Abstract
Advances in hematopoietic cell transplantation (HCT) have led to an increasing number of transplant survivors. In order to adequately support their healthcare needs, there is a need to know the prevalence of HCT survivors. We used data on 170,628 recipients of autologous and allogeneic HCT reported to the Center for International Blood and Marrow Transplant Research from 1968 to 2009 to estimate the current and future number of HCT survivors in the United States. Stacked cohort simulation models were used to estimate the number of HCT survivors in the US in 2009 and make projections for HCT survivors by the year 2030. There were 108,900 (range, 100,500–115,200) HCT survivors in the United States in 2009. This included 67,000 autologous HCT and 41,900 allogeneic HCT survivors. The number of HCT survivors is estimated to increase by 2.5 times by the year 2020 (242,000 survivors) and 5 times by the year 2030 (502,000 survivors). By 2030, the age at transplant will be <18 years for 14% of all survivors (N=64,000), 18–59 years for 61% survivors (N=276,000) and 60 years and older for 25% of survivors (N=113,000). In coming decades, a large number of individuals will be HCT survivors. Transplant center providers, hematologists, oncologists, primary care physicians and other specialty providers will need to be familiar with the unique and complex health issues faced by this population.
Keywords: Hematopoietic cell transplantation, Allogeneic, Autologous, Survivors, Prevalence
INTRODUCTION
Of the 11.7 million people living with cancer in the United States (US) in 2007, 8% had hematologic malignancies.1 For selected patients with hematologic malignancies and other disorders, hematopoietic-cell transplantation (HCT) offers the best chance of long-term survival. In the US, approximately 20,000 patients receive HCT each year.2 Transplant activity has steadily increased over time with introduction of safer transplant regimens, newer indications, and alternative graft sources for hematopoietic stem cells. Early and long-term survival after HCT has also improved over time.3–7 Patients who survive in remission for the first few years after transplantation have an 80–90% probability of surviving over the following 10–15 years.7–14 However, they continue to experience increased morbidity and mortality from late complications related to pre-, peri- and post-transplant treatment exposures and need life-long surveillance for their screening and prevention.15–17
A better understanding of the prevalence of HCT survivors, which is presently not known, will help inform policy and research in HCT survivorship. Issues that will require attention include availability of healthcare resources required for survivor care, appropriate models of delivery of care, integration of primary care providers in survivor care, efficacy and acceptability of various screening and preventive practices, and healthcare costs associated with survivor care. We used data from a national HCT clinical outcomes registry, the Center for International Blood and Marrow Transplant Research (CIBMTR), to calculate the current and future prevalence of HCT survivors in the United States (US).
METHODS
Data source
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP). Established in 2004, it comprises a voluntary group of more than 450 centers worldwide that contribute detailed data on consecutive HCTs to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis.2 Patients are followed longitudinally with yearly follow-up. Computerized checks for errors, physician reviews of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review by the National Marrow Donor Program’s Institutional Review Board.
We included all first HCT recipients in the US from 1968–2009 that were registered with the CIBMTR in our analysis. All patients were included, irrespective of diagnosis, transplant type (autologous or allogeneic) or donor source.
Because of varying policies, submission of data to the CIBMTR varied by year since its inception, but generally increased over time. For the time period considered, the CIBMTR captured data on 50–80% of autologous, 50–100% of related donor and 90–100% of unrelated donor HCT. Data from other databases that capture center activity and hospital discharges were used to apply correction factors for each year to extrapolate our estimates of HCT recipients.2
Statistical analysis
We used a stacked cohort simulation model to estimate the number of HCT recipients and survivors.18 For this, we constructed the current survivorship population by simulating cohorts of patients who received HCT in each year from 1968 to 2009. Survival curves for each cohort were drawn or projected out through 2009 and then “stacked” to estimate the prevalence of HCT survivors.
