Abstract
The Golgi apparatus contains multiple classes of cisternae that differ in structure, composition, and function, but there is no consensus about the number and definition of these classes. A useful way to classify Golgi cisternae is according to the trafficking pathways by which the cisternae import and export components. By this criterion, we propose that Golgi cisternae can be divided into three classes that correspond to functional stages of maturation. First, cisternae at the cisternal assembly stage receive COPII vesicles from the ER and recycle components to the ER in COPI vesicles. At this stage, new cisternae are generated. Second, cisternae at the carbohydrate synthesis stage exchange material with one another via COPI vesicles. At this stage, most of the glycosylation and polysaccharide synthesis reactions occur. Third, cisternae at the carrier formation stage produce clathrin-coated vesicles and exchange material with endosomes. At this stage, biosynthetic cargo proteins are packaged into various transport carriers, and the cisternae ultimately disassemble. Discrete transitions occur as a cisterna matures from one stage to the next. Within each stage, the structure and composition of a cisterna can evolve, but the trafficking pathways remain unchanged. This model offers a unified framework for understanding the properties of the Golgi in diverse organisms.
Keywords: Golgi, cisternal maturation, COPI, clathrin, compartmentation, secretory pathway
The Golgi apparatus is one of the most elaborate organelles in the cell (Farquhar and Palade 1981). It consists of flattened membrane sacs called cisternae, which are usually but not always organized into polarized stacks (Mowbrey and Dacks 2009). Depending on the organism and cell type, a Golgi stack may contain as few as 3 or as many as 20 cisternae (Becker and Melkonian 1996; Mogelsvang et al. 2003; Rambourg and Clermont 1997). Most organisms have individual Golgi stacks, but in many vertebrate cells the Golgi stacks are linked by lateral connections to form a continuous ribbon (Klumperman 2011; Marsh et al. 2001). Despite this variable morphology, the Golgi operates by conserved principles in diverse eukaryotes (Duden and Schekman 1997; Hawes 2005).
Within a Golgi stack, the cisternae differ in structure, composition, and function. Biosynthetic cargo proteins enter the Golgi at the cis face of the stack and depart from the trans face (Farquhar and Palade 1981). During passage through the stack, biosynthetic cargo proteins undergo glycan remodeling and other modifications (Ruiz-May et al. 2012; Stanley 2011). Complex polysaccharides are also synthesized within the Golgi (Dick et al. 2012; Parsons et al. 2012). The trans-most cisternae are designated the trans-Golgi network (TGN), and are responsible for packaging biosynthetic cargo proteins and polysaccharides into transport carriers for delivery to downstream destinations (Anitei and Hoflack 2011; Kang et al. 2011; Mellman and Simons 1992; Viotti et al. 2010). The membranes at the cis and trans faces of the stack are often tubulovesicular structures that may not be closely aligned with the rest of the stack (Rambourg and Clermont 1997; Staehelin and Kang 2008), but for convenience we will refer to all of these structures as cisternae.
The functions of the Golgi stack are carried out by enzymes and trafficking proteins, each of which localizes preferentially to a particular set of cisternae. Similarly, the non-stacked Golgi cisternae of the budding yeast Saccharomyces cerevisiae vary in composition and function (Papanikou and Glick 2009; Preuss et al. 1992). Such findings have led to the idea that the Golgi consists of multiple classes of cisternae. However, no consensus yet exists about the number of such classes or the molecular events that define them.
We suggest that the most useful way to classify Golgi cisternae is to consider trafficking pathways. According to this view, cisternae in a given class employ the same mechanisms for importing and exporting components, and these trafficking pathways reflect conserved core functions. This approach will enable us to integrate experimental findings about Golgi organization in a variety of organisms.
Evidence and Rationale for Different Classes of Golgi Cisternae
In previous classification schemes, the major criterion for differentiating between Golgi cisternae has been the polarized distribution of processing and biosynthetic enzymes. N-linked oligosaccharides are modified by a series of Golgi resident glycosylation enzymes that reside at characteristic locations within the stack (Ruiz-May et al. 2012; Stanley 2011). Density gradient fractionation and immunolocalization studies of the mammalian Golgi revealed that early-acting glycosylation enzymes tend to be concentrated in cisternae at the cis side of the stack while late-acting glycosylation enzymes are concentrated in cisternae at the trans side (Dunphy and Rothman 1985; Kornfeld and Kornfeld 1985; Rabouille et al. 1995). Some glycosylation enzymes in mammalian and plant cells are concentrated in the middle of the Golgi stack, in so-called medial cisternae (Donohoe et al. 2013; Dunphy and Rothman 1985; Rabouille et al. 1995). The polarized localization of glycosylation enzymes presumably boosts efficiency because a biosynthetic cargo protein encounters enzymes in the order of their action. In the TGN of mammalian and fungal cells, another type of processing occurs when peptidases activate prohormones (Rockwell et al. 2002).
Why place Golgi enzymes in different cisternae? This separation enables the cell to optimize the pH, ion composition, and substrate concentrations for each set of enzymes. Moreover, enzyme separation may sometimes be essential to prevent two reactions from competing. For example, mammalian lysosomal hydrolases are modified with a mannose 6-phosphate sorting tag, and this modification must occur before the lysosomal hydrolases encounter Golgi-localized mannosidases (Goldberg and Kornfeld 1983). In plant cells, segregation of the backbone synthesis reactions of pectic and xyloglucan polysaccharides to different cisternae prevents the nascent molecules from becoming entangled before the pectic polysaccharides become methylesterified (Atmodjo et al. 2013; Zhang and Staehelin 1992). Yet these requirements are probably unusual, because most carbohydrate synthesis reactions could occur efficiently in a single mixed compartment (Dunphy and Rothman 1985). A more general explanation for the cisternal structure of the Golgi is that this organelle serves as a “delay timer” (Glick and Malhotra 1998). Biosynthetic cargo proteins take some time to traverse the Golgi, and this delay provides an opportunity for resident ER proteins to be retrieved and for cargo proteins to be fully processed (Becker and Melkonian 1996; Rothman 1981). Thus, in cells with large Golgi stacks, a particular task may be physically and temporally spaced over multiple cisternae.
