Abstract
The diuretic hormone aedeskinin‑III is known to increase the paracellular Cl- conductance in Malpighian (renal) tubules of the mosquito Aedes aegypti via a G protein-coupled receptor. The increase serves the blood-meal-initiated diuresis and is associated with elevated levels of Ca2+ and phosphorylated adducin in the cytosol of tubule. In the present study we have cloned adducin in Aedes Malpighian tubules and investigated its physiological roles. Immunolabeling experiments are consistent with the association of adducin with the cortical cytoskeleton, especially near the apical brush border of the tubule. An antibody against phosphorylated adducin revealed the transient phosphorylation of adducin 2 min after stimulating tubules with aedeskinin‑III. The PKC inhibitor bisindolylmaleimide‑I blocked the phosphorylation of adducin as well as the electrophysiological and diuretic effects of aedeskinin‑III. Bisindolylmaleimide‑I also inhibited fluid secretion in control tubules. Phorbol 12‑myristate 13‑acetate increased phosphorylated adducin levels in Malpighian tubules, but it inhibited fluid secretion. Thus, the phosphorylation of adducin by PKC alone is insufficient to trigger diuretic rates of fluid secretion; elevated levels of intracellular Ca2+ may also be required. The above results suggest that the phosphorylation of adducin, which is known to destabilize the cytoskeleton, may (1) facilitate the traffic of transporters into the apical brush border supporting diuretic rates of cation secretion and (2) destabilize proteins in the septate junction thereby enabling paracellular anion (Cl‑) secretion at diuretic rates. Moreover, PKC and the phosphorylation of adducin play a central role in control and diuretic tubules, consistent with the dynamic behavior of both transcellular and paracellular transport pathways.
Keywords: Malpighian tubule, adducin, aedeskinin, diuresis, diuretic hormone, mosquito, paracellular transport, phospho‑adducin, protein kinase C, septate junction, signaling pathway, transcellular transport
Introduction
Insect kinins are fast‑acting diuretic hormones that increase electrolyte and fluid secretion in renal (Malpighian) tubules of insects.1‑4 The kinins trigger the excretion of excess electrolytes and water which insects ingest, gorging on the blood of vertebrates or the sap of plants.5,6 In Malpighian tubules of the yellow fever mosquito Aedes aegypti, aedeskinin‑III (AK‑III) short‑circuits the epithelium with switch‑like speed by increasing the paracellular Cl‑ conductance.7,8 The increase accelerates the transepithelial secretion of KCl and NaCl and consequently water.9‑11
It is known that kinins bind to G protein-coupled receptors in the plasma membrane of principal cells and stellate cells, leading to elevated levels of Ins(1,4,5)P3 presumably via elevated phospholipase C activity.11‑16 In turn, the elevated levels of Ins(1,4,5)P3 raise intracellular Ca2+ concentrations thereby depleting intracellular Ca2+ stores. Store depletion triggers the opening of plasma membrane L‑type Ca2+ channels which is the essential step in signal transduction.17 In the absence of extracellular Ca2+, the physiological effects of kinins are markedly blunted and transient in Aedes Malpighian tubules.17 How extracellular Ca2+ entering cells brings about the increase in paracellular Cl‑ conductance remains unknown, but the switch‑like response to kinin diuretic peptides indicates a post‑translational mechanism.7,8
In an attempt to identify the proteins of the AK‑III signaling pathway in Aedes Malpighian tubules, we have analyzed the cytosolic proteome of Aedes Malpighian tubules before and after treatment with AK‑III for only 1 minute.18 In this previous study we observed prominent changes to the cytosolic abundance and phosphorylation state of proteins associated with the cytoskeleton. Of interest in the present study was the significant increase in cytosolic adducin in phosphorylated form after stimulating Malpighian tubules with AK‑III.
Adducin was first observed as a 200‑kDa protein of the spectrin cytoskeleton of red blood cells and characterized as a calmodulin-binding protein.19 It is associated with regions of cell‑cell contact in other cells.20 Adducin promotes the binding of spectrin to actin, it binds actin, and it bundles actin filaments. Binding to actin and spectrin stabilizes the spectrin cytoskeleton.21‑24 Significantly, adducin is strongly expressed along the lateral membranes of epithelial cells where it stabilizes epithelial junctions.25‑27
The goal of the present study was to clone the cDNAs encoding adducins in Malpighian tubules of Aedes aegypti and to elucidate the function of the corresponding proteins in the tubule. We identified two splice variants of the adducin gene and found adducin localized primarily to the subapical region of principal cells. Treating isolated Malpighian tubules with AK‑III caused a transient increase in the phosphorylation of the COOH‑terminal MARCKS domain of adducin in a time course that parallels the electrophysiological effects of AK‑III on Malpighian tubules. The PKC agonist, phorbol myristate acetate (PMA), increased the abundance of phosphorylated adducin (phospho‑adducin) in isolated Malpighian tubules, whereas the PKC antagonists staurosporine and bisindolylmaleimide‑I decreased the abundance of phospho‑adducin. Bisindolylmaleimide‑I also blocked the effect of AK‑III on 1) tubule electrophysiology, and 2) the stimulation of fluid secretion in isolated Malpighian tubules. Thus, PKC and adducin are key mediators of the diuresis triggered by AK‑III in Aedes Malpighian tubules.
Results
Molecular cloning of adducin transcripts
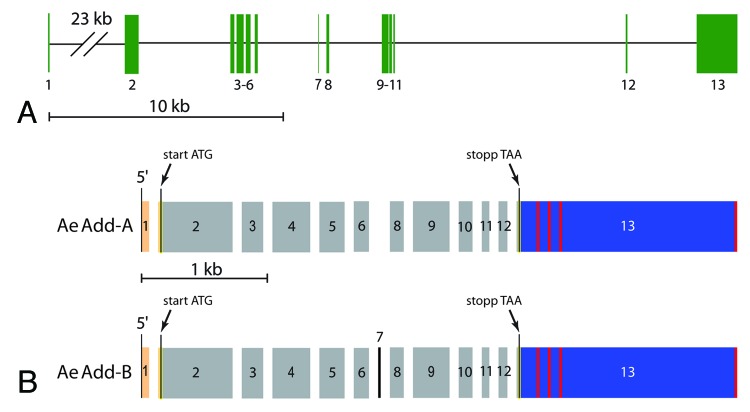
The Aedes genome contains a single gene that encodes a putative adducin, AAEL011105 (www.vectorbase.org). The gene consists of 13 predicted exons distributed along 50 kb of ‘Supercontig 1.541’ at the nucleotide positions 304004–253709 (Fig. 1A). The exact genomic position of each exon is listed in Table 2. As shown in Figure 1B, our RT‑PCR studies of Aedes Malpighian tubules detected the expression of two distinct adducin cDNAs derived from gene AAEL011105 that we designate as AeAdd‑A and AeAdd‑B. The transcripts are identical except for the presence of the 24 bp of exon 7 in AeAdd‑B (Fig. 1B). The length of the 3′‑untranslated region (UTR) in each splice variant is highly variable (red vertical bars in Fig. 1B) which may reflect the several poly‑adenylation sites within exon 13.
Figure 1. Aedes adducin gene. (A) map of Aedes adducin gene as predicted by the Aedes genome.69 Numbered, vertical green bars are exons, horizontal black lines are introns. For more detail see Table 2. (B) fractured exon map of adducin cDNAs cloned from Aedes Malpighian tubules. Numbers refer to the exons in panel A. Orange and blue bars correspond respectively to the 5′‑ and 3′‑untranslated regions which flank the open-reading frame (gray and black). Vertical red bars indicate poly‑adenylation sites. Note the presence of exon 7 in AeAdd‑B and its absence in AeAdd‑A.
Table 2. Genomic locations of the exons of the Aedes adducin gene.
| Exon | Start Position | End Position | Exon Length (base pairs) |
|---|---|---|---|
| 1 |
304004 |
303945 |
60 |
| 2 |
280723 |
280135 |
589 |
| 3 |
276606 |
276439 |
168 |
| 4 |
276376 |
276078 |
299 |
| 5 |
276020 |
275821 |
200 |
| 6 |
275745 |
275622 |
124 |
| 7 |
272652 |
272629 |
24 |
| 8 |
271850 |
271737 |
114 |
| 9 |
269803 |
269518 |
286 |
| 10 |
269439 |
269329 |
111 |
| 11 |
269266 |
269207 |
60 |
| 12 |
259176 |
259109 |
68 |
| 13 | 255447 | 253709 | 1739 |
All positions are relative to Supercontig 1.541 of the Liverpool LVP AaegL1 genomic strain of Aedes aegypti (www.vectorbase.org).
The nucleotide sequences of the adducin cDNAs cloned in our laboratory are identical to the corresponding regions of the Aedes genome with one notable exception. According to the genome, residue 276,127 (in exon 4) is ‘G’, but in 18 of our 25 sequenced RT‑PCR products this residue is ‘A’ (the other 7 products indicated ‘G’). The identity of this residue affects the coding of the 333rd amino acid of the adducin protein. A Gly333 results when the residue is ‘G’, whereas Ser333 results when the residue is ‘A’. Given our sequencing results, we presume that the majority of the adducin cDNAs encode Ser333 rather than Gly333.
