Abstract
Background
The substantial proportion of individuals with Parkinson’s disease (PD) who have or are expected to develop concomitant cognitive impairment emphasizes the need for large, well-characterized participant cohorts to serve as a basis for research into the causes, manifestations, and potential treatments of cognitive decline in those with PD.
Objective
To establish a multi-site clinical core that cognitively and clinically characterizes patients with PD by obtaining quality longitudinal clinical, neuropsychological, and validated biomarker data.
Methods
Six hundred nineteen participants with idiopathic PD (68.0 ± 9.1 years, 7.1 ± 6.2 years since diagnosis, 70% males) were enrolled in the Pacific Northwest Udall Center (PANUC), one of the Morris K. Udall Centers of Excellence for Parkinson’s Research, Clinical Consortium and underwent comprehensive clinical and neuropsychological assessment. Participants were diagnosed with no cognitive impairment (PD-NCI), mild cognitive impairment (PD-MCI), or dementia (PDD) at a diagnostic consensus conference.
Results
A substantial proportion of the overall sample was diagnosed with cognitive impairment at baseline: 22% with PDD and 59% with PD-MCI. A higher rate of cognitive impairment was observed in men than women (87% vs. 68%, p<0.0001), despite a higher level of education. Most patients older than 50 years at the time of diagnosis and with disease duration greater than 10 years were cognitively impaired or demented.
Conclusions
The PANUC Clinical Consortium is a clinically and cognitively well-characterized cohort of patients with PD. Baseline cohort characteristics demonstrate a high rate of cognitive impairment in the sample, as well as potential sex differences with regard to cognitive diagnosis. The PANUC Clinical Consortium, with its access to biomarker, genetic, and autopsy data, provides an excellent foundation for detailed research related to cognitive impairment in PD.
Keywords: cognition, cohort studies, dementia, mild cognitive impairment, movement disorders, Parkinson disease
INTRODUCTION
Motor symptoms historically have defined Parkinson’s disease (PD), yet non-motor symptoms increasingly are recognized as having substantial impact on functionality and quality of life of patients with PD [1]. Of these symptoms, the statistics related to dementia are sobering; dementia prevalence rates among patients with PD are approximately 30–40%, and 80% or greater of patients with PD, who live longer than 20 years, are expected to develop dementia over the course of their disease [2, 3]. In addition, the proportion of individuals with PD who have concurrent cognitive impairment not reaching the severity of dementia can be quite high [4, 5]. However, there is great variability in the nature and course of cognitive symptoms among patients with PD [2] and the methods utilized to assess the extent of cognitive decline. Identification of those patients at greatest risk for rapid decline or severe cognitive impairment could lead to development of specific treatments or measures that prevent or delay the progression of cognitive symptoms. Given the prevalence of cognitive impairment in patients with PD, the burden to caregivers, and the socioeconomic impact of dementia, there is great value in identifying a cohort of PD patients that can be investigated longitudinally with regard to cognitive function and its genetic risk and biomarkers.
Recognizing the importance of establishing a collaborative multidisciplinary effort to understand the causes, diagnosis, and treatment of this multifaceted disease, the National Institute of Neurological Disorders and Stroke (NINDS) first accepted applications to establish the Morris K. Udall Centers of Excellence for Parkinson’s Disease Research in 1997. Currently, there are ten Udall Centers operating across the United States. In 2008, the University of Washington (UW) and Oregon Health and Science University (OHSU) combined efforts to establish the Pacific Northwest Udall Center (PANUC), which focuses research on cognitive impairment and dementia in PD. Within PANUC, a clinical core was established to support research through longitudinal clinical and cognitive characterization of subjects diagnosed with PD, with the following stated goals:
To characterize patients with PD and associated cognitive changes by obtaining longitudinal clinical, genetic, neuropsychological, and validated biomarker data.
To provide well-characterized patients with PD for additional clinical research studies, including biomarker and imaging analyses. Patients will also be approached to consent to autopsy to provide clinicopathologic correlation.
To establish a bank of blood and cerebrospinal fluid from patients with PD that complements existing banks of biofluids and data from patients with Alzheimer’s disease and controls.
We have expanded the PANUC Clinical Core to include collaborators at the University of Cincinnati to increase the number of PD patients characterized using identical clinical and neuropsychological procedures; we call this expanded effort the PANUC Clinical Consortium. In the following sections, we describe the design and baseline cohort characteristics of the PANUC Clinical Consortium.
MATERIALS AND METHODS
Subjects
Two samples were enrolled into the clinical cores across sites (Seattle, Portland, Cincinnati) and comprise the PANUC Clinical Consortium. The “Genetics” sample (GS) was designed to be a large cohort evaluated at local movement disorder clinics, or at a participant’s place of residence, twice during the initial five-year study cycle with additional longitudinal evaluation expected. This sample was enrolled to generate the larger number of blood samples (in addition to careful cognitive and clinical characterization) required for genetics projects and potential future blood-based biomarker efforts. We also assembled a “Main” sample (MS) of GS participants from UW and OHSU who volunteered to undergo more detailed clinical and neuropsychological evaluation on a yearly basis (including blood samples for genetic and biomarker analysis); a subset of MS subjects were also recruited for lumbar puncture and autopsy.