Further evidence for different classes of cisternae has come from morphological studies of Golgi stacks (Donohoe et al. 2013; Klumperman 2011; Rambourg and Clermont 1997; Staehelin and Kang 2008). In plant cells, electron microscopy revealed that the cis-most cisternae often stain much less intensely than medial and trans cisternae, and this staining pattern correlates with the restricted localization of glycosylation and biosynthetic enzymes to medial and trans cisternae (Donohoe et al. 2013). Moreover, in many organisms, cisternae at the trans face are partially or completely separated from the Golgi stack (Kang et al. 2011; Mogelsvang et al. 2003; Mollenhauer and Morré 1991; Rambourg and Clermont 1997; Viotti et al. 2010). As judged by electron tomography, mammalian Golgi stacks show a sharp distinction between the trans-most cisterna, which produces clathrin-coated vesicles, versus earlier cisternae, which produce COPI vesicles (Ladinsky et al. 1999; Mogelsvang et al. 2004).
There is a danger that improvements in analysis methods will yield increasingly finer subdivisions of the Golgi. For example, a recent study of the yeast TGN revealed that the GGA and AP-1 clathrin adaptors are recruited sequentially (Daboussi et al. 2012), yet a division of TGN cisternae into two classes according to adaptor recruitment may not be constructive. In general, structural and compositional differences between cisternae can mask underlying functional commonalities.
How Many Classes of Golgi Cisternae Exist?
The prevailing view is that Golgi cisternae can be divided into four classes: cis, medial, trans, and TGN (Farquhar and Hauri 1997). Each class of cisterna is considered to house a different set of processing enzymes. This model has some experimental support. For example, a study of the yeast pro-α-factor mating pheromone precursor tracked sequential processing by four Golgi enzymes, and the Sec18/NSF vesicle fusion protein was required at each step, suggesting that pro-α-factor passes through four classes of Golgi cisternae (Brigance et al. 2000). But studies of mammalian glycosylation enzymes have not yielded such clear-cut distinctions. As judged by quantitative immunolabeling, individual glycosylation enzymes are most concentrated in particular cisternae, but they have overlapping distributions across the stack (Nilsson et al. 1993; Rabouille et al. 1995). Moreover, a given glycosylation enzyme can show variable intra-Golgi localizations in different cell types (Velasco et al. 1993). The polarized arrangement of Golgi processing enzymes seems to reflect gradients of enzyme localization rather than precise separations (Glick et al. 1997).
In addition to the four putative classes of Golgi cisternae, mammalian cells contain an ER-Golgi intermediate compartment (ERGIC) (Appenzeller-Herzog and Hauri 2006). The ERGIC is stably associated with peripheral ER exit sites, but it generates mobile membrane carriers that travel along microtubules to the Golgi ribbon (Appenzeller-Herzog and Hauri 2006; Lippincott-Schwartz et al. 2000). These ERGIC-derived membranes evidently dock at the cis face of the Golgi stack, where they form a layer that is sometimes termed the cis-Golgi network or CGN (Bannykh and Balch 1997; Ladinsky et al. 1999; Mellman and Simons 1992). This term is misleading because the ERGIC-derived membranes at the cis face of the stack are not actually a network (Ladinsky et al. 1999), so we will refer to these membranes as the “pre-cis layer” (Fig. 1a). In plants and algae, membrane structures termed “cis initiators” at the cis face of the stack have morphological and functional features in common with the mammalian ERGIC/pre-cis layer (Donohoe et al. 2013) (Figs. 1b and 2).
Fig 1. Three-stage models of the mammalian and plant Golgi based on electron tomography of high-pressure frozen/freeze substituted cells.
- Cisternal Assembly Stage: COPII vesicles bud from the ER, and fuse homotypically to form ERGIC elements next to ER exit sites. ER resident proteins are recycled from ERGIC elements to the ER via COPIa vesicles. Mobile ERGIC-derived membranes then traffic to the cis side of a Golgi stack, where they assemble into a pre-cis layer. Fusion of membranes of the pre-cis layer produces a cis cisterna.
- Carbohydrate Synthesis Stage: A transition occurs when a cis cisterna loses the ability to receive COPII vesicles while acquiring the ability to receive glycosylation enzymes and sugar nucleotide transporters in COPIb vesicles. These processes convert the cis cisterna into a medial cisterna. Medial cisternae mature into trans cisternae. Both medial and trans cisternae are involved in carbohydrate synthesis, with early-acting enzymes concentrated in medial cisternae and late-acting enzymes in trans cisternae. Cisternae at the carbohydrate synthesis stage exchange material with one another via COPIb vesicles.
- Carrier Formation Stage: A transition occurs when a trans cisterna loses the ability to receive COPIb vesicles, and subsequently loses the ability to produce COPIb vesicles while acquiring the ability to produce clathrin-coated vesicles. The timing of secretory vesicle formation and the ultimate fate of carrier formation cisternae are still poorly understood. ER membranes attached to trans and/or TGN cisternae probably function in direct lipid transfer between the organelles (Hanada et al. 2009). This diagram depicts the mammalian TGN as the last cisterna in the stack.
- Cisternal Assembly Stage: COPII vesicles bud from the ER, and attach to the cis side of a Golgi stack that is transiently docked at an ER exit site. A cis initiator cisterna is generated when 3–5 of these tethered COPII vesicles fuse homotypically (see Fig. 2). Growth of the initiators is fueled by the fusion of additional COPII vesicles, until the initiators merge into a coherent cis cisterna. The cis cisterna continues to grow through fusion of COPII vesicles, and it nucleates new initiators. ER resident proteins are recycled from cis initiators and cis cisternae to the ER via COPIa vesicles.
