Abstract
OBJECTIVE
Age at onset is an important clinical feature of all disorders. However, no prior studies have focused on this important construct in body dysmorphic disorder (BDD). In addition, across a number of psychiatric disorders, early age at disorder onset is associated with greater illness severity and greater comorbidity with other disorders. However, clinical correlates of age at onset have not been previously studied in BDD.
METHODS
Age at onset and other variables of interest were assessed in two samples of adults with DSM-IV BDD; sample 1 consisted of 184 adult participants in a study of the course of BDD, and sample 2 consisted of 244 adults seeking consultation or treatment for BDD. Reliable and valid measures were used. Subjects with early-onset BDD (age 17 or younger) were compared to those with late-onset BDD.
RESULTS
BDD had a mean age at onset of 16.7 (SD=7.3) in sample 1 and 16.7 (SD=7.2) in sample 2. 66.3% of subjects in sample 1 and 67.2% in sample 2 had BDD onset before age 18. A higher proportion of females had early-onset BDD in sample 1 but not in sample 2. On one of three measures in sample 1, those with early-onset BDD currently had more severe BDD symptoms. Individuals with early-onset BDD were more likely to have attempted suicide in both samples and to have attempted suicide due to BDD in sample 2. Early age at BDD onset was associated with a history of physical violence due to BDD and psychiatric hospitalization in sample 2. Those with early-onset BDD were more likely to report a gradual onset of BDD than those with late-onset in both samples. Participants with early-onset BDD had a greater number of lifetime comorbid disorders on both Axis I and Axis II in sample 1 but not in sample 2. More specifically, those with early-onset BDD were more likely to have a lifetime eating disorder (anorexia nervosa or bulimia nervosa) in both samples, a lifetime substance use disorder (both alcohol and non-alcohol) and borderline personality disorder in sample 1, and a lifetime anxiety disorder and social phobia in sample 2.
CONCLUSIONS
BDD usually began during childhood or adolescence. Early onset was associated with gradual onset, a lifetime history of attempted suicide, and greater comorbidity in both samples. Other clinical features reflecting greater morbidity were also more common in the early-onset group, although these findings were not consistent across the two samples.
Keywords: Body dysmorphic disorder, dysmorphophobia, age at onset, age of onset, comorbidity, symptom severity, quality of life, suicidality
1. Introduction
Body dysmorphic disorder (BDD) is a common and often severe disorder characterized by a distressing or impairing preoccupation with an imagined or slight defect in appearance [1]. In recent years, there have been increased efforts to characterize the phenomenology and course of BDD, and associated morbidity, such as suicidality and psychosocial functioning [2–7]. However, to our knowledge, detailed information about age at onset and clinical correlates of age at onset has not yet been examined in BDD.
Age at onset is an important clinical feature of all disorders. In addition, age at onset has important correlates in many different disorders such as major depressive disorder [8–10], bipolar disorder [11–12], schizophrenia [13–15], panic disorder [16–19], social phobia [20,21] and obsessive-compulsive disorder (OCD; [22–31]). In general, early age at onset has been associated with greater severity of illness (eg, [8,32]), although there are exceptions, particularly for panic disorder, for which some studies have found no differences in symptom severity between early-onset and late-onset patients [16,19]. Furthermore, in many studies early age at onset is associated with increased comorbidity on both Axis I (eg, [8,17]) and Axis II (eg, [9,10,18]), although not all studies have found this (eg [19]). Such findings have clinical implications, in that age at onset could be indicative of a disorder’s severity and comorbidity, and could potentially lead to different therapeutic interventions.
Findings associated with age at onset in social phobia and OCD may be particularly relevant to the present study because these disorders may be closely related to BDD (see eg [33,34]). However, findings in these disorders are inconclusive. Early age at onset was associated with greater symptom severity and increased comorbidity in one study on social phobia [20] but with greater comorbidity but not symptom severity in another study [21]. In OCD, early age at onset is usually associated with greater severity of symptoms [22,24,26,28,31,35,36] and greater comorbidity on both Axis I and Axis II [25,27,29,30,33]. However, Millet et al. [24], Grant et al. [22], and Tükel et al. [31] found comorbidity rates to be similar across groups with different ages of onset. Despite certain discrepancies in the literature, it appears that early age at onset is usually associated with greater symptom severity and increased comorbidity with other disorders. Therefore, we expected to find the same pattern with regard to age at onset in BDD.
Interestingly, studies on age at onset and clinical correlates in other disorders have usually not included measures of psychosocial functioning and quality of life, a lthough there are some exceptions. Grant et al. [22], Rodriguez-Salgado et al. [37], and Pinto et al. [23] found no differences in quality of life or social or work impairment between patients with early-onset versus late-onset OCD. However, Biffin et al. [38] found that early age at onset in bipolar I disorder was associated with more impaired psychosocial functioning and poorer quality of life. Zisook et al. [8] found that early-onset major depressive disorder was associated with more impairment in occupational and social functioning and poorer quality of life, and Bellino et al. [15] found similar results for schizophrenia.
In this report, we examine age at BDD onset in more detail than in previous reports on BDD (we have previously reported only mean, standard deviation, and range of age at onset) [3,39]. There is considerable discrepancy in how early age at onset is defined across studies of other disorders (eg, [22,26]). In this study, we chose a cut-point of 17 and younger for early-onset, and 18 and older for late (or adult) onset, as this is the most common practice in the age at onset literature on other disorders (eg [9,11,17]), especially for OCD (eg [23,35]); thus, this cut-point provides the best comparative data and continuity with this literature. In addition, although such cut-offs are to some extent arbitrary, there is nevertheless a meaningful distinction to be made between adult and child/adolescent samples (eg [9]).
We also examine clinical correlates of age at onset in BDD, which to our knowledge have not previously been examined. Based on the literature in other disorders, we hypothesized that early age at onset would be associated with greater severity of BDD and higher rates of comorbidity on Axis I and Axis II. In addition, although evidence from other disorders is mixed, based on our clinical impressions we hypothesized that early age at onset would be associated with poorer functioning and quality of life.
Much has been written about the need for replication in science (see eg [40]). We address this concern by testing our hypotheses in two separate samples of individuals with BDD. These samples were recruited in different ways, and there were some differences in the measures that were used to assess the two samples (as described below).
2. Methods
2.1. Subjects
Sample 1
Subjects were 184 broadly ascertained adults with current or past DSM-IV BDD who participated in an observational prospective interview study of the course of BDD. Other papers have previously reported on various characteristics of this sample [e.g., 3,41]. This report includes only data from the initial (intake) interview. Inclusion criteria were DSM-IV BDD, including its delusional variant, and ability to be interviewed in person. In this report, study participants younger than 18 were excluded from analyses. The only exclusion criterion was the presence of an organic mental disorder. The study was approved by the hospital institutional review board, and all subjects provided written informed consent for their participation.
The study was conducted in a research and clinical BDD specialty program. Recruitment sources consisted of mental health professionals (48.4%), advertisements (36.4%), brochures and the program website (10.3%), friends and relatives of the subject (3.3%) and nonpsychiatrist physicians (1.6%). All subjects received $50 as compensation for the intake interview. In this sample, 88.6% (n = 163) met current criteria (during the past month) for BDD; the rest of the sample had met full criteria for BDD at some point in their life (7.6% were in partial remission, and 3.8% were in full remission at the time of the intake interview). 75.5% of subjects considered BDD their most problematic lifetime disorder.
