR of the heart: (a) Steady-state free precession (SSFP) cine demonstrates a non-contractile, non-encapsulated hyperintense inter-atrial septal thickening, (b) T1-weighted, (c) T2-weighted, and (d) without enhancement images show hyperintense signal in the area of thickening (white arrows). Findings are compatible with lipomatous hypertrophy.
CONCLUSION
MRI can effectively identify imaging characteristics that suggest the type of cardiac tumor, location, mobility, base thickness, infiltration, and enhancement. MRI can also differentiate a true mass from an intra-cardiac thrombus, which will need very different management. As experience with cardiac MRI continues to increase, the rare cardiac masses will become less of a diagnostic dilemma.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/34/117458
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 2.Burke A, Virmani R. Atlas of Tumor Pathology: Fasc 16, Ser 3. Washington: Armed Forces Institute of Pathology; 1996. Tumors of the heart and great vessels; pp. 1–98. [Google Scholar]
- 3.Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: Diagnosis and management. Lancet Oncol. 2005;6:219–28. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 4.Luna A, Ribes R, Caro P, Vida J, Erasmus JJ. Evaluation of cardiac tumors with magnetic resonance imaging. Eur Radiol. 2005;15:1446–55. doi: 10.1007/s00330-004-2603-y. [DOI] [PubMed] [Google Scholar]
- 5.Webb WR, Higgins CB. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2011. Thoracic imaging: Pulmonary and cardiovascular radiology; pp. 822–4. [Google Scholar]
- 6.Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20:1303–19. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 7.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–83. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 9.Grinda JM, Couetil JP, Chauvaud S, D’Attellis N, Berrebi A, Fabiani JN, et al. Cardiac valve papillary fibroelastoma: Surgical excision for revealed or potential embolization. J Thorac Cardiovasc Surg. 1999;117:106–10. doi: 10.1016/s0022-5223(99)70474-5. [DOI] [PubMed] [Google Scholar]
- 10.Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: Echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784–90. doi: 10.1016/s0735-1097(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 11.Burke AP, Rosado-de-Christenson M, Templeton PA, Virmani R. Cardiac fibroma: Clinicopathologic correlates and surgical treatment. J Thorac Cardiovasc Surg. 1994;108:862–70. [PubMed] [Google Scholar]
- 12.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: Radiologic-pathologic correlation. Radiographics. 2000;20:1073–103. doi: 10.1148/radiographics.20.4.g00jl081073. [DOI] [PubMed] [Google Scholar]
- 13.Jebara VA, Uva MS, Farge A, Acar C, Azizi M, Plouin PF, Corvol P, et al. Cardiac pheochromocytomas. Ann Thorac Surg. 1992;53:356–61. doi: 10.1016/0003-4975(92)91354-c. [DOI] [PubMed] [Google Scholar]
- 14.Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009;2:969–79. doi: 10.1016/j.jcmg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dantzig JM, Delemarre BJ, Bot H, Visser CA. Left ventricular thrombus in acute myocardial infarction. Eur Heart J. 1996;17:1640–5. doi: 10.1093/oxfordjournals.eurheartj.a014746. [DOI] [PubMed] [Google Scholar]
- 16.Roberts WC. Primary and secondary neoplasms of the heart. Am J Cardiol. 1997;80:671–82. doi: 10.1016/s0002-9149(97)00587-0. [DOI] [PubMed] [Google Scholar]