- Carbohydrate Synthesis Stage: A transition occurs when a cis cisterna loses the ability to receive COPII vesicles while acquiring the ability to receive glycosylation enzymes and sugar nucleotide transporters in COPIb vesicles. These processes convert the cis cisterna into a medial cisterna. Both medial and trans cisternae are involved in carbohydrate synthesis, with early-acting enzymes concentrated in medial cisternae and late-acting enzymes in trans cisternae. Medial and trans cisternae can also be distinguished based on their luminal pH. Cisternal acidification causes osmotic shrinkage of the trans cisternae, and squeezes the proteoglycans and complex polysaccharides into the cisternal margins. Cisternae at the carbohydrate synthesis stage exchange material with one another via COPIb vesicles.
- Carrier Formation Stage: A transition occurs when a cisterna loses the ability to receive COPIb vesicles, and subsequently loses the ability to produce COPIb vesicles while acquiring the ability to produce clathrin-coated vesicles. During this transition, the cisterna loses ~35% of its membrane surface area, and it peels away from the stack. The free-floating cisterna then becomes a grape-like cluster of nascent secretory and clathrin-coated vesicles. Release of these vesicles (~30 total) occurs in a concerted manner, leaving only residual membrane fragments. This diagram depicts the plant TGN as the cisternae immediately before and after the transition to the carrier formation stage.
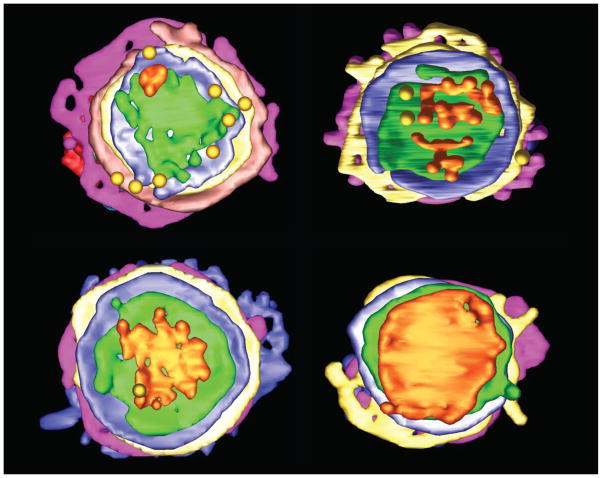
Fig 2. Face-on cis-side views of 3D tomographic reconstructions of Arabidopsis meristem cell Golgi stacks.
Variations in the size and shape of the cis-most (orange) cisterna are consistent with the cisternal maturation model. In the reconstruction at the upper left, 4–5 COPII vesicles have fused to form a cis initator. The other reconstructions presumably correspond to successive time points in the assembly of a full-size cis cisterna. Yellow spheres are COPII vesicles associated with the cis side of the stacks. This figure is courtesy of Byung-Ho Kang.
In addition to describing cisternal structure, composition, and function, a complete picture of Golgi organization needs to address the following questions. How do Golgi cisternae transport biosynthetic secretory cargo while exchanging material with one another and with other organelles? How are resident Golgi proteins localized to particular sets of cisternae? What types of trafficking machinery operate at the Golgi? We will explore these issues as they relate to our proposed approach for classifying Golgi cisternae.
Golgi Organization and Models for Golgi Traffic
The properties of Golgi cisternae are linked to the pathways of Golgi membrane traffic, a topic that is vigorously debated (Glick and Luini 2011; Rabouille and Klumperman 2005). Currently, the predominant model is cisternal maturation, which postulates that Golgi cisternae form de novo and then progressively mature into TGN cisternae. In the simplest version of the maturation model, a TGN cisterna is merely an older version of a cis cisterna, and the Golgi can be viewed as a set of cisternae on a maturation continuum (Glick et al. 1997). A more nuanced view is that maturation occurs in several discrete steps, with the different classes of Golgi cisternae representing successive stages of maturation (Glick and Nakano 2009).
The maturation model can accommodate most of the experimental data from a variety of cell types (Glick and Luini 2011; Rizzo et al. 2013; Staehelin and Kang 2008). According to this model, resident Golgi proteins recycle from older to younger cisternae, thereby staying within the organelle while biosynthetic cargo moves forward. Still uncertain is the mechanism by which resident Golgi proteins recycle. One possibility is that Golgi membrane proteins recycle in COPI vesicles, which bud from Golgi cisternae (Donohoe et al. 2007; Glick and Malhotra 1998; Rabouille and Klumperman 2005). There is good evidence that COPI vesicles contain at least some Golgi membrane proteins, but conflicting data have been obtained about the presence of glycosylation enzymes in COPI vesicles (Cosson et al. 2002; Gilchrist et al. 2006; Kweon et al. 2004; Malsam et al. 2005; Martínez-Menárguez et al. 2001; Orci et al. 2000a; Orci et al. 1997; Sönnichsen et al. 1998). In addition to COPI vesicles, transient tubular connections between cisternae have been postulated to allow recycling of Golgi membrane proteins (Glick and Luini 2011; Marsh et al. 2004; Trucco et al. 2004).
Alternative models for Golgi traffic view the cisternae in a different light. The rapid partitioning model proposes that the Golgi is a continuous structure, with glycosylation and export occurring at all levels of the stack (Patterson et al. 2008). This model treats the Golgi as a single intermixed compartment and makes no distinction between the various cisternae. The cisternal progenitor and rim progression models propose that Golgi cisternae are long-lived structures, and that large portions of the cisternae undergo fission and subsequent fusion to carry biosynthetic cargo forward while resident Golgi enzymes remain in the static portions of the cisternae (Lavieu et al. 2013; Pfeffer 2010).
These various models for Golgi traffic postulate that Golgi cisternae are either transient or static, and either separate or intermixed, so any classification scheme will be influenced by the underlying traffic model. The discussion below builds on our judgment that the cisternal maturation model provides the most compelling interpretations of available data.
