Abstract
Statins have proven their effectiveness in the treatment of cardiovascular disease. This class of drugs has also attracted attention as a potential treatment for dissimilar diseases such as certain types of cancers and neurodegenerative diseases. What appears to be a contradiction is that in the case of cancer, it has been suggested that statins increase apoptosis and alter levels of Bcl-2 family members (e.g., reduce Bcl-2 and increase Bax) whereas, studies mainly using non-cancerous cells report opposite effects. This review examined studies reporting on statin effects on Bcl-2 family members, apoptosis, cell death and cell protection. Much, but not all of the evidence supporting pro-apoptotic effects of statins is based on data in cancer cell lines and the use of relatively high drug concentrations. Studies indicating an anti-apoptotic effect of statins are fewer in number, generally used much lower drug concentrations and normal cells. Those conclusions are not definitive, and certainly there is a need for additional research to determine if statin repositioning is justified for non-cardiovascular diseases.
Keywords: Alzheimer's disease, apoptosis, Bcl-2, cancer, cholesterol, isoprenoids, neuroprotection, neurodegeneration, statins
Statins are well-recognized for their efficacy in the prevention/treatment of cardiovascular disease, a topic which has been extensively reviewed [1]. Statins reduce cholesterol synthesis and increase the uptake of low density lipoproteins. Within the mevalonate pathway, these drugs also have cholesterol-independent effects, namely the reduction of the two isoprenoids farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). Reducing FPP and GGPP decreases prenylation of small GTPases, and it is thought that such a mechanism may contribute to the reduction in morbidity and mortality occurring in cardiovascular disease[2;3]. In addition to the use of statins in the prevention/treatment of cardiovascular disease, it has been suggested albeit with some controversy, that these drugs may have efficacy in treating diseases such as various cancers, ischemic stroke, inflammatory diseases, and certain neurodegenerative diseases [4-8]. One of the proposed mechanisms for the effects of statins in non-cardiovascular diseases involves changes in expression levels of the pro- and anti-apoptotic Bcl-2 family of proteins. Several reports found that statins reduced levels of the anti-apoptotic protein Bcl-2, increased apoptosis and cell death. Some of those studies are summarized in Table 1. In contrast, there is evidence that statins increase Bcl-2 abundance which would favor and in some instances reduce apoptosis and cell death and are listed in Table 2. The purpose of this mini-review will be to focus on studies within the context of what appears to be contradictory findings regarding effects of statins on Bcl-2 expression levels, apoptosis, cell death, and cell protection.
Table 1. In vitro and in vivo studies on statins, cell death, apoptosis, and Bcl-2 family members.
| Treatment | Tissue | Effects | Ref |
|---|---|---|---|
| In vitro, L, 0.1 μM, | Dexamethasone resistant and sensitive human acute T-cell leukemia | ↑AP | 9 |
| In vitro, L, 0.1 μM, 72 h | human glioma cell lines | ↑AP | 11 |
| In vitro, L 10 μM, 12+ h | human promyelocytic HL-60 leukemia cells | ↑AP, but 2 μM no effect | 10 |
| In vitro, L, 50 μM, 48 h | NIH/3T3 fibroblasts overexpressing oncogene HA-ras | ↑AP in HA-ras cells but not low expressing cells. Bcl-2 rescued effects. | 12 |
