Abstract
Generalized lipodystrophy is a rare disorder characterized by marked loss of adipose tissue with reduced triglyceride storage capacity leading to a severe form of metabolic syndrome including, hypertriglyceridemia, insulin resistance, type 2 diabetes mellitus, and hepatic steatosis. Recent echocardiographic studies suggest that concentric left ventricular (LV) hypertrophy is another characteristic feature of this syndrome but the mechanism remains unknown. It has recently been hypothesized that the LV hypertrophy could be an extreme clinical example of “lipotoxic cardiomyopathy” - excessive myocyte accumulation of triglyceride leading to adverse hypertrophic signaling. To test this hypothesis, we performed the first cardiac magnetic resonance study of patients with generalized lipodystrophy, using both magnetic resonance imaging and localized proton spectroscopy to detect excessive triglyceride content in the hypertrophied myocytes. Six patients with generalized lipodystrophy and 6 healthy controls matched for age, gender and body mass index, were studied. As hypothesized, myocardial triglyceride content was 3-fold higher in the patients than controls: 0.6±0.2% vs. 0.2±0.1% (P = 0.004). We also found presence of pericardial fat, representing a previously undescribed adipose depot in generalized lipodystrophy. Patients with generalized lipodystrophy, as compared to controls, also had a striking degree of concentric LV hypertrophy, independent of blood pressure: LV mass index, 101.0±18.3 vs. 69.0±17.7 g/m2, respectively (P = 0.02); LV concentricity, 1.3±0.3 vs. 0.99±0.1 g/mL, respectively (P = 0.04). These findings advance the lipotoxicity hypothesis as a putative underlying mechanism for the dramatic concentric LV hypertrophy found in generalized lipodystrophy.
Keywords: Lipodystrophy, cardiac steatosis, lipotoxicity, magnetic resonance spectroscopy, magnetic resonance imaging.
Introduction
Lipodystrophy is an extremely rare disorder characterized by loss of body fat and thus deficiency of the adipocytokines, such as leptin and adiponectin (1). The etiology can be either inherited or acquired and the loss of body fat may be generalized or partial. Patients with generalized lipodystrophy are markedly leptin deficient and are severely hyperphagic; however, have no adipose tissue to store the excess energy, leading to ectopic deposition of triglyceride in non-adipose tissue-most notably the parenchymal cells of the liver and skeletal muscle (2;3). Consequently, they develop a severe form of metabolic syndrome with insulin resistance, type 2 diabetes mellitus, hypertriglyceridemia and non-alcoholic fatty liver disease (4). Recent echocardiographic studies suggest that concentric left ventricular (LV) hypertrophy constitutes another common feature of patients with either congenital or acquired generalized lipodystrophy (5). While the precise underlying mechanism remains unknown, an attractive hypothesis is that the LV hypertrophy could be an extreme clinical example of “lipotoxic cardiomyopathy” - excessive myocyte accumulation of triglyceride (i.e., “cardiac steatosis”) leading to adverse hypertrophic signaling. However, myocardial triglyceride content has not previously been measured in these patients. We therefore used magnetic resonance imaging (MRI) and localized proton magnetic resonance spectroscopy (MRS) to determine if there was a relation between myocardial hypertrophy and triglyceride content in patients with generalized lipodystrophy.
Methods
Six individuals with generalized lipodystrophy: 2 with congenital generalized lipodystrophy type 1 due to acylglycerol phosphate acyltransferase, isoform 2 gene mutations; 3 with type 2 congenital generalized lipodystrophy due to Berardinelli-Seip congenital lipodystrophy 2 gene mutations; and 1 with acquired generalized lipodystrophy, were recruited for the present investigation. For cross-sectional comparison, 6 healthy control subjects, matched for age, gender and body mass index, without metabolic disease, were selected from an existing database. The study was approved by the Institutional Review Board of UT Southwestern Medical Center and all participants provided written informed consent prior to the study.
Body mass index was calculated as weight (kg) divided by height (m) squared. Body surface area was calculated according to the formula of DuBois and DuBois (6). Blood pressure was measured in the seated position, with a validated oscillometric sphygmomanometer (Series #52,000, Welch Allyn, Inc., Arden, North Carolina), with an appropriately sized cuff placed on the upper left arm. Venous blood samples were obtained from all participants and processed according to standard laboratory procedures (7).