Golgi Organization and Resident Protein Localization
Different Golgi cisternae contain different resident proteins, so a classification scheme should take into account the mechanisms for localizing resident Golgi proteins. Localization signals have been identified for a number of Golgi glycosylation enzymes (Banfield 2011; Machamer 1993). These proteins have a type II topology, with a cytosolic N-terminus and a single transmembrane sequence. The transmembrane sequences, along with flanking sequences, are often necessary and sufficient for Golgi localization. However, the mechanisms of localization have been elusive. According to the maturation model, glycosylation enzymes are continually recycled, perhaps in a COPI-dependent manner. The yeast Vps74 protein has been implicated in linking certain glycosylation enzymes to COPI (Tu et al. 2008). Transmembrane sequence length is important for Golgi localization, suggesting that partitioning into lipid domains plays a role (Sharpe et al. 2010). Depending on the localization mechanism that is envisioned, a given transmembrane sequence length might either promote or inhibit partitioning into a curved vesicle membrane.
The picture is clearer for a class of TGN proteins that includes mammalian TGN38 and yeast Kex2 (Machamer 1993). These proteins have a type I topology, and they localize to the TGN by retrieval mechanisms that involve adaptor-mediated recognition of signals in the C-terminal cytosolic tails.
The division of Golgi resident membrane proteins into type II glycosylation enzymes and type I TGN proteins is unambiguous. By contrast, the type I localization signals of various Golgi glycosylation enzymes all seem to resemble one another (Sharpe et al. 2010). Thus, the known properties of Golgi localization signals point to a major distinction between the TGN and earlier cisternae.
Golgi Organization and the Trafficking Machinery
Trafficking pathways constrain the possibilities for classifying Golgi cisternae. A trafficking step typically involves the following: a coat complex that sorts cargo and sculpts vesicles, a regulatory Rab GTPase, tethering proteins, and a fusogenic SNARE complex (Bonifacino and Glick 2004). The Golgi system employs only a limited number of these components. We will argue that Golgi-associated trafficking components can be divided into three categories based on their site of action either at the ER-Golgi interface, or within the Golgi, or in traffic to and from the TGN.
The conserved coats are COPII, COPI, and clathrin. COPII vesicles carry proteins from the ER to the cis-Golgi, and also fuse with one another to generate ERGIC elements (Barlowe and Miller 2013). COPI vesicles serve two different functions: they recycle proteins from nascent Golgi cisternae to the ER, and they transport proteins between Golgi cisternae (Barlowe and Miller 2013; Popoff et al. 2011). In plant and algal cells, the Golgi-to-ER and intra-Golgi transport functions are carried out by different subtypes of COPI vesicles known as COPIa and COPIb, respectively (Donohoe et al. 2013; Donohoe et al. 2007). COPIa vesicles are confined to the ER-Golgi interface whereas COPIb vesicles surround the medial and trans Golgi cisternae. Mammalian cells also contain COPI vesicle subtypes that we interpret to be analogous to COPIa and COPIb (Lanoix et al. 2001; Malsam et al. 2005; Moelleken et al. 2007; Orci et al. 1997). Clathrin-coated vesicles bud from the TGN to carry proteins to endosomes and lysosomes/vacuoles, and may also recycle proteins from mature to newly formed TGN cisternae (Myers and Payne 2013; Valdivia et al. 2002; Viotti et al. 2010). Thus, a reasonable hypothesis is that different classes of cisternae are marked by COPIa, COPIb, and clathrin.
The number of Golgi-associated Rab proteins varies between cell types. S. cerevisiae is the simplest model organism to examine because it has a streamlined set of Rab proteins. Ypt1 (homologous to mammalian Rab1) operates in ER-to-Golgi transport and also in intra-Golgi transport (Barlowe and Miller 2013; Jedd et al. 1995; Suvorova et al. 2002). Ypt6 (homologous to mammalian Rab6) operates in delivery of membrane carriers from endosomes to the TGN (Liu and Storrie 2012; Storrie et al. 2012). Finally, the Ypt31/Ypt32 pair (homologous to mammalian Rab11) operates in transport from the TGN to the cell surface (Jedd et al. 1997).
During ER-to-Golgi transport, the conserved tethering proteins are the coiled-coil protein known as Uso1 or p115, and the multi-subunit TRAPP complex (Kang and Staehelin 2008; Lord et al. 2013). Tethers of the GRASP family also act during ER-to-Golgi transport in mammalian and fungal cells, but are absent from plant cells (Behnia et al. 2007; Kinseth et al. 2007; Levi et al. 2010; Marra et al. 2001). Retrograde transport of COPIa vesicles to the ER involves the Dsl1 tethering complex (Lerich et al. 2012; Ren et al. 2009; Spang 2012). Intra-Golgi traffic appears to depend mainly on the conserved COG complex (Smith and Lupashin 2008; Ungar et al. 2006), although coiled-coil “golgin” proteins are also involved (Faso et al. 2009; Munro 2011). Delivery of transport carriers from endosomes to the TGN employs the GARP complex plus additional golgins (Munro 2011; Pfeffer 2011). In sum, Golgi-associated tethers mediate transport either between the ER and Golgi, or within the Golgi, or from endosomes to the TGN.
SNARE proteins associate in various combinations to drive membrane fusion. Current evidence suggests that in mammalian and yeast cells, one SNARE complex mediates ER-to-Golgi anterograde traffic, a second SNARE complex mediates Golgi-to-ER retrograde traffic, a third SNARE complex mediates intra-Golgi traffic, and one or two SNARE complexes mediate retrograde traffic to the TGN (Malsam and Söllner 2011). Thus, the SNARE repertoire of the Golgi system is relatively small (Pelham 1998).
This overview of the trafficking machinery argues against the idea that the Golgi should be divided into cis, medial, trans, and TGN classes of cisternae plus a possible ERGIC/pre-cis layer class, because the number of trafficking components seems insufficient. We suggest instead that Golgi cisternae can be divided into three classes (Mellman and Simons 1992), which reflect three structural and functional stages of maturation: cisternal assembly, carbohydrate synthesis, and carrier formation (Fig. 1).
