Abstract
Background
Sorafenib (a VEGFR and multi-targeted kinase inhibitor) and Bortezomib (a proteasome inhibitor) have clinical antineoplastic activities as single agents, and combine synergistically in preclinical models.
Methods
This Phase I study was undertaken to define the toxicity and the maximum tolerated doses (MTD) of the combination in patients with advanced solid tumors. Patients with cytologic or histologic proof of unresectable solid tumors were treated with escalating doses of sorafenib (twice daily) and bortezomib (days 1, 4, 8 and 11 intravenously) with 21-day cycles.
Results
Fourteen patients (7 males, median age 65, range 24–74), with renal (3), lung (3), pancreas (2), and breast, adrenal gland, melanoma, spindle cell tumor, chronic lymphocytic leukemia and multiple myeloma (1 each) were enrolled. All patients are off treatment, 10 due to disease progression. DLT was seen in two patients (one grade 3 abdominal pain and grade 4 lipase elevation; one with grade 3 vomiting) at sorafenib 200 mg twice daily and bortezomib 1.3 mg/m2, establishing the MTD. No grade 4 hematologic or grade 5 toxicities were seen. One patient with renal cell cancer had a partial response and 5 patients attained stable disease.
Conclusions
The combination of sorafenib and bortezomib was tolerated well. The recommended phase 2 doses are sorafenib 200 mg twice daily continuously with bortezomib 1 mg/m2 on days 1, 4, 8, 11 (21 day cycles). The combination shows preliminary signs of efficacy, supporting phase 2 studies.
Keywords: Phase 1 trial, Sorafenib, Bortezomib
INTRODUCTION
Sorafenib (NEXAVAR®, BAY 43-9006) is an orally bioavailable small molecule, belonging to the class of compounds defined as bisaryl ureas, that was designed to specifically target Raf kinase [1–3]. An inhibitor of the adenosine triphosphate binding site of Raf kinase that is active in growth factor over-expressing and mutated K-Ras-bearing cell lines, sorafenib was the first compound of its class to enter clinical trials. In vitro kinase assays suggest that sorafenib is a potent inhibitor of wild type and mutant (V599E) B-Raf and c-Raf Kinase isoforms in vitro. Under cell-free conditions, sorafenib also inhibits several receptor tyrosine kinases (RTKs) that are involved in tumor progression: human VEGFR-2, murine VEGFR-2, murine VEGFR-3, murine PDGFR-β, Flt-3, c-KIT, and p38α (MAPK family). In cellular assays, sorafenib is a potent inhibitor of human and murine VEGFR-2, murine VEGFR-3, and murine PDGFR-β receptor autophosphorylation. Sorafenib exhibits activity against a wide spectrum of malignancies in vitro and in vivo; and it is currently approved for treatment of renal and hepatocellular cancer.
Bortezomib (VELCADE®, PS341) is a small molecule proteasome inhibitor that is currently approved for the treatment of multiple myeloma in patients who have received at least one prior therapy. Bortezomib has a novel pattern of cytotoxicity in in vitro and in vivo assays[4] and in a variety of xenograft tumor models, both as a single agent and in combination with chemotherapy and radiation.[5–7] The anti-neoplastic effect of bortezomib likely involves several distinct mechanisms, including inhibition of cell growth and survival pathways, induction of apoptosis, and inhibition of expression of genes that control cellular adhesion, migration and angiogenesis. Bortezomib is thought to exert its effects in multiple myeloma through inhibition of nuclear factor κB (NF-κB) activation, attenuation of interleukin-6 (IL-6)-mediated cell growth, direct induction of apoptosis, and possibly anti-angiogenic effects.[8]
We previously demonstrated that sorafenib and bortezomib synergistically induced mitochondrial injury and apoptosis in a broad range of solid tumor and leukemia cell lines. [9] Treatment with the combination led to decreased phosphorylation of vascular endothelial growth factor receptor-2, platelet-derived growth factor receptor-beta, and Akt and increased phosphorylation of stress-related c-Jun NH2-terminal kinase (JNK). Another study in hepatocellular cancer suggested that protein phosphatase 2A (PP2A) mediated Akt inhibition was critical for the activity of this combination.[10] Given promising in vitro data, we conducted a phase 1 clinical trial to (1) determine the maximum tolerated dose (MTD) of sorafenib in combination with bortezomib; (2) describe the toxicities associated with the combination and (3) preliminarily evaluate the therapeutic antitumor activity of the combination in the setting of advanced solid tumors.