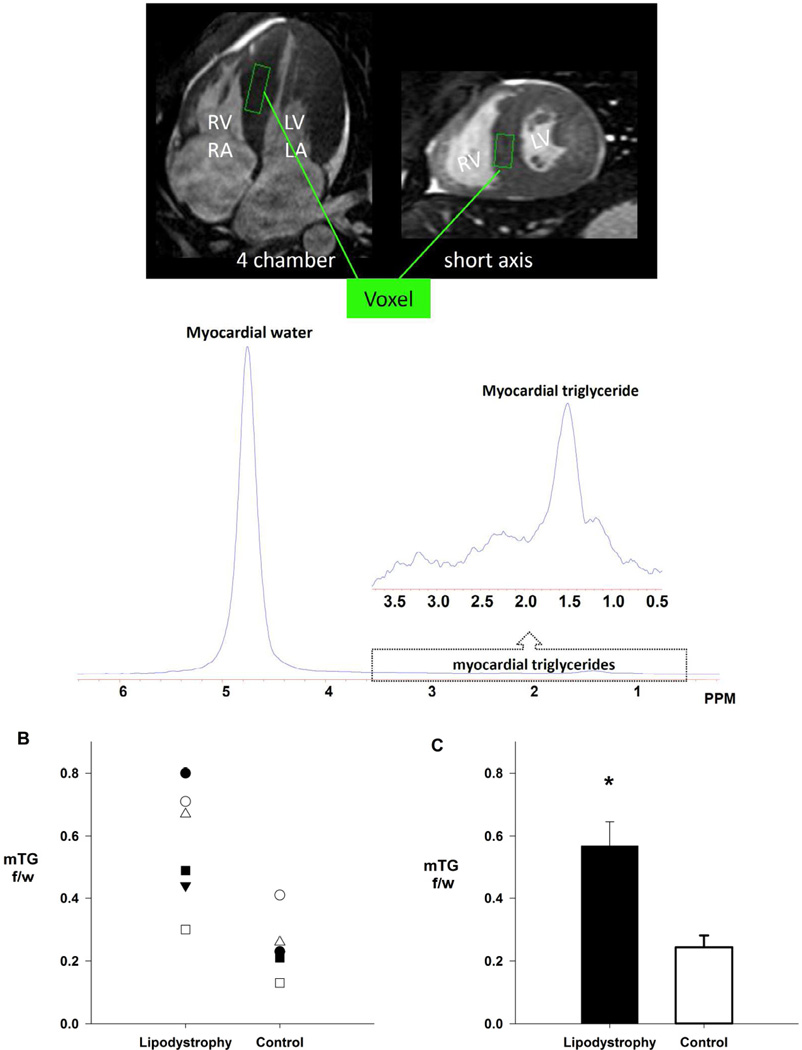
All cardiac MR experiments were performed with a 1.5-T Gyroscan INTERA whole-body MR system (Philips Medical Systems, Best, The Netherlands). Cardiac MRI was used to assess LV morphology, as previously described (8;9). Cardiac MRS was used for the non-invasive quantification of cardiac triglyceride content, as described in detail elsewhere (2;3;10;11). Briefly, image-guided MRS was performed with the following imaging parameters: repetition time of 4 s, echo time of 25 ms, and 1,024 data points over a 1,000-kHz spectral width. The volume of interest (voxel) was centered over the intraventricular septum at end-systole, in order to avoid vascular structures, gross adipose tissue deposits, and to ensure consistent orientation of muscle fibers along the magnetic field (Figure 1A). Spectra were processed and resonances quantified using a standard analysis package (NUTS; ACORNNMR, Fremont, CA). Myocardial triglyceride content is expressed as a percentage of the intensity of the water resonance peak.
Figure 1.