Stage I: Cisternal Assembly
Membranes at the cisternal assembly stage correspond to the ERGIC and pre-cis layer plus one or more cis cisternae in mammalian cells (Farquhar and Hauri 1997) (Fig. 1a), or to the cis initiators plus one or more cis cisternae in plant and algal cells (Donohoe et al. 2013) (Fig. 1b). During cisternal assembly, COPII vesicles fuse homotypically to generate larger structures, which then fuse with each other and with additional COPII vesicles to generate a full-size cis cisterna (Bentley et al. 2006; Donohoe et al. 2013; Zeuschner et al. 2006). This scheme fits with the historical designation of the cis face of the Golgi as the “forming” face (Mollenhauer and Whaley 1963). Fig. 2 shows electron tomography-based models of the cis face of plant Golgi stacks, with the orange structures representing successive stages in cisternal assembly (Donohoe et al. 2013). These images indicate that 3–5 COPII vesicles fuse to produce a cis initiator, which then grows by fusion of additional COPII vesicles to generate a full-size cis cisterna. Little carbohydrate synthesis occurs at the assembly stage, but assembling cisternae are probably responsible for adding mannose 6-phosphate to lysosomal hydrolases in mammalian cells (Pelham 1988) and for assembling scale protein complexes in algae (Donohoe et al. 2013).
The ability to receive COPII vesicles is the defining feature of membranes at the assembly stage. In addition, COPIa vesicles operate at the assembly stage to recycle trafficking machinery components to the ER. COPIa vesicle formation in mammalian cells is apparently restricted to the ERGIC (Ladinsky et al. 1999), suggesting that recycling to the ER occurs prior to transport of ERGIC-derived membranes to the Golgi ribbon (Fig. 1a).
Stage II: Carbohydrate Synthesis
Cisternae at the carbohydrate synthesis stage are commonly referred to as medial and trans (Fig. 1). The main function of these cisternae is glycosylation of proteins and lipids, and synthesis of complex polysaccharides (Atmodjo et al. 2013; Dick et al. 2012; Stanley 2011). In some cell types, the carbohydrate synthesis stage involves a large number of cisternae. Different sets of glycosylation enzymes are often concentrated in younger (medial) or older (trans) cisternae, and these separations can be sharp (Donohoe et al. 2013; Driouich and Staehelin 1997; Dunphy and Rothman 1985).
Traffic between cisternae at the carbohydrate synthesis stage is apparently mediated by COPIb vesicles (Donohoe et al. 2013; Rothman and Wieland 1996; Staehelin and Kang 2008), but the precise role of COPI in intra-Golgi transport is an enduring mystery (Glick and Luini 2011; Rabouille and Klumperman 2005). Some versions of the maturation model account for Golgi polarity by postulating that COPIb vesicles move only in the retrograde direction (Glick et al. 1997). However, a molecular basis for unidirectional traffic of COPIb vesicles has not emerged, and is hard to envision if cisternae at the carbohydrate synthesis stage all use the same trafficking machinery. An alternative is that COPIb vesicles might “percolate” randomly within the Golgi (Orci et al. 2000b). But if COPIb vesicles carry glycosylation enzymes, how could percolating vesicles enable an enzyme to be concentrated in particular cisternae? A possible mechanism involves progressive changes in lipid composition. Lipid biosynthesis and remodeling occur actively in the Golgi (Bankaitis et al. 2012), so older cisternae will have a different lipid composition than younger cisternae. If a given glycosylation enzyme lands in a cisterna where the lipid environment is favorable for the transmembrane sequence (Sharpe et al. 2010), the enzyme would be unlikely to partition into budding COPIb vesicles, so it would become concentrated in that cisterna. This idea is speculative, but it illustrates that stochastic COPIb-mediated transport could generate an asymmetric distribution of resident Golgi proteins.
As older cisternae transition from the carbohydrate synthesis stage to the carrier formation stage, they lose the ability to receive COPIb vesicles. If they continue to produce COPIb vesicles during this time, the cisternae will shrink, as has been observed by electron tomography of plant cells (Kang et al. 2011). Meanwhile, as younger cisternae transition from the cisternal assembly stage to the carbohydrate synthesis stage, they acquire the ability to receive COPI vesicles. The result is a net transfer of material from older to younger cisternae.
Stage III: Carrier Formation
Cisternae at the carrier formation stage correspond to the TGN as originally defined (Griffiths and Simons 1986; Mellman and Simons 1992) (Fig. 1a). However, the boundaries of the TGN have been viewed in different ways, with more recent definitions often including multiple cisternae at the trans face of the stack (Kang et al. 2011; Mogelsvang et al. 2004). To avoid this ambiguity, we propose to define the carrier formation stage by the ability of a cisterna to package biosynthetic cargo into various types of transport carriers (Bard and Malhotra 2006; De Matteis and Luini 2008). Some processing and biosynthesis can also occur at the carrier formation stage. For example, type I TGN proteins catalyze proteolytic cleavage of prohormones (Rockwell et al. 2002), and the trans-most cisternae of the stack house mammalian sialyltransferase and some plant cell wall biosynthetic enzymes (Atmodjo et al. 2013; Rabouille et al. 1995). Carrier formation is a sorting event that is coupled to the condensation of certain cargo proteins, and to changes in pH, Ca2+ concentration, and lipid composition (Bard and Malhotra 2006; Chanat and Huttner 1991; Dettmer et al. 2006; Graham and Burd 2011; Kang et al. 2011; Schmidt and Moore 1995; von Blume et al. 2012). Cisternae peel away from Golgi stacks during the carrier formation stage, becoming “free-floating TGN” in plant cells and in the yeast Pichia pastoris (Bevis et al. 2002; Mogelsvang et al. 2003; Staehelin and Kang 2008). The ultimate fate of Golgi cisternae in mammalian cells has not been fully characterized, but transport carriers form at the trans face of the Golgi and migrate to the plasma membrane (Bard and Malhotra 2006; Polishchuk et al. 2000; Wakana et al. 2012). In plant cells, mature carrier formation cisternae disintegrate into vesicles and residual membrane fragments, thereby completing the life cycle of a Golgi cisterna (Kang and Staehelin 2008) (Fig. 1b).