Sample 2
This sample was obtained from the same site as sample 1. Subjects were 244 adults with current DSM-IV BDD who were referred from a variety of sources for a clinical evaluation or treatment of BDD. They participated in a phenomenology study of the clinical features of BDD (n = 151) [e.g., 42,43] or in pharmacotherapy studies of BDD (n=93) [44–48]. The treatment studies excluded individuals with a current substance use disorder, past or current bipolar disorder, current clinically significant suicidality, or current inpatient or residential treatment status. Other papers have previously reported on various characteristics of this sample [e.g., 42,43]. This study was approved by the hospital institutional review board, and all subjects provided written informed consent.
2.2. Assessments
Measures of illness severity
Severity of BDD symptoms was assessed with three measures: (1) The Yale-Brown Obsessive Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS) [49] is a 12-item reliable and valid semi-structured interview with scores ranging from 0 to 48. (2) The Psychiatric Status Rating Scale for Body Dysmorphic Disorder (BDD-PSR) is based upon PSRs for other disorders, which are included in the Longitudinal Interval Follow-Up Evaluation (LIFE) [50,51]. The BDD-PSR is a reliable 7-item interviewer-rated measure of BDD severity that is based on DSM-IV BDD criteria, with scores ranging from 1 to 7. (3) The Body Dysmorphic Disorder Examination (BDDE) [52] is a 34-item semi-structured interview of BDD symptom severity and negative body image, with scores ranging from 0 to 168. On all of these measures, higher scores indicate greater illness severity.
Demographics and clinical features of BDD
The BDD Form (Phillips KA, unpublished), which has been used in other BDD studies [e.g., 3,42,48], obtained data on demographic characteristics and clinical features of BDD (eg, retrospectively assessed course of illness, suicidality, violent behavior, and lifetime impairment in psychosocial functioning). This measure was also used to determine age at BDD onset. Interviewers carefully questioned participants about the age at which they first met full DSM-IV criteria for BDD; each key DSM-IV criterion (appearance-related preoccupation, distress, and impairment) was inquired about. To enhance the accuracy of age at onset information, subjects were asked about events in their life around the time of reported BDD onset (eg, grade in school, where they lived, memorable events). Participants were also asked about age at onset of subclinical BDD symptoms–i.e., when they first started to dislike and become concerned about their appearance.
The Brown Assessment of Beliefs Scale (BABS), a reliable and valid semistructured interviewer-administered questionnaire, which consists of 7 items (scores range from 0–24), was used to assess current delusionality of beliefs about appearance (eg, “I look ugly”, [53]). The Hamilton Rating Scale for Depression is a 24-item reliable and valid interviewer-administered measure of current depression symptoms (with scores ranging from 0 to 72) [54]. On these measures, higher scores indicate greater symptom severity or more delusional BDD beliefs.
Measures of co-morbidity
In sample 2, most subjects were assessed with the Structured Clinical Interview for DSM-III-R (SCID-III) [55,56] and the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II) [57,58], although some subjects were assessed with the DSM-IV versions of these interviews [59]. Because BDD was not included in the DSM-III-R SCID, BDD was assessed with a reliable semi-structured diagnostic instrument that is similar to the SCID and is based on DSM-IV criteria [60]. In sample 1, the Structured Clinical Interview for DSM-IV, nonpatient version, (SCID-IV) assessed Axis I co-morbidity [61], and the SCID for DSM-IV Axis II Personality Disorders (SCID-II) assessed Axis II disorders [62].
Measures of psychosocial functioning
(1) Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool (LIFE-RIFT) [63] is a reliable and valid semi-structured measure of impairment in various domains (work, school, household duties, recreation, relationships with friends and family, and satisfaction). Total scores range from 4–20. (2) The Social Adjustment Scale–Self-Report (SAS-SR) is a reliable and valid 54-item self-report measure of current functioning in the following domains: work, social and leisure, extended family, primary relationship, parental and family unit [64]. Scores range from 0–4. (3) The Global Assessment of Functioning Scale (GAF) assessed global symptomatology and functioning [1]. (4) The Social and Occupational Functioning Scale (SOFAS) assessed global functioning [1]. Both the GAF and the SOFAS range from 1 to 100. On the LIFE-RIFT and SAS-SR, higher scores reflect poorer functioning; on the GAF and SOFAS, lower scores reflect poorer functioning.
Measures of quality of life
(1) The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) is a reliable and valid self-report questionnaire that measures mental dimensions of health status and associated quality of life [65]. Each subscale has scores ranging from 0 to 100, with lower scores reflecting poorer quality of life. (2) The Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) is a reliable and valid measure of quality of life in eight domains: general activities, physical health, emotional well-being, household, leisure, social, work, and school [66]. We used the “short form,” which yields a total score for these different domains; lower scores reflect poorer quality of life.
As indicated in Table 1, some of the assessments differed across the two samples.
Table 1.
Demographic and Clinical Characteristics of Individuals with Early-Onset BDD vs. Late-Onset BDD
| Variablea | Sample 1 | Sample 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDD onset before age 18 (n=122) | BDD onset age 18 or older (n=62) | Wald or t | p | Effect sizeb | BDD onset before age 18 (n=164) | BDD onset age 18 or older (n=80) | Chi square or t | p | Effect sizeb | |
| Demographics | ||||||||||
| Gender (% female) | 89 (73.0) | 34 (54.8) | 6.09 | .014 | .18 | 84 (51.2) | 46 (57.5) | 0.85 | .356 | .06 |
| Age | 33.8 ± 12.3 | 34.6 ± 9.8 | −0.45 | .632 | .07 | 32.6 ± 10.9 | 32.4 ± 8.0 | 0.23 | .818 | .03 |
