Abstract
Background
In Westernized countries, over 1% of the population is allergic to peanuts or tree nuts, which carries a risk of severe allergic reactions. Several studies support the efficacy of peanut oral immunotherapy (OIT) for reducing the clinical sensitivity of affected individuals; however, the mechanisms of this effect are still being characterized. One mechanism that may contribute is the suppression of effector cells, such as basophils. Basophil anergy has been characterized in vitro as a pathway-specific hyporesponsiveness; however, this has not been demonstrated to occur in vivo.
Objective
To evaluate the hypothesis that basophil anergy occurs in vivo due to chronic allergen exposure in the setting of a clinical oral immunotherapy trial.
Methods
Samples of peripheral blood were obtained from subjects during a placebo-controlled clinical trial of peanut OIT. Basophil reactivity to in vitro stimulation with peanut allergen and controls was assessed by the upregulation of activation markers, CD63 and CD203c, measured by flow cytometry.
Results
The upregulation of CD63 following stimulation of the IgE receptor, either specifically with peanut allergen or non-specifically with anti-IgE antibody, was strongly suppressed by active OIT. However, OIT did not significantly suppress this response in basophils stimulated by the distinct fMLP receptor pathway. In the subset of subjects with egg sensitization, active peanut OIT also suppressed CD63 upregulation in response to stimulation with egg allergen. Allergen OIT also suppressed the upregulation of CD203c including in response to stimulation with IL-3 alone.
Conclusion
Peanut OIT induces a hyporesponsive state in basophils that is consistent with pathway-specific anergy previously described in vitro. This suggests the hypothesis that effector cell anergy could contribute to clinical desensitization.
Keywords: human basophils, desensitization, basophil anergy, CD63, CD203c, oral immunotherapy, peanut allergy
Introduction
Peanut allergy affects over 1% of people in westernized countries [1–3]. The only accepted treatment for food allergy is avoidance and ready access to emergency medications [4]. Unintentional ingestions occur frequently [5], and because peanut allergy is both persistent and more often associated with severe reactions [6, 7], the need for new treatments is substantial [8].
Basophils express the high affinity receptor for IgE (FcεRI) and represent a significant population of antigen-specific cells in IgE-sensitized individuals that are capable of releasing histamine, leukotrienes, cytokines and other mediators [9,10]. Basophils may also be important modulators of adaptive immune responses [11–14]. A significant role for basophils during in vivo allergen exposure is supported by studies documenting basophil activation [15–17]. As such, basophils are both relevant ‘biomarkers’ of IgE-mediated hypersensitivity and potential targets of immunomodulatory interventions.
Phenotyping of basophils during activation has revealed useful markers for flow cytometry-based studies [18]. Anaphylactic degranulation results in the translocation of lysosomal-associated membrane proteins (LAMPs), including CD63, from a predominantly intracellular location to the cell surface [19, 20] resulting in a predominantly bi-modal distribution of CD63 expression corresponding to cells that have undergone degranulation or not. The ectonucleotide pyrophosphatase/phosphodiesterase (ENPP)-3, CD203c, is upregulated during activation as well, but it is also constitutively expressed and its upregulation is kinetically and pharmacologically distinct from the LAMPs [18, 21, 22]. Activation of circulating basophils has been shown to correlate with clinical disease in several contexts including urticaria, anaphylaxis, asthma, food allergy, autoimmune disease and helminth infection [23–27].
Clinical trials indicate that oral immunotherapy (OIT) reduces clinical sensitivity to peanut [28–30]. We hypothesized that with chronic allergen exposure, basophils would become refractory to signaling through the FceRI pathway, as has been shown to occur in vitro [31]. For this mechanistic study, we collected peripheral blood from a subset of peanut allergic subjects enrolled in a double-blind, placebo-controlled trial of peanut OIT [30]. Our objective was to study the effects of OIT on basophil responsiveness in order to better understand mechanisms of OIT and evaluate basophil suppression as a biomarker for OIT.
Methods
Subject Characteristics and Treatment
Twenty-eight subjects from a clinical trial of OIT for peanut allergy that took place at Duke University Medical Center and University of Arkansas for Medical Sciences were included in this mechanistic study. Local Institutional Review Boards approved the protocol and informed consent was obtained from the parents of all subjects. Subjects all had a history of convincing clinical symptoms occurring within 60 minutes of ingesting peanut, a positive skin prick test to peanuts (≥3 mm of negative control), and a peanut CAP-FEIA > 7 kUA/L. The median age at enrollment was 5 years [range 2 — 10]. Clinical and several immunological outcomes have been published separately [30].