Amino acid sequence of Aedes adducin
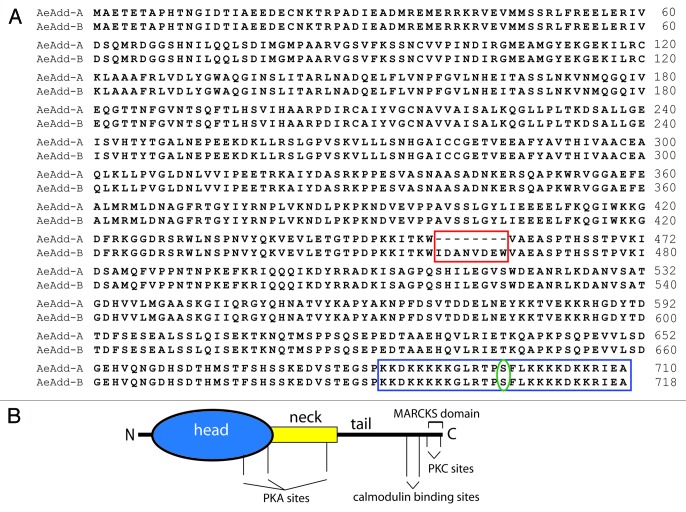
The AeAdd‑A transcript, which lacks exon 7, encodes a protein of 710 amino acids (78.7 kDa), and the AeAdd‑B transcript, which includes exon 7, encodes a protein of 718 amino acids (79.6 kDa). The 8 amino acids encoded by exon 7 are part of the so‑called ‘neck’ domain of the protein (red box, Figure 2). The Aedes adducins share with other adducins a putative MARCKS domain in the COOH‑terminus of the protein (blue box, Figure 2). The MARCKS domain includes a highly‑conserved serine (green oval, Figure 2) that is known to be phosphorylated by PKC and PKA.37‑39
Figure 2. Aedes adducin. (A) Amino‑acid sequence alignment of AeAdd‑A and AeAdd‑B. The red box highlights the 8 amino acids present in AeAdd‑B that are encoded by exon 7. The blue box highlights the C‑terminal MARCKS domain, with a serine (green oval) that is predicted to be phosphorylated. (B). Adducin protein with phosphorylation sites modeled after mammalian β‑adducin.70
Figure 3 illustrates the phylogenetic relationship between the amino‑acid sequence of AeAdd‑B and those of adducins from other organisms. In brief, AeAdd‑B is most closely related to the adducin of Drosophila (DrAdd) and is part of the larger branch that includes adducins from other invertebrates (Caenorhabditis elegans, CaAdd; Schistosoma mansoni, ScAdd). The adducins of humans (HoAdd) cluster in an independent branch of the tree.
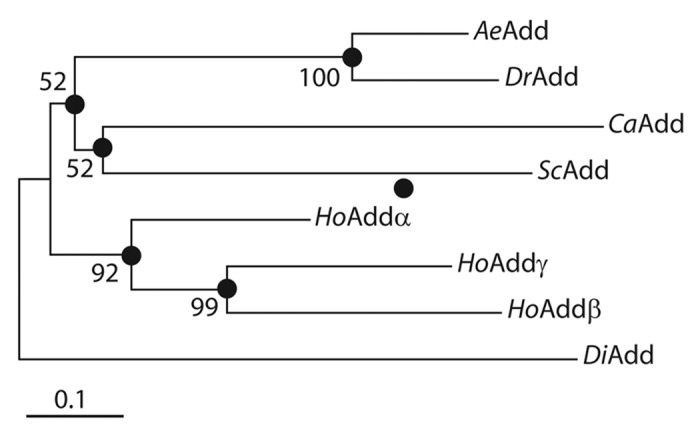
Figure 3. Neighbor‑joining tree of adducin amino‑acid sequences from Aedes aegypti (Ae), Drosophila melanogaster (Dr), Caenorhabditis elegans (Ca), Schistosoma mansoni (Sc), and Homo sapiens (Ho). The tree is rooted to an adducin‑like protein of Dictyostelium discoideum (Di). Filled circles indicate the nodes of branches for which bootstrap scores are provided (from 1000 replicates). The total branch length between two proteins represents the proportion of amino acids that differ between them. The scale bar corresponds to a proportional difference (branch length) of 0.1 (i.e., a 10% difference in amino acids). The tree was constructed with MEGA 4 software71 using Poisson‑corrected distance estimates. Accession numbers are as follows: AeAdd, F705874; DrAdd, NP_001188977; CaAdd, AAD49856; ScAdd, XP_002578303; HoAdd α, NP_001110; HoAdd β, NP_001608; HoAdd γ, NP_058432; DiAdd, XP_640404.
Adducin expression and localization in the Malpighian tubules
Immunoblots were performed to characterize the expression of adducin protein in Malpighian tubules of adult female Aedes mosquitoes. For the sake of comparison we also examined the expression of adducin immunoreactivity in the midgut. Crude lysates of both tissues yield a protein band of ~100 kDa that exhibits adducin immunoreactivity (Fig. 4). Although the band is larger than the expected size of adducin (~79 kDa) based on the cloned cDNAs, it is known that adducins run slightly higher than expected on SDS‑PAGE because of the highly‑charged COOH‑terminal MARCKS domain.29,40
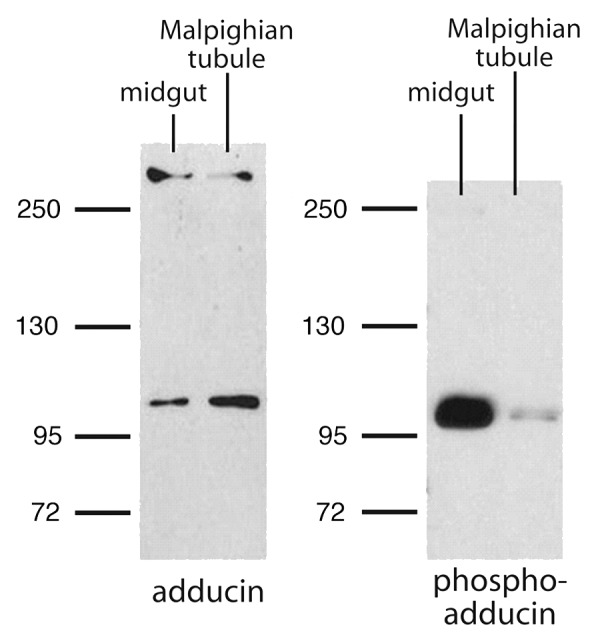
Figure 4. Representative western blots of the immunoreactivity to adducin and phospho‑adducin in lysates of Aedes midgut and Malpighian tubule. Numbers correspond to molecular weight markers in kDa.
A protein band larger than 250 kDa also exhibited adducin immunoreactivity in the Malpighian tubules and midgut (Fig. 4). It is likely that this band represents non‑denatured adducin in complex with other cytoskeletal elements, such as spectrin, which is a protein of ~280 kDa in Aedes aegypti (www.vectorbase.org). Significantly, the antibody against phospho‑adducin detected only the ~100 kDa band of adducin (Fig. 4).
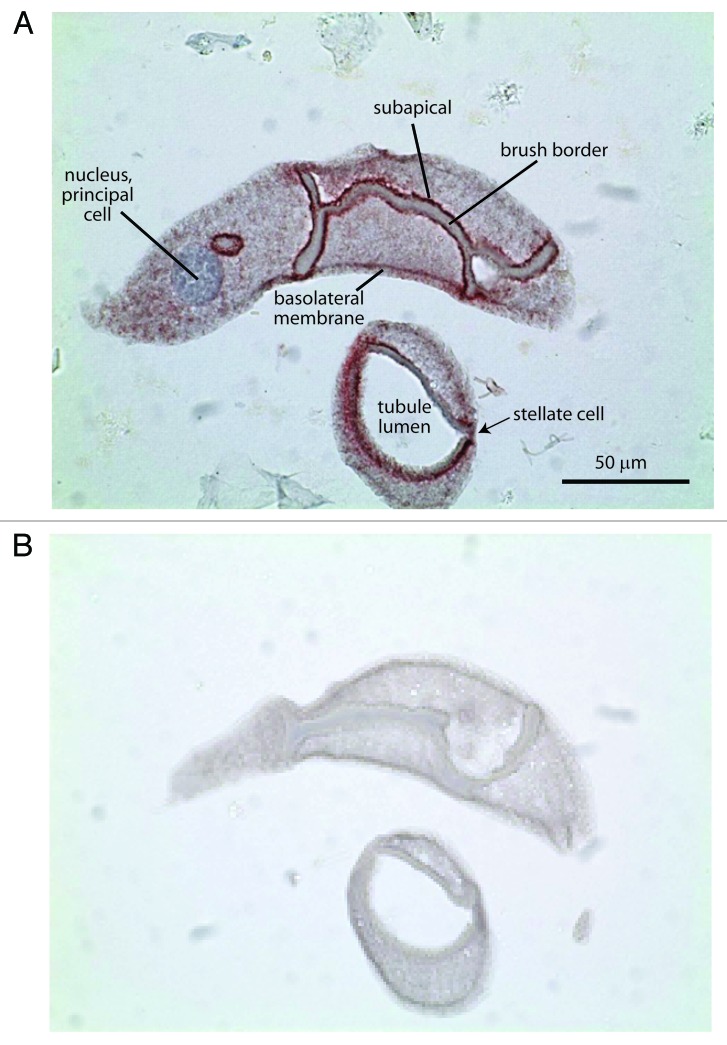
Immunolabeling of sections of paraffin‑embedded Aedes Malpighian tubules revealed strong adducin immunoreactivity along the base of the brush border in principal cells (Fig. 5). Weak adducin immunoreactivity was observed near the basal membrane of principal cells consistent with the presence of adducin in the cortical cytoskeleton. Immunoreactivity was diffuse in the cytoplasm of principal cells. Immunolabeling of stellate cells was also observed, but a precise localization was not possible in view of the small size of these cells.
Figure 5. Representative immunolocalization of adducin in consecutive sections of a Malpighian tubule of Aedes aegypti. (A) Adducin immunoreactivity is indicated by the red staining. Nuclei are stained blue by hematoxylin. (B) Negative control. Tubules undergoing the same adducin staining procedure but without antibody served as negative control.
Time-dependent changes in phospho-adducin
In a previous proteomic study we observed that adducin appears in the cytosol of Malpighian tubules in phosphorylated form after treatment with the diuretic peptide AK‑III for only 2 min.18 It was therefore of interest to examine the time course of the adducin phosphorylation in western blots of tubule lysates. As shown in the representative western blot of Figure 6A, the strongest immunoreactivity to phospho‑adducin was observed 2 min after adding AK‑III (10−7 M) to the peritubular medium of Malpighian tubules. Thereafter, the immunoreactivity to phospho‑adducin progressively diminished in spite of the presence of AK‑III. Notably, the immunoreactivity to total adducin did not change significantly with time (Fig. 6A).
