Abstract
Interleukin-22 (IL-22) plays a key role in promoting antimicrobial immunity and tissue repair at barrier surfaces by binding to the receptors IL-22R1, which is generally thought to be expressed exclusively in epithelial cells, and IL-10R2. Our laboratory previously demonstrated that IL-22 plays an important role in ameliorating liver injury in many rodent models by targeting hepatocytes that express high levels of IL-22R1 and IL-10R2. Recently, we have identified high expression levels of IL-22R1 and IL-10R2 in liver progenitor cells and hepatic stellate cells (HSCs). Overexpression of IL-22 in vivo or treatment with IL-22 in vitro promotes proliferation of liver progenitor cells via a signal transducer and activator of transcription 3 (STAT3)-dependent mechanism. IL-22 treatment also prevents HSC apoptosis in vitro and in vivo. Surprisingly, overexpression of IL-22, via either gene targeting or exogenous administration of adenovirus expressing IL-22, reduces liver fibrosis and accelerates the resolution of liver fibrosis during recovery. The anti-fibrotic effects of IL-22 are mediated via the activation of STAT3 in HSCs and subsequent induction of suppressor of cytokine signaling 3, which induces HSC senescence. Taken together, the hepatoprotective, mitogenic, and anti-fibrotic effects of IL-22 are beneficial in ameliorating alcoholic liver injury. Importantly, due to the restricted expression of IL-22R1, IL-22 therapy is expected to have few side effects, thus making IL-22 a potential candidate for treatment of alcoholic liver disease.
Keywords: hepatic stellate cells, liver fibrosis, liver progenitor cells, senescence
Introduction
Chronic alcohol drinking is a major cause of chronic liver disease worldwide and encompasses a spectrum of liver injuries, including fatty liver, alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma.1,2 Many mechanisms underlying the pathogenesis of alcoholic liver disease (ALD) have been identified.1,2 These include direct hepatotoxicity of ethanol and its metabolites, oxidative stress generated by ethanol metabolism, activation of innate immunity, elevation of pro-inflammatory cytokines and chemokines, and many other mechanisms. Among the cytokines and chemokines involved in progression of ALD, tumor necrosis factor-α and monocyte chemoattractant protein-1 have been shown to play an important role in the development of early alcoholic liver injury,3,4 whereas interleukin-6 (IL-6), via the activation of signal transducer and activator of transcription 3 (STAT3) in hepatocytes during early alcoholic liver injury, plays an important role in ameliorating steatosis and hepatocellular damage.5,6 Although the hepatoprotective function of IL-6 has been well documented, clinical application of IL-6 for ALD treatment is limited by the many potential side effects of IL-6, which likely result from the ubiquitous expression of IL-6 receptor. This led us to explore another hepatoprotective cytokine IL-22, which activates similar signaling pathways (such as STAT3) as those activated by IL-6 in hepatocytes. Importantly, IL-22 receptor expression is restricted to epithelial cells, which suggests that IL-22 treatment would likely produce fewer side effects than IL-6, and is an attractive candidate for ALD therapy.
Initially identified as a gene induced by IL-9 in mouse T lymphocytes, IL-22 shares 22% amino acid identity with IL-10 and belongs to IL-10 family.7 Both IL-10 and IL-22 utilize the ubiquitously expressed IL-10R2, but IL-10 signaling also requires IL-10R1, which is expressed on immune cells, whereas IL-22 signaling needs IL-22R1, which is expressed on epithelial cells.8 Thus, IL-10 targets immune cells and acts as an important anti-inflammatory cytokine; IL-22 targets epithelial cells and plays an important role in promoting tissue repair. After binding to IL-22R1 and IL-10R2, IL-22 predominantly induces STAT3 activation, and to a lesser extent, activation of other signaling pathways such as STAT1, STAT5, mitogen-activated protein kinases in epithelial cells, followed by upregulating expression of many genes that control anti-microbial immunity and tissue repair.9 For example, we and others have previously demonstrated that IL-22 plays an important role in protecting against liver injury,10–15 ameliorating fatty liver disease16–18 and promoting liver regeneration19,20 Recently, we have demonstrated that liver progenitor cells (LPCs) and hepatic stellate cells (HSCs) also express high levels of IL-10R2 and IL-22R1, and are highly responsive to IL-22 stimulation.21,22 In this mini review, we briefly summarize the hepatoprotective functions of IL-22, highlight our recent findings about the effects of IL-22 on LPCs and HSCs, and discuss the therapeutic potential of IL-22 for the treatment of ALD.