| In vivo, F, 92 μM, 6 days | serum form human subjects incubated with human smooth muscle cells | ↑AP, ↓Bcl-2 | 21 |
| In vitro, L 50-200 mM, 24 h | myeloid leukemia cell lines | ↑AP, ↓ Bcl-2 | 60 |
| In vitro, L 1-10 μM, 24 h | immortalized rat brain neuroblasts | ↑AP, ↓Bcl-2, Bcl-xL | 24 |
| In vitro, A, S 10-100 μM, 48 h | rat thoracic vascular smooth muscle cells | ↑AP, ↓Bcl-2, Bax(ne) | 30 |
| In vitro, L 10 and 30 μM, 4 days | myeloma cell lines, not all lines responsive to L | ↑AP, ↓Bcl-2, Bax(ne) | 31 |
| In vitro, C 1 and 3 μM, 20 h | rat aortic vascular smooth muscle cells | ↑AP, ↓Bcl-2 | 61 |
| In vitro, S 0.1-1 μM, 18-24 h | mouse tubular cells w/wo expressing Bcl-xL | ↑AP, ↓Bcl-xL, Bax(ne), Bid(ne), overexpression reduced effects of S | 25 |
| In vitro, L 5 μM, 24-48 h | human glioblastoma cells lines | ↑AP, ↓Bim, no effects Bcl-2, Bcl-xL, Bak, Bid, Bax | 32 |
| In vitro, A, F, L, S, 10 and 20 μM | human vascular endothelial cells | ↑AP, ↓Bcl-2 | 62 |
| In vitro, M 0.003-0.006 μM/ml, 24 and 48 h | U266 human myeloma cells | ↑AP, ↓Bcl-2 mRNA, protein | 23 |
| In vitro, A, C, F, L, S, 30 μM, 24 h | human adult hepatocytes | ↑AP ↓Bcl-2 mRNA, protein, Bax(ne) | 63 |
| In vitro, A, F, 50 μM, 4 days | human breast cancer cells | ↑AP,↓Bcl-2 | 64 |
| In vitro, F 10 μM, 24 h | human CD4+T cells | ↑AP, ↓Bcl-2, Bax(ne) | 33 |
| In vitro, A 10 μM, 24 h | human osteosarcoma cells | ↑AP, ↓Bcl-2 protein, mRNA, Bax(ne) | 34 |
| In vitro, P, S, 0.1, 1.25,5 μM, 48 h | human cardiac myocytes | ↑AP, ↓Mcl-1, Bax(ne), ↑Mcl-1 by P; ↓ Mcl-1 mRNA by S 5μM | 35 |
| In vitro, S 1 and 10 μM, 24 h | Barret's adenocarcinoma cells | ↑AP, ↓Bcl-2 mRNA and protein, Bax(ne) protein, mRNA↑ at 10μM | 65 |
| In vitro, L 1,10, 20 μM, 3-24 h | rat brain neuroblasts | ↑AP, ↑BimEL | 13 |
| In vitro, L 20 μM, 24 or 48 h | human colon cancer cells | ↑AP, no effects on Bcl-2, Bcl-xL | 67 |
| In vitro, S 5μM, 48 h | human breast cancer cells | ↑AP↓Bcl-2 mRNA, no effects on Bcl-xL and Bax | 36 |
| In vitro, L, P, S 20 μM, 24 h | Barret's adenocarcinoma cell lines | ↑AP, ↑Bad, Bax mRNA and protein levels, no effects on Bcl-2, Bcl-xL | 14 |
| In vitro, S 10 μM, 48 h | human colon cancer cells | ↑AP, ↓ Bcl-2 and Bcl-xL mRNA and protein levels | 27 |
| In vitro, L, M, P, S 1-20 μM, 72 h | normal and abnormal human embryonic stem cells; breast adenocarcinoma cells | Inconsistent results on mRNA levels of Bcl-2 and Bax when incubated with S | 68 |
| In vitro, S 1-20 μM, 12-24 h | MethA fibrosarcoma cells | ↑AP, ↑Bax translocation to mitochondria | 69 |
| In vitro, F 5-20 μM, 24 h | human hepatocellular carcinoma cell lines | ↑AP, ↓.Bcl-2 | 66 |
| In vivo, R 20 μM, orally once daily for 6 weeks | CD4(+)C28 (null) T of patients with acute coronary syndromes | ↑AP, ↓.Bcl-2 | 22 |
| In vitro, S 0.6-10 μM 72 h | ARH77multiple myeloma cell line | ↑AP, ↓Bcl-2, Bax(ne) | 37 |
| In vitro, S 25 μM, 16 h | human prostate cancer cell lines | ↑AP, BimL/BimS↓Bcl-2, Bcl-xL, pBad | 28 |
| In vitro, S 20 μM, 24-72 h | MCF7 human breast cancer cells, SAEC human normal small airway epithelial cells, HepG2 human hepatocellular carcinoma cells, NCI-N87 human gastric cancer (NCI gastric cells) and NCiH12299 human non-small cell lung carcinoma (NCH lung) cells | Effects seen in cancer cells but not normal cells: ↑AP, ↑Bax mRNA, ↓Bcl-2 mRNA | 52 |
AP, apoptosis; A, atorvastatin; C, cerivastatin; F, fluvastatin; L, lovastatin; M, mevastatin; ne, no effects; P, pravastatin; S, simvastatin
Table 2. In vivo and in vitro studies on statins, cell protection, apoptosis, and Bcl-2 family members.
