Abstract
Purpose
To compare Reading Center (RC) cup-to-disc ratio (CDR) assessment from stereoscopic photographs with clinician estimation in a uveitis clinical trial.
Methods
Clinical estimation of CDR was performed by ophthalmologists via dilated biomicroscopy. Photographic evaluation was performed at an independent RC by masked, certified evaluators. Quality control was performed by repeat grading of 77 randomly selected images.
Results
Among 481 eyes with uveitis, 353 eyes had clinical and photographic grades for CDR. Agreement between clinical and RC grading was fair, with exact agreement in 29%. Agreement within 0.1 and 0.2 CDR were 70% and 93%, respectively (wkappa=0.34). Inter-grader reproducibility at the RC was better (wkappa=0.59, ICC 0.74).
Conclusion
Morphologic assessment of cup to disc ratio is an important outcome and safety measure for determining glaucomatous damage in clinical trials. Masked RC measurements are more likely to be accurate than biomicroscopic grading in identifying meaningful anatomical change associated with glaucoma.
Keywords: clinical trial, glaucoma, optic nerve head, uveitis
Introduction
The Multicenter Uveitis Steroid Treatment (MUST) Trial is a randomized clinical trial comparing the effectiveness of standard systemic therapy versus the fluocinolone acetonide intraocular implant for treatment of severe non-infectious uveitis.1 Raised intraocular pressure is a common occurrence in eyes with ocular inflammation, resulting primarily from the disease process itself, a response to corticosteroid treatment, or secondary angle closure.2,3 One treatment arm of the MUST trial involves use of fluocinolone acetonide implants, previously associated with a 78% rate of increased intraocular pressure and 40% rate of glaucoma surgery over 3 year follow-up 4, 5. Foster et al define glaucoma as structural damage to the optic nerve associated with functional damage as indicated by visual dysfunction.6 In glaucoma clinical trials, stereoscopic photographs of the optic nerve are traditionally used to assess the vertical cup-disc ratio—a reliable clinical index of glaucomatous damage to the neuroretinal rim.6 Use of this approach, with grading by a masked reading center, in trials where incidence of glaucoma is not the primary outcome is a major commitment, and better understanding of the value of this approach versus simple clinical grading in the context of disease conditions other than glaucoma is needed.
In the MUST Trial, the incidence of glaucoma related to uveitis and/or steroid treatment was an important secondary outcome. The cup-disc ratio was frequently measured by biomicroscopy by a large number of uveitis specialists at different sites over several years and by grading of stereo photographs taken at the same visit by an independent masked reading center. Here we report the reproducibility of standardized Reading Center evaluation of cup-disc ratio, and evaluate agreement between Reading Center grades with biomicroscopic evaluation performed by clinic investigators.
Methods
Study Participants
The design and methodology of the MUST Trial (ClinicalTrials.gov Identifier: NCT00132691) has been described previously.1 The study enrolled patients with severe non-infectious intermediate uveitis, posterior uveitis, or panuveitis at 23 clinical sites in the United States, United Kingdom and Australia. The protocol and informed consent forms are compliant with the Declaration of Helsinki and were approved by the governing institutional review boards. Prior to participation in this study, all clinical centers completed certification of the imaging system and participating photographers through the Fundus Photograph Reading Center (RC), Department of Ophthalmology and Visual Sciences at the University of Wisconsin-Madison. Non-simultaneous stereoscopic color photographs7 of the optic disk were obtained from both eyes at baseline, and de-identified photographs were sent to the Reading Center for evaluation. At the same visit, the study ophthalmologist at each clinic provided a clinical grading of the cup-disc ratio using biomicroscopy through a dilated pupil.
Photography
All patients underwent pharmacologic pupil dilation followed by 3-field modified stereoscopic photography using 30 or 35 degree field with specified capture and export settings. The photographic fields include Field 1M, where the image is centered on the temporal edge of the optic disc, Field 2- centered on the macula, and Field 3M – centered temporal to the center of the macula. These fields were modified from the standard ETDRS photographic protocol8 in order to provide additional views of the macula. Images were obtained in film format and sent as color slides or were obtained digitally (mid 2007 onwards) with certified cameras and saved on a CD or DVD uncompressed, and submitted to the Reading Center according to standard procedures.
Grading
Film sets were viewed upon a standard light box (6500° K color temperature), using a Donaldson stereo viewer (5X). Digital images were displayed upon calibrated 20.5″ LCD monitors and were viewed with hand-held stereo viewers (Screen-Vu Stereoscope, PS Mfg. Co., Portland, OR). Optimum image illumination, contrast, and color balance for digital images were achieved by a standardized procedure at the Reading Center9 where the luminance histograms for each of the red/green/blue (RGB) color channels were analyzed and manually adjusted to enhance color contrast and standardize illumination. Trained graders evaluated each eye utilizing standardized procedures adapted from the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) cup-disc measurement protocol.10,11 Quality of both film and digital images was rated by the graders based upon the ability of the grader to view and grade different lesions of uveitis. Photo quality was graded as Good to fair, if they were good enough to be graded confidently; Borderline when most features could be graded, but some features could not be graded due to defects in image quality such as poor stereoscopic imaging, poor focus or inadequate field definition. Images were considered Ungradable if there was either a very poor view or no view of the fundus.