| Minority (non-white or Hispanic) | 22 (19.1) | 6 (10.2) | 2.32 | .128 | .12 | 8 (14.8) | 2 (8.0) | *c | .490 | .10 |
| Single | 95 (77.9) | 40 (64.5) | 3.75 | .053 | .14 | 132 (80.5) | 65 (81.3) | 0.02 | .887 | .01 |
| Education (at least some college) | 94 (77.0) | 48 (77.4) | 0.00 | .955 | .00 | 121 (80.7) | 63 (82.9) | 0.17 | .684 | .03 |
| Currently employed (full or part time) | 80 (65.6) | 36 (58.1) | 1.00 | .319 | .07 | 61 (53.0) | 32 (51.6) | 0.03 | .856 | .01 |
| Course of BDD | ||||||||||
| Age at onset of BDD | 12.8 ± 2.4 | 24.2 ± 7.7 | −15.07 | <.001 | .55 | 12.8 ± 3.0 | 24.6 ± 6.6 | −15.23 | <.001 | 1.68 |
| Duration of BDD (years) | 20.3 ± 12.7 | 10.1 ± 7.5 | 6.29 | <.001 | .18 | 19.2 ± 11.6 | 7.8 ± 6.6 | 9.59 | <.001 | .99 |
| Continuous course of illnessd | 94 (77.7) | 53 (85.5) | 1.65 | .199 | .58 (.25–1.33) | 139 (85.3) | 66 (84.6) | 0.02 | .893 | .01 |
| Gradual BDD onsete | 110 (90.2) | 49 (79.0) | 4.41 | .036 | 2.54 (1.06–6.08) | 140 (82.0) | 58 (67.6) | 5.22 | .022 | .16 |
| Symptom severity (current) | ||||||||||
| BDD-YBOCS | 30.9 ± 6.3 | 29.3 ± 6.9 | 1.35 | .181 | .00 | 31.1 ± 5.5 | 30.9 ± 6.5 | 0.25 | .803 | .03 |
| BDD PSR | 5.7 ± 0.7 | 5.8 ± 0.7 | −0.03 | .974 | .02 | ---f | --- | --- | --- | --- |
| BDDE | 100.2 ± 12.1 | 87.7 ± 21.5 | 2.11 | .038 | .12 | ---f | --- | --- | --- | --- |
| BABS | 16.1 ± 5.7 | 16.5 ± 5.2 | −0.37 | .712 | .01 | 15.8 ± 5.0 | 17.5 ± 4.7 | −1.61 | .111 | .37 |
| HAM-D (24 item) | 16.5 ± 10.9 | 16.9 ± 10.6 | −0.27 | .791 | .01 | 19.8 ± 8.1 | 19.4 ± 6.3 | 0.23 | .819 | .05 |
| Quality of life (current) | ||||||||||
| SF 36 | ||||||||||
| Mental health | 41.2 ± 18.7 | 39.9 ± 20.0 | .13 | .898 | .00 | 36.6 ± 20.4 | 38.0 ± 15.2 | −0.29 | .775 | .07 |
| Role emotional | 25.7 ± 35.4 | 23.1 ± 34.8 | .27 | .787 | .01 | 29.4 ± 39.0 | 28.8 ± 37.5 | .06 | .955 | .02 |
| Social functioning | 44.2 ± 25.0 | 45.8 ± 28.0 | −0.60 | .551 | .00 | 40.4 ± 27.0 | 34.1 ± 23.2 | 0.95 | .346 | .25 |
| Q-LES-Q | 50.4 ± 16.0 | 49.0 ± 17.4 | 0.35 | .730 | .02 | ---f | --- | --- | --- | --- |
| Functional impairment (current) g | ||||||||||
| LIFE-RIFT | 14.0 ± 3.4 | 13.5 ± 3.5 | 1.15 | .252 | .00 | ---f | --- | --- | --- | --- |
| SAS-SR | 2.4 ± 0.5 | 2.3 ± 0.5 | 1.20 | .234 | .00 | f | --- | --- | --- | --- |
| GAF | 45.6 ± 10.3 | 45.7 ± 12.1 | −0.49 | .625 | .02 | 51.7 ± 12.6 | 50.4 ± 8.5 | 0.57 | .570 | .12 |
| SOFAS | 48.6 ± 11.7 | 47.8 ± 15.2 | 0.19 | .853 | .04 | ---f | --- | --- | --- | --- |
| Not working due to psychopathology | 42 (34.4) | 21 (33.9) | 0.11 | .745 | .89 (.44–1.80) | ---f | --- | --- | --- | --- |
| Not in school due to psychopathology | 38 (31.1) | 16 (25.8) | 0.20 | .657 | .85 (.41–1.75) | ---f | --- | --- | --- | --- |
| Social and occupational impairment due to BDD (lifetime) | 6.2 ± 1.6 | 5.9 ± 1.9 | 0.99 | .325 | .02 | 5.6 ± 1.8 | 5.1 ± 1.6 | 1.58 | .116 | .24 |
| Suicidality (lifetime) | ||||||||||
| Suicidal ideation | 99 (81.1) | 43 (69.4) | 3.54 | .060 | .50 (.24–1.03) | 125 (83.9) | 59 (79.7) | 0.59 | .441 | .05 |
| Suicidal ideation due to BDD | 67 (54.9) | 33 (53.2) | 0.47 | .490 | .80 (.42–1.51) | 112 (70.4) | 57 (73.1) | 0.18 | .673 | .03 |
| Attempted suicide | 37 (30.3) | 9 (14.5) | 5.36 | .021 | .38 (.17–.86) | 47 (29.6) | 13 (16.7) | 4.60 | .032 | .14 |
| Attempted suicide due to BDD | 14 (11.5) | 6 (9.7) | 0.47 | .495 | .70 (.25–1.97) | 33 (20.8) | 5 (6.4) | 8.00 | .005 | .18 |
| History of physical violence | 29 (23.8) | 11 (17.7) | 1.82 | .177 | .57 (.26–1.28) | 18 (22.8) | 7 (20.0) | 0.11 | .740 | .03 |
| History of physical violence due to BDD | 26 (21.3) | 12 (19.4) | 0.72 | .396 | .70 (.32–1.58) | 20 (25.3) | 3 (8.6) | 4.22 | .040 | .19 |
| Psychiatric hospitalization (lifetime) | 47 (38.5) | 22 (35.5) | 0.43 | .511 | .80 (.42–1.54) | 79 (48.8) | 24 (30.4) | 7.34 | .007 | .17 |
Results are presented as n (%) or mean ± standard deviation.
Effect sizes for sample 1 are presented as odds ratio for logistic regressions or adjusted R2 for linear regressions. Effect sizes for sample 2 are presented as Cramer’s V for chi square analyses (.02 = small effect, .03 = medium effect and .05 = large effect) and Cohen’s d for t-tests (.02 = small effect, .05 = medium effect, .08=large effect).
Fishers exact test
Symptoms had not remitted for at least 1 month since onset; assessed retrospectively.
It took longer than 1 week for symptoms to go from nonexistent to meeting clinical threshold.
--- = This measure was either not used in this sample or there exist too few observations to merit the inclusion of analyses.
On the LIFE-RIFT and SAS-SR, higher scores indicate poorer functioning; on the GAF and SOFAS, lower scores indicate poorer functioning
2.3. Statistical analysis
SPSS version 18 was used for analyses. Means, standard deviations, modes, medians, and frequencies were calculated. Differences between early-onset (age 17 or younger) and late-onset (age 18 or older) BDD were examined using chi-square analyses for categorical variables and t tests for continuous variables. Given the exploratory nature of the analyses, the alpha level was .05; we did not correct for multiple comparisons. Thus, due to the number of comparisons, caution should be used in interpreting significant results, as some of them may represent chance associations. All tests were two-tailed. For both studies, we included only participants with current BDD when comparing current BDD and depression symptom severity, BABS score, and current functioning and quality of life. In sample 1, there was a statistically significant difference in gender for subjects with early-onset versus late-onset BDD. As a result, we controlled for this variable in all analyses for sample 1 so the gender difference would not bias the results, especially because we descriptively compare results in the two samples, and there was not a gender difference in sample 2. Thus, we report effect sizes as odds ratios for logistic regressions and adjusted R2 for linear regressions. In sample 2 there were no differences in demographic variables. Therefore, we report effect size estimates using Cohen’s d (d = .2 is a small effect size, .5 is medium, and .8 is large) for continuous variables and the φ coefficient (Cramer’s V; V = .2 is a small effect size, .5 is medium and .8 is large) for categorical variables.