After enrollment, subjects were randomized in a 2:1 ratio to receive either partially defatted peanut flour (Golden Peanut Company, Alpharetta, GA) or placebo (oat flour). Eighteen of the subjects were randomized to receive active peanut treatment at the start of the trial. The remaining ten subjects were randomized to placebo. The peanut OIT protocol consisted of an Initial Escalation Day that was a modified rush desensitization starting at 0.1 mg of peanut protein or placebo. This dose was doubled every 30 minutes until a 6 mg dose was achieved. During the Buildup Phase, the peanut or placebo dose was increased approximately every two weeks until a 4000 mg dose of peanut protein or placebo was reached. The goal maintenance dose was 4000 mg once daily. One month after reaching the maintenance dose all subjects underwent a 5000 mg double-blinded, placebo-controlled food challenge to peanut. Upon completion of the challenge, the treatment status was revealed. Those subjects on treatment continued on the maintenance peanut dose. The subjects on placebo were given the option to undergo desensitization with open label peanut treatment.
At the time of this analysis, five of the placebo subjects elected to start open label active treatment. Of the 23 subjects who received active treatment, three subjects dropped out during build-up prior to a post-baseline assessment of basophil reactivity. Two other subjects had not completed their post-treatment challenge at the time of this analysis; in these cases treatment status was revealed only for this mechanistic analysis — subjects and clinical investigators remained blinded. The median length of time on active therapy was 168 days [1 — 826]; the median length of time on placebo therapy was 358.5 days [68 — 423]. After the baseline assessment, basophil activation was assessed a median of 2 times [0 — 4] prior to unblinding, with a median 182 day interval [84 — 392]. For the entire study (including the open phase), basophil activation was assessed a median of 3 [1 — 7] times. The median interval between assessments for the total study time was 119 days [68 — 326]. Eleven subjects (39%) also had a clinical diagnosis of egg allergy based on a history of convincing clinical reactions and persistent sensitization. All were strictly avoiding egg. Nine of eleven received active peanut OIT treatment.
Reagents
RPMI 1640 with glutamine; N-formyl-methionyl-leucyl-phenylalanine (fMLP) (Fisher Scientific, Pittsburgh, PA); Recombinant human IL-3 (R&D Systems, Minneapolis, MN); polyclonal rabbit anti-human IgE antibody (Bethyl Laboratories, Montgomery, TX); EDTA (Promega Corp, Madison, WI); FACS lysing solution (BD Biosciences, San Jose, CA).
Antibodies
The following mAbs were used: FITC anti-CD63 (clone H5C6, BD Biosciences), PE anti-CD203c (clone 97A6, Serotec, Oxford, UK), PE-Cy5 anti-CD123 (clone 9F5, BD Biosciences), APC anti-CD41a (clone HIP8, BD Biosciences), and PE-Cy7 anti-HLA-DR (clone L243, BD Biosciences).
Assays for IgE and IgG4
Peanut- and egg-specific IgE, IgG, and IgG4 levels were measured in serum by using the ImmunoCAP 100 instrument (Phadia AB) according to the manufacturer’s instructions.
Basophil activation
After overnight shipment to Mount Sinai, whole blood (250 μL) was incubated with equal volumes of basophil stimulation buffer (RPMI plus IL-3 at 2 ng/mL; IL-3 alone control) alone or with the addition of dilutions of peanut antigen (from 1 × 101 to 1 × 10−5 μg/ml total protein prepared from an aqueous extract of defatted roasted peanuts diluted in PBS), dilutions of egg antigen (1 × 100 to 1 × 10−3 μg/ml total protein prepared from powdered egg white diluted in PBS), anti-IgE antibody (0.5 μg/mL, positive control), or fMLP (1 μM, IgE-independent positive control) at 37°C for 30 minutes; RPMI alone was used as an additional negative control. The reaction was stopped with 50 μL of cold PBS plus 20 mM EDTA. Cells were then stained for expression of CD63, CD123, CD203c, CD41a, and HLA-DR at 4°C in the dark for 30 minutes, then washed with cold PBS plus 0.5% BSA plus 2 mM EDTA. Red cells were then lysed and WBC fixed with 4 mL of FACS Lysing Solution to the cell pellet for 20 minutes at room temperature.
