Abstract
In this study we utilised an in vitro model system to gain insight into the potential cellular interactions that quantum dot (QD) nanoparticles may experience while transiting the viable skin epidermis, and we consider the effects of UVB exposure. UVB skin exposure is known to induce a skin barrier defect that facilitates QD stratum corneum penetration. Primary human keratinocytes were cultured in low and high calcium to induce basal and differentiated phenotypes, respectively. Results suggest that differentiation state plays a role in keratinocyte response to UVB exposure and exposure to negatively charged CdSe/ZnS core/shell QD. QD cell uptake increased with QD dose but association with differentiated cells was significantly lower than the basal keratinocyte phenotype. Differentiated keratinocytes were also less sensitive to the cytotoxic effects of UVB exposure. We did not observe an effect of UVB preexposure on QD cytotoxicity level despite the fact that fluorescent microscopy and flow cytometry data suggest that UVB may slightly increase QD uptake in the basal cell phenotype. The implications of these findings for assessing potential risk of human skin exposure are discussed.
Keywords: quantumdots, UVB, primarykeratinocytes, differentiation, nanotoxicology
Introduction
Quantum dots (QDs) are a major class of fluorescent nanoparticles that exhibit advantageous properties including broad optical absorption, narrow emission bandwidth, high quantum yield and easily modified surface chemistry (Dabbousi et al. 1997). These properties are widely exploited in the life sciences and electronics industries, in which QDs are used as biological probes, diagnostic tools, drug delivery vehicles, energy conversion substrates and light emitting diodes (Medintz et al. 2008). These varied uses of QDs have consequently raised environmental health and safety concerns owing to their heavy metal composition and small size which may facilitate their penetration into tissues where they can induce toxic effects. Thus, researchers have begun to investigate the interactions of QDs with tissues associated with the most likely human exposure routes; the gastrointestinal tract (Mohs et al. 2009; Soltesz et al. 2006), the lungs (Geys et al. 2008; Soltesz et al. 2005; Wang et al. 2009) and the skin (Gopee et al. 2009; Mortensen et al. 2008; Zhang & Monteiro-Riviere 2008).
Most research has focused on understanding QD skin penetration and on elucidating the dependence of penetration and systemic translocation on the skin barrier status. Results from studies on ex vivo rat skin (Zhang & Monteiro-Riviere 2008), ex vivo porcine skin (Zhang et al. 2008) and in vivo hairless mouse skin (Gopee et al. 2009; Mortensen et al. 2008) suggest that the stratum corneum (outermost epidermal layer) of intact skin is a formidable barrier to QD penetration. However, QD penetration increases when skin is subjected to barrier impairing conditions such as low-frequency sonophoresis (Paliwal et al. 2006), tape stripping (Ravichandran et al. 2011), dermal abrasion (Gopee et al. 2009; Zhang & Monteiro-Riviere 2008) and UVB exposure (Gulson et al. 2010; Mortensen et al. 2008; Mortensen et al. 2009; Monteiro-Riviere et al. 2011). Studies suggest that if QDs breach the skin barrier they can collect systemically (Gopee et al. 2007) and interact with the local cellular environment in a potentially toxic fashion (Hardman 2006; Lewinski et al. 2008; Rzigalinski & Strobl 2009; Walling et al. 2009). In our previous studies of UVB exposed mouse skin, we observed that QDs pass intercellularly between corneocytes in the stratum corneum (Mortensen et al. 2008). Others similarly observed collection of QDs in the intercellular lipid bilayers of the porcine stratum corneum (Zhang et al. 2008). Transport mechanisms of QDs beyond the stratum corneum are, however, not well characterized. Possible mechanisms include passive paracellular diffusion between keratinocytes (the major epidermal skin cell) or active endocytosis by keratinocytes or other minority cell types such as melanocytes (the pigment producing cells) or Langerhans cells (skin-resident dendritic cells). A mechanistic understanding of how QDs and other nanoparticle (NP) types transport through the viable epidermis, and, in particular, how transport may depend on the specific barrier disrupting condition, is critically important for assessing potential risk from NP skin exposure as well as for optimizing nanoparticle-based transdermal drug and vaccine delivery.
In this study, we used an in vitro model system to gain preliminary insight into the potential cellular interactions that QDs may experience while transiting the viable epidermis. We also consider the effect of UVB exposure which is a common exogenous skin insult. UVB generates DNA damage and a flood of signalling molecules that in vivo induces keratinocyte proliferation (Black et al. 2008; Jans et al. 2006; Kupper et al. 1987), epidermal thickening (Epstein et al. 1970; Haratake et al. 1997; Haratake et al. 1997a; Pentland et al. 2004) and keratinocyte priming to phagocytose melanosomes from neighbouring melanocytes (Belleudi et al. 2010; Cardinali et al. 2005). Approximately 3 days post UVB exposure an inside-out barrier skin defect can be detected as measured by transepidermal water loss (TEWL) (Haratake et al. 1997a; Jiang et al. 2006). It is thought that this defect arises from a disorganisation of stratum corneum lipids stemming from the UVB induced proliferation response (Jiang et al. 2006). This disorganisation may also contribute to an outside-in defect as measured by NP penetration into the viable epidermis. We hypothesised that because UVB skin exposure increases the biological activity of keratinocytes and primes them to uptake melanosomes, UVB may also increase keratinocyte uptake of QDs, a phenomenon we could easily measure using our in vitro model system. To test this hypothesis we cultured primary keratinocytes derived from ex vivo human skin. The established technique of regulating the calcium concentration in the cell culture media (Boyce & Ham 1983; Poumay & Pittelkow 1995; Tamiji et al. 2005; Van Muijen et al. 1987) was used to prepare basal and differentiated (suprabasal) keratinocyte phenotypes. Cells were exposed to UVB immediately before incubation with QDs to avoid the cytotoxic consequence of QD photoactivation (Chang et al. 2009). Previous studies (Ryman-Rasmussen et al. 2007; Ryman-Rasmussen et al. 2007a; Zhang et al. 2008; Zhang & Monteiro-Riviere 2009) have examined the toxicity and uptake mechanisms of various commercially available QDs but with the basal keratinocyte phenotype only. Our studies include the differentiated phenotype, thereby providing a more complete model system to investigate the QD cellular interactions that may occur in the epidermis and the effect of UVB exposure.