Hepatoprotection of IL-22 by targeting hepatocytes
Numerous studies suggest that IL-22 plays key roles in the prevention of hepatocellular damage in a variety of liver injury models.10–15 IL-22 was first found to be hepatoprotective against murine liver injury induced by Concanavalin A (Con A), carbon tetrachloride, and Fas ligand,10,11 and was later confirmed in many other liver injury models.12–15 IL-22 protects against hepatocyte damage and promotes hepatocyte proliferation by activating the STAT3 signaling pathway. Activation of STAT3 subsequently leads to upregulation of a variety of anti-apoptotic (e.g. Bcl-2, Bcl-xL, Mcl-1) and mitogenic (e.g. c-myc, cyclin D1, Rb2, CDK4) genes, resulting in hepatoprotective effects under conditions of liver injury.10 The hepatoprotective functions of IL-22 were further supported in genetically modified mice where IL-22 transgenic mice with overexpression of IL-22 were resistant to Con A-induced liver injury,19 while IL-22 deficient mice were highly susceptible to such injury.12 In addition, IL-22 treatment ameliorated high fat diet (HFD)- or ethanol-induced liver lipogenesis and hepatic steatosis.16,18 IL-22 administration reduced HFD-induced elevation of serum alanine aminotransferase and aspartate aminotransferase (AST) levels, and partially inhibited HFD-induced upregulation of lipogenesis-related genes that are involved in lipid synthesis in the liver. Additionally, IL-22 treatment prevented liver injury in mouse models induced by chronic-binge ethanol feeding16 or acute ethanol challenge.17 Finally, IL-22 was also shown to promote liver cell proliferation in vitro through the activation of AKT and STAT3 signaling; this mitogenic effect was abrogated by overexpression of suppressor of cytokine signaling-1/3, which inhibited STAT3 activation.23 In a liver regeneration model, the levels of serum IL-22 protein and hepatic IL-22R1 mRNA expression were significantly increased after 70% partial hepatectomy. Blockage of IL-22 with administration of an anti-IL-22 antibody before partial hepatectomy significantly decreased hepatocytes proliferation.20 In agreement with the results from partial hepatectomy model, liver ischemia-reperfusion injury is also associated with elevation of hepatic IL-22 and IL-22R1 expression.14 Although injection of an IL-22 neutralizing antibody did not exacerbate liver ischemia-reperfusion injury, treatment of mice with recombinant IL-22 protein markedly ameliorated serum AST levels, improved cardinal histological features of ischemia-reperfusion damage (Suzuki’s score), and abrogated leukocyte sequestration.14
IL-22 is highly upregulated in several liver injury models induced by pathogens;13 however, the roles of IL-22 in the pathogenesis of infection-related liver injury have been inconclusive. Hepatoprotective effects of IL-22 were found in a primary Plasmodium chabaudi infection model,13 but IL-22 did not show any protective effects against liver lesions infected by the parasite Toxoplasma gondii and Mycobacterium avium.24 In addition, in a model of hepatitis B virus (HBV) replication in HBV transgenic mice, blocking IL-22 with a neutralizing antibody ameliorated liver damage by reducing chemokine expression on hepatocytes, and subsequently preventing hepatic recruitment of inflammatory cells, suggesting that IL-22 may contribute to the pathogenesis of HBV-mediated liver inflammation and injury.25
IL-22 promotes liver repair by promoting LPC proliferation in an STAT3-dependent manner
The liver has great regenerative ability after injury induced by hepatotoxins, infections, or loss of tissues. Under most conditions, the liver can regain its original mass through the proliferation of mature healthy hepatocytes. However, when mature hepatocytes are unable to properly proliferate to restore damaged liver during severe or chronic liver injury, LPC-mediated liver repair will be utilized to compensate liver functions.26–31 The beneficial effects of IL-22 on mature hepatocyte survival and proliferation have been well documented.10–20 Recent studies from our lab suggest that IL-22 also promotes LPC growth in patients with chronic viral hepatitis and in mice challenged by feeding a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet or a choline-deficient, ethionine-supplemented diet.21 Increased IL-22 expression was observed in patients with chronic HBV or HCV infection.19,24,32 IL-22 expression correlated with LPC proliferation in HBV patients, which implies that IL-22 may promote LPC growth in those patients.21 IL-22TG mice with IL-22 overexpression in the liver were not associated with increased LPC number under a normal chow, but showed significant higher LPC proliferation rate and total LPC number compared with wild-type (WT) mice after being fed a DDC diet.21 This suggests that IL-22 alone does not initiate LPC activation, but it can promote existing LPC proliferation in vivo in the DDC model.