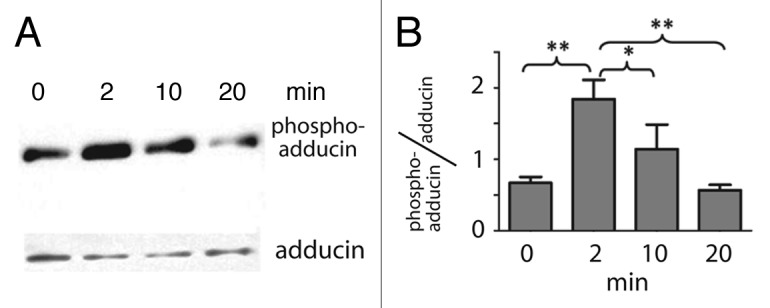
Figure 6. Transient phosphorylation of adducin in the presence of the diuretic peptide AK‑III (10−7 M) in isolated Malpighian tubules of Aedes aegypti. (A) Representative western blots of phospho‑adducin and adducin in Malpighian tubules that were incubated without (time 0) or with AK‑III for the times indicated. (B) Phospho‑adducin immunoreactivity standardized to total adducin immunoreactivity determined in 4 independent western blots. Values are mean ± SE; ** = p < 0.01, * = p < 0.05, one way ANOVA with a Newman‑Keuls post‑test.
Figure 6B summarizes the effects of AK‑III on the immunoreactivity of phospho‑adducin normalized to that of total adducin in 4 separate trials. Again, after adding AK‑III to the peritubular bath of tubules, phospho‑adducin peaked 2 min. After a 10 min exposure to AK‑III, phospho‑adducin levels are not significantly different from control tubules and after a 20 min exposure to AK‑III, phospho‑adducin levels have clearly returned to control levels. Thus, the AK‑III‑mediated phosphorylation of adducin is transient.
The phosphorylation of Aedes adducin by protein kinase C
The signal transduction of AK‑III includes the essential role of Ca2+ as second messenger.11,17,41 For this reason we explored the role of protein kinase C (PKC) in the phosphorylation of adducin. Furthermore, PKC is known to phosphorylate Ser726 in the MARCKS domain of human α adducin,22,38,39 and the homologous Ser is present in Aedes adducin (Fig. 2). Isolated Malpighian tubules were treated with known stimulators and inhibitors of PKC. Lysates of the tubules were then examined for phospho‑adducin immunoreactivity in western blots. Equal protein loading was verified in this series of experiments with an antibody to β‑tubulin (E7) because we had exhausted our supply of the adducin antibody.
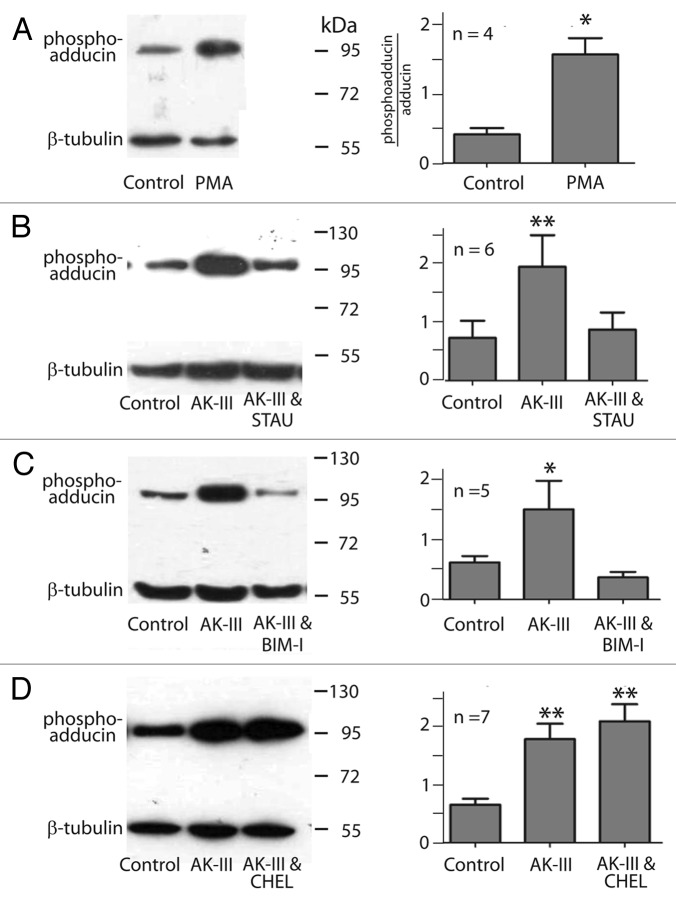
Phorbol myristate acetate (PMA) is an activator of PKC.42,43 Isolated Malpighian tubules were treated with 10−6 M PMA for 20 min to allow sufficient time for the entry of PMA into the cells of the tubule. As shown in Figure 7A, we consistently observed higher levels of phospho‑adducin after treating tubules with PMA. Standardized to β‑tubulin, the increase in phospho‑adducin immunoreactivity is statistically significant (p < 0.05, Figure 7A).
Figure 7. Effects of kinase activators and inhibitors on the phosphorylation of Aedes adducin. Left column, representative western blots; right column, data summary of n (number) of western blots. (A) Effect of phorbol myristate acetate (PMA,10−6 M) in 0.05% DMSO. Phospho‑adducin immunoreactivity is standardized to β‑tubulin. (B) Effects of aedeskinin‑III (AK‑III, 10−7 M) in the absence and presence of staurosporine (STAU, 10−7 M). Tubules were pre‑incubated with STAU in 0.1% DMSO for 20 min before AK‑III was applied for 2 min. (C) Effects of AK‑III (10−7 M) in the absence and presence of bisindolylmaleimide‑I (BIM‑I, 10−5 M). Tubules were pre‑incubated with BIM‑I in 0.1% DMSO for 20 min before AK‑III was applied for 2 min. (D) Effects of AK‑III (10−7 M) in the absence and presence of chelerythrine (CHEL, 5 x 10−6 M). Tubules were pre‑incubated with CHEL in 0.1% DMSO for 20 min before AK‑III was applied (2 min). Values are mean ± SE; analysis was performed using one‑way ANOVA’s with a Newman‑Keuls post tests. (* = p < 0.05, ** = p < 0.01).
Next we investigated the effects of PKC inhibitors on the phosphorylation of adducin. One group of Malpighian tubules served as the control group, a second group was treated with AK‑III (10−7 M) for 2 min, and the third group of tubules was pre‑incubated with the PKC inhibitor of interest for 20 min before they were treated with AK‑III. The treatment of Malpighian tubules with AK‑III for 2 min significantly increased the phospho‑adducin immunoreactivity in all tubules studied (Fig. 7B–D). However, when the tubules were first incubated with staurosporine (10−7 M) or bisindolylmaleimide‑I (BIM‑1, 10−5 M) for 20 min, treatment with AK‑III failed to stimulate the phosphorylation of adducin (Fig. Seven B,C). In contrast, the pre‑incubation of Malpighian tubules with chelerythrine (5 x 10−6 M) did not inhibit the AK‑III‑mediated phosphorylation of adducin (Fig. 7D).
Physiological studies in intact Malpighian tubules
To evaluate the effects of PKC activators and inhibitors on the physiological performance of Malpighian tubules we conducted two‑electrode voltage clamping experiments and fluid secretion assays in isolated Malpighian tubules. The effect of the PKC activator phorbol myristate acetate (PMA) was of interest first. In the typical experiment a Malpighian tubule was bathed in Ringer solution, and a principal cell of the tubule was impaled with voltage and current electrodes for the measurement of the basolateral membrane voltage and the input resistance of the cell. Before applying PMA, the tubule was pre‑pulsed with AK‑III to determine its responsiveness to kinin stimulation.9
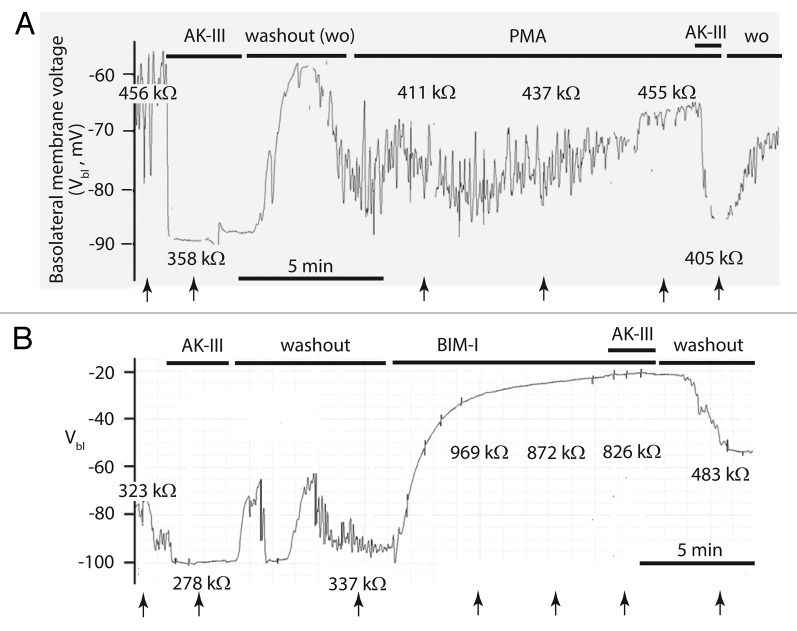
As shown in the representative experiment of Figure 8A, the addition of AK‑III (10−7 M) to the peritubular bath immediately hyperpolarized the basolateral membrane voltage (Vbl) from an oscillating voltage in the vicinity of ‑60 mV to a stable voltage of ‑88 mV. In parallel, the input resistance of the principal cell (Rin) dropped from 456 kΩ to 358 kΩ. The sudden hyperpolarization of Vbl together with the decrease of Rin reflect the well‑known post‑translational diuretic effects of AK‑III in Aedes Malpighian tubules which include 1) the increase in the paracellular Cl‑ conductance, and 2) the activation of Ca2+ channels in the basolateral membrane of principal cells.8,17 The washout of AK‑III returned the tubule to oscillating membrane voltages and previous cell input resistances. The subsequent addition of PMA (10−6M) to the peritubular medium caused a slow depolarization of Vbl together with an increase in Rin (Fig. 8A). In 13 tubule experiments, Vbl significantly (p < 0.02) depolarized from ‑69.5 ± 1.8 mV to ‑59.3 ± 2.4 mV after an average of 10 min in the presence of 10−6 M PMA. At the same time the cell input resistance Rin significantly increased from 439.7 ± 18.4 kΩ to 557.9 ± 19.3 kΩ (p < 0.05). The gradual change of Vbl and Rin suggest the gradual decline in the rate of transepithelial ion secretion. Nevertheless, the tubule still responded to AK‑III in the presence of PMA. After a more than 20 min exposure to PMA, the addition of AK‑III reversibly lead to the prompt hyperpolarization of Vbl from ‑65 mV to ‑86 mV in parallel with the reduction of Rin from 455 k to 405 k (Fig. 8A). Thus, the gradual electrophysiological changes in the presence of PMA did not preclude the usual electrophysiological response of the tubule to AK‑III.