Because of the exclusion criteria for the pharmacotherapy study participants in sample 2 (see above), we included only the phenomenology subgroup (n=151) in analyses of bipolar disorder, substance use disorder, and suicidality. More disorders were assessed in sample 1 than in sample 2 because different versions of the SCID were used for sample 1 versus most of sample 2. There are occasional discrepancies between a group’s total number and the sample size in analyses of a particular variable in both studies; this is due to missing data or because some measures were added later to the studies. Finally, for some variables (symptom severity, quality of life and functional impairment) data are available for only a subset of the subjects included in sample 2; these were either reported or not depending on the number of subjects for whom data were available (see Table 1).
3. Results
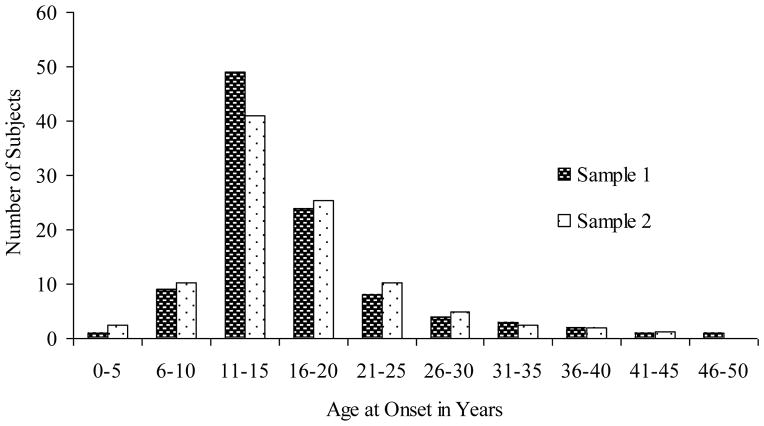
In sample 1, the mean age at BDD onset was 16.7 7.3 (range, 5–49). The median age at onset was 15.0, and the mode was 12.0. In sample 2, the mean age at BDD onset was 16.7 7.2 (range, 4–43). The median age at onset was 15.0, and the mode was 13.0. Figure 1 shows the distribution of age at onset in both studies. 66.3% of subjects in sample 1 and 67.2% in sample 2 had BDD onset before age 18. The mean age at which subjects began to dislike some aspect of their appearance (sub-clinical BDD) was 13.1 6.0 (range, 4 to 43) in sample 1 and 12.6 5.4 (range, 4–30) in sample 2.
Figure 1.
BDD Age at Onset in Two Samples
In sample 1, there was a higher proportion of females with early age at BDD onset (73.0% vs. 54.8%; p=.014), but this was not the case in sample 2. Individuals with early-onset BDD did not significantly differ from those with late-onset BDD on any other demographic variables in either sample (Table 1).
There were more similarities than differences in clinical features among those with early- versus late-onset BDD in both samples 1 and 2 (Table 1). However, those with early-onset BDD were more likely to report a gradual onset of BDD (meaning that it took more than one week for symptoms to become clearly significant) than those with late-onset (90.2% vs. 79.0%, p=.036 in sample 1 and 82.0% vs. 67.6%, p=.022 in sample 2). Contrary to our hypothesis, severity of BDD as measured by scores on the BDD-YBOCS or BDD-PSR was not associated with earlier onset of BDD. However, on another measure of BDD symptom severity (the BDDE), those with earlier onset reported greater BDD severity than those with later-onset BDD (100.2 12.1 vs. 87.7 21.5, p=.038) (the BDDE was used only in study 1).
Psychosocial functioning and quality of life were markedly poor in both groups, but, contrary to our hypothesis, they were not significantly poorer in those with early-onset BDD in either sample (Table 1). Those with earlier onset were more likely to report a history of attempted suicide in sample 1 (30.3% vs. 14.5%, p=.021) and in sample 2 (29.6% vs. 16.7%, p=.032); they were also significantly more likely to report a history of attempted suicide specifically due to their BDD symptoms in sample 2 (20.8% vs. 6.4%, p=.005). A higher proportion of those with early-onset BDD reported a history of suicidal ideation at a trend level in sample 1 (81.1% vs. 69.4%, p=.060). They were also significantly more likely to report a history of physical violence due to BDD (25.3% vs. 8.6%, p=.040) and a history of psychiatric hospitalization (48.8% vs. 30.4%, p=.007) in sample 2.
In sample 1, participants with early-onset BDD had a greater number of lifetime comorbid disorders on both Axis I (4.7 ± 1.8 vs. 3.8 ± 1.7, p=.002) and Axis II (1.0 ± 1.3 vs. 0.5 ± 0.8, p=.001) (Table 2), as predicted. More specifically, those with early-onset BDD were more likely to have any lifetime substance use disorder (59.0% vs. 32.3%, p<.001), including lifetime alcohol use disorders (52.5% vs. 30.6%, p=.001) and lifetime non-alcohol substance use disorders (43.4% vs. 16.1%, p<.001). Those with early-onset BDD were also significantly more likely to have a lifetime eating disorder (anorexia nervosa or bulimia nervosa; 20.5% vs. 6.5%, p=.040) and borderline personality disorder (15.5% vs. 1.6%, p=.032). Significant differences were not found for any other individual Axis I or Axis II disorders.
Table 2.