PATIENTS and METHODS
The study was conducted in collaboration with CTEP/NCI, following approval from the Mayo Clinic Institutional Review Board, and was registered on www.clinicaltrials.gov (NCT00303797). Patients over 18 years of age, with cytologic or histologic proof of unresectable cancer without curative treatment options, were enrolled provided they had adequate hematological (neutrophils ≥ 1500/μL; hemoglobin ≥ 9 g/dl; platelets ≥ 100,000/μL) and organ function (total bilirubin ≤ 1.5 x upper limit of normal (ULN), AST ≤ 3 x ULN or AST ≤ 5 x ULN if liver involvement, creatinine ≤ 1.5 x ULN). Inclusion on the study required an ECOG performance status <= 2, no residual adverse effects from preceding therapy, and no active infection. A New York Heart Association classification III or IV, presence of uncontrolled hypertension, more than grade 1 sensory peripheral neuropathy of any etiology or neuropathic pain of any etiology, need for therapeutic anticoagulation or active HIV infection requiring therapy excluded patients from participating.
Patients received sorafenib orally daily along with bortezomib administered intravenously on days 1, 4, 8, 11 of a 3-week cycle. The dose escalation plan for the phase 1 trial is shown in Table 1. This single arm phase I study was designed to determine the MTD and toxicity of sorafenib and bortezomib administered in combination and used the standard cohort 3+3 design. Three patients were treated at each dose level and observed for a minimum of 3 weeks (i.e. one full cycle) before new patients are treated. No intra-patient dose escalation was allowed. MTD was defined as the dose level below the lowest dose that induces dose-limiting toxicity in at least one-third of patients (at least 2 of a maximum of 6 new patients). The trial was designed to enroll additional patients with hematological malignancies at the MTD.
Table 1.
Dose Escalation Plan
| Dose Level | BAY 43-9006 (mg/dose, taken twice daily) | PS-341 (mg/m2, Each Dose) |
|---|---|---|
| −1 | 200 | 0.5 |
| 0 | 200 | 0.75 |
| 1* | 200 | 1 |
| 2 | 200 | 1.3 |
| 3 | 400 | 1.3 |
| 4 | 400 | 1.5 |
| 5 | 400 | 1.7 |
Starting dose.
DLT was defined as Grade 4 ANC for ≥ 5 days, Grade 4 anemia of any duration, PLT < 25,000 of any duration (hematologic), serum creatinine ≥2 times baseline or > 2 x ULN if baseline levels are within normal range, or any other ≥ grade 3 adverse event as per NCI CTCAE v3.0, except fatigue. Any toxicities that caused dose delay of >2 weeks of the intended next dose were also considered dose-limiting. In addition, grade 3 nausea, vomiting, or diarrhea with maximal supportive treatment (s) was also considered dose-limiting.
RESULTS
Fourteen patients (12 solid tumor patients in the dose escalation cohort and 2 hematological malignancies patients at the MTD) were enrolled and treated between December, 2005 and September, 2008. The patient characteristics are as shown in Table 2. All patients were off treatment as of May, 2009. Ten of these patients (7 treated at dose level 1 and 3 at dose level 2) went off treatment due to disease progression. The other one patient treated at dose level 1 refused further treatment and the other three patients treated at dose level 2 went off treatment due to adverse events.
Table 2.
Patient characteristics
| Overall (N=14) | Dose Level 1 (N=8) | Dose Level 2 (N=6) | |
|---|---|---|---|
| Age, median(range) | 65 (24,74) | 66 (34,72) | 63 (24,74) |
| Gender | |||
| Female | 7 (50%) | 3 (37.5%) | 4 (66.7%) |
| Male | 7 (50%) | 5 (62.5%) | 2 (33.3%) |
| Race | |||
| White | 14 (100%) | 8 (100%) | 6 (100%) |
| Performance Score | |||
| 0 | 6 (42.9%) | 3 (37.5%) | 3 (50%) |
| 1 | 8 (57.1%) | 5 (62.5%) | 3 (50%) |
| Prior Treatments | |||
| Chemotherapy | 12 (85.7%) | 7 (87.5%) | 5 (83.3%) |
| Radiation Therapy | 3 (21.4%) | 2 (25%) | 1 (16.7%) |
| Surgery | 12 (85.7%) | 6 (75%) | 6 (100%) |
| Tumor Site | |||
| Kidney | 3 (21.4%) | 1 (12.5%) | 2 (33.3%) |
| Lung | 3 (21.4%) | 0 (0%) | 3 (50%) |
| Pancreas | 2 (14.3%) | 2 (25%) | 0 (0%) |
| Breast | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
| Adrenal Gland | 1 (7.1%) | 0 (0%) | 1 (16.7%) |
| Multiple Myeloma | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
| CLL | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
| Spindle Cell Tumor | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
| Melanoma | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
All 12 patients enrolled in the solid tumor dose escalation cohort were evaluable for DLT analysis. In this cohort, DLT was seen in two patients at dose level 2 (one patient had a grade 3 abdominal pain and grade 4 lipase and another patient had grade 3 emesis). As a result, dose level 1 was identified as the recommended phase 2 dose. Two additional patients with hematological malignancies were accrued to MTD cohort (dose level 1) before the study was permanently closed.