Flow cytometry
Samples were analyzed on a BD LSRII flow cytometer. Single-color compensation samples were prepared using antibody capture beads (BD Biosciences) according to the manufacturer’s protocol. Fluorescence data were acquired and autocompensated on a modified LSR-II configured for seven color parameters by using FACS Diva software (version 4.0, BD Biosciences). Basophils were identified as CD123+CD203c+HLA-DR-CD41a-. A minimum of 300 CD123+CD203c+HLA-DR-CD41a- events (i.e., basophils) were recorded for each condition or the sample was excluded (83 of 1198 samples, 7%).
Statistical analysis
Values are presented as means and standard deviations. Continuous variables were compared using Student’s t-test and the chi-square test for categorical variables. The outcome of interest was the change over time for CD63 and CD203c between treatment and placebo by stimulant. In particular, we hypothesized that the control group will remain unchanged over time while the treatment group will decrease. This was accomplished using a linear mixed effects model of the nlme package in R analysis (lme function) [32] enabling us to take into account the fact that the measurements were made on the same participants over time and the open-label design. The relationship between the CD63 and peanut concentration was modeled by the modified four parameter log-logistic regression model. The model was fitted to data by nonlinear regression analysis (drm function of the drc package) [33] where EC50 is the concentration producing a response a halfway between the minimum and maximum levels observed. Analyses were performed using the R statistical computing environment [34], P values smaller than 0.05 were considered significant.
Results
Peanut oral immunotherapy suppresses basophil responsiveness to in vitro stimulation with peanut allergen
At baseline, basophils incubated with RPMI or IL-3, which was included with all other stimulants to prime degranulation, expressed low levels of CD63 (<1.5% CD63high). CD63 expression at baseline was strongly and equivalently upregulated in both treatment groups by cross-linking the occupied high affinity IgE receptors (FcεRI) using the control stimulant, anti-human IgE, the distinct G-coupled receptor for fMLP or with a range of soluble peanut allergen concentrations from 10 μg/mL to 100 pg/mL (not shown).
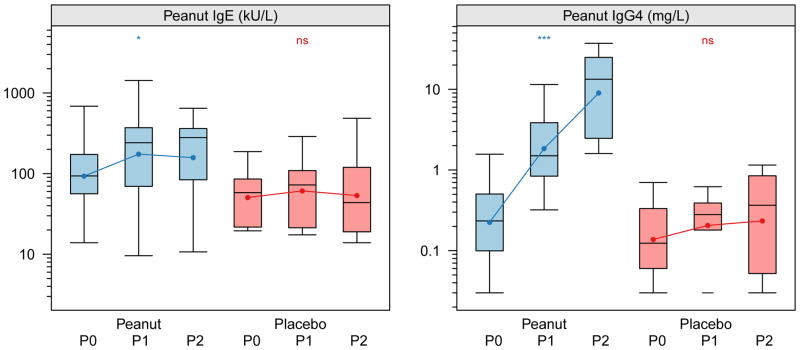
Basophil responses were longitudinally assessed in these subjects during active or placebo immunotherapy treatment (see Methods). As shown in Figure 1, in vitro basophil reactivity to peanut allergen was strongly and significantly suppressed from baseline across the range of allergen stimulation concentrations (see also Table 1). Maximal responses of ~40% CD63high at 100 ng/mL were suppressed to ~10% over time (p <0.001). No significant changes in peanut-allergen induced CD63 upregulation were observed in the placebo group over time (p=0.34). In addition to suppressing the maximal responses, basophil sensitivity to in vitro stimulation was suppressed, as measured by a shift in the half-maximal eliciting concentration (EC50) by about 10-fold, from ~0.4 ng/mL to ~5 ng/mL (Figure 2). The reduced basophil reactivity to peanut allergen coincided with an induction of peanut-specific IgG4, but there was no change in specific IgE (Figure 3).
Figure 1. Allergen-induced CD63 upregulation is suppressed by OIT.
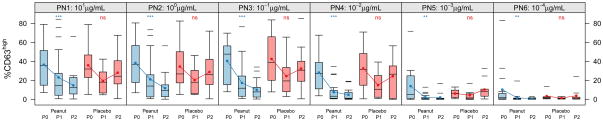
The distribution of percent CD63high basophils after in vitro stimulation from subjects over time by treatment status during the blinded portion of the OIT study. Peanut, active treatment; Placebo, control treatment. Time intervals are indicated in days and were chosen to include roughly equal numbers of assessments over time. Closed circles indicate mean percent CD63high. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers. Closed circles and lines indicate the mean change over time. P0 = day 0, P1 = day 21–156, P2 = day 157–423. *** for <0.001, ** for <0.01, * for <0.05, and ns for =0.05.