Measurement of cup-disc ratio using biomicroscopy
Investigators participating in the MUST Trial were board-certified ophthalmologists, and leading uveitis experts. Patients had dilated fundus examinations at each clinic visit and cup-disc ratio was assessed as part of the regular eye examination. Clinicians did not receive any additional training or guidance in the assessment of cup-disc ratio. Evaluation was performed according to typical ophthalmic practice with cup-disc ratio estimated to the nearest 0.1 (e.g., 0.0–1.0).
Measurement of cup-disc ratio at the Reading Center
Good stereoscopic imaging is optimal for identification of the cup. In some situations, where the diameters could not be measured due to poor photographic quality or due to media opacity obscuring the view of the fundus, then “cannot grade” was assigned to the eye at that visit. In gradable images, the cup was identified by directly visualizing its contour or by tracking the course of blood vessels into the optic nerve head.10,11 In the case of film images, a plastic transparent sheet with various circle sizes was used to measure the vertical diameter of the cup and the disc margins For digital images, the measurements were performed using digital analysis tools available in Topcon Imagenet. Cup-disc ratio was calculated as follows:
Grades were assessed as vertical diameters and the ratio was presented in increments of 0.01; for comparison with clinicians’ grades, these were rounded to the nearest 0.1.
Quality control of Reading Center grading was performed by repeat grading of a random set of 77 images from various subjects and visits by different graders. For purposes of quality control, images were graded independently, without access to the previous visit images or grades assigned. Images were graded according to the procedures described above. Ungradable eyes were excluded prior to analysis for intergrader reproducibility.
Statistical analysis
Calculated measures of agreement included the percent agreement (both exact agreement and agreement +/−0.1), and weighted kappa (κ) statistics. Kappa statistic was weighted as 1 for either exact agreement or disagreement within 0.1, and 0 for all other disagreements. Landis and Koch’s benchmarks12 were used to evaluate simple and weighted kappa statistics, in which κ in the ranges of 0.00–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, 0.81–1.00 respectively indicate slight, fair, moderate, substantial and almost perfect agreement. Only eyes which were graded both at the clinics and the Reading Center were included in calculating measures of agreement. Intergrader reproducibility of the measurement for cup-disc ratio at the Reading Center was assessed by simple and weighted kappa statistics in the same manner and with Intraclass correlation coefficients.
Results
Of the 255 study participants, 463 of 481 (96%) eyes with uveitis had baseline fundus images; 380 of the images (82%) were successfully graded. The 83 eyes scored as ungradeable at the RC had media opacity or poor stereoscopic effects. Clinicians were able to grade the cup-disc ratio in 439 of the 481 eyes with uveitis (91%). Thus, the view limited by small pupils from posterior synechiae and/or lens and vitreous opacities affected the clinicians’ grading significantly less than the camera imaging technique (P<0.0001).
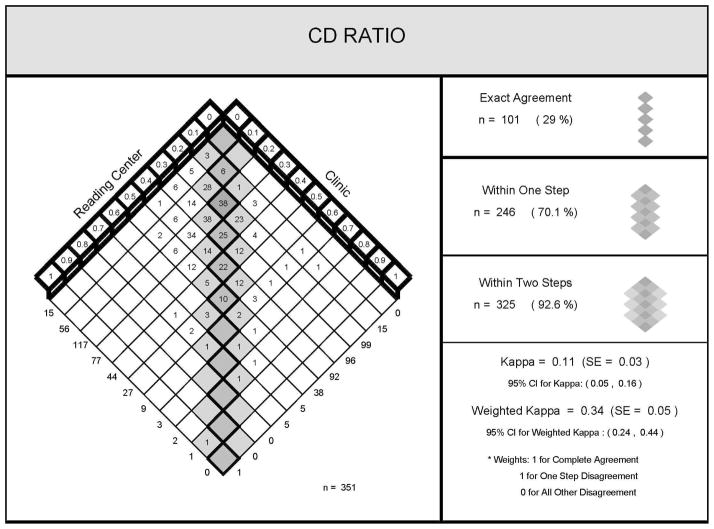
Three hundred fifty-one eyes had both a clinical and Reading Center grading of the cup-disc ratio and the findings are plotted in figure 1 showing the distribution of cup-disc ratios by clinical evaluation and stereoscopic photograph assessment at the Reading Center.
Figure 1.
Comparison of cup-disc ratios between reading center grades and clinician
The clinical grading and the Reading Center grading agreed exactly for 101 of 351 eyes (29%), differed within 0.1 disc diameter (DD) for 246 eyes (70%), and within 0.2 DD for 325 (93%) of eyes.