Figure 8. Representative effects of protein kinase C (PKC) activator and inhibitor on the basolateral membrane voltage (Vbl) and the input resistance (Rin, kΩ) of a principal cell in isolated Malpighian tubules of Aedes aegypti. The tubules were prepulsed with aedeskinin‑III (AK‑III, 10−6 M) to ascertain an active signaling pathway. (A) The PKC activator phorbol myristate acetate (PMA, 10−6M) had minor effects on Vbl and Rin. In the presence of PMA the tubule still responded to AK‑III. (B) The PKC inhibitor bisindolylmaleimide‑I (BIM‑I, 10−5 M) significantly depolarized Vbl and increased Rin. In the presence of BIM‑I the tubule did not respond to AK‑III. PMA and BIM‑I were dissolved in DMSO (final bath concentration of 0.1%). Arrows indicate the times Rin was determined.
In 13 Malpighian tubules studied by the method of Ramsay, the addition of PMA (10−6 M) to the peritubular Ringer bath significantly (p < 0.02) reduced the rate of fluid secretion from 0.92 ± 0.06 nl/min to 0.33 ± 0.03 nl/min (Fig. 9A).
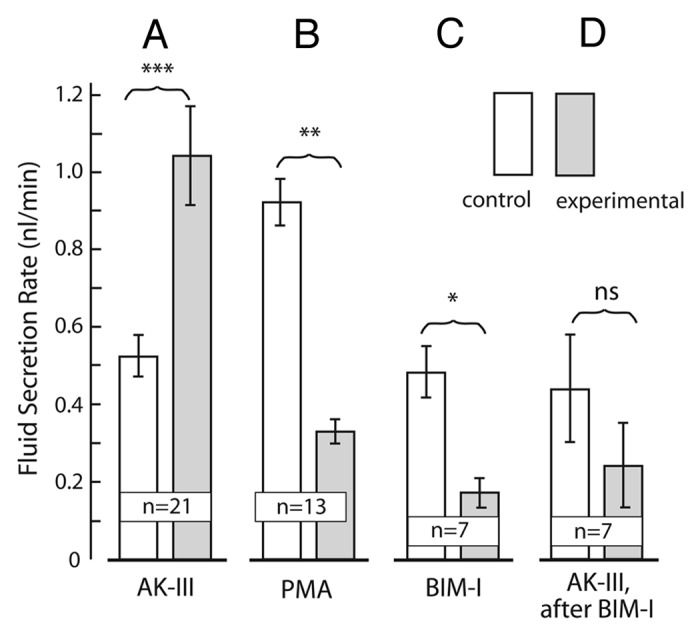
Figure 9. The effects activators and inhibitors of protein kinase C (PKC) on the rate of transepithelial fluid secretion in isolated Malpighian tubules of Aedes aegypti. In view of the variability in spontaneous (control) rates of fluid secretion in Malpighian tubules from different hatches of mosquitoes, each tubule was used as its own control. Accordingly, the paired student t‑test was used to evaluate the significance of the difference between the control and experimental periods. (A) Aedeskinin‑III (AK = III, 10−6 M) significantly increases the rate of fluid secretion. Data are from Schepel et al.72 (B) The PKC activator phorbol myristate acetate (PMA, 10−6 M) significantly reduced transepithelial fluid secretion. (C) The PKC inhibitor bisindolylmaleimide‑I (BIM‑I, 10−5 M) significantly reduced transepithelial fluid secretion. (D) The PKC inhibitor BIM‑I blocked the diuretic effects of aedeskinin‑III (AK‑III). Data are mean ± SE; (n = number of Malpighian tubules tubules); *, p < 0.05; **, p < 0.02; ***, p < 0.001; ns, not significant.
The effects of the PKC inhibitor bisindolylmaleimide‑I was of interest next. Figure 8B illustrates a typical experiment that shows the electrophysiological effects of BIM‑I. Again, it was important to first confirm that the tubule responded to the diuretic peptide AK‑III. The addition of AK‑III (10−6 M) to the peritubular bath hyperpolarized Vbl from ‑80 mV to ‑100 mV in parallel with the drop of Rin from 323 kΩ to 278 kΩ (Fig. 8B). The hyperpolarization of Vbl together with the reduction of Rin is consistently observed when tubules do respond to aedeskinins. In a previous study, AK‑III significantly (p < 0.001) hyperpolarized Vbl from ‑64.3 mV to ‑87.8 mV in parallel with the significant (p < 0.01) reduction of Rin from 343.3 kΩ to 265.2 kΩ.9 The washout of AK‑III recovered pre‑stimulation values (Fig. 8B). Subsequently, the addition of BIM‑I (10−5 M) to the peritubular bath triggered the gradual decay of Vbl together with a large increase of Rin. By the time Vbl had decayed to -23 mV, Rin had increased to 872 kΩ (Fig. 8B). In the presence of BIM‑I, AK‑III (10−6 M) had no significant effect on Vbl and Rin (Fig. 8B). Thus, BIM‑I blocks the electrophysiological effects of AK‑III as it blocks the phosphorylation of adducin (Fig. 7). The washout of both AK‑III and BIM‑I repolarized Vbl to -56 mV and decreased Rin to 483 kΩ. In 9 tubules experiments, BIM‑I significantly (p < 0.01) depolarized Vbl from–63.6 ± 6.0 mV to ‑42.7 ± 4.6 mV while significantly (p < 0.01) increasing Rin from 418.1 ± 53.1 kΩ to 739.0 ± 118.9 kΩ. In the presence of BIM‑I, the effects of AK‑III on Vbl and Rin were consistently blocked.
BIM‑I also reduced rates of fluid secretion in isolated Malpighian tubules. In 7 tubule experiments, the addition of BIM‑I (10−5 M) to the peritubular bath of unstimulated, control Malpighian tubules significantly (p < 0.05) reduced the spontaneous rate of fluid secretion from 0.48 ± 0.07 nl/min to 0.17 ± 0.04 nl/min. (Fig. 9B). Tubules pre‑treated with BIM‑I for 30 min failed to increase the rate of fluid secretion after the addition of AK‑III to the peritubular bath (Fig. 9C). Instead, the average rate of fluid secretion tended to decrease upon the addition of AK‑III from 0.44 ± 0.14 nl/min to 0.24 ± 0.11 nl/min in the presence of BIM‑I, though not significantly. The usual response of tubules to AK‑III is the significant (p < 0.001) increase of the fluid secretion rate from, for example, 0.52 ± 0.06 nl/min to 1.04 ± 0.13 nl/min in 21 tubules (Schepel et al. 2010). Thus, the preincubation with BIM‑I blocked the diuretic effects of AK‑III.
Discussion
Adducin in Malpighian tubules of Aedes aegypti
We have cloned two alternatively‑spliced variants of adducin cDNAs from Aedes Malpighian tubules that differ by the presence or absence of exon 7 (Fig. 1B). The 3′‑untranslated region (UTR) of these cDNAs, which is encoded by exon 13, is characterized by variable lengths. Different lengths of the 3′‑UTR region in the adducin of Drosophila ovaries are thought to influence the trafficking of adducin transcripts within the cell according to specific localization signals found in the UTR sequence.44 Thus, differences in the 3′‑UTR length of AeAdd transcripts in Malpighian tubules may direct the post‑transcriptional trafficking of adducin mRNA.
As shown in Figure 2B, adducin proteins form three broad structural/functional regions: a globular NH2‑terminal head domain, a neck domain, and a COOH‑terminal tail.22,40 The NH2‑terminal head region of Aedes adducin contains an aldolase II superfamily domain similar to human and Drosophila adducin. The head region has been implicated in the interaction of adducin with clathrin coated vesicles through a motif that is present in AeAdd‑A and AeAdd‑B (Fig. 2). Thus, Aedes adducin may be involved in endocytosis.45,46
The neck region in mammalian adducin is necessary for recruiting spectrin and actin22 and for forming hetero‑oligomers of adducin isoforms.21 Exon 7 in AeAdd‑B encodes the 8 amino‑acid residues ‘WIDANVDE’ in the neck domain of the mosquito protein. Thus, the alternative splicing of exon 7 may result in adducins with differential abilities to recruit spectrin and actin and/or to form oligomers.
The COOH‑terminal tail of adducin is thought to directly interact with actin and spectrin thereby regulating the assembly of the spectrin/actin cytoskeleton. The tail includes the putative calmodulin binding site as well as the MARCKS domain (Fig. 2). The latter is the target of protein kinase C (PKC) and protein kinase A (PKA) for regulating the activity of adducin.37‑39 The MARCKS domain is well conserved among Aedes, Drosophila and Homo and includes the serine residue (red box and red highlighted Ser in Figure 2). The phosphorylation of the MARCKS domain causes adducin to dissociate from spectrin and actin, promoting the disassembly of the spectrin cytoskeleton. As a result, proteins of tight and adherens junctions may change conformation, position, or be internalized.38,39,47,48
Immunolocalization of adducin in Aedes Malpighian tubules
In histological sections of Aedes Malpighian tubules, adducin immunoreactivity is observed in both principal and stellate cells (Fig. 5). Notably, prominent immunolabeling occurs along the base of the apical brush border of principal cells, which is strikingly similar to the localization of actin and β spectrin in Aedes Malpighian tubules.49 The presence of these three proteins at the apical base of principal cells indicates an actin/spectrin cytoskeleton that may serve the structure and function of the tall brush border extending into the lumen of the tubule.3 Since adducin caps F‑actin and recruits spectrin to actin,22 adducin may stabilize actin filaments extending into the microvilli of principal cells (Fig. 4). Moreover, the rapid increase in phospho‑adducin immunoreactivity after stimulation with the diuretic peptide AK‑III (Fig. 6) may reflect the destabilization of the apical cytoskeleton, thereby allowing traffic into and out of microvilli.46,50,51 Bradley and Satir have observed in Rhodnius Malpighian tubules that mitochondria move from a position below the cell cortex to one inside the microvilli within 10 min after stimulating tubules with the diuretic hormone serotonin.52,53 The progressive loss of phospho‑adducin 10 min after AK‑III‑stimulation (Fig. 6) suggests similar cytoskeletal dynamics in Aedes Malpighian tubules and reflect the re‑assembly of the cytoskeleton after solute transporters and/or mitochondria have moved into the brush border of Aedes Malpighian tubules.
