Abstract
Angiotensin converting enzyme (ACE) is best known for the catalytic conversion of angiotensin I to angiotensin II. However, the use of gene-targeting techniques has led to mouse models highlighting many other biochemical properties and actions of this enzyme. This review discusses recent studies examining the functional significance of ACE tissue-specific expression and the presence in ACE of two independent catalytic sites with distinct substrates and biological effects. It is these features which explain why ACE makes important contributions to many different physiological processes including renal development, blood pressure control, inflammation and immunity.
Keywords: Angiotensin II, Ac-SDKP, blood pressure, kidney function, MHC class I, antigen processing
Introduction
The 1956 discovery by Skeggs that angiotensin II results from the cleavage of angiotensin I by angiotensin-converting enzyme (ACE) paved the way for a spectacular (and now legendary) success story [1]. Insightful understanding of ACE, a little help from a Brazilian viper, and a touch of luck led to the development of ACE inhibitors which are now powerful tools to treat hypertension, heart failure and other diseases. In part, these drugs are effective because ACE inhibitors prevent angiotensin II formation. However, to understand ACE, one must realize that ACE has many effects beyond angiotensin II production. ACE cleaves many substrates, including angiotensin I, bradykinin, substance P, the tetrapeptide AcSDKP and several others [2-5]. This is one reason that ACE has so many different physiologic actions. Besides wide substrate specificity, there are several biochemical features that make ACE distinctive and contribute to its diverse physiologic effects. For example, the cloning of ACE revealed that the enzyme consists of two independent catalytic domains [6, 7]. These domains share a high degree of amino acid homology and are thought to be the result of an ancient gene duplication [8]. In many respects, the structure of ACE as a single polypeptide chain organized into two independent catalytic domains is the defining physical characteristic of the enzyme. Another important feature of ACE is the physical localization of high quantities of the enzyme on the surface of vascular endothelium, positioning ACE to produce angiotensin II in immediate juxtaposition to vascular smooth muscle [2, 9]. This in turn causes vasoconstriction and raises blood pressure. Large amounts of ACE are also produced by epithelium of the renal proximal tubule [10]. ACE in these and several other locations raise the question as to what are the local effects. Here we will focus on three topics: the local role of ACE in the kidney, novel insights into the function of each of the ACE catalytic domains, and studies on the role of ACE on the immune response. Many studies examining these areas have used mutated mice created through targeted homologous recombination in embryonic stem cells. While such animals are unique and wondrous tools allowing the creation of virtually any type of mutation, it is worth noting that these models do have limitations. For example, an animal lacking a gene from conception has the theoretical possibility of compensating for the genetic change. Further, while mice are surprisingly close to humans in their biology, and mouse genetic models have been generally quite successful in mimicking human syndromes, this is not always the case; there are several instances in which mouse and human physiology and pathology have not proven to be identical [11-14]. Nonetheless, many published studies do show the remarkable and unique biological insights that can be obtained by studying genetically modified mice [15, 16].
The phenotype of ACE null mice
The multiple actions of ACE are demonstrated by the phenotype of mice null for all ACE expression (ACE knockout (KO) mice) [17, 18]. Such animals have profound hypotension with systolic blood pressures nearly 40 mmHg lower than wild type (WT) mice. The ACE KO mice also have renal developmental abnormalities characterized by hypoplastic renal medullas and an expansion of the renal papillae. Knockout mice are also unable to effectively concentrate urine. These hemodynamic and renal abnormalities are common to mice lacking either ACE, angiotensinogen, renin, or the AT1 receptor (AT1R), suggesting that the phenotype is caused by a lack of angiotensin II formation [19-23]. However, ACE KO mice also have phenotypic abnormalities not seen in other models lacking renin-angiotensin system (RAS) components. For example, the absence of ACE by germ cells induces male infertility, a phenotype not present in angiotensinogen KO mice [17, 18]. We note that human males treated with ACE inhibitors are not reported to be infertile, perhaps because of the lack of total ACE inhibition in the testes during drug administration [24]. ACE null mice also have a variety of hematologic abnormalities, including anemia and a shift toward increased production of immature myeloid cells [17, 18, 25]. These abnormalities are more extensive than have been reported for angiotensinogen or renin knockout mice. Some of the phenotypes, such as anemia, have been observed in normal human volunteers administered ACE inhibitors [26]. Further, analysis of rats treated with either an ACE inhibitor or an AT1 receptor antagonist during only the first two weeks of life resulted in adult animals with widening of the renal papillary space and a reduction in the ability to concentrate urine, akin to the changes in ACE KO mice [27, 28]. Finally, in human newborns exposed to ACE inhibitors, there is renal juxtaglomerular hyperplasia, dilatation of Bowman's space, renal tubular dilatation and increased cortical and medullary fibrosis [29, 30].
Importance of endothelial ACE
The high expression level of ACE by vascular endothelium has been known for many years. Indeed, under normal physiologic conditions, virtually all plasma angiotensin I is converted to angiotensin II during a single pass through the lung, an organ rich in endothelium and ACE expression [9]. To examine the role of endothelial ACE promoter ‘swapping’ was used to place the ACE gene under the control of the albumin promoter [31]. These new mice, termed ACE 3/3, produce ACE on the surface of hepatocytes but make no endothelial ACE. Active ACE is released into the plasma by the actions of a cell surface sheddase and, as a result, ACE 3/3 mice have roughly equivalent levels of circulating ACE activity as found in WT mice [32]. Surprisingly, despite a total lack of endothelial ACE, ACE 3/3 mice have normal blood pressure, normal renal morphology and normal renal function. Several other mouse models were made in which ACE expression was targeted to individual tissues while eliminating its expression from endothelium. Yet, similar to what was found in the ACE 3/3 mice, virtually every animal model that makes some ACE is able to maintain a normal blood pressure, even if ACE expression was present in non-traditional tissue types [33, 34]. These data are consistent with studies from Dr. Oliver Smithies, who used both animal models and computer modeling to conclude that the exact levels of ACE in vivo have little long-term effect on basal blood pressure levels [17, 35]. In fact, renin expression is highly variable and can rapidly compensate for differences in ACE expression. Smithies concluded that only when the reduction of ACE activity is greater than 90% will renin production no longer be able to compensate. In such an instance, there is a reduction of blood pressure. Thus, the conclusion from this and a variety of other studies is that while endothelial ACE is a major source of angiotensin II production, the plasticity of the RAS is such that changes in renin expression can compensate for remarkable alterations in both the tissue distribution and the tissue levels of ACE.
Circulating ACE levels are not identical in all humans. Males typically make more ACE than females [36, 37]. Children 4 to 18 years old typically have higher ACE levels than adults. One of the major factors influencing ACE levels is a genetic polymorphism first reported by Rigat et al in 1990 [38]. This group identified a 287 base pair Alu repeat within the 16th intron of the ACE gene (17q23). The presence of this Alu repeat, termed the I (insert) allele, contrasted with the lack of the repeat, the D (deletion) allele. Humans having the D/D genotype have the highest average serum ACE levels (494.1 ± 88.3 μg/L), heterozygous individuals (genotype I/D) have an intermediate level of ACE (392.6 ± 66.8 μg/L), and those that are homozygous I/I in genotype have on average the lowest ACE levels (299.3 ± 49 μg/L). This genetic polymorphism accounts for approximately 47% of the differences in serum ACE levels [38]. Further studies showed that it was probably not the presence of the Alu repeat itself that caused these differences in ACE levels but rather another ACE gene variant in strong linkage disequilibrium with the Alu repeat polymorphism [37]. The presence of other polymorphisms with significant effects on plasma ACE levels and their impact on blood pressure has been reported [39, 40].