Results
In vitro keratinocyte differentiation
Regulating the calcium concentration in the cell culture media produces changes in primary keratinocyte morphology and gene expression. Keratinocytes cultured in low calcium (<0.1 mM) readily proliferate and maintain a dispersed, spread morphology (Figure 1A). In nonconfluent cultures (<80%), cells only express cytokeratins 5/14 (Figure 1B and Figure 1S) consistent with a basal keratinocyte phenotype. Cells cultured in high calcium (1.5 mM) migrate over time (24–48 h) to form desmosomal structures (O’Keefe et al. 1987) and tightly packed stratified layers (Figure 1C). The size of the stratified layers grow with time (Figure 2S). Cells in the top layers appear anucleated and express cytokeratins 1/10 (Figure 1D) consistent with a differentiated keratinocyte phenotype. Under highcalcium conditions it is common (Poumay & Pittelkow 1995; Tamiji et al. 2005), however, to observe regions in the culture dish where cells attached to the plate near differentiated regions express K10/1 weakly and/or continue to express cytokeratins 5/14 (Figure 3S). We chose to conduct experiments with cells incubated in high calcium between 48 and 72 h which is the sufficient time to induce differentiation while ensuring the presence of viable cells.
Figure 1.
Primary keratinocytes cultured in low- (<0.1 mM) and high-calcium (1.5 mM) media. (A) Bright field 10x image of keratinocytes cultured in low calcium showing spread morphology of proliferative cells. (B) Fluorescent image depicting keratin 5 staining (red with Texas Red) of primary keratinocytes cultured in low-calcium media (20x). Blue represents DAPI nuclear stain. (C) Bright field 10x image of keratinocytes cultured in high calcium showing change in cell morphology and migration into tightly packed cell islands. (D) Fluorescent image of keratin 10 staining (green with FITC) of primary keratinocytes cultured in high-calcium media for 48 h (20x). Blue represents DAPI nuclear stain. Keratin 10 patches are expressed in differentiated cells on top of cells growing exhibiting basal (K5) phenotype (see Figure 1S).
Cellular toxicity of UVB
We first examined the cellular response to UVB dose in the absence of QDs. Keratinocytes, cultured in either high or low calcium, were exposed to increasing doses of UVB (0 to 140 mJ/cm2). Cell viability was quantified after 24 h using the MTT assay (n = 4, different skin donors) to test for either increased cell proliferation or cell death. Cell viability for both keratinocyte phenotypes is plotted (Figure 2) normalised to the keratinocytes cultured in low calcium and 0 mJ cm−2 UVB exposure. Results for cells cultured in low calcium indicate that UVB exposure induces a cytotoxicity onset response at 60 mJ cm−2 (p < 0.05). Results for cells cultured in high calcium show a statistically significant lower viability (p < 0.05) at all UVB doses. The data also suggests that UVB may stimulate proliferation under high-calcium conditions, however; a statistical analysis indicates that there is no significant increase or decrease in viability at all UVB doses. When comparisons were made between cells cultured in high and low calcium at each UVB dose, a statistically significant difference (p < 0.05, * with bars) was seen at 0 mJ cm−2, 20 mJ cm−2 and 40 mJ cm−2 UVB. These results are consistent with expectations that differentiated cells would exhibit lower mitochondrial activity than basal cells and that the differentiated phenotype is less sensitive to cytotoxic effects of UVB in this dose range. A 40 mJ cm−2 dose was chosen for subsequent experiments to minimise toxic effects of UVB to the basal phenotype.
Figure 2.
Summary of UVB dose impact on relative cell viability as measured by the MTT assay. For the cells cultured in low calcium, UVB causes a statistically significant decrease in cell viability starting at 60 mJ cm−2 versus their 0 mJ cm−2 dose. For keratinocytes cultured in high calcium a statistically significant difference was observed at all UVB doses relative to the low calcium 0 mJ cm−2 dose control (against which they were normalized), but no statistical difference was observed at all UVB doses when normalised to their own control (high calcium 0 mJ cm−2 UVB). Comparisons made between keratinocytes cultured in high and low calcium at each UVB dose showed a statistical difference a 0, 20, and 40 mJ cm−2 UVB (bar with *). Error bars represent standard error of the mean. Statistical comparisons were made using a Student’s t-test with *p < 0.05 and **p < 0.01. Data represent averages of keratinocyte cultures made from four different skin donors.
Cellular toxicity of QDs
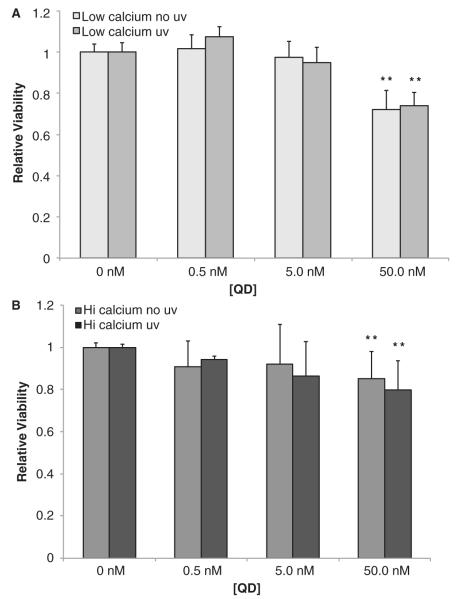
Studies of human skin report that UVB exposure has a more profound effect on basal keratinocytes (Black et al. 2008; Jans et al. 2006; Kupper et al. 1987), which our in vitro findings reported above corroborate. To explore whether the cytotoxic effects of QDs depend on keratinocyte phenotype (basal or differentiated) and if UVB preexposure modulates toxicity, we used a similar experimental strategy as described above. The MTT assay was performed on keratinocytes cultured in high and low calcium, with and without UVB preexposure, for a range of QD doses (Figure 3). In low calcium a QD exposure of 50 nM induced a statistically significant level (p < 0.01) of cytotoxicity (Figure 3A). The QD dose effect on viability was confirmed using Trypan blue exclusion assay (data not shown). The QD toxicity magnitude and the QD dose onset results are consistent with the previous reports (Ryman-Rasmussen et al. 2007; Ryman-Rasmussen et al. 2007a; Zhang et al. 2008; Zhang & Monteiro-Riviere 2009). Interestingly, we did not observe an effect of UVB (40 mJ cm−2) on QD-induced cytotoxicity. Similar results were observed for cells cultured in high calcium (Figure 3B).