Physiology of adducin
The present study was prompted by our previous proteomic study that indicated modifications to the cytoskeleton as one mechanism for regulating the rate of electrolyte and fluid secretion in Malpighian tubules.18 The cytoskeletal protein adducin, actin and actin depolymerizing factor appeared in the cytosol of Malpighian tubules at elevated levels after stimulating Malpighian tubules with aedeskinin‑III for only 1 min. A role of protein kinase C was further implicated by the requirement of Ca2+ for aedeskinin signaling.3,17 Among the first steps in kinin signaling is the activation of Ca2+ channels in the basolateral membrane of principal cells.17,41
One function of Ca2+ is the activation of protein kinase C, as documented in electrophysiological studies and in measurements of fluid secretion (Figs. 8,9). Malpighian tubules respond to kinin diuretic peptides (aedeskinins and leucokinins) with the hyperpolarization of the basolateral membrane voltage of principal cells. The hyperpolarization reflects the short circuit of the transepithelial voltage as kinins trigger the sudden increase of the paracellular Cl‑ conductance.8,9,17,54 In parallel, the cell input resistance decreases due to the activation of basolateral membrane Ca2+ channels (Fig. 8). These electrophysiological effects are absent when tubules have first been treated with the PKC inhibitor bisindolylmaleimide‑I (Fig. 8B). Accordingly, the inhibition of PKC prevents aedeskinin‑III from increasing the paracellular Cl‑ conductance, thereby blocking the key ionic event of the diuresis triggered by kinin diuretic peptides. Studies of fluid secretion confirm this conclusion. Tubules normally respond to aedeskinin or leucokinin by immediately doubling the rate of fluid secretion,55,56 but bisindolylmaleimide‑I prevents the aedeskinin-mediated increase in fluid secretion (Fig. 9C). These findings implicate PKC as playing an integral role in the increase of the paracellular secretion of Cl‑ that leads to a corresponding increase in the transepithelial secretion of cations and water.
The pharmacology of the adducin phosphorylation uncovered in the present study illuminates on the type of PKC that is activated by aedeskinin‑III. The PKC antagonists staurosporine and bisindolylmaleimide‑I block the AK‑III induced phosphorylation of adducin, but chelerythrine does not (Fig. 7). Chelerythrine is an inhibitor of the conventional α and β isoforms of PKC by binding to the catalytic domain of kinases.57 In contrast, bisindolylmaleimide‑I, the most selective inhibitor of conventional α, β, and γ PKC’s and staurosporine are structurally similar and block the ATP binding pocket of PKC’s.58‑61 Thus, the PKC that phosphorylates adducin in Aedes Malpighian tubules may be a variant of the γPKC.
Our use of an antibody specific to the COOH‑terminal MARCKS domain of adducin proves that aedeskinin brings about the phosphorylation of this domain (Figs. 6,7). The MARCKS domain of adducin is required for protein‑protein interactions with actin and spectrin.48,62 Adducin binds to the barbed ends and to the sides of actin filaments thereby enhancing the association of spectrin with actin filaments that stabilizes the spectrin‑actin meshwork.62 Upon phosphorylation of the MARCKS domain, phospho‑adducin dissociates from spectrin and actin, thereby destabilizing the cytoskeleton. In the present study, the phosphorylation of the MARCKS domain took place within 2 min of AK‑III stimulation, confirming the sudden rise in cytosolic phospho‑adducin we have observed in our proteomic study of Aedes Malpighian tubules stimulated with AK‑III for only 1 min.18 Levels of cytosolic phospho‑adducin start to return to control levels two min after stimulation with AK‑III (Fig. 6). The time‑dependent changes in the phosphorylation of adducin are consistent with 1) the known switch‑like on/off effects of leucokinin and aedeskinin on the paracellular shunt resistance of mosquito Malpighian tubules,7,8 2) the transient actin reorganization of the subapical cytoskeleton in Aedes Malpighian tubules after a blood meal,49 and 3) the switch‑like behavior of G protein‑coupled receptors.12
How the adducin‑controlled cytoskeleton may affect the switch‑like increase of the paracellular Cl‑ conductance following treatment with the diuretic hormone AK‑III is explored below.7,8 Importantly, adducin is found concentrated at sites of cell‑cell contact along the lateral membranes of intestinal epithelial cells.25 Here, adducin is a key player in remodeling the tight junction in Ca2+‑ and phosphorylation‑dependent ways.25,63 Phorbol ester causes adducin to leave epithelial contact sites in keratinocytes and MDCK cells, consistent with the phosphorylation of adducin by PKC and the destabilization of junctional complexes along the paracellular pathway.25 Similarly, the phosphorylation of adducin in Aedes Malpighian tubules may destabilize the cytoskeleton along the septate junction, thereby increasing the electrical conductance of the paracellular pathway.8,13,41
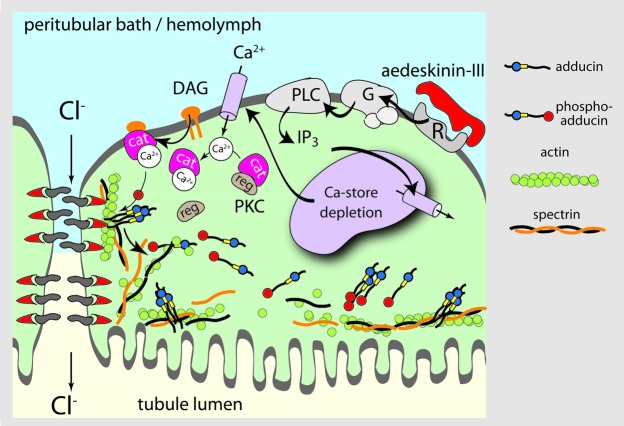
Taken together, the present study illuminates the post‑calcium steps of aedeskinin signaling (Fig. 10). The binding of AK‑III to its G protein-coupled receptor triggers the formation of diacyl glycerol (DAG) and the entry of Ca2+ into the cell. Calcium binding to PKC frees the catalytic domain of PKC which acquires the property of binding to DAG. The activation of PKC by DAG leads to the phosphorylation of proteins nearby, among them adducin. The phosphorylation of adducin for only a few minutes likely allows changes in the cytoskeleton not only at the base of the brush border, but also at the basal and lateral membranes of epithelial cells. The cytoskeletal dynamics along the brush border may fortify the transport activities of the brush border. The cytoskeletal changes at the basolateral membrane may regulate the trafficking of Ca2+ channels and other cation uptake mechanisms, thereby providing additional substrates for the enhanced transcellular secretion of cations. The cytoskeletal changes along the septate junction may modify the junctional complex extending into the paracellular space with the effect of increasing the paracellular secretion of Cl‑.
Figure 10. Hypothetical model of aedeskinin signaling. Aedeskinin‑III (AK‑III) binds to the kinin GPCR located on the basolateral membrane of principal and/or stellate cells. GTP‑α is released and activates phospholipase C (PLC) to produce inositol‑triphosphate (Ins(1,4,5)P3) and diacyl‑glycerol (DAG). Ins(1,4,5)P3 causes the release of intracellular calcium stores. Store depletion triggers the opening of Ca2+ channels in the basolateral membrane.73 Elevated intracellular Ca2+ levels target cytosolic protein kinase C (PKC) to the cell membrane where binding to DAG activates the kinase. PKC phosphorylates adducin causing its dissociation from the actin/spectrin cytoskeleton along the paracellular pathway and the apical membrane. The destabilization of the cytoskeleton along the paracellular pathway may destabilize transmembrane proteins extending into the paracellular space thereby increasing the paracellular electrical conductance. Along the apical membrane, the destabilization of the cytoskeleton may allow the traffic of transporters and cell organelles into and out of the brush border.
Role of PKC in spontaneous, basal fluid secretion
After isolation from the mosquito, Malpighian tubules bathed in Ringer solution secrete fluid for hours without stimulation by extracellular agents. Nevertheless, PKC participates in the mechanism for sustaining control, basal fluid secretion rates. In control tubules, the PKC inhibitor BIM‑I depolarizes the basolateral membrane voltage of principal cells and increases the cell input resistance (Fig. 8B) consistent with the reduction in electrogenic transepithelial ion secretion (Fig. 8B). In parallel, rates of transepithelial fluid secretion decrease (Fig. 9B). The decrease suggests that as much as 65% of the spontaneous fluid secretion rate is dependent on the activity of PKC.
The spontaneous activity of PKC may also account for the spontaneous oscillations of voltage and resistance observed in control, unstimulated tubules.54 The oscillations are dependent on transepithelial Cl‑ gradients, and they reflect spontaneous fluctuations in the paracellular Cl‑ conductance.54,64 The frequency of these oscillations resembles the frequencies of changes in intracellular free Ca2+ that stem from Ca2+ release and reuptake mechanisms at intracellular Ca2+ stores. Moreover, BIM‑I eliminates the oscillations (Fig. 8B), indicating the role of PKC in mediating the spontaneous changes of the paracellular Cl‑ conductance. Supporting this conclusion, we have observed the Ca2+ dependence of spontaneous oscillations.17 Increasing the extracellular Ca2+ concentration progressively reduces the frequency and amplitude of oscillations by increasing the duration of high paracellular Cl‑ conductance.11,17 Moreover, the addition of the Ca2+ ionophore A‑23187 to the peritubular Ringer of Malpighian tubules eliminates oscillations altogether by inducing a high, steady‑state paracellular Cl‑ conductance similar to that induced by kinin diuretic peptides.65 In view of the known activation of PKC by Ca2+, it is tempting to conclude that PKC mediates the spontaneous oscillations of the paracellular Cl‑ conductances via the phosphorylation of adducin and other proteins.