In 1992, a report appeared that indicated that the D/D genotype was found more frequently in patients with myocardial infarction than in control subjects [41]. This initiated many studies examining the association of the I and D genotypes (and associated ACE levels) with a variety of diseases including heart failure, hypertension and even Alzheimer's disease. While the initial studies suggested an important role in cardiovascular pathology, these conclusions became progressively less certain with increased numbers of studies incorporating ever larger numbers of subjects. Several meta-analyses have investigated the role of the I/D polymorphism. For example, a study conducted by Agerholm-Larsen in 2000 reported that in over 40 studies comprising 42,715 Caucasian subjects, plasma ACE was increased in the D/D genotype, but blood pressure, increased risk of myocardial infarction, coronary disease or stroke was not associated with the polymorphism [42]. Thus, the bulk of human investigation to date is consistent with what was found in animals, namely, that apart from the nearly complete inhibition of ACE activity induced by ACE inhibitors, natural variations in plasma ACE levels have little effect on average blood pressure levels or target organ damage [8].
One of the most interesting points of contention in considering ACE is the relative physiologic importance of tissue bound ACE vs. the active enzyme that circulates in plasma. While several groups have considered this question, even today the correct interpretation is quite nuanced. First, it is important to emphasize that the vast majority of ACE is bound to tissues. Lung, testis and kidney contain abundant amounts of ACE. For example, ACE comprises approximately 0.1% of the total protein of lung. In contrast, circulating ACE is only about 0.0017% of total serum proteins [43, 44]. A mouse model that had about 75% normal plasma ACE activity (measured under substrate limiting conditions) but lacked all tissue-bound ACE was still highly deficient in overall ACE activity and presented with a reduced blood pressure equivalent to a complete ACE knockout mouse [45]. Second, both forms of ACE are equally catalytic [46]; in humans, the cell bound sheddase that releases ACE from tissues, cleaves human ACE after Arg1203, releasing a soluble form of the protein containing both ACE catalytic domains [47]. Finally, from the perspective of the receptors that ultimately mediate the effects of angiotensin II and other ACE substrates, it is the concentration of ligand that is important. In other words, receptors make no distinction whether the ligand resulted from circulating or tissue-bound ACE. Thus, under basal circumstances, tissue ACE and, to a lesser degree, the circulating form of ACE combine to produce the final concentration of angiotensin II and other ACE substrates.
The important role of renal ACE
Many scientists have emphasized the important role of the kidney in regulating blood pressure [48, 49]. The kidney comprises 25 different cell types of which ACE expression has been reported in at least 5, including endothelial cells, mesangial cells and epithelial cells from the proximal tubule and distal regions of the nephron [50-53]. By far, the site of highest expression of ACE is the brush border of the proximal tubule [10]. In addition to ACE, angiotensinogen, renin and the AT1 receptor are found within the kidney, raising the question of the specific local effects of angiotensin II production within this organ [54]. The observation that the systemic knockout of either angiotensinogen, renin, ACE or the AT1 receptor results in a common phenotype consisting of renal arteriolar thickening, hypotension, juxtaglomerular cell hypertrophy, hypoplastic renal medulla and papilla, hydronephrosis and the inability to concentrate urine has been used to support the concept that angiotensin II production is important in renal organogenesis [19-23]. In addition, several studies indicate that the expression of ACE in the kidneys is temporally programmed during renal development [55]. However, several mouse models have been created in which ACE is either totally absent or nearly totally absent from renal tissue while being expressed in other locations. Normal renal development and renal function in these mouse models indicate that angiotensin II production by ACE from renal origin is thus not necessary to ensure proper organogenesis [31, 33, 34]. Additional insight was provided by experiments in a mouse model expressing ACE only in renal tissues and nowhere else. These mice, termed ACE 9/9, were created using the KSP-cadherin promoter to substitute for the endogenous ACE promoter, resulting in ACE production only throughout renal tubular epithelium. Because of the restricted expression of ACE to renal tissues, this model still had a profound reduction of total ACE protein including the lack of ACE on any tissues positioned to convert plasma angiotensin I to angiotensin II. Probably because of this, the ACE 9/9 mice develop a phenotype very similar to systemic ACE knockout mice with hypotension, varying degrees of renal medullary thinning and an inability to concentrate urine. Thus, a variety of experiments indicate that local ACE expression and angiotensin II generation within renal tissue is not sufficient to preserve normal kidney morphology and function in the setting of hypotension due to a systemic lack of ACE activity.
Renal ACE and baseline kidney function
The detailed physiologic study of specific functions of renal ACE has been complicated by the low blood pressure present in several ACE genetic models [56]. However, mouse genetic models lacking renal ACE while maintaining normal blood pressure support the concept that, in normotensive animals, renal ACE is not critical for basal renal function. For example, when ACE is only expressed in the heart, or only in the liver or only in myelomonocytic cells, renal concentrating ability is preserved [31, 33, 34]. Further, a compound heterozygous mouse in which one ACE allele was null and a second ACE allele targeted ACE expression to the liver had a normal blood pressure, glomerular filtration rate (GFR), single nephron GFR, renal plasma flow and renal concentrating ability [57]. In this model, only tubuloglomerular feedback was abnormal; it being significantly reduced in the absence of renal ACE.
Renal ACE and hypertension
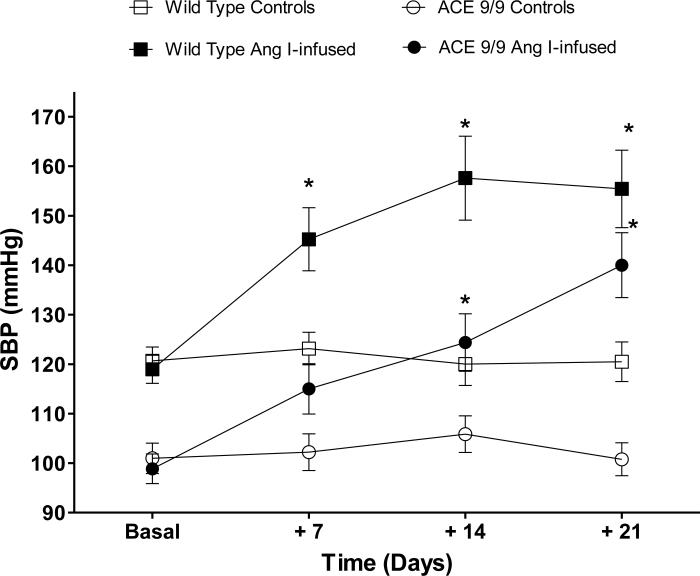
While indisputable evidence supports a role for the RAS in hypertension, a paradox is the absence of consistent signs of systemic RAS activation in most hypertensive subjects [58]. This has focused attention on alterations of organ-specific RAS function as responsible for, or at least permissive of, elevated blood pressure. Evidence from several laboratories supports the ability of an increased intrarenal RAS to elevate blood pressure. For example, transgenic mice over expressing angiotensinogen and renin in proximal tubular cells develop hypertension [59-62]. We recently reported that when ACE 9/9 mice (mice expressing ACE only in renal tubules) were exposed to chronic angiotensin I infusion, they increased renal levels of angiotensin II, increased the rate of urinary angiotensin II excretion and developed hypertension (Figure 1) [52]. In these experiments, regardless of their basal hypotension, ACE 9/9 mice respond to systemic chronic angiotensin I infusion with a blood pressure increase that was similar in magnitude to that observed wild-type mice treated with angiotensin II. Indeed, after three weeks of angiotensin I infusion, ACE 9/9 mice had a blood pressure increase of 41 mmHg over baseline vs. 36 mmHg in wild-type mice. These findings suggest that intrarenal ACE has the capacity to increase local kidney angiotensin II levels and to induce hypertension even in the absence of systemic ACE. Thus, while previous data suggest that under physiological conditions both the systemic and intrarenal RAS work in a concerted manner to maintain homeostasis of blood pressure [63, 64]; in certain pathological states, like the hypertension induced by angiotensin II infusion, there is an independent activation of the intrarenal RAS. Indeed, ACE expression is not only increased during angiotensin II-induced hypertension but also in many other conditions where renal parenchymal function is compromised [65].