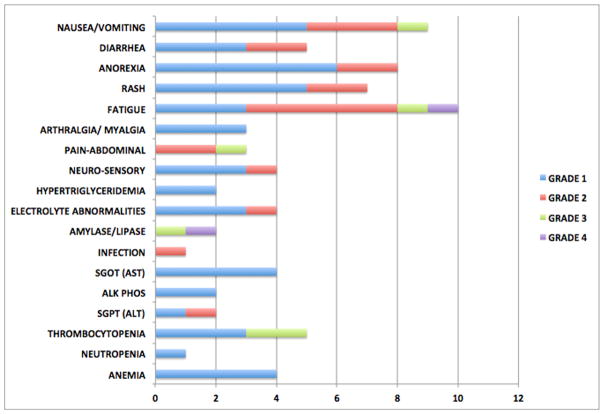
Considering all 14 enrolled patients, no grade 4 hematologic toxicities or grade 5 toxicities were seen in this study. One grade 4 fatigue and one grade 4 lipase (one of the reported DLTs) were observed during cycle 1. The most common cycle 1 toxicities reported included gastrointestinal toxicities (nausea, anorexia), dermatologic (rash, pruritus), fatigue, and hematological (thrombocytopenia) toxicities. The commonly observed toxicities across all cycles for all the enrolled patients are shown in Figure 1.
Figure 1.
Common toxicities seen across all cycles.
The best response was one partial response seen in a patient with renal cell cancer who was treated at dose level 2, which was sustained for 2 cycles. Another patient with renal cell cancer treated at dose level 1 had a stable disease for 2 cycles. Stable disease lasting one cycle was seen in 2 patients treated at dose level 2 (lung, adrenal gland) and 2 patients treated at dose level 1 (pancreas, multiple myeloma). Follow up details of the enrolled patients are provided in Table 3. The median time to progression for the entire cohort was 53 days: 48 days in dose level 1 and 90 days in dose level 2.
Table 3.
Follow up details
| Overall (N=14) | Dose Level 1 (N=8) | Dose Level 2 (N=6) | |
|---|---|---|---|
| Number cycles received, median(range) | 2 (1,5) | 2 (2,5) | 3 (1,4) |
| Follow-up Status | |||
| Alive | 9 (64.3%) | 5 (62.5%) | 4 (66.7%) |
| Dead | 5 (35.7%) | 3 (37.5%) | 2 (33.3%) |
| Progression Status | |||
| Yes | 10 (71.4%) | 7 (87.5%) | 3 (50%) |
| No | 4 (28.6%) | 1 (12.5%) | 3 (50%) |
| Off Active Treatment | |||
| Yes | 14 (100%) | 8 (100%) | 6 (100%) |
| Reason End Treatment | |||
| Progression Onstudy | 10 (71.4%) | 7 (87.5%) | 3 (50%) |
| Refused Further Rx | 1 (7.1%) | 1 (12.5%) | 0 (0%) |
| AEs/Side Effects | 3 (21.4%) | 0 (0%) | 3 (50%) |
DISCUSSION
Sorafenib has exhibited activity in the context of multiple types of cancers and is currently approved for treatment of hepatocellular and renal cell cancer.[11,12] In addition, preclinical studies and ongoing phase 2 trials have suggested activity in other cancer types as well. Bortezomib, the first proteasome inhibitor to enter the clinical arena, has also demonstrated clinical efficacy in multiple cancer types, particularly multiple myeloma and mantle cell lymphoma.[13,14] As with sorafenib, bortezomib appears to have promise in other cancer types and is under active investigation ion multiple settings. Based on the preclinical data demonstrating synergy when these drugs are combined and the delineation of possible mechanisms for the synergy, we undertook this phase 1 trial in patients with solid tumors. While the overall rates and pattern of toxicity were as expected based on the known toxicity profile of the individual drugs, we reached an MTD at doses (sorafenib 200 mg twice daily and bortezomib 1.0 mg/m2 days 1, 4, 8 and 11) lower than what is considered standard for either of the drugs as monotherapy (1.3 mg/m2 days 1, 4, 8 and 11 of 21 days for bortezomib and 400 mg twice daily for sorafenib).[15]
Dose escalation beyond the MTD was associated with GI toxicity. Pancreatitis has been reported with sorafenib and bortezomib monotherapy, even though the frequency is low.[16–20] Previous reports have also suggested that asymptomatic elevations of pancreatic enzymes may be more common than appreciated with sorafenib; and in some instances patients who developed pancreatitis have been able to go back to treatment with sorafenib without additional problems.[16,19] In particular, we observed a high incidence of GI intolerance and fatigue with the combination, even though it is unclear whether the fatigue was related more to the primary cancers rather than therapy. Varying degree of nausea and diarrhea have been reported in the sorafenib trials[21,22,15]. The other adverse events we observed in this trial were consistent with that reported for either of the drugs.