DSM-IV Axis I and Axis II Comorbidity in Individuals with Early-Onset vs. Late-Onset BDD
| Variablea | Sample 1 | Sample 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDD onset before age 18 (n=122) | BDD onset age 18 or older (n=62) | Wald or t | p | Effect sizeb | BDD onset before age 18 (n=164) | BDD onset age 18 or older (n=80) | Chi square or t | p | Effect sizeb | |
| Number of Comorbid Axis I disordersc | 4.7 ± 1.8 | 3.8 ± 1.7 | 3.01 | .002 | .040 | 2.9 ± 1.9 | 2.5 ± 1.4 | 1.68 | .129 | .24 |
| Lifetime mood disorders | 104 (85.2) | 50 (80.6) | 0.53 | .468 | .74 (.33–1.68) | 133 (81.1) | 66 (86.8) | 1.21 | .271 | .07 |
| Major depressive disorder | 88 (72.1) | 48 (77.4) | 0.85 | .358 | 1.41 (.68–2.92) | 123 (75.5) | 62 (82.7) | 1.54 | .214 | .08 |
| Bipolar disorder | 13 (10.7) | 2 (3.2) | 3.08 | .079 | .25 (.05–1.18) | 8 (7.9) | 2 (4.2) | *d | .502 | .07 |
| Lifetime anxiety disorders | 88 (72.1) | 41 (66.1) | 0.55 | .458 | .43 (.18–1.02) | 101 (61.6) | 36 (47.4) | 4.29 | .038 | .13 |
| Panic disorder | 32 (26.2) | 8 (12.9) | 3.71 | .054 | .82 (.37–1.80) | 25 (15.3) | 8 (10.7) | 0.94 | .333 | .06 |
| Agoraphobia | 1 (0.8) | 1 (1.6) | 0.47 | .492 | 2.67 (.16–43.88) | 2 (1.2) | 3 (4.0) | *d | .181 | .09 |
| Social phobia | 47 (38.5) | 23 (37.1) | 0.01 | .905 | .96 (.51–1.83) | 65 (39.6) | 18 (24.3) | 5.26 | .022 | .15 |
| Specific phobia | 25 (20.5) | 10 (16.1) | 0.29 | .591 | .80 (.35–1.81) | 17 (10.4) | 6 (8.1) | 0.31 | .576 | .04 |
| Obsessive-compulsive disorder | 43 (35.2) | 20 (32.3) | 0.44 | .508 | .80 (.41–1.55) | 52 (31.9) | 16 (21.6) | 2.63 | .105 | .11 |
| Posttraumatic-stress disorder | 11 (9.0) | 4 (6.5) | 0.17 | .683 | .79 (.23–2.60) | ---e | --- | --- | --- | --- |
| Lifetime substance use disorders | 72 (59.0) | 20 (32.3) | 13.98 | <.001 | .27 (.13–.54) | 39 (38.6) | 14 (29.2) | 1.27 | .260 | .09 |
| Alcohol use disorders | 64 (52.5) | 19 (30.6) | 10.69 | .001 | .31 (.16–.63) | 23 (22.8) | 9 (18.8) | 0.31 | .576 | .05 |
| Non-alcohol substance use disorders | 53 (43.4) | 10 (16.1) | 14.23 | <.001 | .22 (.10–.48) | 27 (27.0) | 9 (18.8) | 1.20 | .273 | .09 |
| Lifetime eating disorder (anorexia and bulimia only) | 25 (20.5) | 4 (6.5) | 4.21 | .040 | .31 (.10–.95) | 19 (11.6) | 2 (2.6) | 5.22 | .022 | .15 |
| Anorexia nervosa | 15 (12.3) | 3 (4.8) | 1.81 | .179 | .41 (.11–1.50) | 6 (3.7) | 0 (0.0) | *d | .181 | .11 |
| Bulimia nervosa | 11 (9.0) | 1 (1.6) | 2.30 | .130 | .20 (.03–1.60) | 14 (8.6) | 2 (2.7) | *d | .159 | .11 |
| Lifetime somatoform disorders | 1 (0.8) | 2 (3.2) | 1.88 | .170 | 5.50 (.48–62.74) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Number of comorbid Axis II disorders | 1.0 ± 1.3 | 0.5 ± 0.8 | 3.30 | .001 | .048 | 1.1 ± 1.5 | 0.7 ± 1.3 | 1.00 | .322 | .29 |
| Paranoid personality disorder | 14 (12.1) | 2 (3.2) | 3.46 | .063 | .23 (.05–1.08) | 1 (5.3) | 0 (0.0) | *d | 1.000 | .15 |
| Schizoid personality disorder | 3 (2.6) | 1 (1.6) | 0.32 | .568 | .51 (.05–5.23) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Schizotypal personality disorder | 4 (3.4) | 0 (0.0) | .00 | .997 | .00 (.00–undeff) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Antisocial personality disorder | 8 (6.9) | 3 (4.8) | 1.02 | .312 | .48 (.12–1.98) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Borderline personality disorder | 18 (15.5) | 1 (1.6) | 4.61 | .032 | .11 (.01–.82) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Histrionic personality disorder | 1 (0.9) | 0 (0.0) | 0.00 | .997 | .00 (.00–undeff) | 0 (0.0) | 0 (0.0) | *d | 1.000 | .00 |
| Narcissistic personality disorder | 5 (4.3) | 0 (0.0) | 0.00 | .997 | .00 (.00–undeff) | 1 (5.3) | 2 (15.4) | *d | .552 | .17 |
| Avoidant personality disorder | 33 (28.4) | 12 (19.4) | 2.39 | .122 | .54 (.25–1.18) | 7 (36.8) | 4 (30.8) | *d | 1.000 | .06 |
| Dependent personality disorder | 5 (4.3) | 2 (3.2) | .02 | .881 | .88 (.16–4.80) | 2 (10.5) | 0 (0.0) | *d | .502 | .21 |
| Obsessive-compulsive personality disorder | 20 (17.2) | 4 (6.5) | 3.09 | .079 | .36 (.12–1.13) | 1 (5.3) | 1 (7.7) | *d | 1.000 | .05 |
Results are presented as n (%) or mean ± standard deviation.
Effect sizes for sample 1 are presented as Odds Ratio for logistic regressions or Adjusted R2 for linear regressions. Effect sizes for sample 2 are presented as Cramers V for chi square analyses (.02 = small effect, .03 = medium effect and .05 = large effect) and Cohens d for t-tests (.02 = small effect, .05 = medium effect, .08=large effect).
There were more disorders assessed in sample 1 than in sample 2; therefore number of comorbid disorders in the two studies should not be directly compared.
Fishers exact test
This disorder was not assessed in sample 2.
undef = the upper limit is undefined because one of the groups did not have the disorder; thus, the upper limit could not be calculated.
Among participants in sample 2, those with early-onset BDD were more likely to have a lifetime anxiety disorder (61.6% vs. 47.4%, p=.038), lifetime social phobia (39.6% vs. 24.3%, p=.022), and a lifetime eating disorder (11.6% vs. 2.6%, p=.022). Significant differences were not found for any other individual Axis I or Axis II disorders.
4. Discussion
In this study, we examined age at onset of BDD and clinical correlates of early-onset illness in two samples of individuals with lifetime DSM-IV BDD. To our knowledge, this is the first report of detailed information about age at onset and the first to examine clinical correlates of age at onset in BDD. In addition, to our knowledge, the two samples described in this study are the largest samples of clinically evaluated individuals with DSM-IV BDD that have been studied.
The mean, median, mode, and range of age at onset were remarkably similar in the two samples. It is notable that approximately two-thirds of subjects in both samples in this study had BDD onset before age 18. The most common age at onset was 12–13 years. Although illness onset ranged from 4–43 and 5–49 across the two samples, BDD is usually a disorder of child or adolescent onset. Much smaller studies have reported a mean age at BDD onset of 17 to 20 [67–69].
It is interesting that no participant in either sample had onset of the illness after their 40s, even though the later decades of life arguably represent a time when appearance concerns become more legitimate (eg, in terms of hair loss, wrinkles, loss of muscularity, or overall attractiveness). But if BDD has, in part, an evolutionary basis, which may relate to a desire to attract mates [70–72], then its usual age of onset becomes more understandable. In addition, body image is a very important aspect of psychological and interpersonal development in adolescents [73], which may contribute to BDD’s typical onset during adolescence. Furthermore, social status (including inclusion or exclusion from peer groups) is critically important during this period [72]. Research is needed, however, to determine why BDD usually onsets during early adolescence.
It is interesting that females were more likely than males to have early age at onset in sample 1 (although this difference was not found in sample 2). This gender difference is the opposite of the male gender predominance that has been found in OCD [31,36]. However, not all OCD studies have found this difference [22,35].
Contrary to our hypotheses that early-onset of BDD would be associated with more severe BDD symptoms as well as poorer psychosocial functioning and quality of life, this study found that BDD subjects with early-onset and late-onset illness were generally similar with respect to BDD severity, psychosocial functioning, and quality of life, although BDD severity was greater in the early-onset group on one of three BDD severity measures. The finding that BDD severity did not significantly differ between groups on most measures appears to conflict with data from OCD research which has found early age at onset to be associated with greater OCD symptom severity [22,24,26,28,31,35,36]. However, this finding is in accordance with one study on age at onset in social phobia [21] but conflicts with one other social phobia study [20]. The finding, however, that early age at BDD onset was not significantly associated with poorer functioning or quality of life is consistent with some OCD research [22,37].