Figure 2. Both the maximum response and sensitivity of allergen-induced CD63 upregulation is suppressed by OIT.
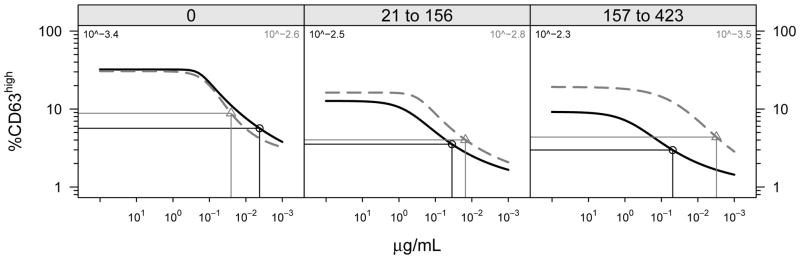
The population fitted dose response for CD63 upregulation by treatment group is shown over time in days (0, baseline; 21–156; 157–423) during the placebo-controlled study period. Horizontal and vertical lines indicate half maximal and 50% eliciting dose, respectively, for each treatment group. Active treatment is shown in blue; placebo is in red.
Figure 3. Active OIT induces changes in peanut-specific IgG4.
The peanut-specific IgE and IgG4 levels were determined using the Phadia UniCap system from samples matched to time points analyzed for basophil reactivity. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers.
Oral immunotherapy specifically suppresses FcεRI-induced upregulation of CD63
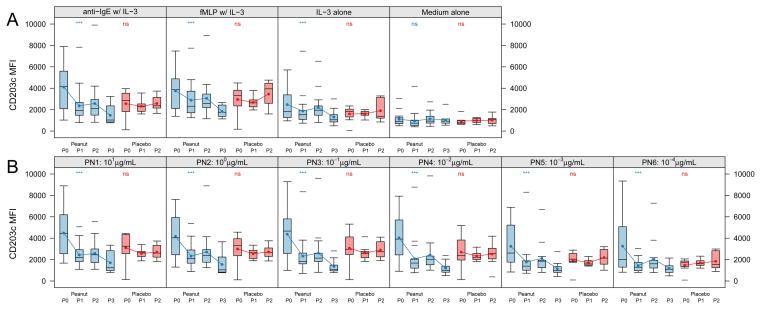
Active therapy not only suppressed basophil reactivity to specific allergen (peanut), but it also significantly suppressed upregulation of CD63 in response to cross-linking of IgE with anti-human IgE (p<0.001, Figure 4). There was no significant change in the placebo group. Active therapy did not suppress basophil responsiveness to fMLP (p=0.19).
Figure 4. Allergen OIT suppresses polyclonal anti-IgE- but not fMLP-induced CD63 upregulation.
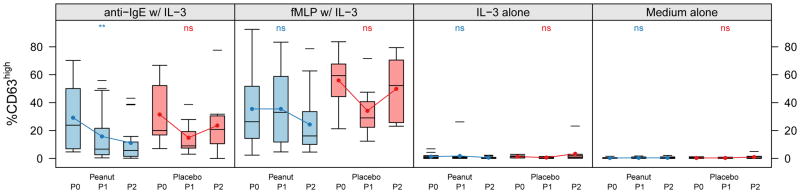
The distribution of percent CD63high basophils after in vitro stimulation from subjects over time by treatment status during the blinded portion of the OIT study. Anti-IgE w/IL-3, 0.5 μg/mL polyclonal rabbit anti-human IgE with 2 ng/mL human recombinant IL-3; fMLP, 1 μM N-formyl-methionyl-leucyl-phenylalanine with IL-3; IL-3 alone at the same 2 ng/mL concentration; Medium alone is RPMI. Time intervals are indicated in days and were chosen to include roughly equal numbers of assessments over time. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers. Closed circles and lines indicate the mean change over time. P0 = day 0, P1 = day 21–156, P2 = day 157–423. *** for <0.001, ** for <0.01, * for <0.05, and ns for ≥0.05.
By study design, those subjects remaining on active therapy during the open-label portion of the study were on treatment longer than any subject that received placebo. Additional data collected beyond the placebo-controlled portion of the study are found in Table I. The findings with respect to basophil suppression are the same: CD63 upregulation by activation via the IgE receptor with both peanut allergen and anti-IgE was suppressed while activation by fMLP was not (not shown).