Subjects were recruited from several sources, including the Washington State Parkinson’s Disease Registry ([WPDR] a statewide PD research registry), the Parkinson’s Disease Research, Education, and Clinical Center (PADRECC) at the Portland and Puget Sound Veterans Affairs (VA) Medical Centers, and the University of Cincinnati James J. and Joan A. Gardner Center for Parkinson’s disease and Movement Disorders.
Inclusion/exclusion criteria
All participants met the United Kingdom Parkinson’s Disease Society Brain Bank (UKBB) clinical diagnostic criteria for idiopathic PD [6]. Potential participants who failed to fully meet UKBB criteria for PD, or those with a history of other neurologic disorders that would significantly impact cognition, e.g., large-vessel stroke or severe traumatic brain injury, were excluded.
Enrollment
From February 2010 to October 2012, a total of 621 participants with idiopathic PD were entered into the Clinical Consortium across the three sites; of these, 619 were given a cognitive diagnosis and included in the baseline analyses. Current enrollment in the GS totals 475, with 144 followed in the MS. All participants underwent detailed informed consent procedures and provided consent in writing in accordance with procedures approved by the institutional review boards at the VA Puget Sound, Portland VA Medical Center, and University of Cincinnati.
Study Procedures
Clinical examination
At baseline and follow up visits, all participants (GS and MS) underwent extensive clinical examination, including a structured assessment of PD motor symptoms (MDS-UPDRS Part III)[7], symptom history, medications, focused past medical history, environmental exposures, and family history. Information pertaining to activities of daily living was gathered via clinical interview of the patient and caregiver. In the MS, a formal Clinical Dementia Rating was also assessed.
Neuropsychological assessment
Following recently published consensus guidelines [8], GS participants underwent comprehensive cognitive examination at baseline and follow-up visits; MS participants were given additional tests to provide a more robust characterization of cognitive function, although these were not necessarily used for diagnostic purposes. Specific test details are provided in Table 1.
Table 1.
Neuropsychological measures
| Clinical Sample | Annual Sample | |
|---|---|---|
| Premorbid estimate | Shipley-2 vocabulary | |
| Global Cognition | Montreal Cognitive Assessment (MoCA) Dementia Rating Scale-2 (DRS-2) Mini Mental State Examination (MMSE) | Cognitive Abilities Screening Instrument (CASI) |
| Attention and working memory | Letter-Number Sequencing (WMS-III) Digit Symbol (WAIS-R) Trail Making Test | Digit Span(WAIS-R) |
| Executive | Clock drawing test (MoCA) Phonemic Fluency | |
| Language | Semantic Fluency | Boston Naming Test |
| Memory | Hopkins Verbal Learning Test-Revised (HVLT-R) | Logical Memory (WMS-R) |
| Visuospatial | Judgment of Line Orientation Cube copy (MoCA) |
Normative data derived from the National Alzheimer's Coordinating Center Uniform Data Set.[48] Non-UDS test norms were derived from the test manuals.
Diagnostic consensus conference
Biweekly diagnostic consensus conferences are held at the Seattle site, and monthly telephone consensus conferences are conducted between the Seattle site and other sites to assess potential clinical core enrollees for motor and cognitive diagnoses. These meetings were designed to ensure that enrollees meet UKBB criteria for PD diagnostic criteria, permit discussion and consensus concerning clinical cognitive diagnosis, and increase inter-rater reliability among clinicians within and across sites. Conferences are attended one or more movement disorders specialist from each site, a neuropsychologist, behavioral neurologist, and study support personnel.
Cognitive diagnostic criteria
Clinical core participants are assigned to one of the following cognitive diagnostic categories: Parkinson’s disease dementia (PDD), mild cognitive impairment (PD-MCI), or no cognitive impairment (NCI). A diagnosis of PDD is made for those participants meeting published diagnostic criteria [9]: 1) presence of a dementia syndrome in the context of established PD, 2) impairment (1.5 SD below the normative mean) on more than one cognitive domain, 3) impairment that represents a decline from premorbid level of cognitive function, and 4) cognitive deficits that are severe enough to impair daily life. In those with estimated premorbid abilities substantially higher or lower than the norm, adjusted means (based on the Shipley-2 Vocabulary test) are used as comparison points. The onset of parkinsonism must precede the diagnosis of dementia by at least one year to warrant the diagnosis of PDD versus dementia with Lewy bodies (DLB).
PD-MCI was diagnosed according to the following criteria: 1) observed objective cognitive decline defined by performance that is one standard deviation below the published normative mean or the participant’s estimate of premorbid ability on two tests (based on education and the Shipley-2 vocabulary score); or 1.5 standard deviations below the normative mean or estimated premorbid ability on one test, provided there is corroborating evidence of impairment in the same domain on the MoCA or DRS-2; 2) reported/observed cognitive decline by the clinician, participant, or collateral/family member; and 3) cognitive impairments that are not sufficient to substantially interfere with activities of daily living. In addition, participants are diagnosed with PD-MCI if they obtain a valid/reliable MoCA score less than 26 (based on suggested MoCA cutoffs for cognitive impairment) and there is a cognitive decline reported/observed by the patient, caregiver, or clinician even if the other tests do not meet the above criteria. Use of the above criteria permits diagnosis in a manner consistent with recently recommended consensus guidelines for Level I or Level II PD-MCI (depending on the amount of cognitive test data available for a given participant) [10].