In terms of efficacy, we did observe a partial response and stabilization of multiple neoplasms in this heavily pretreated population. The partial response was in a patient with renal cell cancer, where sorafenib has reported single-agent activity. In addition a low response rate was seen with bortezomib in a phase 2 trial in patients with renal cell cancer.[23] Stable disease was also observed in one patient each with lung, adrenal and pancreas cancer. Sorafenib has reported activity in the setting of pancreatic cancer in combination with gemcitabine [24], as a single agent in non-small cell lung cancer [25], and in metastatic adrenocortical cancer. [26] Similarly, bortezomib has been reported to have limited efficacy in non-small cell lung cancer [27,28].
In conclusion, we have defined the recommended phase 2 dose of sorafenib and bortezomib used in combination to be sorafenib 200 mg twice daily continuously with bortezomib 1 mg/m2 intravenously on days 1, 4, 8, 11 of a 21 day cycle. Preliminary assessment of the combination suggests an acceptable toxicity profile and clinically relevant activity that needs further exploration. Future clinical trials should also include patients with B-cell malignancies, where preclinical evidence suggests a synergistic combination.[29]
Acknowledgments
FUNDING
This work was supported by National Cancer Institute; CA69912. Clinicaltrials.gov identifier: NCT00303797
We are indebted to patients participating in this trial and to the enrolling investigators for their interest in and dedication to this study. We also thank X for commitment to the completion of this trial and X for administrative assistance.
Footnotes
CONFLICT OF INTEREST STATEMENT
SKK has research funding for clinical trials from Millennium Pharmaceuticals and Onyx Pharmaceuticals, and has participated in advisory boards with Millennium Pharmaceuticals and Onyx Pharmaceuticals.
References
- 1.Lowinger TB, Riedl B, Dumas J, Smith RA. Design and discovery of small molecules targeting raf-1 kinase. Curr Pharm Des. 2002;8 (25):2269–2278. doi: 10.2174/1381612023393125. [DOI] [PubMed] [Google Scholar]
- 2.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8 (3):219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 3.Bollag G, Freeman S, Lyons JF, Post LE. Raf pathway inhibitors in oncology. Curr Opin Investig Drugs. 2003;4 (12):1436–1441. [PubMed] [Google Scholar]
- 4.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59 (11):2615–2622. [PubMed] [Google Scholar]
- 5.LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62 (17):4996–5000. [PubMed] [Google Scholar]
- 6.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS., Jr Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61 (9):3535–3540. [PubMed] [Google Scholar]
- 7.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5 (9):2638–2645. [PubMed] [Google Scholar]
- 8.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277 (19):16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Friday BB, Lai JP, Yang L, Sarkaria J, Kay NE, Carter CA, Roberts LR, Kaufmann SH, Adjei AA. Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther. 2006;5 (9):2378–2387. doi: 10.1158/1535-7163.MCT-06-0235. 5/9/2378 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ, Cheng AL. Synergistic interactions between sorafenib and bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt inactivation. J Hepatol. 2009 doi: 10.1016/j.jhep.2009.10.011. S0168-8278(09)00657-6 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356 (2):125–134. doi: 10.1056/NEJMoa060655. 356/2/125 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359 (4):378–390. doi: 10.1056/NEJMoa0708857. 359/4/378 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O’Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24 (30):4867–4874. doi: 10.1200/JCO.2006.07.9665. JCO.2006.07.9665 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352 (24):2487–2498. doi: 10.1056/NEJMoa043445. 352/24/2487 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, Eisen T, Fishman M, Quinn D. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2010 doi: 10.1016/j.critrevonc.2010.03.006. S1040-8428(10)00075-2 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Kanemitu T, Kamoto A, Satoh M, Mori N, Sekii K, Yoshioka T, Itatani H, Fujimoto T. Painless acute pancreatitis associated with sorafenib treatment: a case report. Med Oncol. 2010 doi: 10.1007/s12032-010-9479-2. [DOI] [PubMed] [Google Scholar]