Figure 3.
Determination of the cytotoxicity levels that result from increasing dosage of DHLA functionalised QD 620 nm and UVB exposure for keratinocytes cultured in basal low-calcium media (A) and differentiated high-calcium media (B). For both charts, MTT assay shows an onset of toxicity occurring at a QD dosage of 50.0 nM (**p < 0.01). Error bars represent standard error of the mean. Data represent averages of keratinocyte cultures made from four different skin donors.
Fluorescence microscopy
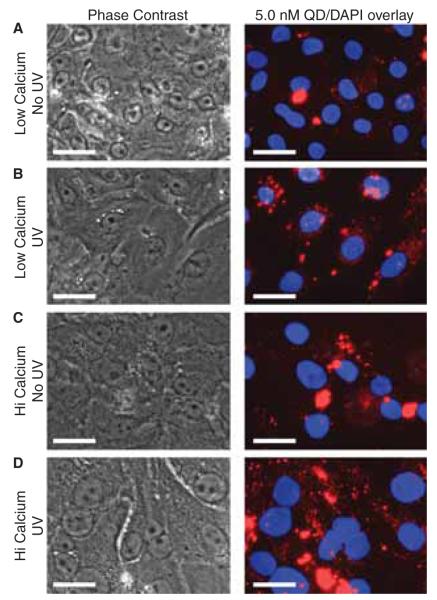
To visualise the interaction of QDs with primary keratinocytes in culture, we imaged the cells 24 h after QD exposure (5 nM) using fluorescence microscopy (Figure 4). Prior to imaging the cultures were gently washed twice with 1X DPBS to remove any precipitated QDs. This treatment yielded an undetectable QD background on the plate for all conditions. For keratinocytes cultured in low calcium and no UVB exposure, there appeared to be QDs clustered in the perinuclear areas observed as punctate spots (Figure 4A). Exposing cells cultured in low calcium to UVB (40 mJ cm−2), prior to introducing QDs, appeared to increase the number punctate QD spots present in the cells (Figure 4B). Interestingly, no difference in cytotoxicity resulting from this association was observed (Figure 3A). For keratinocytes cultured in high-calcium conditions, with or without UVB preexposure, there appeared to be a substantially higher amount of cell-associated QDs (Figure 4C and 4D). Studies investigating the stability of QDs found that high-calcium media did not enhance QD precipitation onto the tissue culture plate over a 24-h period (Figure 4S). Therefore, the increased association of QDs with cells cultured in high calcium is a characteristic of the differentiated phenotype. We examined cell exposure to QD doses of 0.5 nM and 50.0 nM (data not shown) and observed similar trends between low- and high-calcium culture conditions. However, at 50 nM we saw distinct evidence of morphological changes due to cellular toxicity (Figure 3). The qualitative observation that UVB slightly increased QD uptake in basal keratinocyte without a cytotoxic consequence challenges whether the punctate QD spots are truly internalised. Confocal microscopy and transmission electron microscopy (TEM) are methods commonly used to confirm QD cellular uptake. Others have shown using these techniques that internalised QDs do appear as punctate spots (Chang et al. 2009; Geys et al. 2009; Kelf et al. 2010; Zhang & Monteiro-Riviere 2009). We have confirmed using TEM that keratinocytes uptake DLHA-QDs into vesicles (Zheng et al. 2012), but because this technique and confocal microscopy are statistically limited in the number of cells that can be analysed, we chose to use flow cytometry to further investigate the effect of keratinocyte differentiation and UVB exposure on QD cell association.
Figure 4.
Phase contrast and fluorescent microscopy images of the QD-cell interactions. Primary keratinocytes cultured in high- and lowcalcium conditions were either irradiated with 40 mJ cm−2 UVB or sham irradiated and incubated with 5.0 nM QD. After 24 h the media was removed and replaced with 1x DPBS to allow imaging in the plate. UVB appeared to increase the uptake of QDs in keratinocytes cultured in low-calcium conditions (A, B). In high-calcium conditions, there is an increased presence of QD clusters (C, D) which make it difficult to discern an effect of UVB. Control studies indicate that highcalcium conditions do not exacerbate the precipitation of QDs thus suggesting an increased association with the differentiated keratinocyte phenotype. Scale bar = 25 μm.
Flow cytometry and QD cell association
Flow cytometry was used to provide a more quantitative evaluation of the effect of QD dose, calcium concentration in the culture media and UVB preexposure on QD cell association (Figure 5). A standard gating strategy was used to define single cells from debris and multiple cell clusters (Figure 5A). Using the Calcein AM/Sytox Blue stain combination, we separated live cells from dead cells, respectively (Figure 5B). Gating on the live cell population we found a clear increase in mean fluorescence intensity signal in the QD channel with increasing QD dose, as is illustrated in the overlaid histogram distributions (Figure 5C). We found similar trends when comparing both the live and dead populations, but the live cell population consistently yielded a higher differentiation of QD intensity histogram with dose than the dead cell population. We suspect that this is due to the increased variation in cellular membrane permeability within the dead and apoptotic populations.
Figure 5.
An example cell experiment demonstrating flow cytometric gating strategy and the effect on quantum dot exposure. The forward scattering/side scattering plot (A) is gated to eliminate debris and multicell clusters. The Calcein AM/Sytox Blue live/dead scatter plot (B) is segregated into live and dead proportions. An example increase in QD fluorescence with increasing dose of QD (low calcium without UV exposure) can be observed with overlayed histograms (C).