Treating Malpighian tubules with the PKC agonist, PMA, results in the phosphorylation of adducin (Fig. 5). PMA is a phorbol ester that mimics the role of DAG in the activation of conventional PKCs.42,43,66 Although PMA activates PKC and phosphorylate adducin in intact tubules, this effect is insufficient to trigger diuresis. Moreover, PMA brings about the inhibition of fluid secretion, and it depolarizes the basolateral membrane voltage (Figs. 8A and 9). In parallel, the magnitude of the spontaneous voltage oscillations decreases and the cell input resistance increases. These effects indicate the inhibition of transepithelial electrolyte and fluid secretion beyond the phosphorylation of Aedes adducin. PMA is reported to withdraw the Na/K/2Cl cotransporter from the basolateral membrane of principal cells as in T84 cells67 which in Aedes Malpighian tubules is expected to inhibit fluid secretion.36 PMA is also reported to target RasGRP and Munc‑13 which may bring about the inhibition of fluid secretion in the intact tubule independently of PKC.68
In the presence of PMA, the tubules still respond to stimulation by aedeskinin with the usual marked hyperpolarization of the basolateral membrane voltage together with the reduction in cell input resistance (Fig. 8A). These responses present the electrophysiological signature of the kinin diuresis that includes signaling by Ca2+. Accordingly, the full‑blown diuresis triggered by kinin diuretic peptides requires not only PKC and the phosphorylation of adducin, but also additional post‑translational effects of Ca2+ that remain to be elucidated.
Materials and Methods
Mosquito rearing and isolation of Malpighian tubules
The mosquito colony (Aedes aegypti) was maintained as described previously.8 Malpighian tubules and midgut were isolated from female mosquitoes and transferred to freshly prepared Ringer solution containing in mM: NaCl 150, KCl 3.4, CaCl2 1.7, NaHCO3 1.8, MgSO4 1, glucose 5, and HEPES 25. The pH of the solution was adjusted to 7.1. For molecular studies, the tubules and midgut were immediately frozen in liquid nitrogen after experimental treatments and stored at ‑80°C.
Molecular cloning
Malpighian tubule cDNA was generated using a Generacer kit (Invitrogen, Carlsbad, CA) as described previously.28 In brief, 150 Malpighian tubules were isolated from 30 adult female Aedes mosquitoes and placed in a solution of ice‑cold Trizol reagent (Invitrogen). The total RNA was extracted and used as a template to create two independent pools of single‑stranded cDNA for the 5′‑ and 3′‑rapid amplification of cDNA ends (RACE). We refer to these pools, respectively, as the 5′‑cDNA and 3′‑cDNA.
The Aedes aegypti genome (www.vectorbase.org) was referenced to design the primers ‘Ad1‑F’ and ‘Ad‑3R’ (Table 1) which bind to regions of the predicted open‑reading frame (ORF) of a putative adducin gene (accession number AAEL011105). The 5′‑RACE was conducted on 0.5 μl of 5′‑cDNA using the following: Generacer 5′‑primer (Invitrogen, Carlsbad, CA, USA), reverse primer Ad‑3R (Table 1), and Platinum PCR Supermix HF (Invitrogen). The 3′ RACE was conducted on 0.5 μl of 3′‑cDNA using the following: Generacer 3′‑primer (Invitrogen), forward primer Ad‑1F (Table 1) and Platinum PCR Supermix HF (Invitrogen). For both the 5′‑RACE and 3′‑RACE, the total reaction volume was 25 μl, and a touchdown thermocycling protocol was employed as described in the Generacer kit (Invitrogen). All RACE products were run on a 1% agarose gel supplemented with ethidium bromide and visualized with a UV transilluminator.
Table 1. Primers used in the cloning of adducin cDNAs.
| Primer | Use | Primer Sequence | Genomic position |
|---|---|---|---|
| Ad3R |
5′ RACE |
5′ CCG GCG TCG AAG AAT GTG TTG GCG AAG CTT 3′ |
271811271840 |
| Ad1F |
3′ RACE |
5′ CCG GCA CAC CAG ACC CGA AGA AAA T 3′ |
275653275629 |
| AdFL_1F |
Full Length |
5′ GTA GTT GAC GCC GCC GTG AAA AAA CGT TGA 3′ |
303973303944 |
| AdFL_1R | Full Length | 5′ ATC GTC GCT GAG TGC TGT TCA TGT TGT GAT 3′ | 255310255339 |
The genomic positions that the primers anneal to are indicated and are relative to “Supercontig 1.541” of the Aedes aegypti genome (www.vectorbase.org). All primers were synthesized by Integrated DNA Technologies.
The RACE products were ligated into TOPO TA cloning plasmids (Invitrogen) and transformed into TOP10 One Shot E. coli (Invitrogen) following the manufacturer’s protocol. Plasmid DNA was isolated using QIA Spin miniprep kits (Qiagen, Valencia, CA). The purified plasmid DNA was sequenced by the Cornell University Life Sciences Core Laboratory Centers.
Once the 5′ and 3′ ends of the adducin cDNAs were sequenced, two primers were designed to amplify a ‘full‑length’ cDNA containing the entire ORF (‘Ad‑FL1F’ and ‘Ad‑FL1R’ in Table 1). These primers were added to a mixture containing 0.5µl of Malpighian tubule 3′‑cDNA and Platinum PCR Supermix HF (Invitrogen) which was subjected to the following amplification protocol: one cycle at 94°C for 2 min; 35 cycles at 94°C for 30 sec, 65°C for 30 sec, and 68°C for 4 min; and one final cycle at 68°C for 10 min. The PCR products were TOPO cloned and sequenced as described above.
The sequencing data from the RACE experiments and full‑length PCR were assembled to form consensus sequences. As described in the Results, two different splice variants of Aedes adducin were identified in Malpighian tubules: AeAdd‑A (GenBank accession number F705874) and AeAdd‑B (GenBank accession number F705875).
Antibodies
To detect adducin immunoreactivity on western blots and in immunohistochemistry, a polyclonal rabbit antibody—affinity purified against human α and β adducins—was used.29,30 We refer to this antibody as the ‘adducin antibody’. To detect phosphorylated adducin in western blotting, a polyclonal rabbit antibody raised against the phosphorylated human γ‑adducin was purchased from Millipore (Billerica, MA). We refer to this antibody as the ‘phospho‑adducin antibody’. It targets the COOH‑terminal MARCKS domain of adducin. A monoclonal mouse antibody raised against β‑tubulin (E7) was also purchased for use in western blots (Developmental Studies Hybridoma Bank, University of Iowa).
Western blotting
Crude lysates of Malpighian tubules and midguts isolated from 10 adult female mosquitoes were prepared in a 10‑fold dilution of ice‑cold Ringer solution supplemented with 50 μM EDTA, Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL, USA) and Halt Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific), as described in a previous study.28 Total protein content of samples was assessed using a Pierce Bicinchoninic Acid (BCA) Protein Assay (Thermo Fisher Scientific). To denature proteins, the samples were supplemented with an appropriate amount of a 5x Laemmli sample buffer and boiled for 5 min.31
An 8% acrylamide gel was prepared and each lane was loaded with 25 µg of total protein derived from Malpighian tubules or midguts. The proteins were separated by electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane (Bio‑Rad, Hercules, CA). The PVDF membrane was blocked for 90 min at room temperature in a blocking solution consisting of Tween‑Tris‑buffered saline (TTBS; 10 mM TRIS‑HCl, 150 mM NaCl, 0.01% Tween 20, pH 7.4) and non‑fat dry milk powder (5% w/v). The PVDF was then probed with either the adducin antibody or phospho‑adducin antibody (diluted 1:1000 in blocking solution) overnight at 4°C. On the following day the PVDF membrane was washed in TTBS three times (5 min each) and probed with a goat‑anti rabbit secondary antibody conjugated with horseradish peroxidase for 90 min at room temperature. After washing in TTBS three more times, the PVDF was placed in Supersignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) for 5 min. The luminescent signal emitted from the PVDF membrane was detected with X‑ray film in a dark room.
Time course and pharmacology of adducin phosphorylation
In time course studies of the phosphorylation of adducin after stimulating Malpighian tubules with the diuretic peptide aedeskinin‑III (AK‑III), 200 Malpighian tubules were isolated from 40 adult female mosquitoes and distributed equally in four 1.5 ml microcentrifuge tubes containing 200 μl Ringer solution. To three of the tubes, AK‑III was added to a final concentration of 10−7 M. After 2, 10, or 20 min incubation with AK‑III, the Ringer solution was aspirated and the tubules were snap frozen in liquid nitrogen stored at ‑80°C. The fourth tube (control) received no AK‑III.
For identifying the kinase(s) that phosphorylate adducin in Aedes Malpighian tubules, 50 tubules from 10 adult female mosquitoes were isolated per treatment. To test the effects of an agonist of protein kinase C (PKC), two groups of tubules were incubated with either phorbol 12‑myristate 13‑acetate (PMA, 10−6 M in 0.05% DMSO) or the vehicle (0.05% DMSO) for 20 min before freezing the tubules in liquid nitrogen. To test the effects of PKC antagonists on adducin phosphorylation, two groups of tubules were incubated with the vehicle (0.05% or 0.1% DMSO) and one group was incubated with either chelerythrine (Chel, 5 x 10−6 M), bisindolylmaleimide‑I (BIM‑I, 10−5 M), or staurosporine (Stau, 10−7 M). After a 20 min incubation, AK‑III (10−7 M) was added to one of the tubes containing the vehicle and the tube containing the antagonist. After 2 min of incubation with AK‑III, the Ringer solution was aspirated and the tubules were frozen in liquid nitrogen as described above.