Cisternae at the carrier formation stage produce constitutive and regulated secretory vesicles destined for the plasma membrane, as well as clathrin-coated vesicles destined for endosomes and lysosomes/vacuoles (De Matteis and Luini 2008). In addition, older carrier formation cisternae apparently recycle components to younger carrier formation cisternae by a pathway involving the clathrin adaptor AP-1 (Valdivia et al. 2002). The production of clathrin-coated vesicles rather than COPI vesicles is a clear distinction between the carrier formation stage and earlier stages. Moreover, unlike earlier cisternae, the carrier formation cisternae exchange membrane with the endosomal system (Pfeffer 2011; Viotti et al. 2010). This difference is highlighted by the response of mammalian and plant cells to brefeldin A, which causes cis through trans cisternae to fuse with the ER but causes the TGN to fuse with endosomes (Klausner et al. 1992; Nebenführ et al. 2002).
Maturation Versus Micro-maturation
We define Golgi maturation as the transitions that initiate and terminate each of the three stages: cisternal assembly, carbohydrate synthesis, and carrier formation. As described above, these transitions are marked by changes in trafficking pathways. Maturation events that have been visualized by live-cell imaging of yeast presumably reflect the transition between the carbohydrate synthesis and carrier formation stages (Losev et al. 2006; Matsuura-Tokita et al. 2006). Transitions between stages of the Golgi are expected to be relatively fast, but studies of the transition intermediates will likely be informative. An attractive possibility is that a cisterna in transition has a mixed composition (Pfeffer 2010), with a domain corresponding to the earlier stage shrinking while a domain corresponding to the later stage expands.
Some resident Golgi proteins are uniquely present during a particular stage, but others may be present at two or even all three stages. For example, the yeast guanine nucleotide exchange factor Gea2 has been localized to both early and late Golgi cisternae (Chantalat et al. 2004; Spang et al. 2001; Tsai et al. 2013), and the homologous mammalian GBF1 protein is apparently associated with Golgi membranes from the cisternal assembly stage to the carrier formation stage (Lowery et al. 2013). Such overlap reflects the precursor-product relationship between cisternae at successive stages.
Within each stage of Golgi maturation, changes occur as the cisterna ages. Examples include the following:
During the cisternal assembly stage, the membrane structure grows from a few fused COPII vesicles to a full-size cisterna.
During the carbohydrate synthesis stage, the enzymatic and lipid composition of the cisterna evolves.
During the carrier formation stage, clathrin adaptors are sequentially recruited.
We propose that changes occurring within a stage can be termed “micro-maturation”. These relatively minor changes are mechanistically different from each other, and from the major changes in trafficking pathways that occur during the transitions between stages. This concept provides a basis for categorizing the many processes that transform Golgi cisternae.
Ultimately, the three-stage model must be tested and refined by analyzing how Golgi cisternae mature. A process called Rab conversion has emerged as a possible mechanism for Golgi maturation, based partly on analogy to endosome maturation (Glick and Nakano 2009; Mizuno-Yamasaki et al. 2012; Rink et al. 2005). Cascades involving Arf GTPases may also play a role in Golgi maturation (Lowery et al. 2013; Richardson et al. 2012; Stalder and Antonny 2013). The components that drive transitions between the three stages of the Golgi are presumably conserved in most or all eukaryotes. Additional components found in certain organisms, such as species-specific Rab proteins associated with the mammalian and plant Golgi (Liu and Storrie 2012; Woollard and Moore 2008), are likely to regulate micro-maturation events, giving the Golgi its unique properties in each cell type.
Acknowledgments
Thanks to Vivek Malhotra and members of the Glick lab for helpful discussion. This work was supported by U.S. National Institutes of Health grants T32 GM007183 to K.J.D. and R01 GM061156 to B.S.G.
References
- Anitei M, Hoflack B. Exit from the trans-Golgi network: from molecules to mechanisms. Curr Opin Cell Biol. 2011;23:443–451. doi: 10.1016/j.ceb.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D. Evolving views of pectin biosynthesis. Annu Rev Plant Biol. 2013;64:747–779. doi: 10.1146/annurev-arplant-042811-105534. [DOI] [PubMed] [Google Scholar]
- Banfield DK. Mechanisms of protein retention in the Golgi. Cold Spring Harb Perspect Biol. 2011;3:a005264. doi: 10.1101/cshperspect.a005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Garcia-Mata R, Mousley CJ. Golgi membrane dynamics and lipid metabolism. Curr Biol. 2012;22:R414–R424. doi: 10.1016/j.cub.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Malhotra V. The formation of TGN-to-plasma membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- Barlowe CK, Miller EA. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193:383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Melkonian M. The secretory pathway of protists: spatial and functional organization and evolution. Microbiol Rev. 1996;60:697–721. doi: 10.1128/mr.60.4.697-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Barr FA, Flanagan JJ, Barlowe C, Munro S. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J Cell Biol. 2007;176:255–261. doi: 10.1083/jcb.200607151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley M, Liang Y, Mullen K, Xu D, Sztul E, Hay JC. SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J Biol Chem. 2006;281:38825–38833. doi: 10.1074/jbc.M606044200. [DOI] [PubMed] [Google Scholar]
- Bevis BJ, Hammond AT, Reinke CA, Glick BS. De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat Cell Biol. 2002;4:750–756. doi: 10.1038/ncb852. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Brigance WT, Barlowe C, Graham TR. Organization of the yeast Golgi complex into at least four functionally distinct compartments. Mol Biol Cell. 2000;11:171–182. doi: 10.1091/mbc.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL. The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci. 2004;117:711–722. doi: 10.1242/jcs.00896. [DOI] [PubMed] [Google Scholar]
- Cosson P, Amherdt M, Rothman JE, Orci L. A resident Golgi protein is excluded from peri-Golgi vesicles in NRK cells. Proc Natl Acad Sci USA. 2002;99:12831–12834. doi: 10.1073/pnas.192460999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat Cell Biol. 2012;14:239–248. doi: 10.1038/ncb2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G, Akslen-Hoel LK, Grøndahl F, Kjos I, Prydz K. Proteoglycan synthesis and Golgi organization in polarized epithelial cells. J Histochem Cytochem. 2012;60:926–935. doi: 10.1369/0022155412461256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang BH, Gerl MJ, Gergely ZR, McMichael CM, Bednarek SY, Staehelin LA. cis-Golgi cisternal assembly and biosynthetic activation occur sequentially in plants and algae. Traffic. 2013;14:551–567. doi: 10.1111/tra.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang BH, Staehelin LA. Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci USA. 2007;104:163–168. doi: 10.1073/pnas.0609818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Staehelin LA. The plant Golgi apparatus: structural organization and functional properties. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser; Basel: 1997. pp. 275–301. [Google Scholar]