For prevalence estimate, we first stratified our study population into subgroups defined by key prognostic factors (age at HCT, gender, diagnosis and transplant/donor type). Subgroups with similar survival curves that included <200 patients were combined, in a hierarchy of age, gender, diagnosis, and donor source, to obtain sufficient statistical power in survival analysis. Survival probabilities were estimated for a total of 61 such subgroups. Next, for each subgroup, we used the survival curve to estimate the annual probability of dying from all causes in a certain year following HCT. For years where sufficient followup was not available to draw the curve through 2009, age-specific annual mortality rates and disease-specific mortality rates were used to describe survival probability beyond the survival curve. Finally, we calculated the number of survivors for each subgroup accumulated up to the year 2009. After the matrix of survival probabilities was constructed, the number of survivors for each year was derived by multiplying the number of transplant recipients (corrected for incomplete data reporting to CIBMTR) and the survival probability for that year. The total number of survivors was the sum of all survivors for each year up to the year 2009. The standard error of the survival probability was used to estimate the lower and higher range for number of survivors. In final presentation of our estimates, we combined subgroups into categories of age at HCT, gender, diagnosis, and transplant/donor type. Supplemental Material highlights one subgroup to illustrate our methods.
For estimating future number of HCT survivors, we first established a regression equation to describe the pattern of transplant activity in the US from 2000–2009. We chose this year range as transplant rates were most stable during this time period. In addition, this time period reflected the use of HCT for contemporary indications and excludes the influence of major practice changes on transplant utilization (decrease in allogeneic HCT rates for chronic myeloid leukemia with the advent of imatinib and autologous HCT rates for breast cancer). We used this equation to project the future number of HCT recipients until 2030. Next, we used the method described above for prevalence analysis to estimate the future number of survivors. Accumulated numbers of survivors by 2015, 2020, 2025, and 2030 were calculated by multiplying numbers of recipients with survival probabilities for each year. Sensitivity analyses were performed to assess the range of HCT survivors in response to the possible future variation in rates of HCT after the year 2009.
RESULTS
Table 1 describes the prevalence of HCT survivors in the US in 2009. There were 108,900 (range, 100,500–115,200) HCT survivors in the United States in 2009. This included 67,000 autologous HCT and 41,900 allogeneic HCT survivors. Fifteen percent of survivors were <18 years of age at the time of transplantation and 16% were 60 years of age or older.
Table 1.
Prevalent number of hematopoietic cell transplant recipients and survivors in the United States by year 2009
| Characteristics | Number of recipients reported to CIBMTR | Estimated total number of recipients in the US*† | Number of survivors† | |
|---|---|---|---|---|
|
| ||||
| N (%) | N | N | Range | |
| Total number | 170,628 | 266,200 | 108,900 | 100,500–115,200 |
| Age at transplant (years) | ||||
| <18 | 23,972 (14.1%) | 34,100 | 16,800 | 15,800–17,700 |
| 18–44 | 60,382 (35.5%) | 97,100 | 36,600 | 32,300–39,100 |
| 45–59 | 60,428 (35.6%) | 98,500 | 38,200 | 34,900–40,300 |
| ≥ 60 | 25,104 (17.8%) | 36,500 | 17,300 | 16,600–18,100 |
| Missing | 742 (0.4%) | – | – | – |
|
| ||||
| Gender | ||||
| Female | 87,809 (51.5%) | 138,200 | 52,800 | 45,800–56,000 |
| Male | 82,300 (48.2%) | 128,000 | 56,100 | 54,700–59,200 |
| Missing | 519 (0.3%) | – | – | – |
|
| ||||
| Diagnosis | ||||
| Acute leukemia/myelodysplastic syndrome | 44,850 (26.3%) | 60,500 | 22,400 | 20,800–23,900 |
| Chronic myeloid leukemia | 10,585 (6.2%) | 14,400 | 5,400 | 4,300–6,000 |
| Lymphoma | 44,658 (26.2%) | 75,200 | 34,900 | 31,700–36,600 |
| Multiple myeloma | 27,635 (16.2%) | 42,700 | 23,100 | 22,400–23,900 |
| Other | 42,612 (25.0%) | 73,400 | 23,200 | 21,400–24,800 |
| Missing | 288 (0.2%) | – | – | – |
|
| ||||
| Transplant/donor type | ||||
| Autologous | 101,946 (60.0%) | 166,700 | 67,000 | 62,300–70,500 |
| Allogeneic related | 38,899 (22.9%) | 61,000 | 25,100 | 22,500–27,000 |
| Allogeneic unrelated | 29,187 (17.2%) | 38,400 | 16,800 | 15,700–17,700 |
| Missing | 596 (0.3%) | – | – | – |
Correction factors were applied to account for partial reporting of clinical data by transplant centers to the CIBMTR (see Methods)
Estimates rounded to the nearest hundreds
Figure 1 shows the projected number of survivors by year 2030. If the number of transplants performed continues to increase at the current rate, we estimate a 2.5-fold increase in the number of HCT survivors by 2020 and a 5-fold increase by 2030. This figure also demonstrates the estimated growth in the number of survivors by transplant type and by age at transplantation. Figure 2 shows results of sensitivity analyses for how any changes in the future utilization of HCT might affect the estimated survivor projections.