The LPCs that were isolated from the DDC-fed mice expressed high levels of IL-22R1 and IL-10R2. This is not surprising because IL-22R1 is known to be expressed on epithelial cells, and LPCs are the progenitor cells for liver epithelial cells, including hepatocytes and biliary epithelial cells. In vitro treatment with IL-22 promotes proliferation of primary LPCs from the DDC-fed mice or proliferation of the LPC cell line, BMOL (bipotential mouse oval liver) cells,21 suggesting that IL-22 directly stimulates LPC proliferation.
It has previously been well documented that STAT3 activation mediates many functions of IL-22 in hepatocytes. Recently, we have provided several lines of evidence that suggest that STAT3 also plays an important role in IL-22-mediated stimulation of LPC proliferation.21 First, deletion of hepatocyte STAT3 in IL-22TG (double mutant mice with IL-22TG and liver-specific STAT3 knockout) significantly reduced the number of LPCs in the DDC-fed model. Second, administration of adenovirus IL-22 markedly increased the number of LPCs in DDC-fed, wild-type mice but not in liver-specific STAT3 knockout mice. Third, primary wild-type LPCs responded very well to IL-22-induced cell proliferation in vitro, whereas primary STAT3 knockout LPCs poorly responded to such stimulation. Taken together, IL-22 may not only stimulate mature hepatocyte proliferation but also promote liver repair even in patients with severe or chronic liver damage by targeting LPCs.
Anti-fibrotic effects of IL-22
Liver fibrosis, or scarring of the liver, is induced by various types of chronic liver diseases, and is a major cause of morbidity and mortality worldwide. Generally, following liver injury by many etiologies, HSCs undergo activation and transformation. Activation of HSCs is considered the most important event for the production of collagens in hepatic fibrosis, which is controlled by many growth factors (such as platelet-derived growth factor), cytokines (such as transforming growth factor-β), chemokines, and other factors.33 Activated HSCs produce extracellular matrix proteins, thereby leading to liver fibrosis.
Apoptosis or senescence of activated HSCs can limit the fibrogenic response to tissue damage and is an important way to control HSC activation. Many factors have been identified to induce HSC apoptosis and play an important role in inhibiting liver fibrosis. For example, γ-interferon (IFN) binds IFN-γ receptor on HSCs, and subsequently induces STAT1 activation and HSC apoptosis, thereby attenuating liver fibrosis.34 In contrast, the mechanisms by which HSC senescence is regulated remain largely unknown. Senescent HSCs are characterized by expression of β-galactosidase, induction of p53, p21, p16, and matrix-degrading enzymes, and downregulation of matrix production.35,36
Recently, our lab has demonstrated that IL-22 treatment ameliorates liver fibrosis by targeting HSCs in a murine model of CCl4-induced liver fibrosis.22 For the first time, we have demonstrated that HSCs express high levels of IL-10R2 and IL-22R1; the latter one is generally thought to be expressed exclusively in epithelial cells. Overexpression of IL-22 by either gene targeting (e.g. IL-22 transgenic mice) or exogenous administration of adenovirus expressing IL-22 reduced liver fibrogenesis and accelerated the resolution of liver fibrosis during recovery. IL-22 overexpression or treatment increased the number of senescence-associated β-galactosidase-positive HSCs. Further studies suggest that IL-22 treatment directly induces senescence in activated HSCs by activating p53-p21 pathway in a STAT3-dependent manner.22 The anti-fibrotic effect of IL-22 was also demonstrated recently in other mouse models by Dr. Kisseleva’s group.37 This group reported that deletion of IL-22 significantly exacerbated CCl4-induced liver fibrosis; whereas administration of IL-22 ameliorated the bile duct ligation-induced liver fibrosis.37 Finally, negative correlation between fibrosis stage and IL-22 expression was observed in chronic HBV-infected liver samples.38 These data suggest that IL-22 may also play an anti-fibrotic role in human liver diseases. However, further studies are required to clarify this.