Figure 1.
Mice expressing ACE only in renal tubules (ACE 9/9 mice) become hypertensive in response angiotensin I infusion. Wild-type and ACE 9/9 mice were infused with angiotensin I (1000 ng/kg/min via osmotic minipump) for three weeks. Systolic blood pressure was determined by tail-cuff plethysmography. *p<0.05 vs. respective genotype and time-matched controls by two-way ANOVA. Data are presented as means and S.E.M.
The ACE 9/9 mice were envisioned as the ideal approach to dissect the specific effects of renal ACE on blood pressure regulation. However, their renal phenotype and hypotension represented important constrains. For this reason, a second approach was to expose mice with no or minimal amounts of renal ACE to experimental hypertension. Specifically, mice with ACE expression in the liver (termed ACE 3/3) and mice with ACE expression in myelomonocytic cells (termed ACE 10/10) were studied [66]. Both models have normal basal blood pressure, despite markedly reduced or no renal ACE. These studies showed that the absence of kidney ACE blunted the hypertension induced either by angiotensin II infusion or by nitric oxide synthesis inhibition. Further, the renal response to high plasma angiotensin II was fundamentally changed. Indeed, intrarenal angiotensin II elevation, sodium and water retention, and activation of several transporters along the nephron, including NKCC2, NCC and ENaC were substantially reduced or absent in the ACE 3/3 and ACE 10/10 mice. Thus, we found that the ACE within the kidney and the local, renal generation of angiotensin II work as master regulators of sodium transport along the nephron. These actions appear indispensable for mounting an anti-natriuretic response during experimental hypertension, even in the presence of high plasma angiotensin II levels [66].
Function of the N- and C-domains of ACE
When ACE was first cloned, it became apparent that this enzyme is composed of a single polypeptide chain organized into two homologous catalytic domains, the N- and C-domains [6, 7]. These domains are homologous in sequence; each domain independently binds zinc and is capable of ACE catalytic activity [67, 68]. Experimental data has indicated that the catalytic activity of the C-domain is probably negatively impacted by the presence of the N-domain [69, 70]. Although the two domains of ACE share many physical characteristics, including significant amino acid homology, they have some different biochemical properties including thermostability, chloride requirements and substrate specificity (reviewed in [8]). For example, in vitro analysis has demonstrated that the Κcat of the ACE C-domain for angiotensin I is approximately 3-fold higher than that of the ACE N-domain [68]. This was investigated in mouse models in which point mutations were introduced into the mouse genome, selectively inactivating the catalytic activity of either the ACE N- or C-domains [71, 72]. Such mice are referred to as N-KO or C-KO, indicating the functional lack of the N- or C-domains, respectively. The genetic introduction of point mutations is quite selective; it prevents the targeted domain from binding the ACE cofactor zinc [68]. Both the NKO and C-KO mice have a normal sized ACE protein, a normal pattern of ACE tissue expression and a normal tissue abundance of ACE. Under basal conditions, both strains of mice maintain normal blood pressure and have plasma levels of angiotensin II similar to those observed in WT mice. However, analysis of angiotensin I levels and renin activity revealed a more complex picture. The NKO mouse, lacking ACE N-domain activity, had renin and angiotensin I levels that were essentially identical to WT mice. In contrast, the C-KO mouse, lacking C-domain activity, maintained a normal blood pressure only by elevating renin and by increasing 7-fold the plasma levels of angiotensin I. These data indicate that, at least under in vivo basal conditions, it is the ACE C-domain that is mainly responsible for the conversion of angiotensin I to angiotensin II.
If the ACE C-domain is predominantly responsible for angiotensin II production, why has a catalytically active ACE N-domain been preserved over hundreds of millions of years? While the precise answer is not known, what is certain is that there are peptides that are preferentially or exclusively cleaved by the ACE N-domain. Perhaps the most interesting is the tetrapeptide acetyl-SerAspLysPro (AcSDKP) [73]. In the N-KO mouse, the plasma concentration of this peptide is 7.3-fold that measured in a WT mouse [71]. The initial studies of AcSDKP indicated that this peptide inhibited the recruitment of hematopoietic progenitors into active proliferation. AcSDKP has been studied for its effect on a variety of other physiologic processes, including angiogenesis [74]. Several studies from the laboratory of Dr. Oscar Carretero have used models of heart or kidney injury to show that AcSDKP can inhibit fibrosis [75-81]. Our group has studied bleomycin-induced lung injury in the N-KO mouse [82]. Bleomycin is a chemotherapeutic agent that can induce lung fibrosis. When N-KO or WT mice were exposed to equivalent concentrations of intratracheal bleomycin, there was much less inflammation and fibrosis in the N-KO mice. As discussed, these animals have elevated AcSDKP levels and this peptide was implicated as the etiologic cause of the reduced injury response observed in the N-KO model. Thus, several studies implicate an ACE N-domain substrate as important in regulating the injury response.
ACE and immunity
Recently, several lines of investigation have focused on potential roles of ACE in the immune response. This work was the natural outgrowth of three research trends concerning the RAS. First was the realization that angiotensin II can be a pro-inflammatory molecule. Further, recent experiments from Dr. David Harrison and others have emphasized inflammation as an important factor contributing to hypertension [83, 84]. Finally, mouse experiments discussed below indicate that manipulation of myeloid cell ACE levels can have profound effects on the immune response. While a discussion of the complete effects of the ACE on the immune response are beyond this review, here we focus on studies indicating that enhanced ACE expression by myelomonocytic cells can markedly augment the immune response.