| Treatment | Tissue | Effects | Ref |
|---|---|---|---|
| In vivo, S 120 μM/kg, orally, 21 days | mouse, brain, microarray analysis, statin levels determined | ↑Bcl-2 mRNA, protein levels | 40 |
| In vivo, S 120 μM/kg, orally, 21 days | Guinea pig, brain and dissociated brain cells | ↓AP,↑Bcl-2, ↓Bax↑P | 41 |
| In vivo, S 2.4 μM/kg, i.p., 2 weeks | rat quinolinic acid model of Huntington's disease, brain striatum | ↑Bcl-2, ↓Bax, ↑P | 42 |
| In vivo, A 41 μM/kg, orally, 3 weeks | spontaneously hypertensive rats | no effects on AP, Bcl-2, or Bax | 46 |
| In vivo, Pita 0.363 and 0.726 μM/kg, orally, 14 days | rat ischemia model, heart tissue | ↑Bcl-2, ↓Bax, CP | 43 |
| In vivo, S 24 μM/kg, orally, 5 days | rat ischemia model, ventricle tissue | ↑Bcl-2, ↓Bax only in tissue from ischemic rats, CP | 44 |
| In vivo, S 60 μM/kg, orally, 8 weeks | apoE null mice fed high-fat diet, aortic tissue | ↑Bcl-2, ↑Bcl-xlL, Bax(ne), CP | 45 |
| In vitro, S 0.1 μM, 6 days | mouse primary neurons, SH-SY5Y cells | ↓AP, ↑Bcl-2 mRNA, protein, CP | 47 |
| In vitro, F 0.1 μM, 24 h | human umbilical vein endothelial cells incubated with H2O2 | ↑Bcl-2 mRNA, protein, CP | 48 |
| In vitro, A 1.0 μM, 6 h | pig mesenchymal stem cells, hypoxic and serum-free conditions | ↑Bcl-2, ↓Bax, CP | 49 |
| In vitro S 0.001-0.1 μM, | human osteosarcoma cells treated with H2O2 | ↑Bcl-2, ↓.AP, CP | 50 |
| In vitro, P 50 μM, 5 min before and during 15 and 60 min reoxygenation | human atrial trabeculae incubated under hypoxic and reoxygenation | ↑Bcl-2 only during reoxygenation | 51 |
| In vitro, S 5 μM, 48 h | primary human skeletal muscle cells | ↑Bcl-2, Bax, AP | 29 |
AP, apoptosis; A, atorvastatin; CP, cell protection; F, fluvastatin; ne, no effects; Pita, pitavastatin; P, pravastatin; S simvastatin
Statins, Bcl-2 Family Members and Cell Death
One of the earliest studies associating statins with apoptosis and cell death reported on the effects of lovastatin (0.1 μM) on growth in two cell lines, dexamethasone-resistant and dexamethasone-sensitive lines derived from human acute T-cell leukemia patients [9]. Cell death was induced by both lovastatin and dexamethasone, and the observation was made that the cells had “characteristics of apoptosis” but markers of apoptosis were not reported. Since that study, there have been additional [10-14] reports on statin-induced apoptosis and cell death (Table 1). Statin induced apoptosis and/or cell death occurs in cancer lines (e.g. human acute leukemia lines, human promyelocytic HL-60 cells, malignant glioma cells, Barrett's esophageal adenocarcinoma cells) and non-cancer cells (e.g., mouse fibroblasts, rat brain neuroblasts). There is some evidence suggesting that different types of cancer are more susceptible to statins as compared with others [4]. A common feature of many of those studies is that high statin concentrations (μM to mM amounts) were required to cause apoptosis and cell death although there are exceptions [9] including a study showing that lovastatin beginning at 0.1 μM induced DNA degradation in human glioma cells [11]. Although in that study effects of lovastatin on DNA degradation in another cell line, anaplastic astrocytoma, was not apparent until a drug concentration of 1 μM.