One possible explanation for these apparent differences between BDD and studies of OCD is that BDD is a different disorder than OCD, with important differences in clinical features [34] and other aspects of the illness; thus, the same findings should not necessarily be expected in studies of BDD. Another possible explanation for the discrepancies between this study and the OCD literature may be due to the variability in defining age at onset and early-onset in OCD studies. Age at onset has been defined as the age when symptoms begin [26,74], the age at which the person displays significant distress or impairment [36], or the age when the person first meets DSM-IV criteria [27]. In our study, we used the last approach to classify our subjects. Without consistent definitions regarding age at onset phenotypes, comparisons between research studies of early and later onset of illness are hindered.
Some of the comorbidity findings differed between the two samples; reasons for this are unclear, although one sample was assessed using DSM-IV criteria and the other largely with DSM-III-R criteria. Nonetheless, our findings offer some support for our hypothesis that early-onset BDD would be associated with greater psychiatric comorbidity. In sample 1, early-onset BDD subjects had a greater number of comorbid Axis I and Axis II disorders, and they were more likely to have an eating disorder, any substance use disorder, an alcohol use disorder, a non-alcohol use disorder, panic disorder, and borderline personality disorder. Although the total number of comorbid disorders in sample 2 was not significantly greater in the early-onset group, those with early-onset BDD were significantly more likely to have several individual disorders. The markedly higher rates of lifetime substance use disorders in early-onset subjects in sample 1 (for example, 59.0% had a lifetime substance use disorder) is worth noting. Previous research suggests that BDD contributes to substance use disorders, with 68% of individuals with both BDD and a substance use disorder reporting that BDD contributed to their substance use [75]. If BDD in fact does contribute to the development of problematic substance use in many individuals with BDD, then one might expect that the earlier the onset of BDD, the more years a person with BDD has to develop a substance use disorder.
In both studies, early onset was significantly associated with a history of suicide attempts and with a history of suicide attempts due to BDD in sample 2. Nearly twice as many individuals with early-onset BDD had attempted suicide, although effect sizes were in the small to medium range. The association between age at onset and suicidality has been assessed in some previous non-BDD studies, which found that early age at disorder onset is associated with greater suicidality (see eg [8,76–79]. However, suicidality has received little attention in the age at onset literature and should be a focus of future studies, given its clinical importance.
This study has several limitations. Although the use of 18 years as a cutpoint for defining “early-onset” is consistent with much prior research in other disorders [9,11,17,23,35], other age cutpoints are sometimes used (eg [8,24,35]). In addition, subgroups within the early-onset age cohort may exist (eg, prepubertal youth may differ from older adolescents); future research should explore the possible heterogeneity of this early-onset group. In addition, onset of BDD was retrospectively ascertained in this study based on subjects’ recollections. Although the interviewers took great care to ascertain age at onset as accurately as possible, it can be difficult to remember the details of an event that occurred, on average, decades earlier, and some of our subjects may not have accurately recalled age of BDD onset. Prospective, longitudinal studies in high-risk samples are needed to address this limitation in the age at onset literature (see eg [80] for a study involving a prospective, high-risk design). Strengths of the study include examination of two different clinically interviewed, large samples that were ascertained differently, examination of a broad range of clinically important variables, and use of both self-report and interviewer-administered measures with strong psychometric properties and established norms.
There are several clinical implications of this study. The primary one is that clinicians should be aware that BDD is primarily a disorder of childhood or adolescence onset, and that subclinical BDD symptoms begin, on average, several years before individuals experience the full-fledged disorder. In addition, a history of suicide attempts and comorbidity, and possibly other features reflecting greater morbidity, are more common in the early age at onset group. Early screening and intervention efforts targeting children and adolescents with BDD are needed. For clinicians who work with adults, it is important to be aware that this disorder has likely been present since childhood or adolescence and that possible negative effects on attainment of developmental transitions and tasks may be an important part of the case conceptualization and treatment plan.
Although early age at onset was not associated with significantly poorer psychosocial functioning or quality of life at the time of assessment, the disorder may have a critical impact on other areas of development that were not assessed in this study [81]. Developmental transitions and tasks during adolescence include affiliation transitions (greater autonomy from the family, peer affiliations, development of romantic affiliations), achievement transitions (eg, school and work), and identity transitions (eg, changes in self-definition) [82–85]. Severe psychopathology may adversely affect key developmental transitions [81]. Such failures may have adverse repercussions throughout life [86]. Future studies of age at onset in BDD, an often-severe disorder, should examine these important constructs.
Acknowledgments
This study was supported by grant from the National Institute of Mental Health (R01 MH60241 and 5 K24 MH063975)) to Katharine A. Phillips, M.D.
The authors wish to thank Martha Niemiec for her invaluable assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Publishing, Inc; 1994. [Google Scholar]
- 2.Phillips KA, Menard W, Fay C, Pagano ME. Psychosocial functioning and quality of life in body dysmorphic disorder. Compr Psychiatry. 2005;46:254–60. doi: 10.1016/j.comppsych.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005;46:317–25. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry. 2005;66:717–25. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KA, Pagano ME, Menard W, Stout RL. A 12-month follow-up study of the course of body dysmorphic disorder. Am J Psychiatry. 2006;163:907–12. doi: 10.1176/appi.ajp.163.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didie ER, Menard W, Stern AP, Phillips KA. Occupational functioning and impairment in adults with body dysmorphic disorder. Compr Psychiatry. 2008;24:26–8. doi: 10.1016/j.comppsych.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Fontenelle LF, Telles LL, Nazar BP, De Menezes GB, Do Nascimento AL, Mendlowicz MV, et al. A sociodemographic, phenomenological, and long-term follow-up study of patients with body dysmorphic disorder in Brazil. Int J Psychiatry Med. 2006;36:243–59. doi: 10.2190/B6XM-HLHQ-7X6C-8GC0. [DOI] [PubMed] [Google Scholar]