Cytokine expression
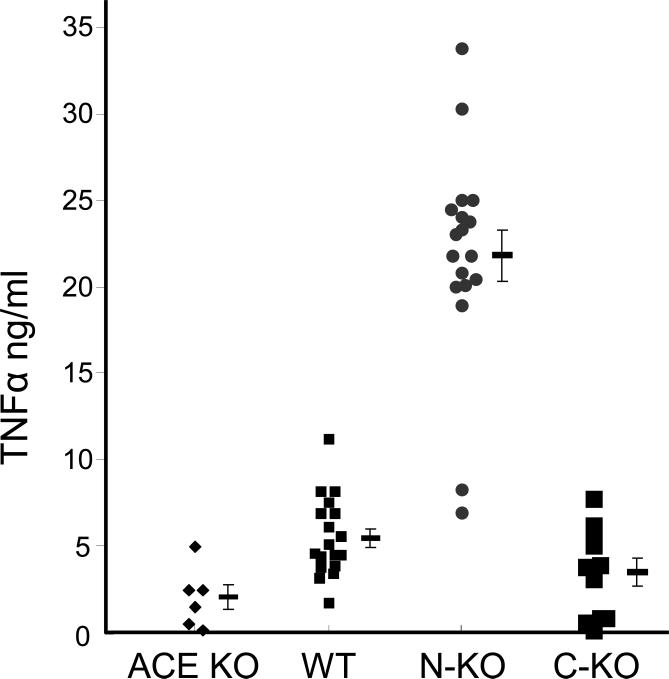
There is significant data suggesting that ACE may play a role in regulating cytokine expression by monocytes and macrophages. These data come from an analysis of the NKO mice discussed above [82, 85]. Figure 2 shows data from an experiment in which thioglycollate-elicited peritoneal macrophages were collected and exposed in vitro to lipopolysaccharide (LPS) for 18 hours. LPS is a well-studied immune activator and macrophages respond to LPS exposure by increased production of a variety of pro-inflammatory cytokines, including IL-12, nitric oxide, and TNFα. Cells derived from C-KO mice (animals lacking ACE C-terminal activity) produce TNFα levels equivalent to cells from WT mice. In contrast, equivalent cells derived from N-KO mice produce substantially higher levels of TNFα. While the precise biochemical differences giving rise to such exaggerated TNFα production by N-KO cells is not understood, it is known that a similar increase of TNFα production is observed in vivo in N-KO mice exposed to LPS. Further, increased TNFα production was found in peritoneal macrophages from N-KO mice made hypertensive by angiotensin II infusion. In vitro, N-KO macrophages over produce the pro-inflammatory cytokine IL-12 in response to LPS but produce less of the anti-inflammatory cytokine IL-10 [85]. While these findings clearly show an effect of the ACE N-domain on cytokine production, what is not clear is whether this is a direct effect on the biochemistry of cytokine production, or whether the biochemical milieu within the ACE N-KO mice somehow instructs macrophages along a developmental program leading to changes in differentiation state and an intrinsically higher inflammatory response to pro-inflammatory stimuli.
Figure 2.
ACE affects macrophage TNFα production. Peritoneal macrophages were collected from ACE knockout (KO), wild type (WT), N-KO and C-KO mice four days after intraperitoneal thioglycollate injection. After purification by adhesion, the cells were cultured for 18 h in the presence of 1 μg/ml LPS. TNFα concentration was then measured by ELISA. Data from individual mice, as well as the group means and S.E.M., are shown. Cells from N-KO mice produce much more TNFα than cells from the other ACE genotypes.
ACE and MHC class I peptide processing
Recently, ACE has been described as affecting another immune phenomenon, the processing of peptides displayed by major histocompatibility (MHC) class I molecules on the surface of cells [86-89]. Under basal conditions, MHC class I molecules display peptides derived from the normal protein complement of a cell. The peptide repertoire displayed by each individual identifies that individual and the immune system of the individual is tolerant of (i.e., non-reactive against) such displayed peptides. However, following viral infection, peptides derived from viral proteins are also displayed on the surface of cells. These are detected as foreign and lead to CD8+ T cell activation and destruction of the infected cells.
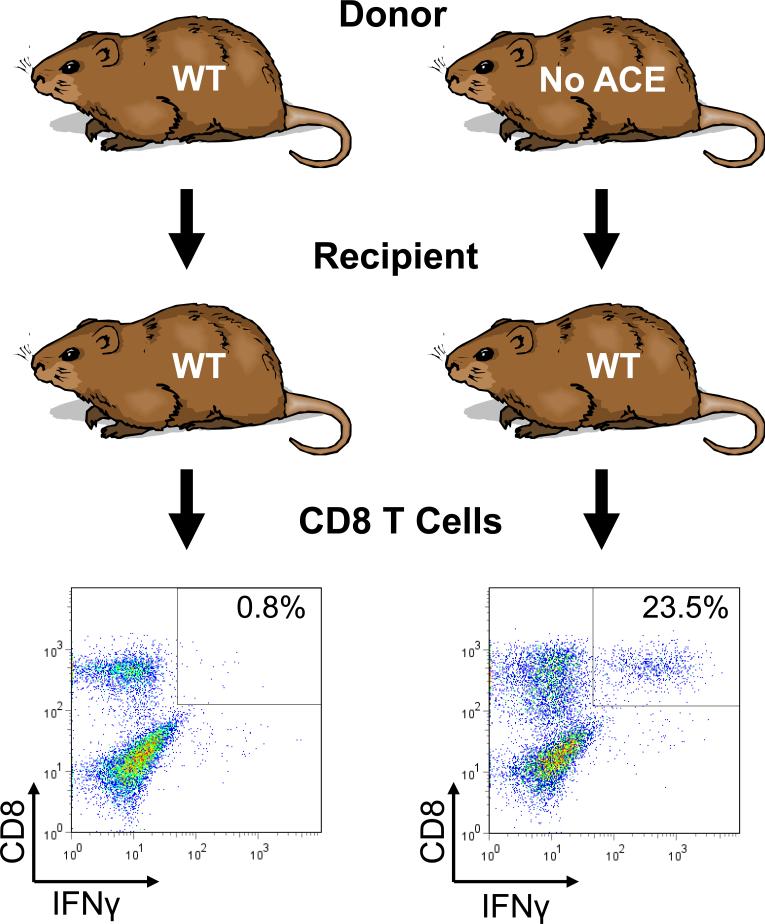
Published studies indicated that when ACE was over expressed in cells, it affected the normal processing of MHC class I peptides [90, 91]. Specifically, peptides undergo trimming in the endoplasmic reticulum before being loaded onto MHC class I molecules and trafficked to the cell surface. While the over expression of ACE can affect such trimming, we wondered what was the role of natural levels of ACE expression on this important immune function. To investigate this, several approaches were used to assess MHC class I peptide repertoire in ACE KO and ACE WT mice. Perhaps most informative were experiments that took advantage of cross-immunization [91]. If a mouse is immunized with cells that are totally identical (syngeneic), the T cells of the recipient mouse will be tolerant and there will be no CD8+ T cell activation. However, if the same experiment is performed in animals that are not identical, the recipient mice will recognize the immunizing (or transplanted) cells as foreign, leading to CD8+ T cell activation. This can be detected because activated CD8+ T cells make interferon-γ (IFN-γ). To assess the role of ACE, we created two strains of mice that were on the same genetic background except for the presence or absence of ACE. Following cross-immunization, it became apparent that these two strains treat each other's tissues as non-identical. As shown in Figure 3, when recipient WT mice receive WT donor tissue, less than 1% of CD8+ T cells produce IFN-γ. In contrast, when WT mice were immunized with cells from a mouse that was an ACE KO, more than 20% of the CD8+ T cells were activated and produced IFN-γ. The opposite experiment (ACE WT donor and ACE KO recipient) showed exactly the same results. Thus, these experiments suggested the very novel finding that the MHC class I peptide repertoire presented by a mouse null for ACE expression is sufficiently different from that of a WT mouse that the two animals recognize each other's tissues as foreign. These conclusions were quite novel since the general consensus was that MHC class I peptides were enzymatically trimmed from their N-termini, and not by a carboxypeptidase such as ACE. Many other experiments and controls were performed to verify the conclusions. One set of experiments used a specific anti-MHC class I antibody that bound to the immunizing cells and partially prevented MHC recognition by the recipient's CD8+ T cells. The specificity of this antibody proved that it was MHC class I (and not other factors such as cytokines) that evoked the immune response. In a second important control, a WT recipient mouse was immunized with cells from an ACE KO mouse. The recipient was then sacrificed and its splenocytes were challenged with one of two syngeneic cell populations: either syngeneic cells from an ACE WT mouse or syngeneic cells from an ACE WT mouse, but from a donor that was previously treated with an ACE inhibitor for several days. When the recipient WT mouse was immunized with ACE KO tissues and then challenged with WT cells (i.e., totally syngeneic cells) but from an animal treated with an ACE inhibitor, the WT recipient's T cells detected foreign epitopes, resulting in T cell activation and IFN-γ production. Human patients taking ACE inhibitors are neither immuno-suppressed nor typically develop autoimmune disease. This may be due to the many controls that normally regulate the immune response. Nonetheless, these data suggest that, at least in mice, the carboxypeptidase ACE does play a role in a very important immune process, the generation of MHC class I peptide diversity.