The frozen tubules were prepared for western blotting experiments as described above. On a given PVDF, the phospho‑adducin immunoreactivity was detected first. After detection, the PVDF was stripped of the phospho‑adducin antibody using Restore PLUS Western Blot Stripping Buffer or Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) for 10 min at room temperature. After confirming the removal of the phospho‑adducin antibody, the PVDF membrane was incubated with the adducin antibody to normalize the phospho‑adducin signal. In some experiments, normalizing the phospho‑adducin signal to that of total adducin was not possible, because the supply of the adducin antibody had been depleted. In these cases it was necessary to use an anti‑β‑tubulin antibody (E7) to normalize levels of phospho‑adducin immunoreactivity.
The resulting X‑ray films from the phosphorylation experiments were digitized with a scanner and the optical densities of the respective immunoreactivities were quantified using ImageJ software (http://rsbweb.nih.gov/ij).
Immunohistochemistry
Sections of paraformaldehyde‑fixed Malpighian tubules embedded in paraffin were rehydrated and treated with methanol/peroxide as described previously.32 Following the methanol/peroxide treatment, the slides were subjected to a ‘wet‑autoclave’ antigen‑retrieval procedure33 and then washed in phosphate buffered saline (PBS; 145 mM NaCl, 3.2 mM NaH2PO4, and 7.2 mM Na2HPO4, pH 7.5) for 5 min. The sections were blocked for 20 min at room temperature with a 4:1 mixture of 10% normal goat serum (Zymed, San Francisco, CA) and 10X Casein solution(Vector Laboratories, Burlingame, CA), then incubated overnight at 4°C with the adducin antibody diluted 1:1000 in PBS supplemented with 1x casein. The following day, the sections were washed with PBS as described above and probed with a 1:100 dilution of a biontylated goat‑anti rabbit secondary antibody (Vector Laboratories) in PBS‑casein for 20 min at room temperature. The sections were then washed in PBS and incubated with a streptavidin‑conjugated horseradish peroxidase (Zymed) for 30 min at room temperature. After washing again with PBS, the sections were stained with Vector AEC (Vector Laboratories) for 15 min at room temperature and then washed with water. A counter‑stain was performed with hematoxylin for 10 sec. Since a pre‑immune serum for the adducin antibody was not available, we consider tubules undergoing the staining procedure without the antibody as negative control.
Electrophysiological studies of isolated Malpighian tubules
After isolation from a 3–5 d old female mosquito, the set of 5 Malpighian tubules (still attached to the midgut) were transferred to a perfusion bath containing 0.5 ml of Ringer solution. The bottom of the bath was covered with a thin sheet of ParafilmR (American National Can, Menasha, WI). Malpighian tubules cling to stretched ParafilmR which stabilizes them during the perfusion of the bath at a rate of 3 ml/min. The midgut served to position the tubules in the center of the perfusion bath for impalement with microelectrodes. Conventional microelectrodes (Omega dot borosilicate glass capillaries, model 30–30–1; Frederick Haer, St. Bowdoinham, ME; or model 1B100F‑4; World Precision Instruments, Sarasota, FL) were pulled on a programmable puller (Model P‑97; Sutter Instruments, Novato, CA) to yield resistances between 20 and 40 MΩ when filled with 3 M KCl. The microelectrodes were bridged to the measuring hardware using an Ag/AgCl junction. The Ag/AgCl junction was prepared by first degreasing a silver wire (OD 0.25 mm) with alcohol and then by Cl‑ plating the wire in a concentrated solution of household Clorox for 15 min. The Ag/AgCl wires were inserted into the back of voltage and current microelectrodes. An Ag/AgCl wire (OD 0.5 mm) was inserted into a 4% agar bridge of Ringer solution to serve as the ground electrode. A single principal cell near the blind (distal) end of one Malpighian tubule was chosen for impalement with both voltage and current electrodes as described previously.34 The voltage electrode measured the basolateral membrane voltage (Vbl) using the Geneclamp 500 electrometer (Axon Instruments, Molecular Devices, Sunnyvale, CA). The input resistance (Rin) of the impaled principal cell was measured from current‑voltage plots generated by voltage clamping the cell in a series of five increasing voltage‑clamp steps: 5 mV, 400 ms each, starting at (Vbl ‑ 10 mV). The voltage‑stepping protocol and data acquisition were executed digitally by using a Digidata 1440 (Molecular Devices) under control of the Clampex module of the pCLAMP software package (version 10; Molecular Devices). The experiment was discontinued if Vbl measured by voltage and current electrodes differed by more than 10 mV. Moreover, the experiment was discontinued if the tubules did not respond to stimulation by AK‑III.9 After washout of AK‑III, new control values of Vbl and Rin were recorded. Thereafter, BIM‑I (10−5 M) or PMA (10−6 M), was added to the bath and values of Vbl and Rin were taken every few min for the next 20 min. AK‑III (10−6 M) was then added to record effects on Vbl and Rin. The experiments concluded with a final washout and final measurements of Vbl and Rin. All experiments were done at room temperature.
Ramsay fluid secretion assays
The rate of fluid secretion was measured at room temperature in isolated Malpighian tubules as developed by Ramsay35 and adopted by us.36 In brief, the distal (blind) end of the tubule was bathed in a Ringer droplet of 50 µl under light mineral oil. The open end of the tubule was pulled into the oil with a glass hook so that secreted fluid exited the tubule lumen as a droplet separate from the bathing Ringer solution. The glass hook was formed on a microforge (Stoelting Co., Wood Dale, IL) using soft glass (R‑6, Drummond Scientific Co., Broomall, PA). It was then washed in an acid solution of K2CrO4 and H2SO4. After rinsing with distilled water and drying, the glass hook was exposed to the vapor of 20 µl dimethyl‑dichlorosilan for 90 sec (Fluka, Buchs, Switzerland) and then baked overnight at 110°C. The silanization prevents fluid secreted by the tubules from spreading out along the glass hook.
Each tubule was used as its own control. The initial 30 min marked the control fluid secretion period. Thereafter, the secreted fluid droplet (nanoliter volumes, 10−9 liter) was removed and 5 µl of Ringer solution was removed from the peritubular bath and replaced with 5 µl of Ringer solution containing the agent of interest. The 30 min experimental period began as soon as the agent had been added to the peritubular bath. In some experiments tubules were first studied under control conditions for 30 min, then in the presence of bisindolylmaleimide‑I for another 30 min, and finally in the presence of bisindolylmaleimide plus AK‑III for a third 30 min interval. Rates of fluid secretion were determined by plotting cumulative volume (nl) secreted by the tubule as a function of time (30 min), each for the control and the experimental period(s). The plots were usually linear, yielding the rate of fluid secretion as the slope of the line (Microsoft Excel 2007, Microsoft, Redmond, WA). Using each tubule as its own control allowed the statistical analysis by the paired t‑test which tests the significance of the difference between the control and experimental secretion periods. The paired comparison eliminates the variability between the secretion rates in tubules isolated from different mosquitoes.
Statistical analyses
Graphpad Prism (Graphpad Software, www.graphpad.com) was used for the statistical analyses of quantitative phosphorylations; one way ANOVA with a Newman‑Keuls post‑test was used to evaluate the significance of multiple treatments, and the paired Student’s t‑test was used in studies of fluid secretion and electrophysiology where each tubule was used as its own control.
Disclosure of Potential Conflicts of Interest
The manuscript contains no issues that would present conflict of interest for the authors.
Acknowledgments
This work was supported by NSF IOB 0542792 awarded to K.W. Beyenbach and by NIH K01 DK080194–01 awarded to P.M. Piermarini.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/23120
References
- 1.Beyenbach KW, Piermarini PM. Osmotic and Ionic Regulation in Insects. In: Evans DH, ed. Osmotic and Ionic Regulation: Cells and Animals: CRC Press, 2009:231‑93. [Google Scholar]
- 2.Coast GM. Neuroendocrine control of ionic homeostasis in blood‑sucking insects. J Exp Biol. 2009;212:378–86. doi: 10.1242/jeb.024109. [DOI] [PubMed] [Google Scholar]
- 3.Beyenbach KW, Piermarini PM. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol (Oxf) 2011;202:387–407. doi: 10.1111/j.1748-1716.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torfs P, Nieto J, Veelaert D, Boon D, van de Water G, Waelkens E, et al. The kinin peptide family in invertebrates. Ann N Y Acad Sci. 1999;897:361–73. doi: 10.1111/j.1749-6632.1999.tb07906.x. [DOI] [PubMed] [Google Scholar]
- 5.Benoit JB, Denlinger DL. Meeting the challenges of on‑host and off‑host water balance in blood‑feeding arthropods. J Insect Physiol. 2010;56:1366–76. doi: 10.1016/j.jinsphys.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post‑bloodmeal diuresis in Aedes aegypti (L.) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1983;153:257–65. [Google Scholar]
- 7.Beyenbach KW. Regulation of tight junction permeability with switch‑like speed. Curr Opin Nephrol Hypertens. 2003;12:543–50. doi: 10.1097/00041552-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol. 1993;132:63–76. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- 9.Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010;299:R612–22. doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veenstra JA, Pattillo JM, Petzel DH. A single cDNA encodes all three Aedes leucokinins, which stimulate both fluid secretion by the malpighian tubules and hindgut contractions. J Biol Chem. 1997;272:10402–7. doi: 10.1074/jbc.272.16.10402. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Beyenbach KW. Leucokinin and the modulation of the shunt pathway in Malpighian tubules. J Insect Physiol. 2001;47:263–76. doi: 10.1016/S0022-1910(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 12.Pietrantonio PV, Jagge C, Taneja‑Bageshwar S, Nachman RJ, Barhoumi R. The mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins. Insect Mol Biol. 2005;14:55–67. doi: 10.1111/j.1365-2583.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 13.Radford JC, Davies SA, Dow JA. Systematic G‑protein‑coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem. 2002;277:38810–7. doi: 10.1074/jbc.M203694200. [DOI] [PubMed] [Google Scholar]
- 14.Radford JC, Terhzaz S, Cabrero P, Davies SA, Dow JA. Functional characterisation of the Anopheles leucokinins and their cognate G‑protein coupled receptor. J Exp Biol. 2004;207:4573–86. doi: 10.1242/jeb.01317. [DOI] [PubMed] [Google Scholar]