Figure 1.
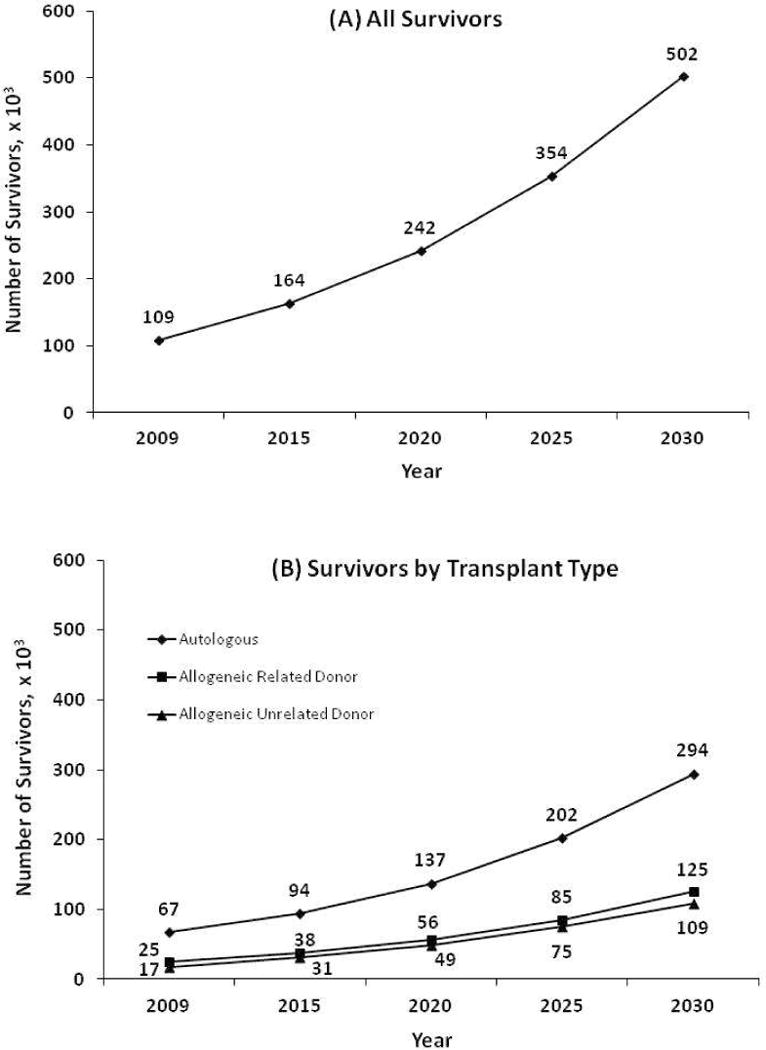
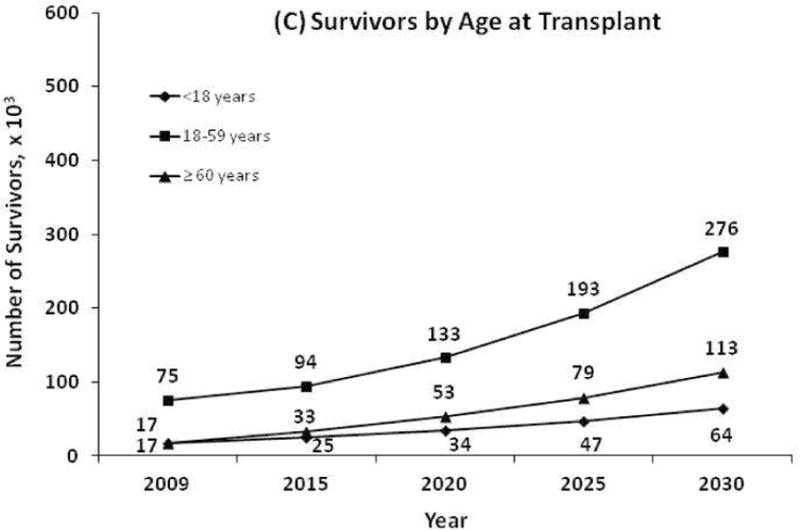
Projected number of hematopoietic cell transplant survivors in the US by year 2030
Figure 2.