To explore the effect of calcium concentration and UVB exposure (40 mJ cm−2) on QD cell association, we quantified the relative QD fluorescence signal as a function of QD dose (Figure 6). The values reported are from the average of four experiments (n = 4, different skin donors). When the median fluorescent intensity values are plotted on a log scale, several trends become apparent (Figure 6). The most notable is a dramatic increase in fluorescence intensity with increasing QD dose. There is a steady increase of median QD fluorescent intensity with the 0.5 nM, 5.0 nM and 50 nM doses, respectively. Another obvious trend is the impact of calcium concentration on QD cell association. At each QD dose, a statistically significant higher QD fluorescence is observed for cells cultured in low calcium (basal phenotype). The effect of calcium is, however, lessened at 50 nM QD dose. We attribute this to the excessive presence of QDs producing a fluorescence intensity level that approaches the saturation limit of the flow cytometry detector. When considering the impact of UVB, the results are less clear. There is no statistically significant effect of UVB at any dose, although the data at 0.5 nM and 5.0 nM for cells cultured in low calcium suggest UVB exposure may slightly enhance uptake, consistent with qualitative findings reported in Figure 4 and expectations based on the UVB priming of basal keratinocytes to uptake melanosomes. Taken together, these results suggest that QD dose and calcium level have a strong influence on QD/keratinocyte association but that UVB preexposure is not a significant contributor, at least at the dosage and time point used in this study. The latter is consistent with the MTT assay data reported in Figure 2.
Figure 6.
Flow cytometry results plotting the average (n = 4 skin donors) relative fluorescence intensity in the QD channel as a function of QD dose and UVB exposure for primary keratinocytes cultured in high- and low-calcium conditions. QD fluorescence intensity increases with QD dose for keratinocytes culture in both low (basal) and high (differentiated) calcium but at each QD dose the intensity is lower under highcalcium conditions. UVB preexposure appears to enhance QD uptake for cells culture in low calcium but the effect in not statistically significant. The lines represent statistical significance between sample means at each QD dose and the individual marks represent statistical significance over control (cells cultured without QD). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The skin is keratinized squamous epithelial tissue composed of basal keratinocytes bound to the basement membrane and suprabasal keratinocytes undergoing terminal processes to form the stratum corneum – the outermost layer of skin that provides the main barrier to inside-out water loss and outside-in penetration of exogenous substances. Under barrier-impaired conditions, we and others have shown that nanoparticles can penetrate into the viable epidermis (Gopee et al. 2009; Mortensen et al. 2008; Mortensen et al. 2009; Paliwal et al. 2006; Ravichandran et al. 2011; Zhang & Monteiro-Riviere 2008). Thus, it is of interest to understand the mechanisms of how nanoparticles may transport through the epidermis and to explore whether keratinocyte differentiation state may impact their association with nanoparticles. This information is critical to advancing our understanding of the risk associated with nanoparticle skin exposure. If nanoparticles that penetrate the stratum corneum barrier interact efficiently with terminally differentiating cells they will be sloughed off within 30 days posing less a cytotoxic or genotoxic concern than if they were endocytosed by more permanent epidermal cell types (basal keratinocytes, melanocytes) or immune dendritic cells, which could traffic them systemically and possibly alter skin immune responses.
In this study, we utilised an in vitro model system to explore the interaction of QD nanoparticles with primary human keratinocytes in basal and differentiated (suprabasal) states, and we inquired into the effects that UVB irradiation may have these interactions. UVB is of interest as in vivo it induces an inside-out barrier defect that peaks 3–4 days after exposure (Haratake et al. 1997a; Jiang et al. 2006; Jiang et al. 2007; Takagi et al. 2004). In previous studies using a mouse model, we found evidence that UVB irradiation increases QD skin penetration (Mortensen et al. 2008). Here, we investigated the effect of QD dose (0.5 nM, 5 nM, 50 nM) and UVB preexposure (40 mJ cm−2) on the cytotoxicity and QD cellular association. Primary human keratinocytes were cultured in either low- or high-calcium conditions to induce a basal or differentiated phenotype (Figure 1). This was confirmed by immunohistochemical staining for cytokeratin K5 and K10, respectively. Results averaged over several human donors suggest that differentiation state plays a role in keratinocyte response to UVB and QD association.
First, we investigated the effect of differentiation state on UVB response. We found that keratinocytes cultured in high calcium were less sensitive to the cytotoxic effects of UVB and overall they exhibited a lower mitochondrial activity than basal cells based on the MTT assay (Figure 2). It has been shown in recent studies that differentiated keratinocytes are more resistant to UVB in terms of apoptosis induction, due to an increase in the Notch signalling pathway and their resistance to reactive oxygen species (Chang et al. 2009; Mandinova et al. 2008; Zuliani et al. 2005). In the skin, it is more critical to limit the maximum allowed damage from UVB on basal keratinocytes than differentiated cells as mutations in the basal cells can have more long-term consequential effects. Our in vitro results corroborate the reported greater sensitivity of basal cells to UVB exposure.
Keratinocyte cytotoxicity response as a function of QD dose, with and without UVB preexposure, was investigated next (Figure 3). UVB preexposure was chosen because QDs absorb UVB and studies suggest that irradiating cells in the presence of QDs can significantly increase the cytotoxicity response (Chang et al. 2009). Our results on QD dose (no UVB) find that the magnitude and onset of QD-induced cytotoxicity are consistent with previous studies using QDs with a similar core-shell structure and surface chemistry (Ryman-Rasmussen et al. 2007; Ryman-Rasmussen et al. 2007a; Zhang et al. 2008; Zhang & Monteiro-Riviere 2009). Primary human epidermal keratinocytes (HEKs) exhibited a cytotoxicity onset at 20.0 nM following exposure to carboxylic acid-coated 565 nm emitting and 655 nm emitting CdSe/ZnS QD for 24 h (Ryman-Rasmussen et al. 2007). The cytotoxicity levels found in keratinocytes are in line with literature for other cell types exposed to similar ZnS-capped and carboxylic acid-functionalised cadmium-based QD (Hardman 2006; Jamieson et al. 2007; Lewinski et al. 2008; Medintz et al. 2008; Rzigalinski & Strobl 2009). We did not observe an effect of UVB preexposure on QD cytotoxicity despite the fact that our fluorescent microscopy (Figure 4) and flow cytometry data (Figure 6) suggest that UVB may slightly increase QD uptake in the basal cell phenotype. QDs were added immediately after UVB exposure and cells were allowed to incubate with QD for 24 h after which the MTT assay was performed. Because the stimulatory effect of UVB is likely resolved quickly (Braun et al. 2006; Cadet et al. 2005), the long incubation of cells with QDs likely masked any effect of UVB on cell uptake as endocytic processes persisted over the 24-h period. It is plausible that a shorter incubation time with QDs would have revealed a greater effect of UVB on QD uptake. Lastly, we did not observe an effect of keratinocyte differentiation state on the QD cytotoxicity magnitude or dose onset. This is not unexpected as MTT assay primarily reports the effect of QDs on the basal keratinocyte phenotype present in both high- and low-calcium culture conditions.