Poor performances on cognitive tests that relate (in the opinion of the clinician) to motor disturbance (e.g., tremor), excessive fatigue, depression/anxiety, poor effort/motivation, English as a second language, learning disability, or non-PD primary medical problems are not considered valid and thus are not used as indicators for cognitive decline. For the purposes of cognitive diagnosis, missing data was dealt with in the following manner: if a test score was missing due to the participant’s inability to complete the test as a result of cognitive difficulty, these scores were recoded as 0 and incorporated into the diagnostic data. If scores were missing due to motor dysfunction, or due to other factors unrelated to cognition, these were coded as missing and not considered in the diagnosis. If we were unable to determine level of cognitive decline due to the above factors, participants were assigned a diagnosis of “other” and excluded from current analyses (n=2).
Participants are categorized as having NCI if they fail to meet the above criteria for PDD or PD-MCI. Diagnoses are established at baseline and each subsequent follow-up visit so that conversions from NCI to PD-MCI/PDD and PD-MCI to PDD, or even revert from MCI to NCI (potentially dependent on anxiety, depression, medication state, etc.) can be captured.
Laboratory measures/CSF collection/imaging
Blood to be used for biomarker assays and DNA is collected from both the GS and MS using standardized procedures that are harmonized across sites. Briefly, uniform sample collection kits, labeled storage tubes and boxes, Sprotte 24 g spinal needles and 20 g spinal introducers are provided to each of the collection sites by the Seattle site. CSF is collected from participants in the MS who agree to undergo lumbar puncture. A videotape demonstrating optimal CSF collection was provided to each site. CSF and blood samples are collected via standard manualized procedures to ensure uniform collection procedures.
Autopsy
Neuropathologic evaluations will be collected as described previously [11, 12], and include measures of total brain weight, hallmark AD neuropathologic features, Lewy body disease, cerebral amyloid angiopathy, and vascular brain injury. Medical records are collected for the two weeks preceding death for clinical core subjects who come to autopsy. Data recorded includes cause of death, medical problem list, medications, and the results of laboratory tests.
Data entry and validation
Cognitive and clinical data are reviewed by the psychometrist and study nurse, and verified at the clinical consensus meeting. Prior to data entry, the protocols are again reviewed for correct data transcription. Data are maintained in a database overseen by the PANUC data core.
Analyses
T-tests, Fisher’s exact tests, and Wilcoxon rank-sum tests were used to determine differences on demographic factors, measures of global cognition and depression, and disease severity and duration. Frequency analyses were conducted to determine the prevalence of cognitive diagnoses. All analyses were conducted using Stata 12 (Stata Corp., College Station, TX).
RESULTS
Cohort Demographic Features
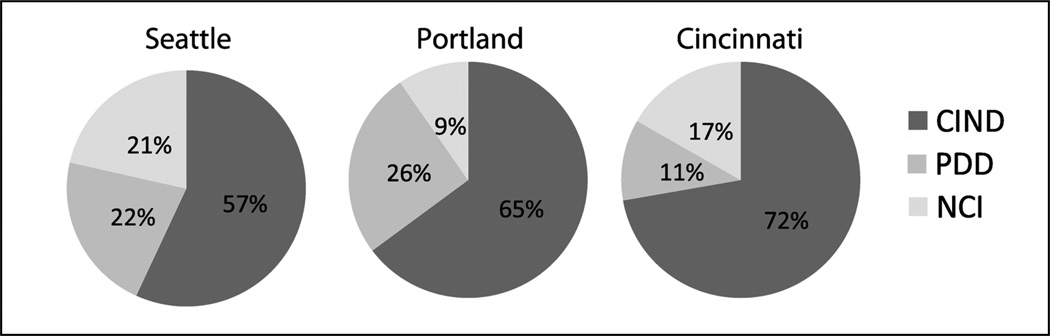
Table 2 provides the demographic and clinical features of the total cohort. Across all sites at the baseline evaluation, 22% of the sample was diagnosed with PDD, 59% with PD-MCI, and the remaining 19% had NCI. Figure 1 shows the breakdown of cognitive diagnoses across sites. The Portland site had a significantly higher number of PD patients diagnosed with cognitive impairment than the Seattle site (p=0.003). When the MS and GS were compared, 67% were diagnosed with PD-MCI and 17% with PDD in the MS, while 57% were diagnosed with PD-MCI and 23% were diagnosed with PDD in the GS. Statistically, the proportions of PD-MCI and PDD in these two samples were not significantly different. A total of 138 participants underwent lumbar puncture procedures; these data will be presented in future publications.
Table 2.