Oral immunotherapy to peanut suppresses basophil reactivity to a bystander allergen
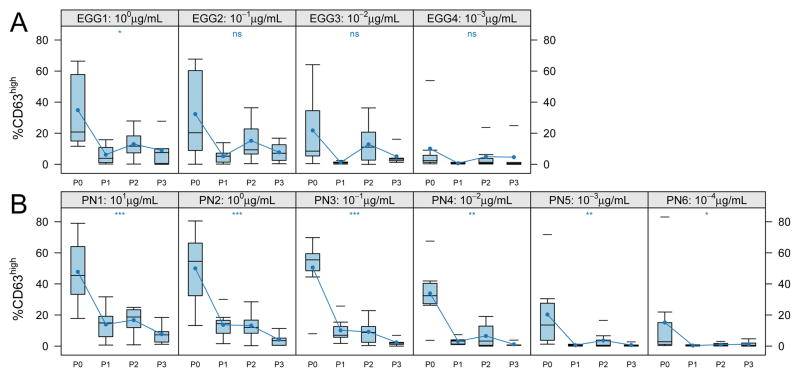
We also measured egg allergen-induced basophil activation in those subjects with a history of clinical reactivity to egg and persistent egg sensitization (11/28, 39% of total subjects). Of those subjects, ten were randomized to receive active peanut OIT treatment, but two dropped out after the first visit. We therefore analyzed the longitudinal change in basophil reactivity within the treated group. Although the subset of egg-sensitized subjects was small and the response to egg more variable, peanut OIT significantly suppressed the in vitro basophil response to egg allergen at the highest concentration (1 μg/mL; median CD63 high expression from ~40% to <10%, p<0.05) and this trend was evident at lower concentrations of egg allergen stimulation as well (Figure 5A). Within the egg sensitized subgroup, the suppression of CD63 upregulation induced by peanut (Figure 5B) and anti-IgE (not shown) was comparable to the overall group. Suppression of basophil activation by egg allergen in this subset of individuals was not associated with any change in egg-specific IgE or IgG4 (Figure 6).
Figure 5. Allergen OIT suppresses bystander allergen-induced CD63 upregulation.
The distribution of percent CD63high basophils after in vitro stimulation from egg-allergic subjects (n=8) receiving active treatment over time. A. CD63 upregulation induced by egg white allergen (EGG1-4) at indicated concentrations. B. CD63 upregulation induced by peanut allergen as in Figure 2 limited to the nine egg sensitized subjects. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers. Closed circles and lines indicate the mean change over time. P0 = day 0, P1 = day 21–156, P2 = day 157–423, P3 = day 424–827. *** for <0.001, ** for <0.01, * for <0.05, and ns for ≥0.05.
Figure 6. Active OIT induced no changes in egg-specific IgG4 or IgE.
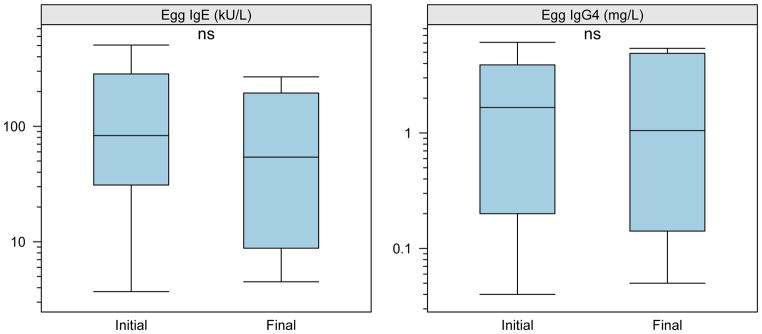
The egg-specific IgE and IgG4 levels were determined using the Phadia UniCap system from samples matched to time points analyzed for basophil reactivity. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers.
Oral immunotherapy also suppresses in vitro upregulation of CD203c
CD203c is constitutively expressed by human basophils and unlike CD63 is upregulated by IL-3 alone [18, 20, 21] and further upregulated by FcεRI and fMLP receptor stimulation. We found that the expression of CD203c following in vitro stimulation with IL-3 alone or together with anti-IgE, allergen or fMLP was also suppressed by peanut OIT (Figure 7). There was no change in the ex vivo CD203c expression as seen in the RPMI control condition.
Figure 7. Expression of CD203c on basophils over time.