Figure 3.
Recipient WT mice were immunized with macrophages from donor mice that were either WT for ACE or ACE KO (no ACE). All mice were on a C57BL/6 genetic background. Ten days later, the recipient mice were sacrificed and recipient splenocytes were restimulated in vitro with cells identical in ACE genotype to those used for the initial immunization. After 7 days in culture, flow cytometry was performed 5 hrs. after a final restimulation. This analysis examined cell surface expression of CD8 and intracellular expression of IFN-γ. The percentage of CD8+ cells that are also IFN-γ+ is indicated in the boxed area. ACE WT mice are essentially tolerant of immunization/restimulation by syngeneic ACE WT cells. However, an equivalent immunization with ACE KO cells induced ACE WT CD8+ cell activation.
The genetic over expression of ACE in myelomonocytic cells
Shen et al. used targeted homologous recombination to place the ACE gene under the control of the c-fms promoter [34]. As a result, mice homozygous for the mutant allele, termed ACE 10/10 mice, express large quantities of ACE in myelomonocytic cells including monocytes, macrophages, Kupfer cells and microglia. Further, these mice lack ACE expression in other tissues, including the endothelium and the kidneys. Despite this distinct pattern of expression, the phenotype of these mice is grossly normal; they maintain normal blood pressure, kidney function and normal histological appearance of the bone marrow and peripheral blood. ACE 10/10 mice have an enhanced innate and adaptive immune response that confers significant anti-tumoral and anti-bacterial protection [34, 92]. When normal mice were challenged with an intradermal inoculation of B16 melanoma cells, they developed a large neoplasm. However, ACE 10/10 mice showed substantial anti-tumoral protection with tumors that averaged only one-sixth (1/6) the size of those in WT mice. This was associated with increased tumor and vascular inflammation, with a large increase in the number of intratumor monocytes and macrophages. There was also a systemic increase in the number of anti-tumor CD8+ T cells. Further, the analysis of the ACE 10/10 macrophages revealed an increased production of pro-inflammatory cytokines. That the observed responses were myelocytic specific and not the result of an absence of ACE in other tissues was confirmed by bone marrow transplantation experiments. Specifically, when ACE 10/10 bone marrow was transplanted into WT mice, the recipient mice showed substantially increased protection against B16 melanoma, as compared to equivalent mice transplanted with WT bone marrow. Importantly, the conferred protection was also independent of angiotensin II. The best evidence for this came from experiments in angiotensinogen knockout mice. When these mice were bred with the ACE 10/10 mice, the double mutants exhibited an anti-tumor immune response significantly better than angiotensinogen mice having WT ACE alleles. Further, ACE 10/10 mice treated with an ACE inhibitor displayed an anti-tumor response equivalent to ACE inhibitor treated WT mice; treatment of ACE 10/10 mice with an AT1 receptor antagonist did not reduce the immune response.
The immune protection conferred by the ACE 10 mutation extended beyond the anti-tumoral response. Follow-up experiments showed that ACE 10/10 mice have an increased resistance to infection by either Listeria monocytogenes, an intracellular pathogen, or methicillin-resistant Staphylococcus aureus (MSRA), an extracellular pathogen [92]. Once again, the reduced bacterial burden observed in ACE 10/10 mice was dependent on the catalytic activity of ACE, not on angiotensin II synthesis. In response to bacterial challenge, the macrophages of ACE 10/10 mice made more inducible nitric oxide synthase and more nitric oxide (NO). This is important because NO production and the generation of reactive nitrogen intermediates are an important part of bacterial killing by mouse macrophages.
Anti-viral memory response
As discussed, a major role of MHC class I peptide presentation is to help defend against viral infection. This was tested in ACE 10/10 mice. Polyoma viruses (PyV) are a family of DNA-based viruses. One type of PyV in particular, named BK, is often a problem in immuno-compromised individuals, especially patients receiving renal and bone marrow transplants [93]. PyV is also a mouse pathogen. It is known that the major PyV MHC class I peptide epitope is a peptide termed LT359-368 [94]. When cells are infected with PyV, they will present this peptide on the cell surface bound to MHC class I. Many other viral peptides are also presented, but it is this peptide that will induce a strong CD8+ T cell response.
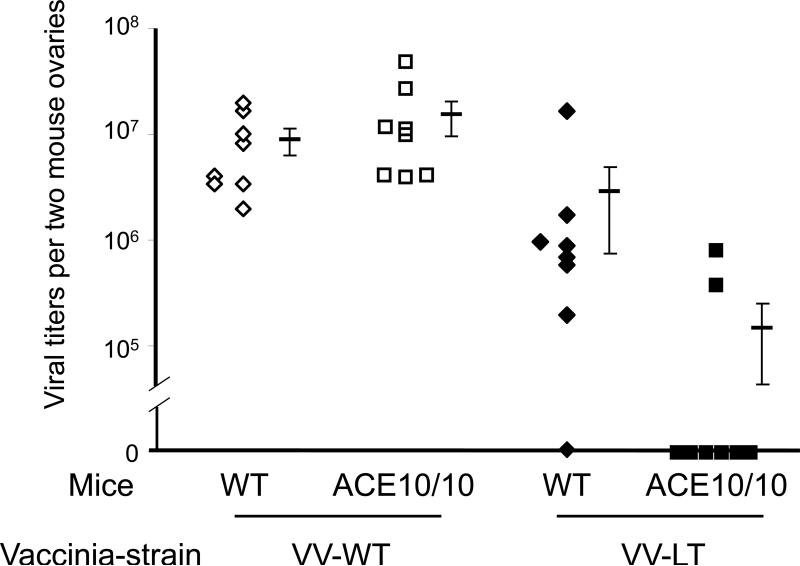
To investigate the immune response in ACE 10/10, we first infected female WT and ACE 10/10 mice with PyV. This causes a chronic, low level infection [95]. At 28 days, the same mice were re-challenged with either WT vaccinia virus or LT359-368 embedded vaccinia virus (VV-LT). Normally, vaccinia virus is not immunologically related to PyV, and indeed, as shown in Figure 4, when ACE 10/10 or WT mice were exposed to WT vaccinia (VV-WT), there were no differences in viral titers. However, for this experiment, we also used the vaccinia strain VV-LT which contains the LT359-368 PyV amino acid sequence against which the mice had previously been exposed during the original PyV infection. In other words, this is a chimeric vaccinia virus containing the critical MHC class I epitope from PyV. Four days after vaccinia infection, mouse ovary vaccinia viral titers were measured by a plaque assay. There was no difference between ACE 10/10 and WT mice exposed to VV-WT (i.e., wild-type vaccinia: Fig. 4: lane 1 and 2). However, with VV-LT, 6 of the 8 ACE 10/10 mice had no detectable vaccinia virus (lane 4). In contrast, only 1 of the 8 WT mice had a similar clearance (lane 3). This dramatically demonstrates the improved immune response present in ACE 10/10 mice.