Cohort demographic and clinical characteristics.
| Total (n=619) |
PD-NCI (n=116) |
PD-MCI (n=368) |
PDD (n=135) |
|
|---|---|---|---|---|
| Age at visit | ||||
| m (sd) | 68.0 (9.1) | 64.1 (8.6) | 67.8(8.8)*** | 72.0(9.1)*** |
| Years of education | ||||
| m (sd) | 16.0 (2.8) | 16.6 (2.8) | 16.1 (2.7)‡ | 15.3(3.0)*** |
| Gender | ||||
| % male | 70.0 | 49.1 | 70.4*** | 86.7*** |
| Yrs since diagnosis | ||||
| m (sd) | 7.1 (6.2) | 5.4 (4.3) | 6.6 (6.9)‡ | 9.8 (7.5)*** |
| UPDRS, part 3 | ||||
| m (sd) | 28.2 (13.0) | 21.6 (10.5) | 27.5 (12.4)*** | 35.8 (13.2)*** |
| Hoehn & Yahr stage | ||||
| median (range) | 2 (1–5) | 2 (1–4) | 2(1–5) | 2.5(1.5–5)*** |
| MoCA | ||||
| m (sd) | 24.1 (3.8) | 27.4 (2.0) | 24.7(2.5)*** | 19.5 (4.2)*** |
| GDS | ||||
| m (sd) | 6.0 (1.8) | 5.5 (1.6) | 5.9 (1.8)* | 6.8 (1.9)*** |
| Medications % current use | ||||
| L-Dopa | 60.6 | 57.8 | 60.9 | 62.2 |
| Dopamine agonist | 31.2 | 38.8 | 31.3 | 24.4*** |
| MAO inhibitor | 21.2 | 34.5 | 21.2** | 9.6*** |
| COMT inhibitor | 12.4 | 17.2 | 9.8* | 15.6 |
| Anticholinergic | 1.8 | 2.6 | 1.6 | 1.5 |
| Antipsychotic | 0.3 | 0 | 0.5 | 0 |
| Antidepressant | 19.1 | 23.3 | 19.3 | 14.8 |
| Cognition enhancer | 4.0 | 0 | 0.8 | 16.3*** |
Statistical comparisons are as related to subjects with NCI
p<0.10 (statistical trend),
p≤ 0.05,
p≤ 0.01,
p≤ 0.001
Figure 1.
PANUC Clinical Consortium cognitive diagnoses by study site.
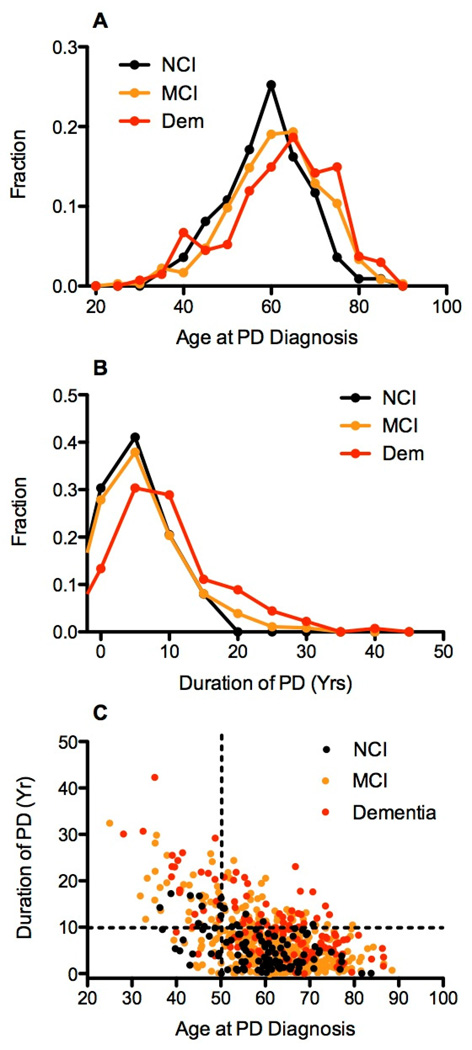
Overall, study participants with cognitive impairment (PD-MCI or PDD) were older, had lower education, higher scores on a depression screen, and lower total scores on global cognitive screening (MoCA) than those participants without cognitive impairment (Table 2). Cognitively impaired patients (PD-MCI or PDD) were older at diagnosis than those with NCI (NCI mean age at diagnosis = 58.7 [9.6] versus 62.4 [11.6] for PDD subjects [p=0.005] and 60.8 [10.4] for PD-MCI [p=0.06, statistical trend], Figure 2, panel A). Patients with PDD and PD-MCI, had a significantly longer time since PD diagnosis than those with NCI (NCI mean disease duration = 5.4 [4.3] versus 9.8 [7.4] for PDD subjects [p<0.0001] and 7.4 [6.1] for PD-MCI [p=0.001], Figure 2, panel B). Based on visual inspection of Figure 2, panel C, we set cut off values of 50 years of age and 10 years disease duration. Using these cutoffs, we found significantly different proportions of cognitively normal PD subjects within the four groups (Fisher's exact test P < 0.0002), indicating that in the PANUC Clinical Consortium, it was unusual for a patient who was older than 50 years at the time of diagnosis to remain cognitively normal after 10 years of disease duration.
Figure 2.
Panel A shows the frequency distribution of age at diagnosis of PD for PANUC consortium subjects stratified by cognitive status at time of entry into PANUC. Panel B shows the frequency distribution for duration of PD at the time of entry into PANUC. Panel C shows the plot of age at PD diagnosis vs. duration of PD for subjects stratified by cognitive status. Dashed lines establish age and duration cutoffs that define four groups. Fisher's exact test for the four groups of cognitively normal PD subjects had p < 0.0002.