- 8.Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, et al. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164:1539–46. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- 9.Fava M, Alpert JE, Borus JS, Nierenberg AA, Rosenbaum J. Patterns of personality disorder comorbidity in early-onset versus late-onset major depression. Am J Psychiatry. 1996;153:1308–12. doi: 10.1176/ajp.153.10.1308. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Sakado K, Uehara T, Narita T, Hirano S. Personality disorder comorbidity in early-onset versus late-onset major depression in Japan. J Nerv Ment Dis. 1999;187:237–42. doi: 10.1097/00005053-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Suominen K, Mantere O, Valtonen H, Arvilommi P, Leppämäki S, Paunio T, et al. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007;9:698–705. doi: 10.1111/j.1399-5618.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg JF, Garno JL. Age at onset of bipolar disorder and risk for comorbid borderline personality disorder. Bipolar Disord. 2009;11:205–8. doi: 10.1111/j.1399-5618.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: Meta-analysis. Br J Psychiatry. 2009;195:286–93. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- 14.Luoma S, Hakko H, Ollinen T, Järvelin M-R, Lindeman S. Association between age at onset and clinical features of schizophrenia: The northern Finland 1966 birth cohort study. Eur Psychiatry. 2008;23:331–5. doi: 10.1016/j.eurpsy.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Bellino S, Rocca P, Patria L, Marchiaro L, Rasetti R, Di Lorenzo R, et al. Relationships of age at onset with clinical features and cognitive functions in a sample of schizophrenia patients. J Clin Psychiatry. 2004;65:908–14. doi: 10.4088/jcp.v65n0705. [DOI] [PubMed] [Google Scholar]
- 16.Venturello S, Barzega G, Maina G, Bogetto F. Premorbid conditions and precipitating events in early-onset panic disorder. Compr Psychiatry. 2002;43:28–36. doi: 10.1053/comp.2002.29844. [DOI] [PubMed] [Google Scholar]
- 17.Segui J, Marquez M, Garcia L, Canet J, Salvador-Carulla L, Ortiz M. Differential clinical features of early-onset panic disorder. J Affect Disord. 1999;54:109–17. doi: 10.1016/s0165-0327(98)00148-7. [DOI] [PubMed] [Google Scholar]
- 18.Iketani T, Kiriike N, Stein MB, Nagao K, Minamikawa N, Shidao A, et al. Patterns of Axis II comorbidity in early-onset versus late-onset panic disorder in Japan. Compr Psychiatry. 2004;45:114–20. doi: 10.1016/j.comppsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein RB, Wickramaratne PJ, Horwath E, Weissman MM. Familial aggregation and phenomenology of ‘early’-onset (at or before age 20 years) panic disorder. Arch Gen Psychiatry. 1997;54:271–8. doi: 10.1001/archpsyc.1997.01830150097014. [DOI] [PubMed] [Google Scholar]
- 20.Dalrymple KL, Herbert JD, Gaudiano BA. Onset of illness and developmental factors in social anxiety disorder: Preliminary findings from a retrospective interview. J Psychopathol Behav Assess. 2007;29:101–10. [Google Scholar]
- 21.Menezes GB, Fontenelle LF, Versiani M. Early-onset social anxiety disorder in adults: Clinical and therapeutic features. Rev Bras Psiquiatr. 2005;27:32–6. doi: 10.1590/s1516-44462005000100009. [DOI] [PubMed] [Google Scholar]
- 22.Grant JE, Mancebo MC, Pinto A, Williams KA, Eisen JL, Rasmussen SA. Late-onset obsessive compulsive disorder: Clinical characteristics and psychiatric comorbidity. Psychiatry Res. 2007;152:21–7. doi: 10.1016/j.psychres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Pinto A, Mancebo MC, Eisen JL, Pagano ME, Rasmussen SA. The Brown longitudinal obsessive compulsive study: Clinical features and symptoms of the sample at intake. J Clin Psychiatry. 2006;67:703–11. doi: 10.4088/jcp.v67n0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millet B, Kochman F, Gallarda T, Krebs MO, Demonfaucon F, Barrot I, et al. Phenomenological and comorbid features associated in obsessive-compulsive disorder: Influence of age of onset. J Affect Disord. 2004;79:241–6. doi: 10.1016/S0165-0327(02)00351-8. [DOI] [PubMed] [Google Scholar]
- 25.De Mathis MA, Do Rosario MC, Diniz JB, Torres AR, Shavitt RG, Ferrão YA, et al. Obsessive-compulsive disorder: Influence of age at onset on comorbidity patterns. Eur Psychiatry. 2008;23:187–94. doi: 10.1016/j.eurpsy.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Do Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Da Silva Prado H, Sada P, et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158:1899–903. doi: 10.1176/appi.ajp.158.11.1899. [DOI] [PubMed] [Google Scholar]
- 27.Chabane N, Delorme R, Millet B, Mouren M-C, Leboyer M, Pauls D. Early-onset obsessive-compulsive disorder: A subgroup with a specific clinical and familial pattern? J Child Psychol Psychiatry. 2005;46:881–7. doi: 10.1111/j.1469-7610.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 28.Lomax CL, Oldfield VB, Salkovskis PM. Clinical and treatment comparisons between adults with early- and late-onset obsessive-compulsive disorder. Behav Res Ther. 2009;47:99–104. doi: 10.1016/j.brat.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Hemmings SMJ, Kinnear CJ, Lochner C, Niehaus DJH, Knowles JA, Moolman-Smook JC, et al. Early- versus late-onset obsessive-compulsive disorder: Investigating genetic and clinical correlates. Psychiatry Res. 2004;128:175–82. doi: 10.1016/j.psychres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Geller DA, Biederman J, Faraone SV, Bellordre CA, Kim GS, Hagermoser L, et al. Disentangling chronological age from age of onset in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2001;4:169–78. doi: 10.1017/S1461145701002395. [DOI] [PubMed] [Google Scholar]
- 31.Tukel R, Ertekin E, Batmaz S, Alyanak F, Sozen A, Aslanta B, et al. Influence of age of onset on clinical features in obsessive-compulsive disorder. Depress Anxiety. 2005;21:112–7. doi: 10.1002/da.20065. [DOI] [PubMed] [Google Scholar]
- 32.Campbell LA, Brown TA, Grisham JR. The relevance of age of onset to the psychopathology of generalized anxiety disorder. Behav Ther. 2003;34:31–48. [Google Scholar]
- 33.Pinto A, Phillips KA. Social anxiety in body dysmorphic disorder. Body Image. 2005;2:401–5. doi: 10.1016/j.bodyim.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips KA, Stein DJ, Rauch SL, Hollander E, Fallon BA, Barsky A, et al. Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depress Anxiety. 2010;27:528–55. doi: 10.1002/da.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobin C, Blundell ML, Karayiorgou M. Phenotypic differences in early- and late-onset obsessive-compulsive disorder. Compr Psychiatry. 2000;41:373–9. doi: 10.1053/comp.2000.9009. [DOI] [PubMed] [Google Scholar]
- 36.Fontenelle L, Mendlowicz MV, Marques C, Versiani M. Early- and late-onset obsessive-compulsive disorder in adult patients: An exploratory clinical and therapeutic study. J Psychiatr Res. 2003;37:127–33. doi: 10.1016/s0022-3956(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Salgado B, Dolengevich-Segal H, Arrojo-Romero M, Castelli-Candia P, Navio-Acosta M, Perez-Rodriguez MM, et al. Perceived quality of life in obsessive-compulsive disorder: Related factors. BMC Psychiatry. 2006;6:20. doi: 10.1186/1471-244X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biffin F, Tahtalian S, Filia K, Fitzgerald PB, De Castella AR, Filia S, et al. The impact of age at onset of bipolar I disorder on functioning and clinical presentation. Acta Neuropsychiatrica. 2009;21:191–6. doi: 10.1111/j.1601-5215.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- 39.Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry. 2003;44:270–6. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt S. Shall we really do it again? The powerful concept of replication is neglected in the social sciences. Rev Gen Psychol. 2009;13:90–100. [Google Scholar]
- 41.Phillips KA, Menard W, Fay C. Gender similarities and differences in 200 individuals with body dysmorphic disorder. Compr Psychiatry. 2006;47:77–87. doi: 10.1016/j.comppsych.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips KA, Mcelroy SL, Keck PE, Pope HG, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry. 1993;150:302–8. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- 43.Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. J Nerv Ment Dis. 1997;185:570–7. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Phillips KA, Dwight MM, Mcelroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 1998;59:165–71. doi: 10.4088/jcp.v59n0404. [DOI] [PubMed] [Google Scholar]