The pivotal roles that Bcl-2 family members play in apoptosis and cell death are well-recognized, and that large body of work has been extensively reviewed [15-20]. There have been several studies showing that statins alter expression levels of Bcl-2 family members. This section will examine reports indicating that statins alter levels of proteins such as Bcl-2, Bcl-xL, and Bax. Reductions in Bcl-2 and Bcl-xL and an increase in Bax favor a pro-apoptotic cell environment. An early study reported that serum from normal human subjects receiving fluvastatin (92 μM/day for 6 days) added to human smooth muscle cells in vitro reduced Bcl-2 proteins levels and increased apoptosis [21]. Similar findings were seen in T-cells of patients with acute coronary syndromes who received rosuvastatin (20 μM/day for 6 weeks) [22]. There have been several in vitro studies using different statins (lovastatin, atorvastatin, simvastatin, pravastatin, cerivastatin) and different non-cancer and cancer cell lines demonstrating that statins generally at high concentrations reduced Bcl-2 protein levels (Table 1). A notable exception to the observation that high statin concentrations are needed to act on Bcl-2 was a study that found that Bcl-2 protein and mRNA levels were reduced by lovastatin at concentrations of 2.4 and 6.2 nM/ml [23] although in that study a Western blot showed that the lower lovastatin concentration had a larger reducing effect on Bcl-2 as compared with the higher concentration. The data did not appear to be semi-quantified (scanned), only a single experiment was shown and so it is not clear if differences were significant. Protein levels of another anti-apoptotic member of the Bcl-2 family, Bcl-xL were also reduced by statins (lovastatin, simvastatin) in different cell types (rat brain neuroblasts, mouse tubular cells, human myeloid KBM-5 cells, human colon cancer cells, human prostate cancer cells PC3) [24-28]. While most studies using relatively high concentrations of statins have found that Bcl-2 levels were reduced, a recent study found opposite results [29]. Simvastatin (5 μM) significantly increased Bcl-2 protein levels in primary human skeletal myotubes which was associated with decreased cell viability and enhanced oxidative stress [29]. A conclusion reached in that study was that the simvastatin-induced increase in Bcl-2 protein expression might have been a protective response to drug-induced cell death. In the same study, levels of the pro-apoptotic protein Bax were also significantly increased. Several studies have reported that statins did not alter Bax levels [25;30-37].
Generally at high statin concentrations apoptosis is increased, and Bcl-2 expression levels and cell viability are reduced. The mechanisms for the statin-induced reduction of Bcl-2 protein levels have not been forthcoming. Statins reduce cholesterol, FPP, GGPP and protein prenylation but how those reductions trigger an attenuation of the anti-apoptotic protein Bcl-2 and increase abundance of pro-apoptotic proteins such as Bax and Bim is not understood. There is evidence that statins can act outside of the mevalonate pathway. Statins for example bind to the lymphocyte function-associated antigen-1 (LFA-1) which is a heterodimeric glycoprotein, and it is a member of the β2 integrin family [38;39]. Directly related to the issue of statins and Bcl-2 is work discussed later in this review on Bcl-2 and cell protection showing that statins stimulate Bcl-2 gene expression and protein levels, which do not involve the mevalonate pathway.
Statins, Bcl-2 Family Members and Cell Protection
In the previous section, studies were reviewed that found that statins reduced Bcl-2 protein levels. This section will examine in vivo and in vitro studies which found that statins increase Bcl-2 levels, and some of those studies are listed in Table 2. In 2005, our laboratory was the first to report that a statin, simvastatin, significantly increased Bcl-2 gene expression in brain tissue of mice receiving the drug orally (120 μmol/kg for 21 days) [40]. Separate groups of mice treated with lovastatin and pravastatin also showed increased Bcl-2 gene expression but those differences were not significant. Simvastatin-induction of Bcl-2 gene expression was detected using the Affimytric DNA array and confirmed using RT-PCR. Bcl-2 protein levels were also significantly increased in simvastatin-treated mice. There were several other genes whose expression levels were also altered by statins (e.g., Igfbp3, Hk1,c-fos, c-myc, Npy1r, MCT2, Sdc4). In a subsequent study in collaboration with Walter Muller and Gunter Eckert, we replicated our findings on simvastatin induction of brain Bcl-2 protein levels but this time in the Guinea pig demonstrating that the drug on increased Bcl-2 protein levels in another species [41]. In the same study, Bax protein levels were significantly reduced. Dissociated brain cells from the Guinea pigs administered simvastatin in vivo exhibited neuroprotection when challenged ex vitro with sodium nitroprusside and the Bcl-2 protein inhibitor HA14-1. In an in vivo rat quinolinic acid model of Huntington's disease, simvastatin (2.4 μmol/kg i.p./day, 2 or 8 weeks) was neuroprotective [42]. Bcl-2 protein levels were increased whereas levels of the proapoptotic protein Bax were reduced, results which are similar to what we observed in brain tissue of simvastatin-treated Guinea pigs [41]. Other in vivo studies [43-45] reported that statins increased Bcl-2 abundance and reduced apoptosis and they are summarized in Table 2. An exception to those findings is a study showing that administration of atorvastatin (41 μmol/kg for 3 weeks) did not significantly alter levels of Bcl-2 and Bax in aortic smooth muscle cells from spontaneously hypertensive rats [46]. Markers of apoptosis were not affected by atorvastatin treatment in those animals.