- Duden R, Schekman R. Insights into Golgi function through mutants in yeast and animal cells. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 219–246. [Google Scholar]
- Dunphy WG, Rothman JE. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Hauri H-P. Protein sorting and vesicular traffic in the Golgi apparatus. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 63–129. [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus (complex) - (1954–1981) - from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C, Boulaflous A, Brandizzi F. The plant Golgi apparatus: last 10 years of answered and open questions. FEBS Lett. 2009;583:3752–3757. doi: 10.1016/j.febslet.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- Glick BS, Elston T, Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3:a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Glick BS, Nakano A. Membrane traffic within the Golgi stack. Annu Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DE, Kornfeld S. Evidence for extensive subcellular organization of asparagine-linked oligosaccharide processing and lysosomal enzyme phosphorylation. J Biol Chem. 1983;258:3159–3165. [PubMed] [Google Scholar]
- Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011;21:113–121. doi: 10.1016/j.tcb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hawes C. Cell biology of the plant Golgi apparatus. New Phytol. 2005;165:29–44. doi: 10.1111/j.1469-8137.2004.01218.x. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson CJ, Litt RJ, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic. 2011;12:313–329. doi: 10.1111/j.1600-0854.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- Kang BH, Staehelin LA. ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma. 2008;234:51–64. doi: 10.1007/s00709-008-0015-6. [DOI] [PubMed] [Google Scholar]
- Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. The Golgi associated protein GRASP is required for unconventional secretion during development. Cell. 2007:524–534. doi: 10.1016/j.cell.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J. Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol. 2011;3:a005181. doi: 10.1101/cshperspect.a005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kweon HS, Beznoussenko GV, Micaroni M, RSP, Trucco A, Martella O, Di Giandomenico D, Marra P, Fusella A, Di Pentima A, Berger EG, Geerts WJ, Koster AJ, Burger KN, Luini A, Mironov AA. Golgi enzymes are enriched in perforated zones of Golgi cisternae but are depleted in COPI vesicles. Mol Biol Cell. 2004;15:4710–4724. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J, Ouwendijk J, Stark A, Szafer E, Cassel D, Dejgaard K, Weiss M, Nilsson T. Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J Cell Biol. 2001;155:1199–1212. doi: 10.1083/jcb.200108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Zheng H, Rothman JE. Stapled Golgi cisternae remain in place as cargo passes through the stack. eLife. 2013;2:e00558. doi: 10.7554/eLife.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerich A, Hillmer S, Langhans M, Scheuring D, van Bentum P, Robinson DG. ER import sites and their relationship to ER exit sites: a new model for bidirectional ER-Golgi transport in higher plants. Front Plant Sci. 2012;3:143. doi: 10.3389/fpls.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi SK, Bhattacharyya D, Strack RL, Austin JRI, Glick BS. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11:1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Storrie B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol Life Sci. 2012;69:4093–4106. doi: 10.1007/s00018-012-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Ferro-Novick S, Miller EA. The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the Golgi. Cold Spring Harb Perspect Biol. 2013;5:a013367. doi: 10.1101/cshperspect.a013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;22:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Lowery J, Szul T, Styers M, Holloway Z, Oorschot V, Klumperman J, Sztul E. The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN) J Biol Chem. 2013;288:11532–11545. doi: 10.1074/jbc.M112.438481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE. Targeting and retention of Golgi proteins. Curr Opin Cell Biol. 1993;5:606–612. doi: 10.1016/0955-0674(93)90129-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- Malsam J, Söllner TH. Organization of SNAREs within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3:a005249. doi: 10.1101/cshperspect.a005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra P, Maffucci T, Daniele T, Di Tullio G, Ikehara Y, Chan EKL, Luini A, Beznoussenko G, Mironov A, De Matteis MA. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat Cell Biol. 2001;3:1101–1113. doi: 10.1038/ncb1201-1101. [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci USA. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BJ, Volkmann N, McIntosh JR, Howell KE. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet beta cells. Proc Natl Acad Sci USA. 2004;101:5565–5570. doi: 10.1073/pnas.0401242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Menárguez JA, Prekeris R, Oorschot VMJ, Scheller R, Slot JW, Geuze HJ, Klumperman J. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J Cell Biol. 2001;155:1213–1224. doi: 10.1083/jcb.200108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;22:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Mellman I, Simons K. The Golgi complex: in vitro veritas? Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Rivera-Molina FPN. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelleken J, Malsam J, Betts MJ, Movafeghi A, Reckmann I, Meissner I, Hellwig A, Russell RB, Söllner T, Brügger B, Wieland FT. Differential localization of coatomer complex isoforms within the Golgi apparatus. Proc Natl Acad Sci USA. 2007;104:4425–4430. doi: 10.1073/pnas.0611360104. [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell. 2003;14:2277–2291. doi: 10.1091/mbc.E02-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE. Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic. 2004;5:338–345. doi: 10.1111/j.1398-9219.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ. Perspectives on Golgi apparatus form and function. J Electron Microsc Tech. 1991;17:2–14. doi: 10.1002/jemt.1060170103. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Whaley WG. An observation on the functioning of the Golgi apparatus. J Cell Biol. 1963;17:222–225. doi: 10.1083/jcb.17.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583:3738–3745. doi: 10.1016/j.febslet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Munro S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol. 2011;3:a005256. doi: 10.1101/cshperspect.a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MD, Payne GS. Clathrin, adaptors and disease: insights from the yeast Saccharomyces cerevisiae. Front Biosci. 