Flow cytometry was further used to investigate the effect of QD dose, keratinocyte differentiation state and UVB exposure on QD keratinocyte association. Results (Figure 6) show that calcium level in the media and QD dose had statistically significant effects on QD cell association. QD cell association increased with QD dose but association with the differentiated phenotype (cells cultured in high calcium) was significantly lower than the basal phenotype (cells cultured in low calcium). Fluorescent microscopy images (Figure 4), however, suggest the opposite, showing an increased presence of cell-associated QDs and QD clusters for cells cultured in high-calcium media. Control studies showed that high-calcium media did not exacerbate QD precipitation over a 24-h period at 37°C (Figure 4S). Therefore, it is plausible that the observed QD clustering on differentiated cells reflects a physiochemical effect of cell–cell junction formation and stratification (Yuki et al. 2007). It is known that keratinocyte differentiation is regulated by cadherin-mediated cell–cell contact formation (Charest et al. 2009), which is dependent on the calcium ion concentration (Nagar et al. 1996). Thus, incorporation of calcium in the stratified regions suggests that electrostatic interactions may induce the accumulation of negative-charged DLHA QDs on the differentiated cells. The extra wash steps needed to prepare samples for flow cytometry was likely more effective at removing these QD deposits from the cells than the simple wash step used before fluorescent microscopy imaging. Thus, we conclude from the flow cytometry results on the live cell population that QDs are more likely to be endocytosed by the basal keratinocytes rather than the differentiating cells. This is consistent with expectations based on the profound biological differences that exist between basal and differentiated keratinocytes. For example, basal keratinocytes have increased expression of epidermal growth factor receptors (El-Abaseri et al. 2006; Hanover et al. 1985; Kansra et al. 2002) and low-density lipoprotein receptors (Goldstein et al. 1985; Ponec et al. 1992). The clathrin-coated pitmediated endocytosis pathway of these receptors has been shown to be important in nanoparticle uptake (Jiang et al. 2008; Kelf et al. 2010; Zhang et al. 2009; Zhang & Monteiro-Riviere 2009).
Conclusions
An in vitro model system was used to explore the effects of UVB exposure, differentiation state and QD dose on the interactions of negative-charged QDs with primary keratinocytes. This study has yielded several intriguing results that provide mechanistic insight on how nanoparticles may transit the epidermis. Conclusions that can be drawn from this study are that the basal keratinocyte phenotype is more susceptible to effects of UVB and the uptake of QDs than the differentiated keratinocytes, the latter of which comprise the majority of cells in the epidermis. Flow cytometry studies showed QD nanoparticles do associate with the differentiated keratinocytes, but to a much lesser extent. These findings imply that in vivo, if QD nanoparticles penetrate the stratum corneum barrier, they would be more likely to be endocytosed by basal keratinocytes than terminally differentiating ones. However, paracellular diffusion to the basal layer in the epidermis would likely be hindered by cell–cell junctions and interactions with suprabasal cells. UVB skin exposure in vivo primes keratinocytes to uptake melanosomes; however, our in vitro results showed that UVB exposure increased only slightly the uptake of QDs by the basal keratinocyte phenotype but the enhancement level was not statistically significant. We conclude that different QD dosing conditions may be needed to observe an effect of UVB exposure. Nonetheless, it is interesting to note that primary keratinocytes cultured in low calcium (basal phenotype) can uptake high quantities of QDs without suffering significant acute cytotoxicity. The long-term effects of this uptake are unclear. The potential for nanoparticles to be endocytosed by basal keratinocytes suggests that further studies are needed to examine the potential long-term effects of QDs to induce cell dysfunction or transformation. The insights gleaned from this study will be confirmed using our ex vivo human skin and in vivo mouse models. Our future work will seek to validate the differences observed in the uptake levels of negative-charged QD between basal and differentiated keratinocytes. We will examine different UVB and QD dosing protocols as well as investigate the effects of QD surface charge on the interactions of QDs and other nanoparticle types with various skin-resident cell types. These interactions warrant consideration for developing a detailed understanding of the risk from human nanoparticle skin exposure and will provide useful insight for optimising nanoparticle-based transdermal drug and vaccine delivery systems.
Experimental section
Quantum dot functionalisation
We functionalised 620 nm emitting CdSe/ZnS QD (6.55 ± 1.17 nm) purchased in toluene at a concentration of ~10 μM (NN-Labs LLC) with dihydrolipoic acid (DHLA) for use in an aqueous environment as described previously (Mortensen et al. 2009). Briefly, a 250-μL aliquot of organic QDs (1.25 mg) is removed from their solvent by addition of 1.5 mL of a 50/50 methanol/acetone mixture and centrifugation at 14,000 rpm (~23,000 g) for 5 min. The excess solvent is removed using nitrogen gas and the QDs resuspended in 250 μL tetrahydrofuran (THF). Meanwhile, a 10,000× molar excess of pure DHLA (50 μL) is added to 1 mL methanol in a different glass vial and the pH adjusted using tetramethylammonium hydroxide pentahydrate powder (Sigma-Aldrich Inc.) to pH 11. The QDs in THF are added dropwise to the methanol mixture and incubated at 60°–C with stirring for 3 h. After 3 h, the temperature is reduced to room temperature and the reaction mixture is stirred overnight. The bottom methanol layer is then removed, shaken with excess ether and centrifuged at 14,000 rpm for 5 min. The liquid is decanted, the pellet dried using nitrogen gas and the QDs resuspended in 250 μL double distilled water. To remove any excess ligand, the functionalised QDs are dialysed using a 5-kD molecular weight cut-off DispoDialyzer filter (Harvard Apparatus Inc.) and an excess volume of water for 72 h. After dialysing, the concentration is determined by measuring the absorption at the first exciton and using an extinction coefficient from the literature with Lambert–Beer’s law (Yu et al. 2003). The DLHA-coated QDs exhibited a hydrodynamic radius of 19 ± 2 nm and surface charge of −45 ± 5 mV determined by dynamic light scattering and zeta potential measurements (Malvern ZetaSizer ZS).