- 15.Lu HL, Kersch C, Pietrantonio PV. The kinin receptor is expressed in the Malpighian tubule stellate cells in the mosquito Aedes aegypti (L.): a new model needed to explain ion transport? Insect Biochem Mol Biol. 2011;41:135–40. doi: 10.1016/j.ibmb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cady C, Hagedorn HH. Effects of putative diuretic factors on intracellular second messenger levels in the Malpighian tubules of Aedes aegypti. J Insect Physiol. 1999;45:327–37. doi: 10.1016/S0022-1910(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 17.Yu MJ, Beyenbach KW. Leucokinin activates Ca(2+)‑dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol. 2002;283:F499–508. doi: 10.1152/ajprenal.00041.2002. [DOI] [PubMed] [Google Scholar]
- 18.Beyenbach KW, Baumgart S, Lau K, Piermarini PM, Zhang S. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J Exp Biol. 2009;212:329–40. doi: 10.1242/jeb.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner K, Bennett V. A new erythrocyte membrane‑associated protein with calmodulin binding activity. Identification and purification. J Biol Chem. 1986;261:1339–48. [PubMed] [Google Scholar]
- 20.Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin‑regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–91. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin‑actin complexes. J Biol Chem. 1995;270:18990–6. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–95. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mische SM, Mooseker MS, Morrow JS. Erythrocyte adducin: a calmodulin‑regulated actin‑bundling protein that stimulates spectrin‑actin binding. J Cell Biol. 1987;105:2837–45. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baines AJ. Evolution of the spectrin‑based membrane skeleton. Transfus Clin Biol. 2010;17:95–103. doi: 10.1016/j.tracli.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser HW, O’Keefe E, Bennett V. Adducin: Ca++‑dependent association with sites of cell‑cell contact. J Cell Biol. 1989;109:557–69. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdi KM, Bennett V. Adducin promotes micrometer‑scale organization of beta2‑spectrin in lateral membranes of bronchial epithelial cells. Mol Biol Cell. 2008;19:536–45. doi: 10.1091/mbc.E07-08-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E‑cadherin‑mediated cell‑cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–85. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piermarini PM, Grogan LF, Lau K, Wang L, Beyenbach KWA. A SLC4‑like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol. 2010;298:R642–60. doi: 10.1152/ajpregu.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruenbaum LM, Gilligan DM, Picciotto MR, Marinesco S, Carew TJ. Identification and characterization of Aplysia adducin, an Aplysia cytoskeletal protein homologous to mammalian adducins: increased phosphorylation at a protein kinase C consensus site during long‑term synaptic facilitation. J Neurosci. 2003;23:2675–85. doi: 10.1523/JNEUROSCI.23-07-02675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilligan DM, Lozovatsky L, Gwynn B, Brugnara C, Mohandas N, Peters LL. Targeted disruption of the beta adducin gene (Add2) causes red blood cell spherocytosis in mice. Proc Natl Acad Sci U S A. 1999;96:10717–22. doi: 10.1073/pnas.96.19.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol. 2009;296:F730–50. doi: 10.1152/ajprenal.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankfalvi A, Navabi H, Bier B, Böcker W, Jasani B, Schmid KW. Wet autoclave pretreatment for antigen retrieval in diagnostic immunohistochemistry. J Pathol. 1994;174:223–8. doi: 10.1002/path.1711740312. [DOI] [PubMed] [Google Scholar]
- 34.Masia R, Aneshansley D, Nagel W, Nachman RJ, Beyenbach KW. Voltage clamping single cells in intact malpighian tubules of mosquitoes. Am J Physiol Renal Physiol. 2000;279:F747–54. doi: 10.1152/ajprenal.2000.279.4.F747. [DOI] [PubMed] [Google Scholar]
- 35.Ramsay JA. Active transport of potassium by the Malpighian tubules of insects. J Exp Biol. 1953;30:358–69. [Google Scholar]
- 36.Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide‑sensitive electrolyte transport in Malpighian tubules. Am J Physiol. 1991;261:C521–9. doi: 10.1152/ajpcell.1991.261.3.C521. [DOI] [PubMed] [Google Scholar]
- 37.Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, et al. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem. 1995;270:25534–40. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka Y, Hughes CA, Bennett V. Adducin regulation. Definition of the calmodulin‑binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271:25157–66. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS‑related domain inhibits activity in promoting spectrin‑actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–97. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V. Primary structure and domain organization of human alpha and beta adducin. J Cell Biol. 1991;115:665–75. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu MJ, Beyenbach KW. Effects of leucokinin‑VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol. 2004;207:519–26. doi: 10.1242/jeb.00772. [DOI] [PubMed] [Google Scholar]
- 42.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–42. doi: 10.1016/S0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 43.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–96. [PubMed] [Google Scholar]
- 44.Whittaker KL, Ding D, Fisher WW, Lipshitz HD. Different 3′ untranslated regions target alternatively processed hu‑li tai shao (hts) transcripts to distinct cytoplasmic locations during Drosophila oogenesis. J Cell Sci. 1999;112:3385–98. doi: 10.1242/jcs.112.19.3385. [DOI] [PubMed] [Google Scholar]
- 45.Mangmool S, Haga T, Kobayashi H, Kim KM, Nakata H, Nishida M, et al. Clathrin required for phosphorylation and internalization of beta2‑adrenergic receptor by G protein‑coupled receptor kinase 2 (GRK2) J Biol Chem. 2006;281:31940–9. doi: 10.1074/jbc.M602832200. [DOI] [PubMed] [Google Scholar]
- 46.Torielli L, Tivodar S, Montella RC, Iacone R, Padoani G, Tarsini P, et al. alpha‑Adducin mutations increase Na/K pump activity in renal cells by affecting constitutive endocytosis: implications for tubular Na reabsorption. Am J Physiol Renal Physiol. 2008;295:F478–87. doi: 10.1152/ajprenal.90226.2008. [DOI] [PubMed] [Google Scholar]
- 47.Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK‑II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Matsuoka Y, Bennett V. Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS‑related domain and a newly defined oligomerization domain. J Biol Chem. 1998;273:19329–38. doi: 10.1074/jbc.273.30.19329. [DOI] [PubMed] [Google Scholar]
- 49.Karas K, Brauer P, Petzel D. Actin redistribution in mosquito malpighian tubules after a blood meal and cyclic AMP stimulation. J Insect Physiol. 2005;51:1041–54. doi: 10.1016/j.jinsphys.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Manunta P, Barlassina C, Bianchi G. Adducin in essential hypertension. FEBS Lett. 1998;430:41–4. doi: 10.1016/S0014-5793(98)00457-8. [DOI] [PubMed] [Google Scholar]
- 51.Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, et al. Hypertension‑associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest. 1996;97:2815–22. doi: 10.1172/JCI118737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradley TJ, Satir P. 5‑hydroxytryptamine‑stimulated mitochondrial movement and microvillar growth in the lower malpighian tubule of the insect, Rhodnius prolixus. J Cell Sci. 1981;49:139–61. doi: 10.1242/jcs.49.1.139. [DOI] [PubMed] [Google Scholar]
- 53.Bradley TJ, Satir P. Evidence of microfilament‑associated mitochondrial movement. J Supramol Struct. 1979;12:165–75. doi: 10.1002/jss.400120203. [DOI] [PubMed] [Google Scholar]
- 54.Beyenbach KW, Aneshansley DJ, Pannabecker TL, Masia R, Gray D, Yu M. Oscillations of voltage and resistance in Malpighian tubules of Aedes aegypti. J Insect Physiol. 2000;46:321–33. doi: 10.1016/S0022-1910(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 55.Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol. 2003;206:3845–56. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- 56.Hayes TK, Pannabecker TL, Hinckley DJ, Holman GM, Nachman RJ, Petzel DH, et al. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Malpighian tubules. Life Sci. 1989;44:1259–66. doi: 10.1016/0024-3205(89)90362-7. [DOI] [PubMed] [Google Scholar]
- 57.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–9. doi: 10.1016/0006-291X(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 58.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand‑Perret T, Ajakane M, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–81. [PubMed] [Google Scholar]
- 59.Davis PD, Hill CH, Keech E, Lawton G, Nixon JS, Sedgwick AD, et al. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989;259:61–3. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- 60.Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, et al. Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–22. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294:335–7. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CL, Hsieh YT, Chen HC. Phosphorylation of adducin by protein kinase Cdelta promotes cell motility. J Cell Sci. 2007;120:1157–67. doi: 10.1242/jcs.03408. [DOI] [PubMed] [Google Scholar]
- 63.Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell. 2010;21:3506–17. doi: 10.1091/mbc.E10-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blumenthal EM. Characterization of transepithelial potential oscillations in the Drosophila Malpighian tubule. J Exp Biol. 2001;204:3075–84. doi: 10.1242/jeb.204.17.3075. [DOI] [PubMed] [Google Scholar]
- 65.Clark TM, Hayes TK, Holman GM, Beyenbach KW. The concentration‑dependence of CRF‑like diuretic peptide: mechanisms of action. J Exp Biol. 1998;201:1753–62. doi: 10.1242/jeb.201.11.1753. [DOI] [PubMed] [Google Scholar]
- 66.Nishizuka Y, Kikkawa U. Early studies of protein kinase C: a historical perspective. Methods Mol Biol. 2003;233:9–18. doi: 10.1385/1-59259-397-6:9. [DOI] [PubMed] [Google Scholar]
- 67.Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB. Activated PKCdelta and PKCepsilon inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+‑K+‑2Cl‑ cotransporter NKCC1. J Biol Chem. 2010;285:34072–85. doi: 10.1074/jbc.M110.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 69.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–23. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pariser H, Perez‑Pinera P, Ezquerra L, Herradon G, Deuel TF. Pleiotrophin stimulates tyrosine phosphorylation of beta‑adducin through inactivation of the transmembrane receptor protein tyrosine phosphatase beta/zeta. Biochem Biophys Res Commun. 2005;335:232–9. doi: 10.1016/j.bbrc.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 71.Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, et al. Megaintestine in claudin‑15‑deficient mice. Gastroenterology. 2008;134:523–34. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 72.Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010;299:R612–22. doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu MJ, Beyenbach KW. Leucokinin activates Ca(2+)‑dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol. 2002;283:F499–508. doi: 10.1152/ajprenal.00041.2002. [DOI] [PubMed] [Google Scholar]