2013;18:862–891. doi: 10.2741/4149. [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Pypaert M, Hoe MH, Slusarewicz P, Berger EG, Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Amherdt M, Ravazzola M, Perrelet A, Rothman JE. Exclusion of Golgi residents from transport vesicles budding from Golgi cisternae in intact cells. J Cell Biol. 2000a;150:1263–1270. doi: 10.1083/jcb.150.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Volchuk A, Engel T, Gmachl M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Anterograde flow of cargo across the Golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc Natl Acad Sci USA. 2000b;97:10400–10405. doi: 10.1073/pnas.190292497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Papanikou E, Glick BS. The yeast Golgi apparatus: insights and mysteries. FEBS Lett. 2009;583:3746–3751. doi: 10.1016/j.febslet.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons HT, Christiansen K, Knierim B, Carroll A, Ito J, Batth TS, Smith-Moritz AM, Morrison S, McInerney P, Hadi MZ, Auer M, Mukhopadhyay A, Petzold CJ, Scheller HV, Loqué D, Heazlewood JL. Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 2012;159:12–26. doi: 10.1104/pp.111.193151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988;7:913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. How the Golgi works: a cisternal progenitor model. Proc Natl Acad Sci USA. 2010;107:19614–19618. doi: 10.1073/pnas.1011016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Entry at the trans-face of the Golgi. Cold Spring Harb Perspect Biol. 2011;3:a005272. doi: 10.1101/cshperspect.a005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between the Golgi apparatus and plasma membrane. J Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff V, Adolf F, Brügger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3:a005231. doi: 10.1101/cshperspect.a005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–817. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y. Three-dimensional structure of the Golgi apparatus in mammalian cells. In: Berger EG, Roth J, editors. The Golgi Apparatus. Birkhäuser Verlag; Basel: 1997. pp. 37–61. [Google Scholar]
- Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, Hughson FM. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139:1119–1129. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BC, McDonold CM, Fromme JC. The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback. Dev Cell. 2012;22:799–810. doi: 10.1016/j.devcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Parashuraman S, Mirabelli P, Puri C, Lucocq J, Luini A. The dynamics of engineered resident proteins in the mammalian Golgi complex relies on cisternal maturation. J Cell Biol. 2013;201:1027–1036. doi: 10.1083/jcb.201211147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by Kex2/furin proteases. Chem Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- Rothman JE. The Golgi apparatus: two organelles in tandem. Science. 1981;213:1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Ruiz-May E, Kim SJ, Brandizzi F, Rose JK. The secreted plant N-glycoproteome and associated secretory pathways. Front Plant Sci. 2012;3:117. doi: 10.3389/fpls.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WK, Moore HP. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol Biol Cell. 1995;6:1271–1285. doi: 10.1091/mbc.6.10.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe HJ, Stevens TJ, Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142:158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Lupashin VV. Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation. Carbohydr Res. 2008;343:2024–2031. doi: 10.1016/j.carres.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B, Lowe M, Levine T, Jämsä E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. The DSL1 complex: the smallest but not the least CATCHR. Traffic. 2012;13:908–913. doi: 10.1111/j.1600-0854.2012.01362.x. [DOI] [PubMed] [Google Scholar]
- Spang A, Herrmann JM, Hamamoto S, Schekman R. The ADP ribosylation factor-nucleotide exchange factors Gea1p and Gea2p have overlapping, but not redundant functions in retrograde transport from the Golgi to the endoplasmic reticulum. Mol Biol Cell. 2001;12:1035–1045. doi: 10.1091/mbc.12.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Kang BH. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 2008;147:1454–1468. doi: 10.1104/pp.108.120618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder D, Antonny B. Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett. 2013;587:2028–2035. doi: 10.1016/j.febslet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Stanley P. Golgi glycosylation. Cold Spring Harb Perspect Biol. 2011;3:a005199. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Micaroni M, Morgan GP, Jones N, Kamykowski JA, Wilkins N, Pan TH, Marsh BJ. Electron tomography reveals Rab6 is essential to the trafficking of trans-Golgi clathrin and COPI-coated vesicles and the maintenance of Golgi cisternal number. Traffic. 2012;13:727–744. doi: 10.1111/j.1600-0854.2012.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco A, Polishchuk RS, Martella O, Pentima AD, Fusella A, Giandomenico DD, Pietro ES, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts WJ, Koster AJ, Burger KN, Mironov AA, Luini A. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- Tsai PC, Hsu JW, Liu YW, Chen KY, Lee FJ. Arl1p regulates spatial membrane organization at the trans-Golgi network through interaction with Arf-GEF Gea2p and flippase Drs2p. Proc Natl Acad Sci USA. 2013;110:E668–677. doi: 10.1073/pnas.1221484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Tai WC, Chen L, Banfield DK. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321:404–407. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16:113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Valdivia RH, Baggott D, Chuang JS, Schekman R. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Velasco A, Hendricks L, Moremen KW, Tulsiani DRP, Touster O, Farquhar MG. Cell type-dependent variations in the subcellular distribution of α-mannosidase I and II. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, Jürgens G, de Vries SC, Robinson DG, Schumacher K. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22:1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol. 2012;199:1057–1066. doi: 10.1083/jcb.201207180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M, Malhotra V. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 2012;31:3976–3990. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard AA, Moore I. The functions of Rab GTPases in plant membrane traffic. Curr Opin Plant Biol. 2008;11:610–619. doi: 10.1016/j.pbi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8:377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA. Functional compartmentation of the Golgi apparatus of plant cells: immunocytochemical analysis of high-pressure frozen and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 1992;99:1070–1083. doi: 10.1104/pp.99.3.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]