Figure 4.
All mice were infected with mouse polyoma virus on day 0. On day 28, mice were challenged with either wild type vaccinia virus (VV-WT) or a genetically modified vaccinia virus expressing the polyoma amino acid sequence LT359-368 (VV-LT). Four days after the vaccinia infection, the mice were sacrificed and the titer of vaccinia virus in the ovaries of each mouse was measured by a plaque assay. ACE 10/10 mice are not intrinsically resistant to WT vaccinia virus. However, once immunized with polyoma virus, the ACE 10/10 mice were substantially more resistant to VV-LT vaccinia virus that expressed the major MHC class I epitope of polyoma. The figure shows data from individual mice, as well as the group means and S.E.M.
The above experiments reflect data obtained in a mouse model over-expressing ACE. In some senses, this is equivalent to the pharmacologic use of ACE. In humans, the immune system is composed of many different components and it appears that under normal conditions, the treatment with ACE inhibitors has no appreciable negative effects on the immune response to either infection or malignancy. This was closely examined following a 2010 article suggesting that there may be increased risk of cancer associated with the use of AT1 receptor blockers [96]. Several meta-analyses have now concluded that the use of ACE inhibitors is not associated with increased incidence of cancer [97-99]. In addition, the bulk of these studies suggest no overall risk or mortality for angiotensin II receptor antagonists. However, the incidence of cancer with the combination of receptor antagonists and ACE inhibitors cannot be ruled out.
Conclusion
The outline of the RAS was known in the 1950s when ACE was first discovered. ACE inhibitors were approved for human use in the early 1980s, but the enzyme was not cloned until 1988-1989. It was only then that people realized that ACE was composed of two similar but independently catalytic domains, the N- and C-domains. More than anything, this is the defining characteristic of ACE. The combination of these two domains, relatively nonspecific substrate specificity, and wide tissue distribution, has positioned ACE as an important enzyme in many different physiologic processes. Doctors routinely decrease ACE activity with ACE inhibitors. However, we now know that an increase in ACE activity can have important effects. While ACE has been investigated for over 50 years, the modern understanding of this enzyme has motivated groups to synthesize and study ACE domain specific inhibitors [100, 101]. The clinical utility of such inhibitors are not yet known, yet we would predict that these drugs will eventually offer a repertoire of agents to manipulate ACE, and that such drugs will eventually find their place in clinical practice.
ACKNOWLEDGMENTS
The authors are supported by grants from the National Heart, Lung and Blood Institute (R01HL110353 to K.E.B and W-L.B.B), the National Institute for Diabetes, Digestive and Kidney Diseases (T32DK007770 to K.H.S, R00DK083455 to R.A.G-V), and the American Heart Association (AHA Scientist Development Grant 11SDG6770006 to P.D.S, Beginning Grant-in-Aid 13BGIA14680069 to X.Z.S).
Footnotes
DISCLOSURE
The authors declare that they have no conflict of interests.
REFERENCES
- 1.Skeggs LT, Jr., Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdos EG, Skidgel RA. The angiotensin I-converting enzyme. Lab Invest. 1987;56:345–348. [PubMed] [Google Scholar]
- 3.Skidgel RA, Engelbrecht S, Johnson AR, Erdos EG. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- 4.Skidgel RA, Erdos EG. Novel activity of human angiotensin I converting enzyme: release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1985;82:1025–1029. doi: 10.1073/pnas.82.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizi M, Ezan E, Nicolet L, Grognet J-M, Menard J. High Plasma Level of N-Acetyl-Seryl-Aspartyl-Lysyl-Proline : A New Marker of Chronic Angiotensin-Converting Enzyme Inhibition. Hypertension. 1997;30:1015–1019. doi: 10.1161/01.hyp.30.5.1015. [DOI] [PubMed] [Google Scholar]
- 6.Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein KE, Martin BM, Edwards AS, Bernstein EA. Mouse angiotensin-converting enzyme is a protein composed of two homologous domains. J Biol Chem. 1989;264:11945–11951. [PubMed] [Google Scholar]
- 8.Bernstein KE, Ong FS, Blackwell WL, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, Touyz RM. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev. 2013;65:1–46. doi: 10.1124/pr.112.006809. DOI 10.1124/pr.112.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng KK, Vane JR. Conversion of angiotensin I to angiotensin II. Nature. 1967;216:762–766. doi: 10.1038/216762a0. [DOI] [PubMed] [Google Scholar]
- 10.Alhenc-Gelas F, Baussant T, Hubert C, Soubrier F, Corvol P. The angiotensin converting enzyme in the kidney. J Hypertens Suppl. 1989;7:S9–13. doi: 10.1097/00004872-198909007-00003. discussion S14. [DOI] [PubMed] [Google Scholar]
- 11.Dorin JR. Development of mouse models for cystic fibrosis. Journal of inherited metabolic disease. 1995;18:495–500. doi: 10.1007/BF00710060. [DOI] [PubMed] [Google Scholar]
- 12.Chegary M, Brinke H, Ruiter JP, Wijburg FA, Stoll MS, Minkler PE, van Weeghel M, Schulz H, Hoppel CL, Wanders RJ, Houten SM. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta. 2009;1791:806–815. doi: 10.1016/j.bbalip.2009.05.006. DOI 10.1016/j.bbalip.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King-Underwood L, Little S, Baker M, Clutterbuck R, Delassus S, Enver T, Lebozer C, Min T, Moore A, Schedl A, Pritchard-Jones K. Wt1 is not essential for hematopoiesis in the mouse. Leukemia research. 2005;29:803–812. doi: 10.1016/j.leukres.2004.11.020. DOI 10.1016/j.leukres.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. DOI 10.1189/jlb.0310149. Journal of leukocyte biology. 2010;88:1157–1162. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- 15.Keller C, Capecchi MR. New genetic tactics to model alveolar rhabdomyosarcoma in the mouse. Cancer research. 2005;65:7530–7532. doi: 10.1158/0008-5472.CAN-05-0477. DOI 10.1158/0008-5472.CAN-05-0477. [DOI] [PubMed] [Google Scholar]
- 16.Makki N, Capecchi MR. Cardiovascular defects in a mouse model of HOXA1 syndrome. Hum Mol Genet. 2012;21:26–31. doi: 10.1093/hmg/ddr434. DOI 10.1093/hmg/ddr434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. DOI 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 18.Esther CR, Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 19.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 21.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T, et al. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. DOI 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. DOI 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N, Lopez ML, Cowhig JE, Jr., Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 24.Manolis A, Doumas M. Sexual dysfunction: the ‘prima ballerina’ of hypertension-related quality-of-life complications. J Hypertens. 2008;26:2074–2084. doi: 10.1097/HJH.0b013e32830dd0c6. DOI 10.1097/HJH.0b013e32830dd0c6. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE, Shen XZ. Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 2011;25:1145–1155. doi: 10.1096/fj.10-169433. DOI 10.1096/fj.10-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratt MC, Lewis-Barned NJ, Walker RJ, Bailey RR, Shand BI, Livesey J. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol. 1992;34:363–365. doi: 10.1111/j.1365-2125.1992.tb05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA. Renin-angiotensin system in neonatal rats: induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int. 1994;45:485–492. doi: 10.1038/ki.1994.63. [DOI] [PubMed] [Google Scholar]