The distribution of CD203c median fluorescence (MFI) on basophils after in vitro stimulation of study subject basophils over time by treatment status. A. CD203c expression of basophils stimulated with control stimulants as indicated above each panel. B CD203c expression of basophils stimulated with peanut allergen. Box plot represents the interquartile range (25th and 75th percentile) with a line indicating the median. Whiskers extend to 1.5 times the interquartile range. Dashes represent the outliers. Closed circles and lines indicate the mean change over time. P0 = day 0, P1 = day 21–156, P2 = day 157–423, P3 = day 424–827. *** for <0.001, ** for <0.01, * for <0.05, and ns for ≥0.05. IL-3, 2 ng/mL recombinant human IL-3; anti-IgE, 0.5 μg/mL anti-human IgE with IL-3; 1 μM fMLP, N-formyl-methionyl-leucyl-phenylalanine with IL-3; PN1-6, 1 × 101 to 1 × 10−4 μg/mL total peanut protein with IL-3.
Discussion
Successful immunomodulatory may theoretically prevent mediator release from effector cells such as basophils by suppressing signaling events upstream or at various stages downstream of IgE binding. Induction of allergen-specific IgG, which occurs during both subucutaneous and oral immunotherapy may competitively inhibit allergen interaction with cell-bound IgE (upstream) or lead to co-ligation of FcγRIIb inhibitory receptors with FcεRI (early downstream) and both of these mechanisms induce allergen-specific suppression of effector cells due to the specificity of the inhibitory IgG [35, 36].
Several very general mechanisms of cellular adaptation downstream of repeated receptor stimulation have also been demonstrated to occur in basophils and/or mast cells in vitro and these vary in degree of specificity from highly allergen-specific, to pathway- but not allergen-specific [31, 37]. Our data suggest that OIT may induce basophil anergy — a pathway-specific, but antigen non-specific, refractory state that is inducible in vitro by FcεRI stimulation [31]. CD63 (LAMP-3) upregulation is a marker of basophil granule fusion to the plasma membrane during degranulation [20]. Consistent with anergy, CD63 upregulation that was induced by specific allergen stimulation as well as non-specific stimulation with anti-IgE and bystander allergen was suppressed by active OIT, while fMLP-induced CD63 upregulation remained unaffected. A testable consequence of FcεRI-stimulation induced anergy would be an expected loss of Syk expression [38], and we plan to evaluate this in future mechanistic studies.
Consistent with previous reports, our data (Supplemental Figures 1 and 2) demonstrate that CD203c is both constitutively expressed and further upregulated following activation [39]. The regulation of CD203c expression has been distinguished from CD63 in numerous ways including by kinetics, pharmacological inhibitors and the capacity of IL-3 to independently induce its expression [18, 22, 40]. Treatment with peanut OIT suppresses the IL-3 induced CD203c upregulation to the level of resting basophils. Given a recent report that the IL-3 receptor complex includes the ITAM-containing FcRγ chain [41], reduced expression of Syk following FcεRI stimulation could theoretically be involved in both the suppression of CD63 upregulation downstream of FcεRI stimulation as well as the suppression of IL-3 induced upregulation of CD203c. To our knowledge, Syk dependence of CD203c upregulation downstream of IL-3 stimulation has not been shown, though it has been reported that IL-3 induced IL-13 expression is not sensitive to the Btk inhibitor, PCI-32765 [40] and that IL-3 does not significantly affect Syk phosphorylation [42]. Because IL-3 directly induces CD203c upregulation and because we chose, as is often done, to include it as a priming agent with all stimulants when this study was designed, we cannot now determine whether peanut OIT independently suppresses CD203c upregulation induced by allergen, anti-IgE or fMLP. It must also be noted that it is possible that OIT suppression of CD63 upregulation may be due to an effect on IL-3 signaling that is specific to its priming of the IgE pathway. However, IL-3 primes basophil degranulation induced by multiple secretagogues including anti-IgE, fMLP, C5a and calcium ionophore and, given the differences of the signaling pathways involved, is thought to effect common distal signaling mediators [43]. Based on this, we believe that our data are most consistent with the interpretation that peanut OIT suppresses FcεRI signaling.
A significant limitation of the current study is our inability to correlate basophil reactivity and suppression with clinical responsiveness to OIT, due to a lack of pre-treatment challenge data, and an unanticipated absence of post-treatment response variability due to the fact that all active treatment subjects tolerated the final 5000 mg challenge dose. In a previously published study of milk allergic subjects, an association was found between clinical sensitivity and basophil reactivity, and in that study IgE pathway-specific suppression of basophils was also observed among individuals tolerating chronic oral exposure of milk in the diet [27].