Therapeutic potential of IL-22 for the treatment of ALD
ALD, which embodies a wide spectrum of disorders ranging from simple fatty liver to cirrhosis and hepatocellular carcinoma, represents a major health issue worldwide. At present, there are no treatments for ALD that are approved by the Food and Drug Administration. Abstinence and nutritional intervention have been shown to be beneficial in early stages of ALD, curing most patients with mild alcoholic liver injury but not those with severe forms of ALD, such as severe alcoholic hepatitis. Steroids have been widely used for the treatment of severe alcoholic hepatitis for over 35 years, but their benefit still remains controversial. Several studies have shown that treatment of severe alcoholic hepatitis with steroids improves the short-term (30 days) survival but has no benefit for the long-term survival.39–45 The beneficial effect of steroids in the early stage of Alcoholic Hepatitis (AH) may be related to their immunosuppressive functions, which ameliorate liver inflammation and systemic inflammatory responses. However, steroid treatment inhibits liver regeneration and does not promote liver repair in patients with ALD, which may contribute to the lack of long-term survival benefit in patients with severe alcoholic hepatitis.
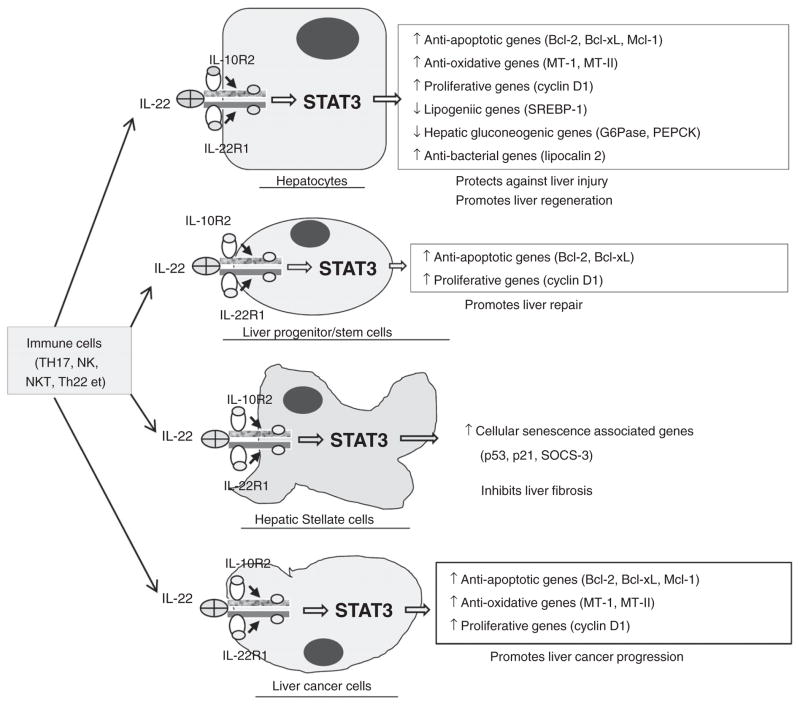
The beneficial effects of IL-22 that were elucidated from animal models of liver injury are summarized in Figure 1. IL-22 treatment ameliorated steatosis and liver damage in several models of liver injury, including chronic-binge ethanol feeding,16 acute ethanol feeding,17 and high-fat diet-induced fatty liver disease.18 Overexpression of IL-22 in vivo19 or in vitro incubation with IL-22 promoted liver regeneration or hepatocyte proliferation, respectively.10 Additionally, IL-22 treatment may potentially augment liver repair by promoting LPC proliferation and survival.21 It is reasonable to speculate that the anti-fibrotic effect of IL-22 may be also beneficial for treatment of ALD that is always associated with fibrosis.22 Hepatic expression of IL-22R1 was upregulated in patients with alcoholic hepatitis without elevation of IL-22, suggesting that those patients may be sensitive to IL-22 treatment.16 Finally, more importantly, IL-22 therapy may have minimal side effects due to the restricted expression of IL-22R1 on epithelial cells (e.g. hepatocytes and LPCs) and HSCs. It is important to note that although IL-22 itself does not initiate liver tumor development, IL-22 is able to promote existing liver cancer cell proliferation and survival.19,46 Therefore, IL-22 treatment should be safe for alcoholic hepatitis patients without liver cancer, used with caution in those with liver cirrhosis, and should not be used for those with liver cancer. Clinical trials examining IL-22 and/or steroids for the treatment of patients with severe alcoholic hepatitis are warranted.