Primary keratinocyte isolation, culture and differentiation
We obtained fresh human skin from deidentified adult donors following mammoplasty (Strong and Highland Hospitals, University of Rochester, NY), stored it at 4°C and used it within 6 h of surgery. To limit melanin content variability, experiments were limited to skin donors of Type I and Type II pigmentation. Skin samples were approved for usage by the University of Rochester Research Subjects Review Board (RSRB). To harvest keratinocytes, a modified version of the protocol outlined by Pentland and Needleman was used (Pentland & Needleman 1986). First, skin samples were rinsed with sterile 1X phosphate buffered saline (PBS), treated with 0.4 mL fungizone (Invitrogen) in 500 mL sterile 1X PBS for 10 min and rinsed again thoroughly with 1X PBS. Subcutaneous fat was removed and the dermis thinned using a sterile blade and scalpel. The skin samples were then transferred to fresh 60 mm sterile tissue culture plates with gauze and incubated overnight at room temperature in 6 mL of 0.25% Trypsin-EDTA in a sterile cell culture hood with the stratum corneum exposed to the air. The next day the epidermis was carefully separated from the dermis and mixed well with serum free keratinocyte growth media (KGM-SF) (Gibco Inc.) and 1% penicillin/streptomycin (Gibco Inc.) plus 10% foetal bovine serum (FBS). After mixing, the cells were strained using a 100-μm filter and plated in collagen-coated 12-well plates (Purecol 1:5 diluted in 1X PBS). After 24 h, the media was changed to KGM-SF without FBS, and 3 days later differential trypsin (~35–45 sec incubation with 0.25% Trypsin-EDTA) was performed to remove residual melanocytes and fibroblasts while leaving behind the keratinocytes. After 1 week, the media was changed again. When the plates reached ~80% confluence (~2 weeks), the cells were split into two groups, one with low calcium (<0.1 mM calcium chloride KGM-SF only) and one with high calcium (KGM-SF supplemented with 1.5 mM calcium chloride) to induce differentiation.
UV irradiation
Experiments were performed 48 h after the addition of calcium to half of the harvested cells. The media was removed from both basal and differentiated cells and replaced with 300 μL 1X PBS. Cells were then exposed to UVB through a Schott WG 295 nm long-pass glass filter (BES Optics) using FS20 sunlamps (Westinghouse) as described previously (Brouxhon et al. 2007). The FS20 sunlamp emits primarily in the UVB spectrum (290–320 nm), with low amounts of UVA. Lamp output was calibrated using an IL1700 light meter (International Light) with an SED 240 probe to detect light output from 255 to 320 nm (wavelengths shorter than 295 nm removed by Schott filter). Doses from 20 to 140 mJ cm−2 UVB were established using this technique. Sham irradiation was performed by exposing the cells to the sunlamp for equivalent amounts of time with a foil cover to block light. To determine the impact of UVB dose on cellular viability, three wells of each calcium state were exposed to a UVB dose ranging from 0 to 140 mJ cm−2 and mitochondrial reductase activity measured 24 h later by the MTT assay (Invitrogen). Briefly, 100 μL yellow MTT reagent (1.2 mg/mL) was added to each well and incubated for 4 h. The reactive solution was then removed, replaced with 1 mL isopropanol to solubilise the resultant formazan crystals and the absorbance at 600 nm measured. UVB dose impact on viability using MTT was measured on four separate occasions with cells grown from unique donors and viability was statistically compared to unexposed control using Student’s paired t-test. Parallel experiments were performed using the same UV protocols, but the flow cytometric preparation described below.
Quantum dot application
To determine the impact of exposure to increasing doses of DHLA QD 620 nm on cell viability, basal and differentiated primary keratinocytes were incubated with QDs for 24 h with and without UVB exposure. Half of the cells were exposed to 40 mJ cm−2 UVB, a dose that did not demonstrate statistically significant killing of the primary keratinocytes, but that has been shown by previous work to induce biological response (Brouxhon et al. 2007; Chaturvedi et al. 2004). Doses of 0 nM, 0.5 nM, 5.0 nM and 50.0 nM DHLA QD 620 nm were then introduced into the cell media and incubated for 24 h in triplicate (3 wells of a 12-well plate). The effect of QDs on mitochondrial reductase activity was measured as described above using the MTT assay on four separate occasions with cells grown from four different skin donors and viability was statistically compared to unexposed control using Student’s paired t-test. QD interaction with cells was imaged using phase contrast and fluorescence microscopy (Olympus IX70 with QImaging Retiga EXi camera) with a mercury lamp excitation source (360/30 bandpass filter) and narrow emission (620/10 bandpass filter). Images were analysed using ImageJ (NIH). Parallel experiments were performed using the same QD exposure protocols, but the flow cytometric preparation described below.
Flow cytometric analysis
To prepare the UV-irradiated and QD-exposed cell samples for flow cytometric analysis, the media was collected from each cell sample, pooled together with each of their three repeats and the cells were trypsinised from their plates. After addition of FBS, the cells from each condition were added to their respective media and centrifuged. This procedure was enacted to preserve any cells that may have been released from the plate into the media due to cytotoxicity over the course of the experiment. The cell samples were then washed with 1X PBS, centrifuged and stained using a Calcein AM live stain (Invitrogen, Oregon, USA) and Sytox Blue dead stain (Invitrogen, Oregon, USA). Each cell sample was incubated for 10 min in the dark in a 600-μL mixture of the two stains, both of which were at 1 μM concentration. The samples were then centrifuged and the resuspended in 3% formalin (VWR International) for analysis. The appropriate single-stain controls were prepared for Sytox, Calcein and QD using HaCaT keratinocyte cells to allow fluorescence compensation. The samples were analysed using an 18-color BD LSRII flow cytometer with filters for Calcein AM (488 nm ex. 515/20 nm em.), Sytox Blue (405 nm ex. 450/50 nm em.) and QDs (405 nm ex. 660/40 nm em.), and results processed using Flow Jo (Version 7.6) software. We gated the forward scattering (FSC) and side scattering (SSC) plot to eliminate debris and multicellular events, and the Calcein/Sytox plot to allow separation of live and dead cellular fractions. The QD median intensity values were measured from four different skin donors. The results of each independent measurement were averaged and compared using the Student’s t-test.