In addition, a significantly greater proportion of men than women were diagnosed with cognitive impairment in this cohort (87% vs. 68%, p<0.0001). Average disease duration, age at PD diagnosis, modified Hoehn and Yahr stage, or Geriatric Depression Scale score failed to differ based on gender; however, males had a higher level of education (mean years of education = 15.5 for women vs. 16.2 for men; p=0.004), higher UPDRS scores (p<0.0001), and lower total MoCA scores (mean score = 25.4 for women vs. 23.6 for men; p<0.001). Women were younger at the baseline study visit (mean age= 66.8 for women vs. 68.5 for men; p=0.03).
Table 3 shows demographic and clinical features by study site. There were no differences across study sites with regard to age, years of education, or depression score. The Portland site showed a lower average global cognitive score (based on the MoCA) than the Seattle site (p=0.0001) and the Cincinnati site (p=0.10, statistical trend), and higher modified Hoehn and Yahr score when compared to Seattle (p=0.08, statistical trend) and Cincinnati (p=0.001). Duration (in years) from the time of PD diagnosis was significantly shorter at the Cincinnati site (3.8 ± 3.2 years) than the other sites (7.1 ± 6.3 and 7.8 ± 6.1, p values<0.01). Portland subjects were more likely to be treated with levodopa, dopamine agonists, COMT inhibitors, and antidepressants, likely related to more advanced motor and cognitive symptoms (Table 2).
Table 3.
Demographic and clinical characteristics by site.
| Seattle (n=458) |
Portland (n=125) |
Cincinnati (n=36) |
|
|---|---|---|---|
| Age at visita | |||
| m (sd) | 67.9 (9.4) | 69.4 (7.9) | 65.0 (8.8) |
| Years of education | |||
| m (sd) | 16.1 (2.7) | 15.9 (3.2) | 15.5 (2.9) |
| Genderb | |||
| % male | 64.4 | 91.2 | 66.7 |
| Yrs since diagnosisc | |||
| m (sd) | 7.1 (6.3) | 7.8 (6.1) | 3.8 (3.2) |
| UPDRS, part 3 | |||
| m (sd) | 27.4 (12.7) | 32.9 (13.9) | 21.6 (8.4) |
| Hoehn & Yahr staged | |||
| median (range) | 2 (1–5) | 2.5 (1–4) | 2 (2–3) |
| MoCAe | |||
| m (sd) | 24.5 (3.6) | 22.9 (4.2) | 24.2 (4.1) |
| GDS | |||
| m (sd) | 6.0 (1.8) | 6.1 (1.7) | 5.8 (2.2) |
| Medications % current usef | |||
| L-Dopa | 55.7 | 79.2 | 58.3 |
| Dopamine agonist | 29.0 | 42.4 | 19.4 |
| MAO inhibitor | 25.0 | 12.0 | 5.6 |
| COMT inhibitor | 11.1 | 20.0 | 2.8 |
| Anticholinergic | 1.8 | 2.4 | 0 |
| Antipsychotic | 0.4 | 0 | 0 |
| Antidepressant | 17.7* | 28.8 | 2.8 |
| Cognition enhancer | 3.9 | 5.6 | 0 |
Cincinnati subjects were younger than Seattle (p=0.07, trend) and Portland (p=0.005) subjects
A greater proportion of subjects were male at the Portland site vs. Seattle and Cincinnati (p values < 0.001)
Cincinnati subjects had a shorter disease duration than Seattle (p=0.008) or Portland (p=0.001) subjects
Portland subjects were at a more advanced Hoehn & Yahr stage and had higher mean UPDRS scores than Seattle (p=0.08, trend; p<0.0001, respectively) or Cincinnati (p values < 0.001) subjects
Portland subjects had lower total MoCA scores than Seattle (p=0.0001) or Cincinnati (p=0.10, trend) subjects
A greater proportion of Portland subjects were current users of L-Dopa, dopamine agonists, COMT inhibitors, and antidepressants (p values < 0.05), while a greater proportion of Seattle subjects were current users of MAO inhibitors (p values <0.01). Both Seattle and Portland subjects had a greater proportion of subjects prescribed antidepressants (p values <0.05) as compared to Cincinnati.
DISCUSSION
The PANUC Clinical Consortium establishes a cohort of PD patients characterized using uniform methods for PD clinical diagnosis and cognitive evaluation. Baseline characteristics identified a substantial proportion of the cohort as cognitively impaired. The prevalence of PDD in the sample (22%) was somewhat lower than point prevalence rates generally described by others (30–40%) [9, 13]. However, an additional 59% of the sample was identified with PD-MCI, a rate that is substantially higher than a recent pooled prevalence estimate of 26% [14]. There are several potential contributors to this finding. As with the study of MCI in AD, diagnostic categorization of cognitive decline the field of PD research remains challenging. The variability in research design, assessment methods, diagnostic, inclusion/exclusion criteria and cohort-specific factors (e.g., disease duration and severity, level of education, research setting, sample size, and concomitant psychiatric symptoms) across studies can lead to substantial variability in reported prevalence rates of mild neurocognitive disorders in PD [15].