- 17.Minami H, Kawada K, Ebi H, Kitagawa K, Kim YI, Araki K, Mukai H, Tahara M, Nakajima H, Nakajima K. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008;99 (7):1492–1498. doi: 10.1111/j.1349-7006.2008.00837.x. CAS837 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Srinivas S. Acute pancreatitis associated with sorafenib. South Med J. 2007;100 (9):909–911. doi: 10.1097/SMJ.0b013e31813c695d. [DOI] [PubMed] [Google Scholar]
- 19.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, Sridhara R, Garvey P, Justice R, Pazdur R. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12 (24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. 12/24/7271 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Elouni B, Ben Salem C, Zamy M, Sakhri J, Bouraoui K, Biour M. Bortezomib-induced acute pancreatitis. JOP. 2010;11(3):275–276. v11i03a17 [pii] [PubMed] [Google Scholar]
- 21.Laber DA, Mushtaq M. Compassionate use of sorafenib in patients with advanced renal cell cancer. Clin Genitourin Cancer. 2009;7 (1):34–38. doi: 10.3816/CGC.2009.n.006. G483104382074124 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Ng R, Chen EX. Sorafenib (BAY 43-9006): review of clinical development. Curr Clin Pharmacol. 2006;1 (3):223–228. doi: 10.2174/157488406778249325. [DOI] [PubMed] [Google Scholar]
- 23.Kondagunta GV, Drucker B, Schwartz L, Bacik J, Marion S, Russo P, Mazumdar M, Motzer RJ. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22(18):3720–3725. doi: 10.1200/JCO.2004.10.155. doi:10.1200/JCO.2004.10.155 22/18/3720. [DOI] [PubMed] [Google Scholar]
- 24.Siu LL, Awada A, Takimoto CH, Piccart M, Schwartz B, Giannaris T, Lathia C, Petrenciuc O, Moore MJ. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12 (1):144–151. doi: 10.1158/1078-0432.CCR-05-1571. 12/1/144 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Blumenschein GR, Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27 (26):4274–4280. doi: 10.1200/JCO.2009.22.0541. JCO.2009.22.0541 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Butler C, Butler WM, Rizvi AA. Sustained remission with the kinase inhibitor sorafenib in Stage IV metastatic adrenocortical carcinoma. Endocr Pract. 2009:1–19. doi: 10.4158/EP09295.CR. G4067H83X77180PH [pii] [DOI] [PubMed] [Google Scholar]
- 27.Lara PN, Jr, Chansky K, Davies AM, Franklin WA, Gumerlock PH, Guaglianone PP, Atkins JN, Farneth N, Mack PC, Crowley JJ, Gandara DR. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327) J Thorac Oncol. 2006;1(9):996–1001. 01243894-200611000-00013 [pii] [PubMed] [Google Scholar]
- 28.Scagliotti GV, Germonpre P, Bosquee L, Vansteenkiste J, Gervais R, Planchard D, Reck M, De Marinis F, Lee JS, Park K, Biesma B, Gans S, Ramlau R, Szczesna A, Makhson A, Manikhas G, Morgan B, Zhu Y, Chan KC, von Pawel J. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer. 2010;68 (3):420–426. doi: 10.1016/j.lungcan.2009.07.011. S0169-5002(09)00421-8 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan V, Timm M, Haug JL, Kimlinger TK, Wellik LE, Witzig TE, Rajkumar SV, Adjei AA, Kumar S. Sorafenib, a dual Raf kinase/vascular endothelial growth factor receptor inhibitor has significant anti-myeloma activity and synergizes with common anti-myeloma drugs. Oncogene. 2010;29 (8):1190–1202. doi: 10.1038/onc.2009.403. onc2009403 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]