(A) Measurement of myocardial triglyceride content by localized1H-MRS in a representative lipodystrophy patient. Top s: cine four-chamber and short axis cardiac image. The volume of interest (voxel) is placed within the interventricular septum (green rectangle). Below, spectrum from myocardial tissue collected simultaneously at end-expiration and end-systole with respiratory gating and ECG triggering, respectively. (B) Individual cross-sectional comparison of intramyocellular triglyceride content in six patients with generalized lipodystrophy compared to six age, gender and BMI match controls [●, patient 1, pair 1; ■, patient 2, pair 2; ○, patient 3, pair 3; □, patient 4, pair 4; ▼, patient 5, pair 5; Δ, patient 6; pair 6] . (C) Group average cross-sectional comparison of myocardial triglyceride content, demonstrating a three-fold difference in myocardial triglyceride content in patients versus controls. Data reported as mean ± SEM. P < 0.05.
Cross-sectional comparisons (patients vs. controls) were performed using independent samples t-tests. The level of significance was set a priori at P ≤ 0.05. Data are reported as mean ± SE, unless otherwise specified.
Results
Patient specific characteristics are presented in Table 1. Patients and controls were well matched for age, height, and weight, and therefore no differences in body surface area or body mass index were found. As expected, patients had elevated circulating triglycerides and fasting glucose, with 4 of the 6 patients being treated with insulin (Patients from Pair 1, 3, 4, and 6 – Table 1). One patient (Pair 6) was being treated with a lipid lowering medication at the time of the study. No difference in serum cholesterol level was found, while high-density lipoprotein-cholesterol was significantly lower in patients versus controls. Blood pressure was similar between the two groups, and in the normotensive range, except for two lipodystrophy patients (Pair 3 and 6) whose blood pressure was elevated at the time of study.
Table 1.
Cross-sectional participant characteristics: Patients versus controls
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Pair 6 | Mean (SD) | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Control | Patient | Control | Patient | Control | Patient | Control | Patient | Control | Patient | Control | Patient | Control | ||