The agreement between the clinical and Reading Center grading was in the fair range adjusting for the expected level of agreement given the distributions of grades with each approach and despite giving full credit for agreement within one grade (weighted kappa = 0.34 (95 % CI 0.24, 0.44)). Among 481 eyes from 255 study participants, 467 (97%) had baseline fundus images submitted to the RC in the MUST trial.
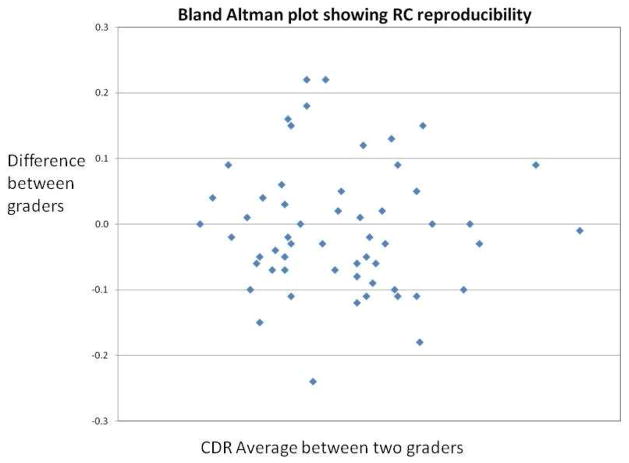
Figure 2 shows inter-grader reproducibility of cup-disc ratios at the Reading Center. For the 58 gradable eyes (75%), agreement within 0.1 DD was moderate (wK = 0.59). The intraclass correlation coefficient for inter-grader agreement was 0.74.
Figure 2.
Reproducibility of cup-disc ratio grades at the reading center.
Discussion
The evaluation of the optic nerve head in uveitis patients is particularly important as a significant proportion of the study population is at risk of developing glaucoma due to sequelae of uveitis or the use of chronic corticosteroid therapy.
Vertical cup-disc ratio, reflecting neuroretinal rim loss at the superior and inferior poles, is the most sensitive and specific variable to differentiate between normal and glaucomatous eyes.13,14,15 Clinicians have the disadvantage of working under time pressure with patients with pain and photophobia due to uveitis. These factors may make assessing the cup-disc ratio more difficult; Furthermore, clinicians rely on an internal standard for estimating cup-disc ratio resulting in limited reproducibility between clinicians and by clinicians with themselves.16,17,18 The photographic method of locating anatomic boundaries, measuring the vertical diameter of the optic disc and cup using grids or digital calipers and calculating the cup-disc ratio is anticipated to be more accurate and reproducible, and indeed was in the current study.19,20 Agreement between graders at the Reading Center was significantly stronger than clinician-Reading Center agreement.
The fair level of agreement between clinician assessment of cup-disc ratio and Reading Center grades of stereoscopic images we observed is consistent with previous studies comparing clinician and reading center assessment of lens opacity, diabetic retinopathy and cytomegalovirus lesions.21–24 Clinician assessment of cup-disc ratio tended to be lower than the Reading Center measurement for smaller ratios and higher than Reading Center assessment for larger ratios (see Figure 1). This finding has been reported previously, 25 suggesting a tendency for clinical graders to over-call extreme results. Possible reasons for such discrepancies between clinicians and Reading Center include less time to fully assess the nerve in a time pressured clinical setting; identification of the cup margin using pallor, instead of contour, and inclusion of peripapillary atrophy into the optic disc margin. There also may be inherent biases in how clinicians look at small and large cups such that they tend to underestimate the size of the former and overestimate the size of the latter. The systematic approach used at the Reading Center avoids this bias.
Graders at the Reading Center had good reproducibility. Evaluation of vertical cup-disc ratio by stereoscopic fundus photography remains the commonest method to evaluate progression of glaucoma in clinical trials. The Ocular Hypertension Treatment Study (OHTS) images were read by multiple graders at a masked reading center. Intraclass correlation between two graders was 0.89. The images were regraded annually and the intraclass correlations were 0.92 or higher over 3 years.26 Varma et al27 reported high intraobserver agreement (wkappa, 0.79 with weights assigned such that larger agreements were weighted more heavily than smaller agreements) and moderate interobserver agreement (weighted kappa, 0.67) for vertical cup-disc ratio for reading center grades of cross-sectional population data. A recent study by Breusegem et al, compared the interobserver agreement for CDR between glaucoma specialists (K 0.51, 95% CI 0.33–0.69) and non expert ophthalmologists (K=0.20, 95% CI 0.19–0.21). The non-experts did not show much improvement in agreement following a training session (K=0.27, 95% CI 0.26–0.28).28 In our study, the intraclass correlation was substantial (0.73), even though MUST Trial enrolled uveitic eyes with many having media limitation such as vitritis, cataract, and pupillary synechia. These results suggest that agreement can be improved substantially over clinical grading using a protocol-driven Reading Center approach. An additional advantage of the Reading Center approach is that Reading Center personnel can be masked to patient history and treatment regimens and hence are unbiased in assessment, whereas ethical constraints may prevent masking of clinician-graders, as in the MUST Trial.