Despite the tendency to incorporate more thorough neuropsychiatric testing to better capture early changes in fronto-subcortical mediated processes in many studies addressing cognition in PD, there is little agreement on specific test components within selected cognitive domains, making cross-study comparisons difficult and contributing to the disparity in reported MCI prevalence rates. The PANUC Clinical Consortium thus incorporates cognitive tests that arose from a collaborative effort between PANUC and the University of Pennsylvania’s Udall Center to harmonize neuropsychiatric tests across sites involved in the study of PD and cognition [8]. Moving forward, it is our hope to include more sites in the PANUC Clinical Consortium, as well as to promote the use of the recommended multilevel cognitive assessment in order to encourage cross-study comparisons.
In addition to specific assessment tools, the procedures used to identify level and extent of impairment can differ substantially across studies. For example, the level of impairment required to diagnose cognitive dysfunction ranges from one to two standard deviations below the normative mean depending on the study [13, 16, 17]. As we have shown previously [18], simply changing the level of impairment can lead to dramatic differences in the reported rate of cognitive impairment. Extent of impairment required also differs across cohorts, with some sites requiring impairment on only one test, while others require impairment either on an average of tests within a domain or on more than one test in a given battery [13, 19–21]. In the PANUC cohort, we chose to set the level of impairment in accordance with the number of tests affected, such that a higher level of impairment was required to diagnose MCI if only one test within a cognitive domain was affected.
Another significant source of variance when considering cognitive diagnosis in older populations relates to lifelong intellectual opportunities, most commonly identified by level of education. Higher education is consistently associated with lower levels of dementia [22–24], which may in part account for the lower level of dementia identified in the PANUC cohort. Given the higher average level of education (consistent with the greater Seattle area demographics) in the PANUC cohort, we chose to incorporate a premorbid estimate by which to compare cognitive test performance, as opposed to the more common method of establishing impairment which consists of comparing individual performance to a standard normative mean [18]. This standard method assumes that all cohort members have average lifelong intellectual abilities, and thereby may be insensitive to detect mild cognitive deficits in people with higher than average premorbid cognitive abilities. Adjusting for premorbid status for those with above average estimated premorbid performance thus allowed for the detection of cognitive decline that did not reach the severity of dementia in higher functioning individuals, thereby increasing PD-MCI prevalence estimates in this cohort.
Other issues related to specific features of the cohort may also have impacted the prevalence of MCI in the sample. For example, the PANUC Clinical Consortium does not restrict enrollment to newly diagnosed participants. Even among early PD patients, a significant proportion may demonstrate cognitive impairment [21, 25]. Studies that include participants with longer average disease durations tend to report higher prevalence rates, including Janvin et al. [17] who found evidence of cognitive impairment in 55% of a sample with an average disease duration of 4 years. In patients followed longitudinally from initial diagnosis, over 50% demonstrated cognitive decline by 5 years post-diagnosis [26]. As it is expected that upwards of 80% of PD patients will eventually develop PDD [2], the high rate of cognitive impairment in the PANUC cohort is not unexpected.
An interesting finding from the PANUC baseline cohort pertains to potential sex differences in cognitive impairment. Although this is a cross-sectional examination of a clinic-based cohort, and thus may not be fully representative of the community at large, it is worth noting that we found a significantly greater proportion of men in the sample who were characterized as having cognitive impairment. It has been established that male gender confers a greater risk for PD [27]; however the relationship between sex and cognitive impairment is not clear. It has been suggested that both sex and side of onset of PD symptoms may interact to influence performance on spatial tasks [28, 29], although this is not found consistently across studies [27]. Non-cognitive sex differences have also been described, such that higher levels of rigidity and REM behavior disorders have been reported in men, while dyskinesia and depression are more common in women with PD [27]. Some studies suggest that onset of PD may be later in women [30, 31]; however, this is not the case with regard to the PANUC cohort. The precise mechanisms underlying sex differences in PD are to date unknown, however, a preponderance of studies in experimental models suggest that estrogen may exert a specific neuroprotective effect against dopaminergic neurodegeneration [27]. Although women in the PANUC cohort were on average two years younger than men at the time of assessment and had higher UPDRS motor scores, because men and women did not differ with regard to disease duration or severity on the Hoehn and Yahr scale, our results related to sex differences and cognitive impairment certainly warrant further longitudinal investigation into rate and extent of cognitive decline and progression to dementia in men and women.
Longitudinally, the aims of the PANUC Clinical Consortium are to provide well-characterized patients with PD for additional clinical research studies (such as biomarker and imaging analyses), genetic analyses, clinicopathologic correlation, and the banking of plasma and CSF for future analyses of promising biomarkers. Although many of the long-term goals of the PANUC Clinical Consortium are perhaps best served by identifying a large group of prevalent PD cases, use of a study design that is not population-based can limit the generalizability of certain results to the wider population. For example, the study enrolls participants with varying levels of disease severity, which restricts our ability to track the natural history of cognitive impairment in PD from the time of disease onset. In addition, patients are referred for study participation from local clinics; as a result, we do not have details pertaining to those patients who were not referred. However, the large sample size will undoubtedly allow us to describe the cognitive impairment trajectory for people with varying levels of motor and cognitive impairment.