- 45.Phillips KA. An open-label study of escitalopram in body dysmorphic disorder. Int Clin Psychopharmacol. 2006;21:177–9. doi: 10.1097/01.yic.0000194378.65460.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips KA, Najar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry. 2003;64:715–20. doi: 10.4088/jcp.v64n0615. [DOI] [PubMed] [Google Scholar]
- 47.Phillips KA, Menard W. A prospective pilot study of levetiracetam for body dysmorphic disorder. CNS Spectr. 2009;14:252–60. doi: 10.1017/s1092852900025414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo-controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59:381–8. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 49.Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR. A severity rating scale for body dysmorphic disorder: Development, reliability, and validity of a modified version of the Yale-Brown obsessive compulsive scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- 50.Keller MB, Lavori PW, Friedman B, Nielsen E. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–8. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 51.Warshaw MG, Keller MB, Stout RL. Reliability and validity of the longitudinal interval follow-up evaluation for assessing outcome of anxiety disorders. J Psychiatr Res. 1994;28:531–45. doi: 10.1016/0022-3956(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 52.Rosen JC, Reiter J. Development of the body dysmorphic disorder examination. Behav Res Ther. 1996;34:755–66. doi: 10.1016/0005-7967(96)00024-1. [DOI] [PubMed] [Google Scholar]
- 53.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: Reliability and validity. Am J Psychiatry. 1998;155:102–8. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- 54.Miller IW, Bishop SB, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: Reliability and validity. Psychiatry Res. 1985;14:131–42. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 55.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID): I. History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 56.Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 57.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R personality disorders (SCID-II). I: Description. J Pers Disord. 1995;9:83–91. [Google Scholar]
- 58.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R personality disorders (SCID-II): II. Multi-site test-retest reliability study. J Pers Disord. 1995;9:92–104. [Google Scholar]
- 59.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P) New York: Biometrics Research; 2002. [Google Scholar]
- 60.Phillips KA. The broken mirror: Understanding and treating body dysmorphic disorder. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 61.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders: Non-patient edition (SCID-N/P) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 62.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II) Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- 63.Leon A, Solomon D, Mueller T, Turvey C, Endicott J, Keller M. The range of impaired functioning tool (LIFE-RIFT): A brief measure of functional impairment. Psychol Med. 1999;29:869–78. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- 64.Weissman M, Prusoff B, Thompson W, Harding P, Myers J. Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis. 1978;166:317–26. doi: 10.1097/00005053-197805000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Ware JE. SF-36 health survey manual and interpretation guide. Boston, MA: New England Medical Center; 1993. [Google Scholar]
- 66.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- 67.Hollander E, Cohen LJ, Simeon D. Body dysmorphic disorder. Psychiatr Ann. 1993;23:359–64. [Google Scholar]
- 68.Neziroglu FA, Yaryura-Tobias JA. Exposure, response prevention, and cognitive therapy in the treatment of body dysmorphic disorder. Behav Ther. 1993;24:431–8. doi: 10.1016/s0005-7967(96)00082-4. [DOI] [PubMed] [Google Scholar]
- 69.Yamada M, Kobashi K, Shigemoto T, Ota T. On dismorphophobia. Bull Yamaguchi Med Sch. 1978;25:47–54. [Google Scholar]
- 70.Stein DJ, Carey PD, Warwick J. Beauty and the beast: Psychobiologic and evolutionary perspectives on body dysmorphic disorder. CNS Spectr. 2006;11:419–22. doi: 10.1017/s1092852900014590. [DOI] [PubMed] [Google Scholar]
- 71.Feusner JD, Hembacher E, Phillips KA. The mouse who couldn’t stop washing: Pathologic grooming in animals and humans. CNS Spectr. 2009;14:503–13. doi: 10.1017/s1092852900023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips KA. Understanding body dysmorphic disorder: An essential guide. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 73.Levine M, Smolak M. Body image development in adolescence. In: Cash T, Pruzinsky T, editors. Body image: A handbook of theory, research, and clinical practice. New York, NY: The Guilford Press; 2002. pp. 74–82. [Google Scholar]
- 74.Delorme R, Golmard JL, Chabane N, Millet B, Krebs MO, Mouren-Simeoni MC, et al. Admixture analysis of age at onset in obsessive-compulsive disorder. Psychol Med. 2005;35:237–43. doi: 10.1017/s0033291704003253. [DOI] [PubMed] [Google Scholar]
- 75.Grant JE, Menard W, Pagano ME, Fay C, Phillips KA. Substance use disorders in individuals with body dysmorphic disorder. J Clin Psychiatry. 2005;66:309–16. doi: 10.4088/jcp.v66n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. 2004;129:127–40. doi: 10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Joa I, Johannessen JO, Langeveld J, Friis S, Melle I, Opjordsmoen S, et al. Baseline profiles of adolescent vs. adult-onset first-episode psychosis in an early detection program. Acta Psychiatr Scand. 2009;119:494–500. doi: 10.1111/j.1600-0447.2008.01338.x. [DOI] [PubMed] [Google Scholar]
- 78.Sax KW, Strakowski SM, Keck PE, Jr, Mcelroy SL, West SA, Bourne ML, et al. Comparison of patients with early-, typical-, and late-onset affective psychosis. Am J Psychiatry. 1997;154:1299–301. doi: 10.1176/ajp.154.9.1299. [DOI] [PubMed] [Google Scholar]
- 79.Dalrymple KL, Zimmerman M. Age of onset of social anxiety disorder in depressed outpatients. J Anxiety Disord. 2011;25:131–7. doi: 10.1016/j.janxdis.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, et al. High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. J Abnorm Psychol. 2012;121:339–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown B, Dolcini M, Leventhal A. Transformations in peer relationships at adolescence: Implications for health-related behavior. In: Schulenberg J, Maggs J, Hurrelmann K, editors. Health risks and developmental transitions during adolescence. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 82.Igra V, Irwin C. Theories of adolescent risk-taking behavior. In: DiClemente R, Hansen W, Ponton L, editors. Handbook of adolescent health risk behavior. New York, NY: Plenum Press; 1996. [Google Scholar]
- 83.Maggs J. Alcohol use and binge drinking as goal-directed action during the transition to postsecondary education. In: Schulenberg J, Maggs J, Hurrelmann K, editors. Health risks and developmental transitions during adolescence. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 84.Phillips KA, Rogers J. Cognitive-Behavioral Therapy for youth with body dysmorphic disorder: Current status and future directions. Child Adolesc Psychiatr Clin N Am. 2011;20:287–304. doi: 10.1016/j.chc.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schulenberg J, Maggs J, Hurrelmann K. Negotiating developmental transitions during adolescence and young adulthood: Health risks and opportunities. In: Schulenberg J, Maggs J, Hurrelmann K, editors. Health risks and developmental transitions during adolescence. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 86.Evans D, Seligmann M. Introduction. In: Evans D, Foa E, Gur R, Hendin H, O’Brian C, Seligman M, et al., editors. Treating and preventing adolescent mental health disorders: What we know and what we don’t know. A research agenda for improving the mental health of our youth. New York, NY: Oxford University Press; 2005. p. xxv. [Google Scholar]