In this study, the clinicians graded cup-disc ratio in more eyes than at the Reading Center (91% versus 80%). This is attributed to more stringent rules of assessment followed at the Reading Center, where the cup and the disc margins have to be clearly visualized with good stereopsis in order to be measured. Lens opacity, pupillary synechia, and vitreous haze impact the ability to obtain high quality photographic images. Clinicians using a narrow slit beam may have been able to make a greater number of attempts to obtain an adequate view of the optic nerve, which could have contributed to their more frequent success in assigning a grade by biomicroscopy. Limitations of the study include the lack of inter-clinician reproducibility data, and lack of information as to why eyes could not be graded in the clinic. Strengths include the prospective, protocol-driven data collection, and an appropriate sample size.
Conclusion
Reliable grading of cup-disc ratio is difficult in patients with uveitis. Clinical grading by ophthalmologists often shows significant inter-observer variability. Even using the Reading Center approach, agreement was less than in population-based and glaucoma-specific studies suggesting poor media clarity in the inflamed eye affects both clinical and reading center approaches. Nevertheless, grading of stereo photographs at a Reading Center is a suitable approach for clinical trials of active uveitis and allows masking of the cup-disc ratio, an important outcome measure. These results suggest that when cup-disc ratio is an important outcome in a multi-center clinical study, a Reading Center approach to the determination of cup-disc ratio is preferable to clinical grading, even for conditions like uveitis where media limitations on photography are frequent.
Acknowledgments
Financial support: Supported by cooperative agreements from the National Eye Institute to Mount Sinai School of Medicine (U10 EY 014655), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 014660), and the University of Wisconsin, Madison, School of Medicine (U10 EY 014656).
Credit Roster
Participating Clinical Centers
Duke University, Durham, NC: Glenn J. Jaffe, MD (Director); Shelley Day, MD; Annie Lee, MD; Cindy Skalak. Former Members: Claxton Baer, MD; Sai Chavala, MD; Michael Cusick, MD; Pouya Dayani, MD; Justis Ehlers, MD; Muge Kesen, MD Alex Melamud, MD; Jawad A. Qureshi, MD; Adrienne Williams Scott; Robert F. See, MD; Robert K. Shuler, MD.
Emory University, Atlanta, GA: Steven Yeh, MD (Director); Alicides Fernandes, MD. Former Members: Deborah Gibbs, COMT; Daniel F. Martin, MD; Sunil Srivastava, MD.
Johns Hopkins University, Baltimore, MD: James P. Dunn, MD (Director); Ellen Arnold, BS; Jeff Boring, COA; Diane M. Brown, MSN, RN; Alison G. Livingston, BSN, RN; Terry Reed, COA. Former Members: Marie-Lynn Belair, MD; Stephen G. Bolton, CRNP; Joseph B. Brodine; Lisa M. Brune, BSN, RN; Anat Galor, MD; Adam Jacobowitz, MD; Meera Kapoor; Sanjay Kedhar, MD; Stephen Kim, MD; Henry A. Leder, MD; Yavette Morton; Kisten Nolan, BSN, MPH, RN; George B. Peters, MD, MBA; Priscilla Soto; Ricardo Stevenson, MD; Michelle Tarver-Carr, MD, PhD; Yue Wang, MD.
Massachusetts Eye Research and Surgery Institute, Cambridge, MA: C. Stephen Foster, MD (Director); Linda Bruner; David M. Hinkle, MD; Jyothir Johnson; Danielle Marvell; Sana S. Siddique. Former Members: Sarah Acevedo, MD; Fahd Anzaar, MD; Tom Cesca; Angelica Contero; Kayleigh Fitzpatrick; Karina Q. Lebron, MD; Chandra Morgan; Nita Patel, MSW; Jennifer Pinto; Janet Sprague; Taygan Yilmaz.
National Eye Institute, Bethesda, MD: H. Nida Sen, MD, MHSc (Director); Michael Bono; Denise Cunningham, CRA; Darryl Hayes, COA; Dessie Koutsandreas, BS, COA; Nupura Krishnadev, MD; Theresa Larson, MD; Annal D. Meleth, MD; Robert B. Nussenblatt, MD; Patti R. Sherry, BSN; Gregory L. Short, COMT; Wendy Smith, MD; Alana Temple, BSN. Former Members: Allison Bamji, RN; Hanna Coleman, MD; Geetaniali Davuluri, MD; Lisa Faia, MD; Chloe Gottlieb, MD; Guy V. Jirawuthiworavong, MD; Julie C. Lew, MD; Richard Mercer, COT; Dominic Obiyor, BSN; Cheryl H. Perry, BSN; Natalia Potapova, MD; Eric Weichel, MD; Keith J. Wroblewski, MD; Steven Yeh, MD.
New York Eye & Ear Infirmary, New York, NY: Paul A. Latkany, MD (Director); Jason Badamo; Jenny Gallardo; Monica Lorenzo-Latkany, MD; Robert Masini; Susan Morell; Ann Nour; Meredith Sanchez; Kate Steinberg. Former Members: Kenneth M. Boyd; Jacek Jarczynski; Mirjana McGrosky.