Supplementary Material
Acknowledgements
All flow cytometry experiments were executed at the University of Rochester Medical Center Flow Core, which is headed by Dr. Timothy Bushnell. This work was supported by the National Science Foundation (CBET 0837891), the National Institute of Health (NIDA K25AI060884) and the Centers for Disease Control and Prevention (R21OH009970).
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary material available online Supplementary Figures 1S–4S.
References
- Belleudi F, Purpura V, Scrofani C, Persechino F, Leone L, Torrisi MR, FASEB J. Expression and signaling of the tyrosine kinase FGFR2b/KGFR regulates phagocytosis and melanosome uptake in human keratinocytes. Advance online publication; 2010. [DOI] [PubMed] [Google Scholar]
- Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis. 2008;29(1):219–225. doi: 10.1093/carcin/bgm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Braun S, Krampert M, Bodó E, Kümin A, Born-Berclaz C, Paus R, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119:4841–4849. doi: 10.1242/jcs.03259. [DOI] [PubMed] [Google Scholar]
- Brouxhon S, Kyranides S, O’Banion MK, Johnson R, Pearce DA, Centola GM, et al. Sequential Down-regulation of E-Cadherin with Squamous Cell Carcinoma Progression: Loss of E-Cadherin via a Prostaglandin E2-EP2 Dependent Posttranslational Mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR, et al. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol. 2005;125:1190–1199. doi: 10.1111/j.0022-202X.2005.23929.x. [DOI] [PubMed] [Google Scholar]
- Chang S, Dai Y, Kang B, Han W, Mao L, Chen D. UV-enhanced cytotoxicity of thiol-capped CdTe quantum dots in human pancreatic carcinoma cells. Toxicol Lett. 2009;188:104–111. doi: 10.1016/j.toxlet.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Charest JL, Jennings JM, King WP, Kowalczyk AP, García AJ. Cadherin-mediated cell–cell contact regulates keratinocyte differentiation. J Invest Dermatol. 2009;129(3):564–572. doi: 10.1038/jid.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Qin J, Stennett L, Choubey D, Nickoloff BJ. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol. 2004;198:100–109. doi: 10.1002/jcp.10392. [DOI] [PubMed] [Google Scholar]
- Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Mattoussi H, Ober R, Jensen KF, et al. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B. 1997;101:9463–9475. [Google Scholar]
- El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27:225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]
- Epstein JH, Fukuyama K, Fye K. Effects of ultraviolet radiation on the mitotic cycle and DNA, RNA and protein synthesis in mammalian epidermis in vivo. Photochem Photobiol. 1970;12:57–65. doi: 10.1111/j.1751-1097.1970.tb06038.x. [DOI] [PubMed] [Google Scholar]
- Geys J, DeVos R, Nemery B, Hoet PHM. In vitro translocation of quantum dots and influence of oxidative stress. Am J Physiol Lung C. 2009;297:L903–L911. doi: 10.1152/ajplung.00029.2009. [DOI] [PubMed] [Google Scholar]
- Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, et al. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect. 2008;116:1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Latendresse JR, et al. Quantitative determination of skin penetration of PEG-coated CdSe quantum dots in dermabraded but not intact SKH-1 hairless mouse skin. Toxicol Sci. 2009;111:37–48. doi: 10.1093/toxsci/kfp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Warbritton AR, et al. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol Sci. 2007;98:249–257. doi: 10.1093/toxsci/kfm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulson B, McCall M, Korsch M, Gomez L, Casey P, Oytam Y, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118(1):140–149. doi: 10.1093/toxsci/kfq243. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Beguinot L, Willingham MC, Pastan IH. Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. J Biol Chem. 1985;260:15938–15945. [PubMed] [Google Scholar]
- Haratake A, Uchida Y, Mimura K, Elias P, Holleran W. Intrinsically aged epidermis displays diminished UVB-induced alterations in barrier function associated with decreased proliferation. J Invest Dermatol. 1997;108:319–323. doi: 10.1111/1523-1747.ep12286474. [DOI] [PubMed] [Google Scholar]
- Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein J, et al. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997a;108:769–773. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- Hardman R. A toxicological review of quantum dots: toxicity depends on physico-chemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Jans J, Garinis GA, Schul W, van Oudenaren A, Moorhouse M, Smid M, et al. Differential role of basal keratinocytes in UV-induced immunosuppression and skin cancer. Mol Cell Biol. 2006;26(22):8515–8526. doi: 10.1128/MCB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SJ, Chen JY, Lu ZF, Yao J, Che DF, Zhou XJ. Biophysical and morphological changes in the stratum corneum lipids induced by UVB irradiation. J Dermatol Sci. 2006;44:29–36. doi: 10.1016/j.jdermsci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF, Zhou XJ. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol. 2007;16:985–992. doi: 10.1111/j.1600-0625.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- Kansra S, Stoll SW, Elder JT. Differential cytoskeletal association of ErbB1 and ErbB2 during keratinocyte differentiation. Biochem Biophys Res Commun. 2002;295:1108–1117. doi: 10.1016/s0006-291x(02)00805-7. [DOI] [PubMed] [Google Scholar]
- Kelf TA, Sreenivasan VKA, Sun J, Kim EJ, Goldys EM, Zvyagin AV. Non-specific cellular uptake of surface-functionalized quantum dots. Nanotechnology. 2010;21:285105. doi: 10.1088/0957-4484/21/28/285105. [DOI] [PubMed] [Google Scholar]