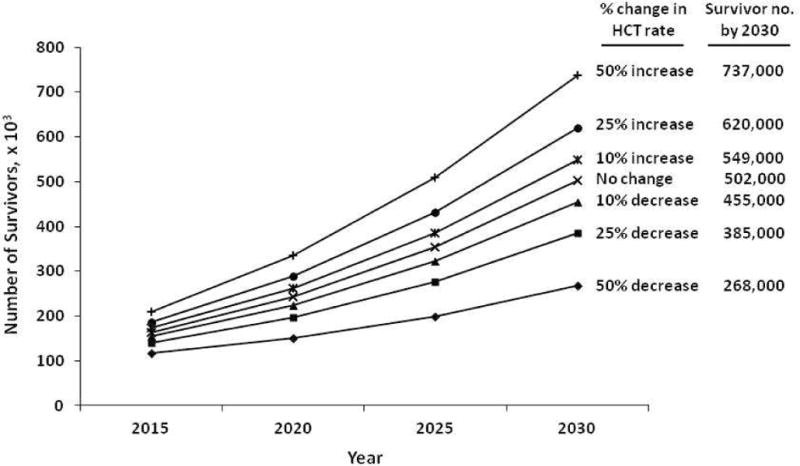
Sensitivity analyses showing how any future changes in the rate of transplant activity would affect the projected estimates for survivors in the US by year 2030
DISCUSSION
The cancer survivor population in the US is expected to grow to 18 million by 2022,19 and based on our analysis, approximately 2% of that population will consist of HCT survivors. HCT survivors can face future lifetimes of potential health problems. Extensive research has been performed and is presently ongoing to better understand the incidence, risk factors, morbidity and mortality associated with late complications such as secondary cancers, infections, bone loss, and cardiac, endocrine, renal and pulmonary dysfunction.15,20–23 Risk for many complications increases with time since transplantation and lifelong surveillance and followup of HCT survivors is recommended for their prevention and timely management.15,16 Adequate personnel, infrastructure and resources will be needed to in order to meet the long-term healthcare needs of transplant survivors. Our estimates will assist transplant centers, other providers and policy makers plan for the present number and future growth in survivor population.
With the increasing number of patients who need transplantation, many transplant centers have to focus their limited resources towards the early post-transplant care of HCT recipients. Centers frequently lack the capacity and physician and other personnel support for the long-term followup of HCT survivors.24–26 Hence, close coordination between transplant centers, referring hematologist-oncologists, primary care and specialty providers, and patients is needed for long-term survivor care. It is also important that non-transplant providers be familiar with the unique and complex health issues faced by this population and guidelines for their preventive care.16,17 Innovative patient-centered models for delivery of long-term care for HCT survivors that integrate transplant and non-transplant providers need to be studied.
HCT is a rapidly evolving field and research advances can substantially decrease (e.g., introduction of imatinib for treatment of chronic myeloid leukemia) or increase (e.g., introduction of alternate graft sources and non-myeloablative/reduced-intensity conditioning regimens) transplant utilization. In fact, the rates of HCT for older patients using non-myeloablative/ reduced-intensity regimens have increased dramatically even since 2009, the last year of HCT included in our analysis. Overall, HCT activity in the US has steadily increased over the last decade. As transplant centers and policy makers plan for survivor care, our sensitivity analyses will provide them with estimates on how any changes in HCT utilization will impact future survivor prevalence. Furthermore, research advances continuously impact HCT practice and the profile of HCT recipients, and our analysis will need to be updated periodically to keep abreast with these changes.
Chronic graft-versus-host disease is an important determinant of late mortality in allogeneic HCT recipients and also contributes significantly to the development of late complications.7,8,15 We were not able to estimate survivor prevalence based on chronic graft-versus-host disease status and how any future advancements in prevention and treatment of chronic GVHD might impact predictions of number of transplant survivors. Future research will have to address this issue.
In conclusion, our analysis, which represents the first estimate of prevalence of HCT survivors in the US, will greatly facilitate policy and research in HCT survivorship.
Supplementary Material
Acknowledgments
We would like to thank David Howard, PhD, Emory University, for his review and constructive feedback on the manuscript.