Royal Victorian Eye & Ear Hospital, East Melbourne, Australia: Richard J. Stawell, MD (Director); Lisa Breayley; Carly D’Sylva; Julie Ewing; Lauren Hodgson; Ignatios Koukouras; Lyndell Lim; Cecilia Ling; Rachel McIntosh; Andrew Newton; Richard Smallwood; Ehud Zamir. Former Members: Nicola Hunt; Lisa Jones; Suzanne Williams.
Rush University Medical Center, Chicago, IL: Pauline T. Merrill, MD (Director); Pam Hulvey; Elaine Kernbauer; Scott Toennessen; Denise L. Voskuil-Marre, BS. Former Members: Bruce Gaines; Christina Giannoulis; Heena S. Khan, BA; Sarah J. Levine.
Texas Retina Associates, Dallas, TX: Robert C. Wang, MD (Director); Hank Aguado; Sally Arceneaux; Kimberly Cummings; Gary E. Fish, MD; Keith Gray; Nick Hesse; Susie Howden; Diana Jaramillo; Michael Mackens; Karin Mutz; Brenda Sanchez. Former Members: Jean Arnwine; David Callanan, MD.
United Kingdom Institute of Ophthalmology, London, UK: Susan Lightman, PhD (Director); Lavanish Joshi; Simon Taylor; Hamish Towler; Rebecca Tronnberg. Former Members: Kate Edwards; Timothy Stubbs.
University of California at Los Angeles, Los Angeles, CA: Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Jose Castellanos, COT; Jean Pierre Hubschman, MD; Ann K. Johiro, NP; Parthu Kalyani; Michael A. Kapamajian, MD; Ralph D. Levinson, MD; Susan S. Ransome, MD. Former Members: Christine R. Gonzales, MD; Anurag Gupta, MD; Peter J. Kappel, MD.
University of California at San Diego, San Diego, CA: William R. Freeman, MD (Director); Igor Kozak, MD; Vivian Nguyen; Debbie Powell, BBA. Former Members: Tom Clark, BSc, CRA; Denine E. Cochran, COT, CCRC, Joshua Hedaya, MD; Tiara Kemper; Jacqueline M. LeMoine; Megan E. Loughran, BA; Luzandra Magana; Francesca Mojana, MD; Victoria Morrison, MD; Stephen F. Oster, MD.
University of California at San Francisco, San Francisco, CA: Ira G. Wong, MD (Director); Nisha Acharya, MD; David Clay; Claire M. Khouri, BA; Mary Lew, BA; Todd P. Margolis, MD, Jay Stewart, MD. Former Members: Salena Lee, BA, OD.
University of Illinois at Chicago, Chicago, IL: Debra A. Goldstein, MD (Director); Marcia Niec; Anna Castro-Malek; Howard H. Tessler, MD. Former Members: Catherine E. Crooke; Dimitry Pyatetsky, MD; Misel Ramirez.
University of Miami, Miami, FL: Janet L. Davis, MD (Director); Thomas Albini, MD; David A. Pinto. Former Members: Daniela Castaño; Marie Chin; Macy Ho; Jaclyn L. Kovach, MD; Richard C-S Lin, MD; Efrem Mandelcorn; Jackie K-D Nguyen, MD; Aura Pacini; Susan Pineda; Jose Rebimbas; Kimberly E. Stepien, MD; Claudia Teran.
University of Michigan, Ann Arbor, MI: Susan G. Elner, MD (Director); Rebecca Brown, COA; Linda Fournier, COA; Julie R. Gothrup, COA; Richard Hackel; Moella Hesselgrave, COA; Robert Prusak; Stephen J. Saxe, MD. Former Members: Melissa Bergeron, COA; Reneé Blosser, COMT; Carrie Chrisman-McClure; Deanna Sizemore, COA.
University of Pennsylvania, Philadelphia, PA: John H. Kempen, MD, PhD (Director); James Berger; Sheri Drossner; Joan C. DuPont; Albert M. Maguire, MD; Dawn McCall; Janice Petner; Laurel Weeney. Former Members: Tim Hopkins, MD; Monique McRay; Daniel Will, MD; Wei Xu.
University of South Florida, Tampa, FL: Peter Reed Pavan, MD (Director); JoAnn Leto; Lori Mayor; Kim McDonald; Scott E. Pautler, MD; Wyatt Saxon; Judy Soto. Former Members: Maria Ortiz; Dee Dee Szalay.
University of Southern California, Los Angeles, CA: Amani Fawzi, MD; (Director); Lupe Cisneros, COA; Elizabeth Corona; Jackie Douglass; Dean Eliott, MD; Margaret Padilla; Narsing A. Rao, MD. Former Members: Alexia Aguirre; Lawrence Chong, MD; Rahul Khurana, MD; Jennifer Lim, MD; Rachel Mead; Sylvia Ramos; Julie H. Tsai, MD.