In conclusion, we show here evidence supporting the hypothesis that peanut OIT induced pathway-specific basophil anergy, suggesting a novel mechanism that together with other established immunomodulatory effects including the induction of specific IgG and changes in antigen-specific T cell phenotypes may contribute to clinical desensitization. The sum of these changes on the clinical response may be purely allergen-specific. However, if chronic allergen exposure induces pathway anergy, there may be a measure of clinical non-specificity with high dose continuous allergen exposure that has not been adequately examined. If basophil responses are blunted at least in part due to anergy, and not only secondary to the induction of allergen-specific IgG, this indicates that basophils are directly engaging with allergen via specific IgE in vivo. Data in murine models support a role for basophils in capturing low concentrations of antigen and modulating B and T cell immunity [11–14, 44]. Whether or not they can function as primary APCs [45, 46] – if they are directly interacting with allergen, their participation in the immune response during OIT deserves further exploration.
Supplementary Material
Acknowledgments
Funding support from the Food Allergy & Anaphylaxis Network, Food Allergy Project, Gerber Foundation, NIH Grant 1 R01-AI06874-01A1, NIH Grant 1 UL1 RR024128-01, NIH Grant R03-AI079544, Dorothy & Frank Robins Family, NIH T32 Grant 5T32-AI007062-32, and the National Peanut Board.
Abbreviations
- OIT
oral immunotherapy
- LAMP
lysosomal associated membrane protein
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- IL-3
Interleukin-3
- ENNP3
ectonucleotide pyrophosphatase/phosphodiesterase (ENPP)3, CD203c
- PN
peanut
- anti-IgE
polyclonal anti-human IgE antibody
- ITAM
immune related tyrosine containing activation motifs
- Syk
spleen tyrosine kinase
- Btk
Bruton’s tyrosine kinase
References
- 1.Ben-Shoshan M, Kagan RS, Alizadehfar R, Joseph L, Turnbull E, Pierre YS, Clarke AE. Is the prevalence of peanut allergy increasing? A 5-year follow-up study in children in Montreal. J Allergy Clin Immunol. 2009 Apr;123(4):783–8. doi: 10.1016/j.jaci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Mullins RJ, Dear KBG, Tang MLK. Characteristics of childhood peanut allergy in the Australian Capital Territory, 1995 to 2007. J Allergy Clin Immunol. 2009 Mar;123(3):689–93. doi: 10.1016/j.jaci.2008.12.1116. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 May; doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009 Jan;60:261–77. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 5.Clark AT, Ewan PW. Good prognosis, clinical features, and circumstances of peanut and tree nut reactions in children treated by a specialist allergy center. J Allergy Clin Immunol. 2008 Aug;122(2):286–9. doi: 10.1016/j.jaci.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 7.Järvinen KM, Amalanayagam S, Shreffler WG, Noone S, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J Allergy Clin Immunol. 2009 Dec;124(6):1267–72. doi: 10.1016/j.jaci.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chafen JJS, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, Sundaram V, Paige NM, Towfigh A, Hulley BJ, Shekelle PG. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010 May;303(18):1848–56. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs BF. Human basophils as effectors and immunomodulators of allergic inflammation and innate immunity. Clin Exper Med. 2005 Jul;5(2):43–9. doi: 10.1007/s10238-005-0064-5. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder JT, MacGlashan DW, Lichtenstein LM. Human basophils: mediator release and cytokine production. Adv Immunol. 2001 Jan;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 11.Denzel A, Maus UA, Gomez MR, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008 Jul;9(7):733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 12.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009 Jan;9(1):9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009 Sep;183(5):3033–9. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 14.Sokol CL, Chu N, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009 Jul;10(7):713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, MacGlashan DW, Saini SS. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010 Apr;125(4):889–895.e7. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989 Jul;321(4):228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, Jolie PL. Increased plasma histamine concentrations after food challenges in children with atopic dermatitis. N Engl J Med. 1984 Aug;311(6):372–6. doi: 10.1056/NEJM198408093110605. [DOI] [PubMed] [Google Scholar]
- 18.Hennersdorf F, Florian S, Jakob A, Baumgärtner K, Sonneck K, Nordheim A, Biedermann T, Valent P, Bühring H. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005 May;15(5):325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak AM. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem. 2005 Sep;53(9):1043–70. doi: 10.1369/jhc.5R6647.2005. [DOI] [PubMed] [Google Scholar]