| Anthropometrics | |||||||||||||||
| Age (years) | 16 | 23 | 18 | 33 | 20 | 19 | 21 | 22 | 23 | 23 | 30 | 27 | 21 (5) | 24 (5) | 0.288 |
| Gender | F | F | F | F | M | M | M | M | F | F | F | F | --- | --- | |
| Height (cm) | 160 | 158 | 162 | 160 | 175 | 173 | 163 | 184 | 176 | 160 | 171 | 168 | 168 (7) | 167 (0.1) | 0.941 |
| Weight (kg) | 50 | 51 | 47 | 46 | 83.4 | 86 | 66 | 81 | 72 | 55.9 | 71.3 | 65.8 | 64.9 (14.0) | 64.3 (16.3) | 0.897 |
| BMI (kg/m2) | 19.5 | 20.4 | 17.9 | 18.0 | 27.2 | 28.7 | 24.8 | 23.9 | 23.2 | 21.8 | 24.4 | 23.3 | 22.9 (3.5) | 22.7 (3.6) | 0.944 |
| BSA (m2) | 1.5 | 1.5 | 1.5 | 1.5 | 2.0 | 2.0 | 1.7 | 2.0 | 1.9 | 1.6 | 1.8 | 1.8 | 1.7 (0.2) | 1.7 (0.3) | 0.924 |
| Hemodynamics | |||||||||||||||
| Systolic blood pressure (mmHg) | 120 | 112 | 106 | 118 | 142 | 141 | 125 | 122 | 122 | 106 | 166 | 136 | 130 (21) | 122 (14) | 0.470 |
| Diastolic blood pressure (mmHg) | 76 | 74 | 60 | 69 | 71 | 71 | 78 | 67 | 76 | 63 | 98 | 86 | 77 (12) | 72 (8) | 0.440 |
| Mean arterial pressure (mmHg) | 91 | 87 | 75 | 85 | 95 | 94 | 94 | 85 | 91 | 77 | 121 | 102 | 94 (145) | 89 (9) | 0.427 |
| Heart Rate (beats min−1) | 85 | 84 | 80 | 86 | 82 | 67 | 85 | 56 | 75 | 60 | 105 | 78 | 85 (10) | 69 (12) | 0.038 |
| Left ventricular morphology | |||||||||||||||
| End-diastolic volume (mL) | 120 | 111.6 | 103.4 | 77.7 | 178.1 | 141 | 142.6 | 169.5 | 137.9 | 121.2 | 127.5 | 93.6 | 135.0 (25.3) | 119.1 (33.0) | 0.372 |
| End-systolic volume (mL) | 45.3 | 51.7 | 33.5 | 27.2 | 76.5 | 53.2 | 50.6 | 44.5 | 56.5 | 46.5 | 51.9 | 27.9 | 52.4 (14.2) | 41.8 (11.5) | 0.188 |
| Stroke volume (mL) | 75.1 | 60 | 69.9 | 50.5 | 101.6 | 87.8 | 92 | 125.0 | 81.4 | 74.7 | 75.6 | 65.7 | 82.6 (12.0) | 77.3 (26.6) | 0.665 |
| Ejection Fraction (%) | 62 | 54 | 67.7 | 67 | 57 | 62 | 65 | 73.7 | 59 | 61.7 | 59 | 70 | 62 (4) | 65 (7) | 0.372 |
| Left ventricular mass (g) | 147.5 | 99.5 | 110.7 | 65.4 | 212.0 | 140.4 | 196 | 195 | 163.4 | 126.3 | 229.1 | 98.6 | 176.4 (44.2) | 120.9 (44.6) | 0.055 |
| Left ventricular mass index (g/m2) | 98.2 | 66.3 | 75.0 | 45.1 | 106.5 | 70.2 | 114.5 | 95.6 | 87 | 80.3 | 125.1 | 56.4 | 101.0 (18.3) | 69.0 (17.7) | 0.012 |