University of Utah, Salt Lake City, UT: Albert Vitale, MD (Director); Paul S. Bernstein, MD, PhD; Bonnie Carlstrom, COA; James Gilman, CRA; Sandra Hanseen, COA; Paula Morris, CRA; Diana Ramirez; Kimberley Wegner, BS, CRC.
Virginia Eye Consultants, Norfolk, VA: John D. Sheppard, MD, MMSc (Director); Brianne Anthony; Amber Casper; Lisa Felix-Kent, COA; Jeanette Fernandez, COMT; Stephen V. Scoper, MD. Former Members: R. Denise Cole; Nancy Crawford; Rebecca De La Garza; Lisa Franklin; Krista Hamelin; Jen Martin; Rebecca Marx; Gregory Schultz, DD; Joseph Webb, BS; Pamela Yeager.
Vitreoretinal Consultants, Houston, TX: Rosa Y. Kim, MD (Director); Matthew S. Benz, MD; David M. Brown, MD; Eric Chen, MD; Richard H. Fish, MD; Shayla Hay; Jame Major, MD, PhD; Laura Shawver; Tien P. Wong, MD.
Washington University, St. Louis, MO: P. Kumar Rao, MD (Director); Rhonda Weeks. Former Members: Rajendra S. Apte, MD; Kevin J. Blinder, MD; Ashley Hartz; Pam Light; Gaurav K. Shah, MD; Russell VanGelder, MD, PhD.
Committees
Executive Committee: Douglas A. Jabs, MD, MBA (Chair); Michael M. Altaweel, MD; Janet T. Holbrook, PhD; John H. Kempen, MD, PhD; Natalie Kurinij, PhD.
Steering Committee: Douglas A. Jabs, MD, MBA (Chair); Robert D. Almanzor, COA; Michael M. Altaweel; Diane Brown; James P. Dunn, MD; James Gilman; Janet T. Holbrook, PhD; Gary N. Holland, MD; John H. Kempen, MD, PhD; Rosa Y. Kim, MD; Natalie Kurinij, PhD; Nancy Prusakowski, MS; Jennifer E. Thorne, MD, PhD. Former Members: Stephen G. Bolton, RN, BSN; Lisa M. Brune, RN, BSN; Tom Clark, CRA; Larry Hubbard, MAT; Daniel F. Martin, MD; Robert B. Nussenblatt, MD.
Data, Safety and Monitoring Committee: Janet Wittes, PhD (Chair); Michael M. Altaweel, MD; William E. Barlow, PhD; Marc Hochberg, MD; Janet T. Holbrook, PhD; Douglas A. Jabs, MD, MBA; Natalie Kurinij, PhD; Alice T. Lyon, MD; Alan G. Palestine, MD; Lee S. Simon, MD; Harmon Smith, PhD. Former Members: James T. Rosenbaum, MD.
Surgical Quality Assurance Committee: John H. Kempen, MD, PhD (Chair); James P. Dunn, MD; Glenn J. Jaffe, MD. Former Members: Daniel F. Martin, MD.
Medical Therapy Quality Assurance Committee: Jennifer E. Thorne, MD, PhD (Chair); Nisha Acharya, MD; Douglas A. Jabs, MD, MBA; John H. Kempen, MD, PhD; Paul A. Latkany, MD; Robert B. Nussenblatt, MD; Albert Vitale, MD. Former Members: Russell VanGelder, MD, PhD.
Visual Function Quality Assurance Committee: Robert D. Almanzor, COA (Chair); Judith Alexander, BA, CCRP; Jeffrey A. Boring, COA; Deborah Gibbs, COMT; Salena Lee, OD, FAAO; Jennifer E. Thorne, MD, PhD (Advisor). Former Members: Wai Ping Ng, BS.
Glaucoma Committee: David S. Friedman, MD (Chair); Anna Adler, MS; Judith Alexander, BA, CCRP; Alyce Burke, MPH; Janet T. Holbrook, PhD; Joanne Katz, ScD; John H. Kempen, MD, PhD; Nancy Prusakowski, MS; Susan Reed; Jennifer E. Thorne, MD, PhD. Former Members: Nicholas Cohen; Sanjukta Modak, MS; Wai Ping Ng, BS.
Statistical Analysis Committee: Elizabeth A. Sugar, PhD (Chair); Lea Drye, PhD; Kevin Frick, PhD; Janet T. Holbrook, PhD; JoAnn Katz, ScD; Thomas Louis, PhD; Mark L. Van Natta, MHS. Former Members: Sanjukta Modak, MS; David Shade, JD.
Resource Centers
Chairman’s Office: Mount Sinai School of Medicine, New York, NY: Douglas A. Jabs, MD, MBA (Study Chairman); Yasmin Hilal, MHS; Melissa A. Nieves, BA; Karen Pascual, MBA; Jill S. Slutsky, MPA. Former Members: Colby Glomp; Maria Stevens, CM.
University of Pennsylvania, Philadelphia, PA: John H. Kempen, MD, PhD (Study Vice-Chairman).