- Kupper TS, Chua AO, Flood P, McGuire J, Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987;80:430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski NA, Colvin VL, Drezek RA. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- Mandinova A, Lefort K, TommasidiVignano A, Stonely W, Ostano P, Chiorino G, et al. The FoxO3a gene is a key negative target of canonical Notch signalling in the keratinocyte UVB response. EMBO J. 2008;27:1243–1254. doi: 10.1038/emboj.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medintz IL, Mattoussi H, Clapp AR. Potential clinical applications of quantum dots. Int J Nanomed. 2008;3:151–167. doi: 10.2147/ijn.s614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs AM, Duan H, Kairdolf BA, Smith AM, Nie S. Proton-Resistant Quantum Dots: Stability in Gastrointestinal Fluids and Implications for Oral Delivery of Nanoparticle Agents. Nano Res. 2009;2:500–508. doi: 10.1007/s12274-009-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123(1):264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- Mortensen LJ, Oberdorster G, Pentland AP, DeLouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: the effect of UVR. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen LJ, Zheng H, Faulknor R, DeBenedetto A, Beck L, DeLouise LA. Increased in vivo skin penetration of quantum dots with UVR and in vitro quantum dot cytotoxicity. Proc SPIE: Colloidal Quantum Dots for Biomedical Applications IV. 2009;7189:718919. [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380(6572):360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe EJ, Briggaman RA, Herman B. Calcium-induced assembly of adherens junctions in keratinocytes. J Cell Biol. 1987;105:807–817. doi: 10.1083/jcb.105.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S, Menon GK, Mitragotri S. Low-frequency sonophoresis: ultrastructural basis for stratum corneum permeability assessed using quantum dots. J Invest Dermatol. 2006;126:1095–1101. doi: 10.1038/sj.jid.5700248. [DOI] [PubMed] [Google Scholar]
- Pentland AP, Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986;77:246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentland AP, Scott G, VanBuskirk J, Tanck C, LaRossa G, Brouxhon S. Cyclooxygenase-1 deletion enhances apoptosis but does not protect against ultraviolet light-induced tumors. Cancer Res. 2004;64:5587–5591. doi: 10.1158/0008-5472.CAN-04-1045. [DOI] [PubMed] [Google Scholar]
- Ponec M, te Pas MF, Havekes L, Boonstra J, Mommaas AM, Vermeer BJ. LDL receptors in keratinocytes. J Invest Dermatol. 1992;98:50S–56S. doi: 10.1111/1523-1747.ep12462204. [DOI] [PubMed] [Google Scholar]
- Poumay Y, Pittelkow MR. Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins. J Invest Dermatol. 1995;104(2):271–276. doi: 10.1111/1523-1747.ep12612810. [DOI] [PubMed] [Google Scholar]
- Ravichandran S, Mortensen LJ, DeLouise LA. Quantification of human skin barrier function and susceptibility to quantum dot skin penetration. Nanotoxicology Early Online. 2011;5(4):675–86. doi: 10.3109/17435390.2010.537381. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators. Nano Lett. 2007a;7:1344–1348. doi: 10.1021/nl070375j. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharm. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz EG, Kim S, Kim S, Laurence RG, DeGrand AM, Parungo CP, et al. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann Surg Oncol. 2006;13:386–396. doi: 10.1245/ASO.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005;79:269–277. doi: 10.1016/j.athoracsur.2004.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Nakagawa H, Kondo H, Takema Y, Imokawa G. Decreased Levels of Covalently Bound Ceramide Are Associated with Ultraviolet B-Induced Perturbation of the Skin Barrier. J Invest Dermatol. 2004;123:1102–1109. doi: 10.1111/j.0022-202X.2004.23491.x. [DOI] [PubMed] [Google Scholar]
- Tamiji S, Beauvillain JC, Mortier L, Jouy N, Tual M, Delaporte E, et al. Induction of apoptosis-like mitochondrial impairment triggers antioxidant and Bcl-2-dependent keratinocyte differentiation. J Invest Dermatol. 2005;125(4):647–658. doi: 10.1111/j.0022-202X.2005.23885.x. [DOI] [PubMed] [Google Scholar]
- Van Muijen GN, Warnaar SO, Ponec M. Differentiation-related changes of cytokeratin expression in cultured keratinocytes and in fetal, newborn, and adult epidermis. Exp Cell Res. 1987;171(2):331–345. doi: 10.1016/0014-4827(87)90166-2. [DOI] [PubMed] [Google Scholar]
- Walling MA, Novak JA, Shepard JRE. Quantum dots for live cell and in vivo imaging. Int J Mol Sci. 2009;10:441–491. doi: 10.3390/ijms10020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lu M, Wang X, Yin R, Song Y, Le XC, et al. Quantum dots enhanced ultrasensitive detection of DNA adducts. Anal Chem. 2009;81:10285–10289. doi: 10.1021/ac9021105. [DOI] [PubMed] [Google Scholar]
- Yu WW, Qu L, Guo W, Peng X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem Mater. 2003;15:2854–2860. [Google Scholar]
- Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp Dermatol. 2007;16(4):324–330. doi: 10.1111/j.1600-0625.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Monteiro-Riviere NA. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol Appl. 2008;21:166–180. doi: 10.1159/000131080. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Monteiro-Riviere NA. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol Sci. 2009;110:138–155. doi: 10.1093/toxsci/kfp087. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Yu WW, Colvin VL, Monteiro-Riviere NA. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol Appl Pharm. 2008;228:200–211. doi: 10.1016/j.taap.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-Dependent Endocytosis of Nanoparticles. Adv Mater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Mortensen LJ, Ravichandran S, Bentley K, DeLouise LA. Endocytosis of positively and negatively charged quantum dots nanoparticle in HaCaT keratinobytes. 2012. Manuscript submitted.
- Zuliani T, Denis V, Noblesse E, Schnebert S, Andre P, Dumas M, et al. Hydrogenperoxide-inducedcelldeathinnormalhumankeratinocytes is differentiation dependent. Free Radical Bio Med. 2005;38:307–316. doi: 10.1016/j.freeradbiomed.2004.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.