| Left ventricular concentricity (g/mL) | 1.2 | 0.9 | 1.1 | 0.8 | 1.2 | 1.0 | 1.4 | 1.2 | 1.2 | 1.0 | 1.8 | 1.0 | 1.3 (0.3) | 0.99 (0.1) | 0.023 |
| Blood Chemistry | |||||||||||||||
| Glucose (mg/dL) | 237 | 89 | 81 | 86 | 303 | 99 | 182 | 90 | 81 | 80 | 105 | 94 | 164.8 (91.9) | 89.7 (6.5) | 0.07 |
| Triglycerides (mg/dL) | 1420 | 123 | 66.3 | 72 | 2270 | 193 | 90 | 42 | 663 | 34 | 702 | 82 | 968.0 (765.1) | 91.0 (59.2) | 0.02 |
| Cholesterol (mg/dL) | 291 | 192 | 196 | 167 | 448 | 204 | 129 | 129 | 196 | 137 | 203 | 180 | 243.8 (112.6) | 168.2 (30.0) | 0.143 |
| High density lipoproteins (mg/dL) | 32.4 | 61.3 | 27 | 62 | 46.0 | 34.5 | 28.1 | 35 | 27 | 49.4 | 26.4 | 56.7 | 31.2 (7.6) | 49.8 (12.5) | 0.01 |
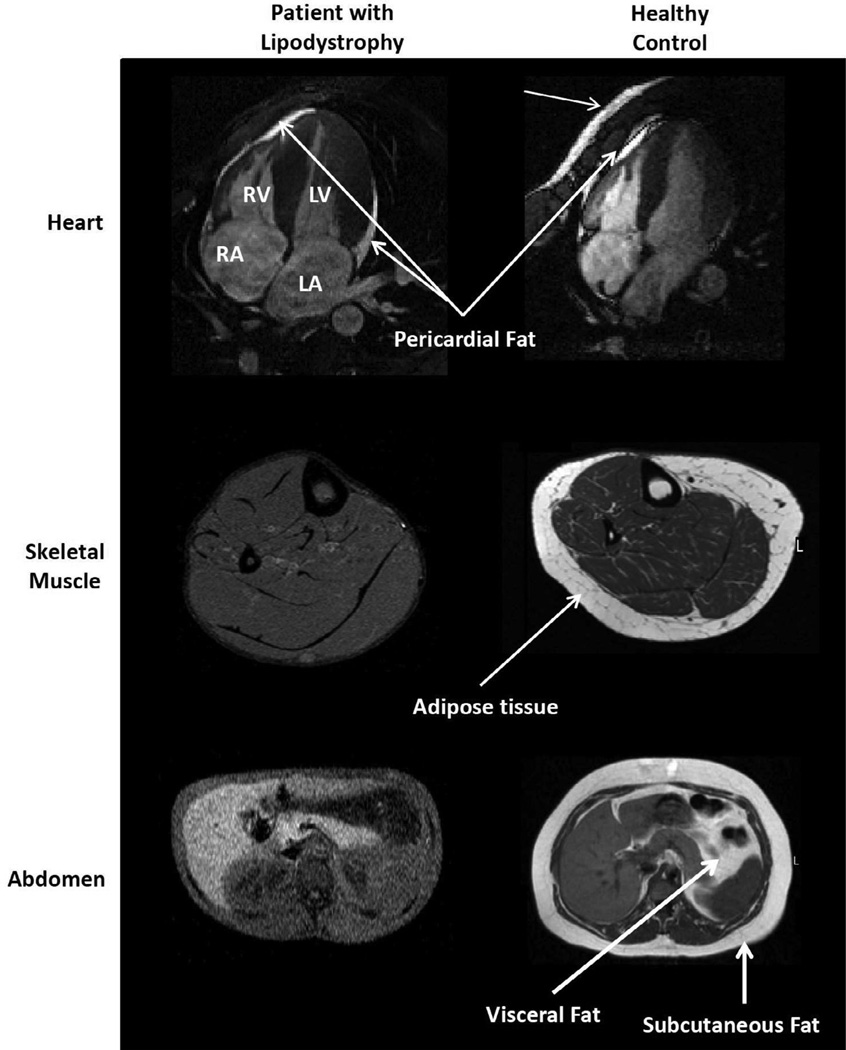
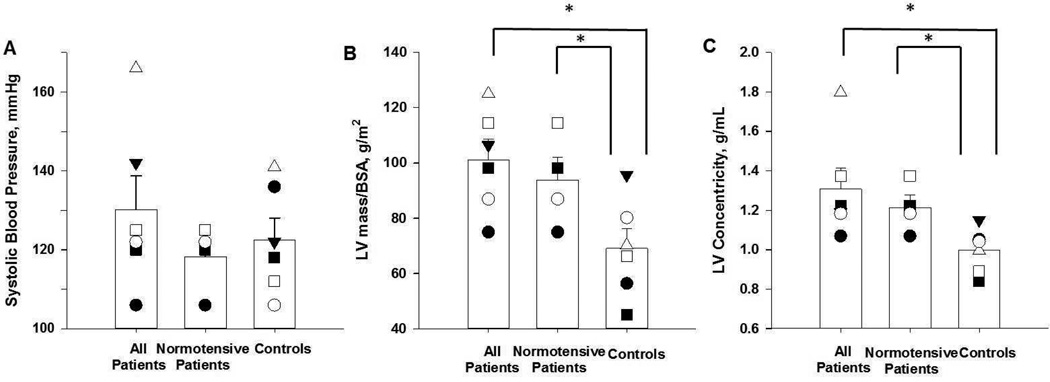
The major novel finding of our study is that intra-myocardial triglyceride content was threefold higher in patients with lipodystrophy compared to controls (Figure 1, P = 0.004). Unexpectedly, cardiac MRI also detected pericardial adipose tissue in the patients, despite their general lack of adipose tissue elsewhere in the body (Figure 2). Lastly, LV mass indexed to body surface area and LV concentricity were dramatically increased in patients over controls (Table 1), regardless of whether the two patients with elevated blood pressure were excluded from analysis (Figure 3). No group differences were found in LV end-diastolic volume, end-systolic volume, stroke volume or ejection fraction between groups (Table 1).
Figure 2.
High resolution magnetic resonance images. Top, cine four-chamber cardiac MR images. Note the presence of pericardial fat in both the patient and control. The control also has chest wall fat, whereas the patient does not. Middle, Axial T1-weighted MR image of calf. Increased typical intensity indicates fat, demonstrating a general lack of adipose tissue in the patient, whereas the control has normal levels of adipose tissue surrounding skeletal muscle. Bottom, axial abdominal MR images at the level of liver. Liver appears bright due to hepatic steatosis in patient with lipodystrophy. Note the general lack of subcutaneous and visceral adipose tissue in the patient with Generalized Lipodystrophy.