Coordinating Center, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD: Janet T. Holbrook, PhD, MPH (Director); Anna L. Adler, MS; Judith Alexander, BA; Jeff A. Boring, COA; Alyce E. Burke, MPH; Karen Collins; John D. Dodge; Lea T. Drye, PhD; Cathleen S. Ewing; Kevin D. Frick, PhD; David S. Friedman, MD, PhD; Rosetta Jackson; Joanne Katz, ScD; Andrea T. Lears, BS; Hope Livingston; Thomas A. Louis, PhD; Curtis L. Meinert, PhD; Jill L. Meinert; Vinnette Morrison, BS; Deborah J. Nowakowski; Nancy Prusakowski, MS; Dave M. Shade, JD; Rochelle E. Smith, BS; Karen Steuernagle; Elizabeth A. Sugar, PhD; Jennifer E. Thorne, MD, PhD; James A. Tonascia, PhD; Mark L. Van Natta, MHS; Richard Zheng, BS. Former Members: Paul Chen; Nicholas Cohen, MS; Sanjukta Modak, MS; Wai Ping Ng, BS; Weijiang Shen, BS; Charles Shiflett, BS; Ada Tieman, MBA.
University of Wisconsin Reading Center, Madison, WI: Michael M. Altaweel, MD (Director); Wendy K. Benz, PhD; Geoffrey Chambers, MS; Debra J. Christianson, BS; Amitha Domalpally, MD; Jacquelyn Freund, MS; Vonnie Gama; Sapna Gangaputra, MD, MPH; Kathleen E. Glander, BBA; Anne Goulding, BA; Jeffrey M. Joyce, MS; Christina N. Kruse, BA; Dawn J. Myers, BS; Susan Reed, BS; James L. Reimers, BA; Amy Remm, BS; Ruth A. Susman, BS; Dennis Thayer; Erika Treichel, DVM; Kelly J. Warren, RN, BS, MSES; Sheila M. Watson, BS, DVM; James K. White, BME; Tara Wilhelmson, BS. Former Members: Margaret A. Fleischli, AB, DVM; Dennis Hafford; Susan E. Harris, MS; Larry D. Hubbard, MAT; Kristine A. Johnson; Lauren Nagle; Gwyn E. Padden-Lechten, BS; Alyson Pohlman, BA; Peggy Sivesind; Mary K. Webster, BS; Grace Zhang, BA.
National Eye Institute, Bethesda, MD: Natalie Kurinij, PhD.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
Off label use of drugs: Not applicable
ClinicalTrials.gov Identifier: NCT00132691
References
- 1.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA Multicenter Uveitis Steroid Treatment Trial Research Group. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010 Apr;149(4):550–561. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallam A, Sheth HG, Habot-Wilner Z, Lightman S. Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am J Ophthalmol. 2009 Aug;148(2):207–213. doi: 10.1016/j.ajo.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Nazm N, Dubey S, Gandhi M, Pegu J. Re: Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am J Ophthalmol. 2010 Mar;149(3):525–6. doi: 10.1016/j.ajo.2009.10.013. author reply 526–7. [DOI] [PubMed] [Google Scholar]
- 4.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008 Sep;126(9):1191–201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006 Jun;113(6):1020–7. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002 Feb;86(2):238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen L. Ocular fundus photography. Am J Ophthalmol. 1964;57:13–28. [PubMed] [Google Scholar]
- 8.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 9.Hubbard LD, Danis RP, Neider MW, Thayer DW, Wabers HD, White JK, Pugliese AJ, Pugliese MF Age-Related Eye Disease 2 Research Group. Brightness, contrast, and color balance of digital versus film retinal images in the age-related eye disease study 2. Invest Ophthalmol Vis Sci. 2008 Aug;49(8):3269–82. doi: 10.1167/iovs.07-1267. [DOI] [PubMed] [Google Scholar]
- 10.Klein BE, Moss SE, Magli YL, Klein R, Hoyer C, Johnson J. Optic disc cupping: prevalence findings from the WESDR. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):304–9. [PubMed] [Google Scholar]
- 11.Klein BE, Moss SE, Magli YL, Klein R, Hoyer C, Johnson J. Optic disc cupping: four year follow-up from the WESDR. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):310–5. [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 13.Jonas JB, Bergua A, Schmitz-Valckenberg P, Papastathopoulos KI, Budde WM. Ranking of optic disc variables for detection of glaucomatous optic nerve damage. Invest Ophthalmol Vis Sci. 2000 Jun;41(7):1764–73. [PubMed] [Google Scholar]
- 14.Crowston JG, Hopley CR, Healey PR, Lee A, Mitchell P. Blue Mountains Eye Study. The effect of optic disc diameter on vertical cup to disc ratio percentiles in a population based cohort: the Blue Mountains Eye Study. Br J Ophthalmol. 2004 Jun;88(6):766–70. doi: 10.1136/bjo.2003.028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healey PR, Mitchell P, Smith W, Wang JJ. Relationship between cup-disc ratio and optic disc diameter: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1997 May;25( Suppl 1):S99–101. doi: 10.1111/j.1442-9071.1997.tb01771.x. [DOI] [PubMed] [Google Scholar]