- 28.Guron G, Adams MA, Sundelin B, Friberg P. Neonatal angiotensin-converting enzyme inhibition in the rat induces persistent abnormalities in renal function and histology. Hypertension. 1997;29:91–97. doi: 10.1161/01.hyp.29.1.91. [DOI] [PubMed] [Google Scholar]
- 29.Pryde PG, Sedman AB, Nugent CE, Barr M., Jr. Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol. 1993;3:1575–1582. doi: 10.1681/ASN.V391575. [DOI] [PubMed] [Google Scholar]
- 30.Quan A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev. 2006;82:23–28. doi: 10.1016/j.earlhumdev.2005.11.001. DOI S0378-3782(05)00268-9 [pii]10.1016/j.earlhumdev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE. Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure. Circ Res. 2002;90:87–92. doi: 10.1161/hh0102.102360. [DOI] [PubMed] [Google Scholar]
- 32.Hooper NM, Turner AJ. Isolation of two differentially glycosylated forms of peptidyldipeptidase A (angiotensin converting enzyme) from pig brain: a re-evaluation of their role in neuropeptide metabolism. Biochem J. 1987;241:625–633. doi: 10.1042/bj2410625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr., Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. DOI 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol. 2007;170:2122–2134. doi: 10.2353/ajpath.2007.061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smithies O, Kim HS, Takahashi N, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. DOI 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- 36.Beneteau-Burnat B, Baudin B, Morgant G, Baumann FC, Giboudeau J. Serum angiotensin-converting enzyme in healthy and sarcoidotic children: comparison with the reference interval for adults. Clin Chem. 1990;36:344–346. [PubMed] [Google Scholar]
- 37.Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 38.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. DOI 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Bouzekri N, Southam L, Cooper RS, Adeyemo A, McKenzie CA, Luke A, Chen G, Elston RC, Ward R. Linkage and association analysis of angiotensin I-converting enzyme (ACE)-gene polymorphisms with ACE concentration and blood pressure. Am J Hum Genet. 2001;68:1139–1148. doi: 10.1086/320104. DOI 10.1086/320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy D, DeStefano AL, Larson MG, O'Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 41.Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, Luc G, Bard JM, Bara L, Ricard S, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. DOI 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 42.Agerholm-Larsen B, Nordestgaard BG, Tybjaerg-Hansen A. ACE gene polymorphism in cardiovascular disease: meta-analyses of small and large studies in whites. Arterioscler Thromb Vasc Biol. 2000;20:484–492. doi: 10.1161/01.atv.20.2.484. [DOI] [PubMed] [Google Scholar]
- 43.Cushman DW, Cheung HS. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971;250:261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- 44.Soffer RL. John Wiley and Sons; New York: 1981. Angiotensin-converting enzyme. [Google Scholar]
- 45.Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. DOI 10.1172/jci119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das M, Hartley JL, Soffers RL. Serum angiotensin-converting enzyme. Isolation and relationship to the pulmonary enzyme. J Biol Chem. 1977;252:1316–1319. [PubMed] [Google Scholar]
- 47.Woodman ZL, Oppong SY, Cook S, Hooper NM, Schwager SL, Brandt WF, Ehlers MR, Sturrock ED. Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem J. 2000;347(Pt 3):711–718. [PMC free article] [PubMed] [Google Scholar]
- 48.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 49.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–1409. doi: 10.1038/nm.2541. DOI 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 50.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. DOI 10.1161/01.HYP.0000081221. 36703.01 01. HYP.0000081221.36703.01 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–459. doi: 10.1681/ASN.2010060624. DOI 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–157. doi: 10.1152/ajprenal.00477.2009. DOI 00477.2009 [pii] 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular Renin-Angiotensin System in Hypertension. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.163519. DOI HYPERTENSIONAHA.110.163519 [pii] 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yosipiv IV, Dipp S, el-Dahr SS. Ontogeny of somatic angiotensin-converting enzyme. Hypertension. 1994;23:369–374. doi: 10.1161/01.hyp.23.3.369. [DOI] [PubMed] [Google Scholar]
- 56.Traynor T, Yang T, Huang YG, Krege JH, Briggs JP, Smithies O, Schnermann J. Tubuloglomerular feedback in ACE-deficient mice. Am J Physiol. 1999;276:F751–757. doi: 10.1152/ajprenal.1999.276.5.F751. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto S, Adams JW, Bernstein KE, Schnermann J. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol. 2005;288:F445–452. doi: 10.1152/ajprenal.00297.2004. DOI 00297.2004 [pii] 10.1152/ajprenal.00297.2004. [DOI] [PubMed] [Google Scholar]
- 58.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 60.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 61.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–945. doi: 10.1152/ajprenal.00146.2007. DOI 00146.2007 [pii] 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ying J, Stuart D, Hillas E, Gociman BR, Ramkumar N, Lalouel JM, Kohan DE. Overexpression of mouse angiotensinogen in renal proximal tubule causes salt-sensitive hypertension in mice. Am J Hypertens. 2012;25:684–689. doi: 10.1038/ajh.2012.16. DOI 10.1038/ajh.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT(1A) receptors. Am J Physiol. 1999;277:F303–311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 64.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002;283:F1003–1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 65.Vio CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl. 2003:S57–63. doi: 10.1046/j.1523-1755.64.s86.11.x. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Villalobos RA, Janjulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth D, Fuchs S, Eldari D, Picard N, Bachman S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013 doi: 10.1172/JCI65460. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehlers MR, Riordan JF. Angiotensin-converting enzyme: zinc- and inhibitor-binding stoichiometries of the somatic and testis isozymes. Biochemistry. 1991;30:7118–7126. doi: 10.1021/bi00243a012. [DOI] [PubMed] [Google Scholar]
- 68.Wei L, Alhenc-Gelas F, Corvol P, Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 69.Binevski PV, Sizova EA, Pozdnev VF, Kost OA. Evidence for the negative cooperativity of the two active sites within bovine somatic angiotensin-converting enzyme. FEBS Lett. 2003;550:84–88. doi: 10.1016/s0014-5793(03)00825-1. [DOI] [PubMed] [Google Scholar]
- 70.Woodman ZL, Schwager SL, Redelinghuys P, Carmona AK, Ehlers MR, Sturrock ED. The N domain of somatic angiotensin-converting enzyme negatively regulates ectodomain shedding and catalytic activity. Biochem J. 2005;389:739–744. doi: 10.1042/BJ20050187. DOI 10.1042/BJ20050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, Michaud A, Zhao H, Keshelava G, Capecchi MR, Corvol P, Bernstein KE. Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem. 2004;279:15946–15953. doi: 10.1074/jbc.M400149200. DOI 10.1074/jbc.M400149200 M400149200 [pii] [DOI] [PubMed] [Google Scholar]