- 20.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991 Sep;88(3 Pt 1):328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 21.Bühring HJ, Seiffert M, Giesert C, Marxer A, Kanz L, Valent P, Sano K. The basophil activation marker defined by antibody 97A6 is identical to the ectonucleotide pyrophosphatase/phosphodiesterase 3. Blood. 2001 May;97(10):3303–5. doi: 10.1182/blood.v97.10.3303. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan D. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010 Sep;40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falcone FH, Telford G, Hooi D, Brown AP, Seabra R, Feary J, Venn A, Britton J, Pritchard DI. Antigen-driven basophil activation is indicative of early Necator americanus infection in IgE-seronegative patients. J Allergy Clin Immunol. 2009 Dec;124(6):1343–50.e7. doi: 10.1016/j.jaci.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Gober LM, Eckman JA, Sterba PM, Vasagar K, Schroeder JT, Golden DBK, Saini SS. Expression of activation markers on basophils in a controlled model of anaphylaxis. J Allergy Clin Immunol. 2007 May;119(5):1181–8. doi: 10.1016/j.jaci.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Lourenço FD, Azor MH, Santos JC, Prearo E, Maruta CW, Rivitti EA, Duarte AJS, Sato MN. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br J Dermatol. 2008 May;158(5):979–86. doi: 10.1111/j.1365-2133.2008.08499.x. [DOI] [PubMed] [Google Scholar]
- 26.Ono E, Taniguchi M, Higashi N, Mita H, Kajiwara K, Yamaguchi H, Tatsuno S, Fukutomi Y, Tanimoto H, Sekiya K, Oshikata C, Tsuburai T, Tsurikisawa N, Otomo M, Maeda Y, Hasegawa M, Miyazaki E, Kumamoto T, Akiyama K. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010 Feb;125(2):483–489.e3. doi: 10.1016/j.jaci.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 27.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009 Apr;123(4):789–94.e20. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LCL, Shreffler WG, Sampson HA, Niggemann B, Wahn U, Beyer K. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jun; doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, Francis JM, Durham S, Vickery BP, Zhong X, Burks AW. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300. 300.e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, Kulis M, Pons L, Vickery B, Burks AW. A randomized controlled study of peanut oral immunotherapy: Clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011 Mar;127(3):654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacGlashan DW, Vilariño N. Nonspecific desensitization, functional memory, and the characteristics of SHIP phosphorylation following IgE-mediated stimulation of human basophils. J Immunol. 2006 Jul;177(2):1040–51. doi: 10.4049/jimmunol.177.2.1040. [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro JC, Bates DM. ISBN. 2000. Mixed-models in S and S-PLUS. [Google Scholar]
- 33.Ritz C, Streibig JC. Bioassay analysis using R. Journal of Statistical Software. 2005;12:1–22. [Google Scholar]
- 34.R Development Core Team. ISBN. 2010. R: A language and environment for statistical computing. [Google Scholar]
- 35.Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, Oliver JM. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000 Aug;106(2):337–48. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 36.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, Pitkin L, Pilette C, Nouri-Aria K, Durham SR. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010 Apr;40(4):598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 37.Sancho-Serra MDC, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcεRI internalization. European journal of immunology. 2011 Jan; doi: 10.1002/eji.201040810. [DOI] [PubMed] [Google Scholar]
- 38.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005 Sep;138(1):29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 39.Hauswirth AW, Sonneck K, Florian S, Krauth MT, Bohm A, Sperr WR, Valenta R, Schernthaner GH, Printz D, Fritsch G, Buhring HJ, Valent P. Interleukin-3 promotes the expression of E-NPP3/CD203C on human blood basophils in healthy subjects and in patients with birch pollen allergy. International journal of immunopathology and pharmacology. 2007 Jan;20(2):267–78. doi: 10.1177/039463200702000207. [DOI] [PubMed] [Google Scholar]
- 40.Macglashan D, Honigberg L, Smith A, Buggy J, Schroeder JT. Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. International immunopharmacology. 2011 Jan; doi: 10.1016/j.intimp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, Karasuyama H, Sugane K, Saito T, Taki S. Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009 Feb;10(2):214–22. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 42.Vilariño N, Miura K, MacGlashan DW. Acute IL-3 priming up-regulates the stimulus-induced Raf-1-Mek-Erk cascade independently of IL-3-induced activation of Erk. J Immunol. 2005 Sep;175(5):3006–14. doi: 10.4049/jimmunol.175.5.3006. [DOI] [PubMed] [Google Scholar]
- 43.Lyngholm JM, Nielsen HV, Holm M, Schiøtz PO, Johnsen AH. Calreticulin is an interleukin-3-sensitive calcium-binding protein in human basophil leukocytes. Allergy. 2001 Jan;56(1):21–8. doi: 10.1034/j.1398-9995.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 44.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010 Jun;16(6):701–7. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010 Sep; doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010 Jul;11(7):608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.