- 16.Durmus M, Karadag R, Erdurmus M, Totan Y, Feyzi Hepsen I. Assessment of cup-to-disc ratio with slit-lamp funduscopy, Heidelberg Retina Tomography II, and stereoscopic photos. Eur J Ophthalmol. 2009 Jan-Feb;19(1):55–60. doi: 10.1177/112067210901900108. [DOI] [PubMed] [Google Scholar]
- 17.Jayasundera T, Danesh-Meyer HV, Donaldson M, Gamble G. Agreement between stereoscopic photographs, clinical assessment, Heidelberg retina tomograph and digital stereoscopic optic disc camera in estimating vertical cup:disc ratio. Clin Experiment Ophthalmol. 2005 Jun;33(3):259–63. doi: 10.1111/j.1442-9071.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 18.Tielsch JM, Katz J, Quigley HA, et al. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology. 1988;95:350–356. doi: 10.1016/s0161-6420(88)33177-5. [DOI] [PubMed] [Google Scholar]
- 19.Parrish RK, 2nd, Schiffman JC, Feuer WJ, Anderson DR, Budenz DL, Wells-Albornoz MC, Vandenbroucke R, Kass MA, Gordon MO Ocular Hypertension Treatment Study Group. Test-retest reproducibility of optic disk deterioration detected from stereophotographs by masked graders. Am J Ophthalmol. 2005 Oct;140(4):762–4. doi: 10.1016/j.ajo.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Feuer WJ, Parrish RK, 2nd, Schiffman JC, Anderson DR, Budenz DL, Wells MC, Hess DJ, Kass MA, Gordon MO. The Ocular Hypertension Treatment Study: reproducibility of cup/disk ratio measurements over time at an optic disc reading center. Am J Ophthalmol. 2002 Jan;133(1):19–28. doi: 10.1016/s0002-9394(01)01338-1. [DOI] [PubMed] [Google Scholar]
- 21.Chew EY, Kim J, Sperduto RD, Datiles MB, 3rd, Coleman HR, Thompson DJ, Milton RC, Clayton JA, Hubbard LD, Danis RP, Ferris FL., 3rd Evaluation of the Age-Related Eye Disease Study Clinical Lens Grading System AREDS Report No. 31. Ophthalmology. 2010 Jun 17; doi: 10.1016/j.ophtha.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott IU, Bressler NM, Bressler SB, Browning DJ, Chan CK, Danis RP, Davis MD, Kollman C, Qin H Diabetic Retinopathy Clinical Research Network Study Group. Agreement between clinician and reading center gradings of diabetic retinopathy severity level at baseline in a phase 2 study of intravitreal bevacizumab for diabetic macular edema. Retina. 2008 Jan;28(1):36–40. doi: 10.1097/IAE.0b013e31815e9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg DV, Holbrook JT, Hubbard LD, Davis MD, Jabs DA, Holland GN Studies of Ocular Complications of AIDS Research Group. Clinician versus reading center assessment of cytomegalovirus retinitis lesion size. Ophthalmology. 2005 Apr;112(4):559–66. doi: 10.1016/j.ophtha.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Emanuele N, Klein R, Moritz T, Davis MD, Glander K, Anderson R, Reda D, Duckworth W, Abraira C VADT Study Group. Comparison of dilated fundus examinations with seven-field stereo fundus photographs in the Veterans Affairs Diabetes Trial. J Diabetes Complications. 2009 Sep-Oct;23(5):323–9. doi: 10.1016/j.jdiacomp.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Klein BE, Moss SE, Magli YL, Klein R, Johnson JC, Roth H. Optic disc cupping as clinically estimated from photographs. Ophthalmology. 1987 Nov;94(11):1481–3. doi: 10.1016/s0161-6420(87)33265-8. [DOI] [PubMed] [Google Scholar]
- 26.Feuer WJ, Parrish RK, 2nd, Schiffman JC, Anderson DR, Budenz DL, Wells MC, Hess DJ, Kass MA, Gordon MO. The Ocular Hypertension Treatment Study: reproducibility of cup/disk ratio measurements over time at an optic disc reading center. Am J Ophthalmol. 2002 Jan;133(1):19–28. doi: 10.1016/s0002-9394(01)01338-1. [DOI] [PubMed] [Google Scholar]
- 27.Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992 Feb;99(2):215–21. doi: 10.1016/s0161-6420(92)31990-6. [DOI] [PubMed] [Google Scholar]
- 28.Breusegem C, Fieuws S, Stalmans I, Zeyen T. Agreement and accuracy of non-expert ophthalmologists in assessing glaucomatous changes in serial stereo optic disc photographs. Ophthalmology. 2011 Apr;118(4):742–6. doi: 10.1016/j.ophtha.2010.08.019. [DOI] [PubMed] [Google Scholar]