The PANUC Clinical Consortium is a clinically and cognitively well-characterized cohort of patients with idiopathic PD. To date, the enrolled cohort has provided baseline, cross-sectional data that enhance our understanding of the extent of cognitive impairment present in PD, as well as a foundation for longitudinal examination of the relationship between cognition, biomarkers, and genetic factors. Baseline cohort characteristics demonstrate a high rate of cognitive impairment in the sample, as well as potential sex differences with regard to cognitive diagnosis. Such baseline results raise many questions concerning the nature and extent of cognitive impairment in PD. The PANUC Clinical Consortium, through longitudinal follow up and access to biomarker, genetic, and autopsy data is uniquely positioned to address these questions and many more. Finally, the Consortium is expandable and thereby provides an opportunity for other investigators focused on clinical research into cognitive impairment in PD to join this collaborative effort.
ACKNOWLEDGEMENTS
This research was supported by NS062684 and NS065070, the Nancy and Buster Alvord Endowment, and the Department of Veterans Affairs. The funding sources did not provide scientific input for the study.
Footnotes
CONFLICT OF INTEREST
Dr. Leverenz is a consultant for Bayer Pharmaceuticals, Navidea Biopharmaceuticals, and Piramel Healthcare.
REFERENCES
- 1.Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M. Nonmotor symptoms in Parkinson's disease in 2012: relevant clinical aspects. Parkinsons Dis. 2012;2012:198316. doi: 10.1155/2012/198316. PubMed PMID: 22888466. Pubmed Central PMCID: 3410355. Epub 2012/08/14.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiol Dis. 2012 Jun;46(3):590–596. doi: 10.1016/j.nbd.2012.03.029. PubMed PMID: 22484304. Epub 2012/04/10.eng. [DOI] [PubMed] [Google Scholar]
- 3.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008 Mar 25;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. PubMed PMID: 18362281. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010 Sep 21;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. PubMed PMID: 20855849. Pubmed Central PMCID: 2942065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monastero R, Di Fiore P, Ventimiglia GD, Ventimiglia CC, Battaglini I, Camarda R, et al. Prevalence and profile of mild cognitive impairment in Parkinson's disease. Neurodegenerative diseases. 2012;10(1–4):187–190. doi: 10.1159/000335909. PubMed PMID: 22398358. [DOI] [PubMed] [Google Scholar]
- 6.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 1988 Jun;51(6):745–752. doi: 10.1136/jnnp.51.6.745. PubMed PMID: 2841426. Pubmed Central PMCID: 1033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008 Nov 15;23(15):2129–2170. doi: 10.1002/mds.22340. PubMed PMID: 19025984. [DOI] [PubMed] [Google Scholar]
- 8.Watson GS, Cholerton BA, Gross RG, Weintraub D, Zabetian CP, Trojanowski JQ, et al. Neuropsychologic assessment in collaborative Parkinson's disease research: A proposal from the National Institute of Neurological Disorders and Stroke Morris K. dall Centers of Excellence for Parkinson's Disease Research at the University of Pennsylvania and the University of Washington. Alzheimers Dement. 2012 Nov 16; doi: 10.1016/j.jalz.2012.07.006. PubMed PMID: 23164549. Epub 2012/11/21.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2007 Sep 15;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 837. PubMed PMID: 17542011. Epub 2007/06/02.eng. [DOI] [PubMed] [Google Scholar]
- 10.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement disorders : official journal of the Movement Disorder Society. 2012 Mar;27(3):349–356. doi: 10.1002/mds.24893. PubMed PMID: 22275317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, Schantz A, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007 Aug 28;69(9):878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. PubMed PMID: 17724290. [DOI] [PubMed] [Google Scholar]
- 12.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of neurology. 2007 Oct;62(4):406–413. doi: 10.1002/ana.21208. PubMed PMID: 17879383. [DOI] [PubMed] [Google Scholar]
- 13.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2005 Oct;20(10):1255–1263. doi: 10.1002/mds.20527. PubMed PMID: 16041803. Epub 2005/07/26.eng. [DOI] [PubMed] [Google Scholar]
- 14.Goldman JG, Litvan I. Mild cognitive impairment in Parkinson's disease. Minerva Med. 2011 Dec;102(6):441–459. PubMed PMID: 22193376. Pubmed Central PMCID: 3370887. Epub 2011/12/24.eng. [PMC free article] [PubMed] [Google Scholar]
- 15.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, et al. MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Movement disorders : official journal of the Movement Disorder Society. 2011 Aug 15;26(10):1814–1824. doi: 10.1002/mds.23823. PubMed PMID: 21661055. Pubmed Central PMCID: 3181006. Epub 2011/06/11.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004 Mar;127(Pt 3):550–560. doi: 10.1093/brain/awh067. PubMed PMID: 14691062. Epub 2003/12/24.eng. [DOI] [PubMed] [Google Scholar]
- 17.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson's disease without dementia. Dementia and geriatric cognitive disorders. 2003;15(3):126–131. doi: 10.1159/000068483. PubMed PMID: 12584427. Epub 2003/02/14.eng. [DOI] [PubMed] [Google Scholar]