Sources of Support
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship and Conflict of Interest Statement
None of the authors has any relevant financial conflicts of interest to disclose. Navneet Majhail had full access to all of the data in the study and had final responsibility for the integrity of the data, the accuracy of the data analysis, and the responsibility for the decision to submit for publication. Authors designed research (all authors), collected data (NM), performed statistical analysis (LT, KK), interpreted data (all authors), drafted the manuscript (NM, LT), and critically revised the manuscript (all authors). All authors have reviewed and approved the final version of the manuscript.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2007. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010 (accessed 01/01/2013) [Google Scholar]
- 2.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2011 Available at: http://www.cibmtr.org.
- 3.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29(7):805–813. doi: 10.1200/JCO.2010.32.5001. Prepublished on 2011/01/12 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. Prepublished on 2010/11/26 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn T, McCarthy P, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased utilization, older age of recipients and use of unrelated donors. Journal of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.46.6193. In press. [DOI] [PMC free article] [PubMed]
- 6.McCarthy PL, Jr, Hahn T, Hassebroek A, et al. Trends in utilization and survival after autologous hematopoietic cell transplantation in North America from 1995 to 2005: Significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.04.027. Prepublished on 2013/05/11 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. Prepublished on 2007/08/03 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–4222. doi: 10.1182/blood-2005-01-0035. Prepublished on 2005/02/11 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28(11):1888–1895. doi: 10.1200/JCO.2009.26.7757. Prepublished on 2010/03/10 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147(1):129–139. doi: 10.1111/j.1365-2141.2009.07798. Prepublished on 2009/07/04 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majhail NS, Bajorunaite R, Lazarus HM, et al. High probability of long-term survival in 2-year survivors of autologous hematopoietic cell transplantation for AML in first or second CR. Bone Marrow Transplant. 2011;46(3):385–392. doi: 10.1038/bmt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. Prepublished on 1999/07/01 as. [DOI] [PubMed] [Google Scholar]
- 15.Majhail NS, Douglas Rizzo J. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2012.258. Prepublished on 2013/01/08 as. [DOI] [PubMed] [Google Scholar]
- 16.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348–371. doi: 10.1016/j.bbmt.2011.12.519. Prepublished on 2011/12/20 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(3):337–341. doi: 10.1038/bmt.2012.5. Prepublished on 2012/03/08 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. doi: 10.1177/0272989X9301300409. Prepublished on 1993/10/01 as DOI. [DOI] [PubMed] [Google Scholar]
- 19.de Moor JS, Mariotto AB, Parry C, et al. Cancer Survivors in the United States: Prevalence across the Survivorship Trajectory and Implications for Care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–570. doi: 10.1158/1055-9965.EPI-12-1356. Prepublished on 2013/03/29 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majhail NS. Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Haematol. 2011;154(3):301–310. doi: 10.1111/j.1365-2141.2011.08756.x. Prepublished on 2011/05/28 as. [DOI] [PubMed] [Google Scholar]
- 21.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19(7):1073–1080. doi: 10.1016/j.bbmt.2013.04.002. Prepublished on 2013/04/16 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savani BN, Griffith ML, Jagasia S, Lee SJ. How I treat late effects in adults after allogeneic stem cell transplantation. Blood. 2011;117(11):3002–3009. doi: 10.1182/blood-2010-10-263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syrjala KL, Martin PJ, Lee SJ. Delivering care to long-term adult survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30(30):3746–3751. doi: 10.1200/JCO.2012.42.3038. Prepublished on 2012/09/26 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majhail NS, Murphy EA, Omondi NA, et al. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17(7):956–961. doi: 10.1016/j.bbmt.2011.03.008. Prepublished on 2011/05/05 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajewski JL, LeMaistre CF, Silver SM, et al. Impending challenges in the hematopoietic stem cell transplantation physician workforce. Biol Blood Marrow Transplant. 2009;15(12):1493–1501. doi: 10.1016/j.bbmt.2009.08.022. Prepublished on 2009/09/29 as. [DOI] [PubMed] [Google Scholar]
- 26.Denzen EM, Majhail NS, Stickney Ferguson S, et al. Hematopoietic cell transplantation in 2020: summary of year 2 recommendations of the National Marrow Donor Program’s System Capacity Initiative. Biol Blood Marrow Transplant. 2013;19(1):4–11. doi: 10.1016/j.bbmt.2012.10.005. Prepublished on 2012/10/20 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