Figure 1.
Hepatoprotective functions of IL-22 and its therapeutic potential in the treatment of alcoholic liver disease. IL-22 has many beneficial effects to promote liver repair and protect against liver injury and fibrosis by targeting hepatocytes, liver progenitor cells, and hepatic stellate cells. In addition, IL-22 itself does not initiate liver cancer development but may promote liver cancer progression. IL-22 does not target immune cells. Therefore, the therapeutic application of IL-22 in patients should be safe for the treatment of alcoholic hepatitis without liver cancer, but should be cautious for those with liver cirrhosis, and should not be used for those with liver cancer. IL, interleukin; SOCS-3, suppressor of cytokine signaling-3; STAT 3, signal transducer and activator of transcription 3.
Acknowledgments
This work was supported by the Intramural Program of NIAAA, NIH.
Footnotes
Conflict of interest
No conflict of interest has been declared by the authors.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 3.Yin M, Wheeler MD, Kono H, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 4.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–97. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27 (Suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Tachibana S, Wang H, et al. Interleukin-6 is an important mediator for mitochondrial DNA repair after alcoholic liver injury in mice. Hepatology. 2010;52:2137–47. doi: 10.1002/hep.23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–19. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 8.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–24. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 10.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 11.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–9. [PubMed] [Google Scholar]
- 12.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastelic B, do Rosario AP, Veldhoen M, et al. IL-22 protects against liver pathology and lethality of an experimental blood-stage malaria infection. Front Immunol. 2012;3:85. doi: 10.3389/fimmu.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chestovich PJ, Uchida Y, Chang W, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–92. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing WW, Zou MJ, Liu S, et al. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011;56:174–9. doi: 10.1016/j.cyto.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing WW, Zou MJ, Liu S, Xu T, Wang JX, Xu DG. Interleukin-22 protects against acute alcohol-induced hepatotoxicity in mice. Biosci Biotechnol Biochem. 2011;75:1290–4. doi: 10.1271/bbb.110061. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Zhang Y, Wang L, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339–47. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology. 2011;54:252–61. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74–80. doi: 10.1152/ajpgi.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng D, Kong X, Weng H, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–98. e7. doi: 10.1053/j.gastro.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis. Hepatology. 2012;56:1150–9. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand S, Dambacher J, Beigel F, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–28. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Cobleigh MA, Lian JQ, et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MS, Feng CG, Barber DL, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–90. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–9. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–45. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roskams T, Katoonizadeh A, Komuta M. Hepatic progenitor cells: an update. Clin Liver Dis. 2010;14:705–18. doi: 10.1016/j.cld.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Rountree CB, Mishra L, Willenbring H. Stem cells in liver diseases and cancer: recent advances on the path to new therapies. Hepatology. 2012;55:298–306. doi: 10.1002/hep.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–63. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. 2011;149:231–9. doi: 10.1093/jb/mvr001. [DOI] [PubMed] [Google Scholar]
- 32.Dambacher J, Beigel F, Zitzmann K, et al. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41:209–16. doi: 10.1016/j.cyto.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–51. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 35.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–64. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 36.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng F, Wang K, Aoyama T, et al. IL-17 signaling in inflammatory cells, Kupffer cells and Hepatic Stellate cells exacerbates liver fibrosis. Gastroenterology. 2012;143:765–76. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang X, Gui H, King NJ, et al. IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol. 2012;90:611–19. doi: 10.1038/icb.2011.79. [DOI] [PubMed] [Google Scholar]
- 39.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 40.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–8. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 42.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–7. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 43.Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:24–34. doi: 10.1038/ncpgasthep0683. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–9. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 45.Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–60. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 46.Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–9. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]