- 72.Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, Capecchi MR, Corvol P, Bernstein KE. Angiotensin-Converting Enzyme C-Terminal Catalytic Domain Is the Main Site of Angiotensin I Cleavage In Vivo. Hypertension. 2008;51:267–274. doi: 10.1161/HYPERTENSIONAHA.107.097865. DOI 10.1161/hypertensionaha.107.097865. [DOI] [PubMed] [Google Scholar]
- 73.Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 74.Lenfant M, Wdzieczak-Bakala J, Guittet E, Prome JC, Sotty D, Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci U S A. 1989;86:779–782. doi: 10.1073/pnas.86.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao TD, Yang XP, D'Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. N-acetyl-serylaspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research. Hypertension. 2010;55:459–467. doi: 10.1161/HYPERTENSIONAHA.109.144568. DOI 10.1161/HYPERTENSIONAHA.109.144568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CX, Rhaleb NE, Yang XP, Liao TD, D'Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. American journal of physiology. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. DOI 10.1152/ajpheart.00481.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, Andre S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. American journal of physiology. 2009;296:H404–412. doi: 10.1152/ajpheart.00747.2008. DOI 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng H, Carretero OA, Peterson EL, Yang XP, Santra K, Rhaleb NE. N-Acetyl-serylaspartyl-lysyl-proline inhibits ET-1-induced collagen production by preserving Src homology 2-containing protein tyrosine phosphatase-2 activity in cardiac fibroblasts. Pflugers Arch. 2012;464:415–423. doi: 10.1007/s00424-012-1150-7. DOI 10.1007/s00424-012-1150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens. 2004;22:593–603. doi: 10.1097/00004872-200403000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhaleb NE, Pokharel S, Sharma U, Carretero OA. Renal protective effects of N-acetyl-Ser-Asp-Lys-Pro in deoxycorticosterone acetate-salt hypertensive mice. J Hypertens. 2011;29:330–338. doi: 10.1097/HJH.0b013e32834103ee. DOI 10.1097/HJH.0b013e32834103ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma U, Rhaleb N-E, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. American journal of physiology. 2008;294:H1226–1232. doi: 10.1152/ajpheart.00305.2007. DOI 10.1152/ajpheart.00305.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P, Xiao HD, Xu J, Ong FS, Kwon M, Roman J, Gal A, Bernstein KE, Fuchs S. Angiotensin-converting enzyme N-terminal inactivation alleviates bleomycin-induced lung injury. Am J Pathol. 2010;177:1113–1121. doi: 10.2353/ajpath.2010.081127. DOI 10.2353/ajpath.2010.081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. DOI jem.20070657 [pii] 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. DOI 346/12/913 [pii]10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 85.Ong FS, Lin CX, Campbell DJ, Okwan-Duodu D, Chen X, Blackwell WL, Shah KH, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, Bernstein KE. Increased angiotensin II-induced hypertension and inflammatory cytokines in mice lacking angiotensin-converting enzyme N domain activity. Hypertension. 2012;59:283–290. doi: 10.1161/HYPERTENSIONAHA.111.180844. DOI HYPERTENSIONAHA.111.180844 [pii]10.1161/HYPERTENSIONAHA.111.180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sherman LA, Burke TA, Biggs JA. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med. 1992;175:1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kozlowski S, Corr M, Takeshita T, Boyd LF, Pendleton CD, Germain RN, Berzofsky JA, Margulies DH. Serum angiotensin-1 converting enzyme activity processes a human immunodeficiency virus 1 gp160 peptide for presentation by major histocompatibility complex class I molecules. J Exp Med. 1992;175:1417–1422. doi: 10.1084/jem.175.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakagawa Y, Takeshita T, Berzofsky JA, Takahashi H. Analysis of the mechanism for extracellular processing in the presentation of human immunodeficiency virus-1 envelope protein-derived peptide to epitope-specific cytotoxic T lymphocytes. Immunology. 2000;101:76–82. doi: 10.1046/j.1365-2567.2000.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisenlohr LC, Bacik I, Bennink JR, Bernstein K, Yewdell JW. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992;71:963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 90.Shen XZ, Lukacher AE, Billet S, Williams IR, Bernstein KE. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J Biol Chem. 2008;283:9957–9965. doi: 10.1074/jbc.M709574200. DOI 10.1074/jbc.M709574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol. 2011;12:1078–1085. doi: 10.1038/ni.2107. DOI ni.2107 [pii]10.1038/ni.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okwan-Duodu D, Datta V, Shen XZ, Goodridge HS, Bernstein EA, Fuchs S, Liu GY, Bernstein KE. Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J Biol Chem. 2010;285:39051–39060. doi: 10.1074/jbc.M110.163782. DOI 10.1074/jbc.M110.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennett SM, Broekema NM, Imperiale MJ. BK polyomavirus: emerging pathogen. Microbes and infection / Institut Pasteur. 2012;14:672–683. doi: 10.1016/j.micinf.2012.02.002. DOI 10.1016/j.micinf.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kemball CC, Lee ED, Vezys V, Pearson TC, Larsen CP, Lukacher AE. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J Immunol. 2005;174:7950–7960. doi: 10.4049/jimmunol.174.12.7950. [DOI] [PubMed] [Google Scholar]
- 95.Swanson PA, 2nd, Lukacher AE, Szomolanyi-Tsuda E. Immunity to polyomavirus infection: the polyomavirus-mouse model. Seminars in cancer biology. 2009;19:244–251. doi: 10.1016/j.semcancer.2009.02.003. DOI 10.1016/j.semcancer.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. The lancet oncology. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. DOI 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. The lancet oncology. 2011;12:65–82. doi: 10.1016/S1470-2045(10)70260-6. DOI 10.1016/S1470-2045(10)70260-6. [DOI] [PubMed] [Google Scholar]
- 98.Coleman CI, Baker WL, Kluger J, White CM. Antihypertensive medication and their impact on cancer incidence: a mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens. 2008;26:622–629. doi: 10.1097/HJH.0b013e3282f3ef5e. DOI 10.1097/HJH.0b013e3282f3ef5e. [DOI] [PubMed] [Google Scholar]
- 99.Singh A, Bangalore S. Which, if any, antihypertensive agents cause cancer? Current opinion in cardiology. 2012;27:374–380. doi: 10.1097/HCO.0b013e328353bc4f. DOI 10.1097/HCO.0b013e328353bc4f. [DOI] [PubMed] [Google Scholar]
- 100.Dive V, Georgiadis D, Matziari M, Makaritis A, Beau F, Cuniasse P, Yiotakis A. Phosphinic peptides as zinc metalloproteinase inhibitors. Cell Mol Life Sci. 2004;61:2010–2019. doi: 10.1007/s00018-004-4050-y. DOI 10.1007/s00018-004-4050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Redelinghuys P, Nchinda AT, Sturrock ED. Development of domain-selective angiotensin I-converting enzyme inhibitors. Annals of the New York Academy of Sciences. 2005;1056:160–175. doi: 10.1196/annals.1352.035. DOI 10.1196/annals.1352.035. [DOI] [PubMed] [Google Scholar]