Figure 3.
Left ventricular (LV) concentricity and LV mass are independent of arterial blood pressure. Note that with the 2 patients with the highest blood pressure removed, systolic blood pressure (A) decreases below that of the controls, yet LV mass indexed to body surface area (BSA) (B) and LV concentricity (C) remain elevated above controls. Subject specific data presented [●, patient 1, pair 1; ■, patient 2, pair 2; ○, patient 3, pair 3; □, patient 4, pair 4; ▼, patient 5, pair 5; Δ, patient 6; pair 6] , along with the mean and standard error (open bars). * indicates P < 0.05.
Discussion
This is the first cardiac magnetic resonance imaging study of patients with generalized lipodystrophy. The MRI data confirm a high degree of concentric LV hypertrophy suggested by previous echocardiographic studies and document two major new findings. First, consistent with the lipotoxicity hypothesis, we found that myocardial triglyceride content is markedly elevated in the patients’ hypertrophied cardiomyocytes. Second, we unexpectedly found pericardial fat to be present in all the patients, representing a previously undescribed depot of adipocytes preserved in generalized lipodystrophy.
Generalized lipodystrophy has been associated with ectopic accumulation of triglyceride in the parenchymal cells of the liver and skeletal muscle (2;3). The three-fold elevation in myocardial triglyceride content in our patients shows that the heart is another steatotic target organ in this disease. From a mechanistic standpoint, generalized lipodystrophy is a perfect storm for cardiac steatosis. In patients with congenital generalized lipodystrophy, widespread failure of adipogenesis causes both leptin deficiency and a greatly reduced adipocyte pool to store triglyceride. In patients with acquired generalized lipodystrophy, autoimmune or other unknown mechanisms cause loss of adipose tissue. These patients therefore lack both the central neural action of leptin, which suppresses appetite, and the peripheral anti-steatotic action of leptin, which upregulates fatty acid oxidation of non-adipose tissue, so as to oxidize any lipid spillover that may have occurred due to over-nutrition (12). Furthermore, secondary insulin resistance would also be expected to disrupt the balance of cardiac substrate utilization, favoring greater uptake of fatty acids (13;14).
In addition to the new spectroscopy data, our imaging data not only confirmed a dramatic degree of concentric LV hypertrophy in generalized lipodystrophy but also document the consistent presence of pericardial fat. The latter finding was quite unexpected because pericardial fat represents a protected pool of adipocytes in a disease characterized by widespread failure of adipogenesis. This in vivo cardiac MRI finding differs from autopsy studies concluding that patients with generalized lipodystrophy have no pericardial fat (15;16). We interpret our data to suggest that the beating heart produces repetitive stimulation of mechanosensitive transcriptional elements of genes involved in adipogenesis. Mechanosensitive adipogenesis is indeed an accepted explanation for the residual adipose tissue patients with congenital generalized lipodystrophy, type 1 have in the intra-orbital region, palms, and soles (17). Thus, while generalized lipodystrophy results in poor growth and development of metabolically active adipose tissue, “mechanical adipose tissue” (reducing friction) appears preserved with pericardial fat constituting yet another example.
The major strength of this study is the application of cardiac MRI/MRS in an extremely rare human disease that provides a unique experiment of nature to study cardiac lipotoxicity in the absence of generalized obesity. While these data in patients with generalized lipodystrophy are entirely consistent with the concentric LV hypertrophy being an extreme human example of lipotoxic cardiomyopathy, the major limitation of this work is that these cross-sectional data cannot prove causal attribution. Importantly, our patients’ LV hypertrophy appears independent of hypertension and thus is not pressure overload hypertrophy. Likewise, none of the patients were actively engaged in regular exercise training, excluding the possibility of exercise-induced cardiac remodeling. We also did not observe a systematic relationship between those with impaired glucose tolerance, whom were receiving insulin injections, and the development of LV hypertrophy, suggesting that the hypertrophy observed in our patients may be independent of insulin. Lastly, pericardial fat has recently been implicated in the pathogenesis of LV hypertrophy (18–20); however, the amount of pericardial fat in our patients appears no higher than normal. Thus, it is tempting to speculate that the development of LV hypertrophy in patients with generalized lipodystrophy is mechanistically linked to their dramatic cardiac steatosis. Indeed, in transgenic mouse models, cardiac-specific overexpression of fatty acid transport proteins, or enzymes involved in triglyceride synthesis, produce severe cardiac steatosis, with the excess fatty acid causing concentric LV hypertrophy (21;22). Thus, in addition to providing a potential mechanistic explanation for the well described cardiomegaly described in generalized lipodystrophy, the present results may also provide important insight into the development of obesity related cardiomyopathy.