- 18.Trittschuh EH, Crane PK, Larson EB, Cholerton B, McCormick WC, McCurry SM, et al. Effects of varying diagnostic criteria on prevalence of mild cognitive impairment in a community based sample. J Alzheimers Dis. 2011;25(1):163–173. doi: 10.3233/JAD-2011-101821. PubMed PMID: 21368379. Pubmed Central PMCID: 3146555. Epub 2011/03/04.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalrymple-Alford JC, Livingston L, MacAskill MR, Graham C, Melzer TR, Porter RJ, et al. Characterizing mild cognitive impairment in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2011 Mar;26(4):629–636. doi: 10.1002/mds.23592. PubMed PMID: 21287603. Epub 2011/02/03.eng. [DOI] [PubMed] [Google Scholar]
- 20.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2007 Jul 15;22(9):1272–1277. doi: 10.1002/mds.21453. PubMed PMID: 17415797. Epub 2007/04/07.eng. [DOI] [PubMed] [Google Scholar]
- 21.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005 Oct 25;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. PubMed PMID: 16247051. Epub 2005/10/26.eng. [DOI] [PubMed] [Google Scholar]
- 22.Cohen OS, Vakil E, Tanne D, Nitsan Z, Schwartz R, Hassin-Baer S. Educational level as a modulator of cognitive performance and neuropsychyatric features in Parkinson disease. Cogn Behav Neurol. 2007 Mar;20(1):68–72. doi: 10.1097/WNN.0b013e3180335f8e. PubMed PMID: 17356347. Epub 2007/03/16.eng. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002 Sep 1;156(5):445–453. doi: 10.1093/aje/kwf074. PubMed PMID: 12196314. Epub 2002/08/28.eng. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003 Aug;25(5):671–679. doi: 10.1076/jcen.25.5.671.14584. PubMed PMID: 12815504. Epub 2003/06/20.eng. [DOI] [PubMed] [Google Scholar]
- 25.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009 Mar 31;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. PubMed PMID: 19020293. [DOI] [PubMed] [Google Scholar]
- 26.Muslimovic D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in Parkinson's disease: a prospective longitudinal study. J Int Neuropsychol Soc. 2009 May;15(3):426–437. doi: 10.1017/S1355617709090614. PubMed PMID: 19402929. Epub 2009/05/01.eng. [DOI] [PubMed] [Google Scholar]
- 27.Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Movement disorders : official journal of the Movement Disorder Society. 2010 Dec 15;25(16):2695–2703. doi: 10.1002/mds.23388. PubMed PMID: 20925068. Pubmed Central PMCID: 3003756. Epub 2010/10/07.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res. 2005 May;45(10):1285–1296. doi: 10.1016/j.visres.2004.11.006. PubMed PMID: 15733961. Epub 2005/03/01.eng. [DOI] [PubMed] [Google Scholar]
- 29.Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson's disease. Brain. 2008 Nov;131(Pt 11):2882–2893. doi: 10.1093/brain/awn237. PubMed PMID: 18957454. Pubmed Central PMCID: 2577802. Epub 2008/10/30.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves G, Muller B, Herlofson K, HogenEsch I, Telstad W, Aarsland D, et al. Incidence of Parkinson's disease in Norway: the Norwegian ParkWest study. Journal of neurology, neurosurgery, and psychiatry. 2009 Aug;80(8):851–857. doi: 10.1136/jnnp.2008.168211. PubMed PMID: 19246476. Epub 2009/02/28.eng. [DOI] [PubMed] [Google Scholar]
- 31.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2007 Aug;78(8):819–824. doi: 10.1136/jnnp.2006.103788. PubMed PMID: 17098842. Pubmed Central PMCID: 2117736. Epub 2006/11/14.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shipley WC, Gruber CP, Martin TA, Klein AM. Shipley-2 Manual. Los Angeles, CA: Western Psychological Services; 2009. [Google Scholar]
- 33.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. PubMed PMID: 15817019. Epub 2005/04/09.eng. [DOI] [PubMed] [Google Scholar]
- 34.Mattis S. Dementia Rating Scale-2: Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. PubMed PMID: 1202204. Epub 1975/11/01.eng. [DOI] [PubMed] [Google Scholar]
- 36.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994 Spring;6(1):45–58. doi: 10.1017/s1041610294001602. discussion 62. PubMed PMID: 8054493. Epub 1994/01/01.eng. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. WMS-III® Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation Harcourt Brace & Company; 1997. [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale-Revised manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 39.Army Individual Test Battery: Manual of directions and scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 40.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3rd ed. xvii. Oxford ; New York: Oxford University Press; 2006. p. 1216. [Google Scholar]
- 41.Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 42.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 43.Kaplan E, Goodglass HSW. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 44.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and inter-rater reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 45.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 46.Benton AL. Revised Visual Retention Test. 4th edition. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 47.Benton AL, Sivan AB, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment-A Clinical Manual. Lutz, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- 48.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer disease and associated disorders. 2009 Apr-Jun;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. PubMed PMID: 19474567. Pubmed Central PMCID: 2743984. [DOI] [PMC free article] [PubMed] [Google Scholar]