Acknowledgments
Funding support: MD Nelson is a Heart and Stroke Foundation of Canada Research Fellow. This work was supported by grants from the National Institutes of Health (R01-DK081524, R01-DK54387, M01-RR00633, and UL1-RR-024982), the Southwest Medical Foundation, and the Lincy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Garg A. Lipodystrophies: Genetic and Acquired Body Fat Disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol Endocrinol Metab. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 3.Simha V, Szczepaniak LS, Wagner AJ, DePaoli AM, Garg A. Effect of Leptin Replacement on Intrahepatic and Intramyocellular Lipid Content in Patients With Generalized Lipodystrophy. Diabetes Care. 2003;26:30–35. doi: 10.2337/diacare.26.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Garg A, Misra A. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin of North Am. 2004;33:305–331. doi: 10.1016/j.ecl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in Congenital and Acquired Generalized Lipodystrophy: A Clinical Assessment. Medicine. 2010;89:245–250. doi: 10.1097/MD.0b013e3181e9442f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBois D, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 7.Ahmad Z, Subramanyam L, Szczepaniak L, Simha V, Adams-Huet B, Garg A. Cholic acid for hepatic steatosis in patients with lipodystrophy: a randomized, controlled trial. Eur J Endocrinol. 2013;168:771–778. doi: 10.1530/EJE-12-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac Steatosis in Diabetes Mellitus: A 1H-Magnetic Resonance Spectroscopy Study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 9.Riley-Hagan M, Peshock RM, Stray-Gundersen J, Katz J, Ryschon TW, Mitchell JH. Left ventricular dimensions and mass using magnetic resonance imaging in female endurance athletes. Am J Cardiol. 1992;69:1067–1074. doi: 10.1016/0002-9149(92)90865-v. [DOI] [PubMed] [Google Scholar]
- 10.Szczepaniak LS, Dobbins RL, Stein DT, McGarry JD. Bulk magnetic susceptibility effects on the assessment of intra- and extramyocellular lipids in vivo. Magn Reson Med. 2002;47:607–610. doi: 10.1002/mrm.10086. [DOI] [PubMed] [Google Scholar]
- 11.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. Liporegulation in Diet-induced Obesity: The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276:5629–5635. doi: 10.1074/jbc.M008553200. [DOI] [PubMed] [Google Scholar]
- 13.Finck BN, Kelly DP. Peroxisome Proliferator-activated Receptor alpha (PPARalpha) Signaling in the Gene Regulatory Control of Energy Metabolism in the Normal and Diseased Heart. J Mol Cell Cardiol. 2002;34:1249–1257. doi: 10.1006/jmcc.2002.2061. [DOI] [PubMed] [Google Scholar]
- 14.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arky RA, McCully KS. Case 1-1975. N Engl J Med. 1975;292:35–41. [Google Scholar]
- 16.Chandalia M, Garg A, Vuitch F, Nizzi F. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1995;80:3077–3081. doi: 10.1210/jcem.80.10.7559900. [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Fleckenstein JL, Peshock RM, Grundy SM. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1992;75:358–361. doi: 10.1210/jcem.75.2.1639935. [DOI] [PubMed] [Google Scholar]
- 18.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 20.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity. 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 21.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, Zhang Y, Yeghiazarians Y, Gardner DG. A Murine Model of Isolated Cardiac Steatosis Leads to Cardiomyopathy. Hypertension. 2011;57:216–222. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]