There is a body of data from in vitro studies showing that statins increase Bcl-2 and reduce apoptosis (Table 2) which is in agreement with the majority of in vivo studies discussed in this section. We reported that simvastatin (0.1 μM) significantly increased Bcl-2 mRNA and protein levels and provided neuroprotection in mouse primary neurons when challenged with oligomeric amyloid β-protein(42) (Aβ42) [47]. When Bcl-2 expression was inhibited by the antisense oligonucleotide G3139, simvastatin neuroprotection was abolished in cells. The finding that inhibition of Bcl-2 eliminates protective effects of simvastatin was replicated using another statin fluvastatin (0.01-0.1 μM) and a different cell type, human vascular endothelial cells which were challenged with H2O2 [48]. In that study it was also observed that fluvastatin increased Bcl-2 mRNA expression and protein levels which is consistent with the earlier study using simvastatin and mouse primary neurons [47]. Treatment of different cell types (mesenchymal stem cells, human osteosarcoma cells, human atrial trabeculae) with statins (atorvastatin, simvastatin, pravastatin) increased Bcl-2 protein levels and reduced markers of apoptosis [49-51] and those studies are summarized in Table 2.
Biphasic Effects of Statins on Bcl-2 Family Members
Statins reduce Bcl-2 mRNA and protein levels, increase apoptosis and cell death (Table 1). In stark contrast, Table 2 list studies reporting that statins increase Bcl-2 mRNA and protein levels, reduce apoptosis and are protective. Many of the in vitro studies supporting a detrimental effect of statins used cancer cell lines, suggesting that cancer cells may respond differently to statins as compared to normal cells. It was recently reported that simvastatin (20 μM) reduced Bcl-2 mRNA and increased apoptosis in different cancer cell lines (MCF7 human breast cancer cells, HepG2 human hepatocellular carcinoma cells, NCI-N87 human gastric cancer NCI gastric cells and NCiH12299 human non-small cell lung carcinoma NCH lung cells), but normal cells (SAEC human normal small airway epithelial cells) were unaffected [52]. However, in view of the fact that a high concentration of simvastatin was employed, the absence of an effect in the epithelial cells may be a unique property of those cells. The majority of studies showing that statins increase Bcl-2 mRNA and proteins levels and reduce apoptosis have used normal cells (Table 2). Exceptions, have been studies using human neuroblastoma cells (SH-SY5Y cells) [47] and human osteosarcoma cells (MG63 cells) [50].
The two studies using cancer cell lines cited above [47;50] used low statin concentrations to stimulate Bcl-2 expression. The study with SH-SY5Y cells used a simvastatin concentration of 0.1 μM and the study with osteosarcoma cells used simvastatin at concentrations ranging from 0.001 to 0.1 μM. The question is raised if statin concentration is a determining factor in whether Bcl-2 levels are increased or reduced. Figure 1 plots in vitro studies showing statins reducing or increasing Bcl-2 mRNA and protein levels as a function of statin concentration. There are more studies showing that Bcl-2 levels are reduced by statins as compared with those studies showing an increase. The majority of studies showing that statins reduce Bcl-2 levels used statins concentrations of 5 μM or greater. Studies showing that statins increase Bcl-2 levels used concentrations of 1 μM or less. Certainly there were exceptions but a guarded conclusion is that whether statins increase or decrease Bcl-2 such effects are dependent on statin concentrations.
Figure 1. Effects of statin concentration on Bcl-2 mRNA and protein levels in vitro.
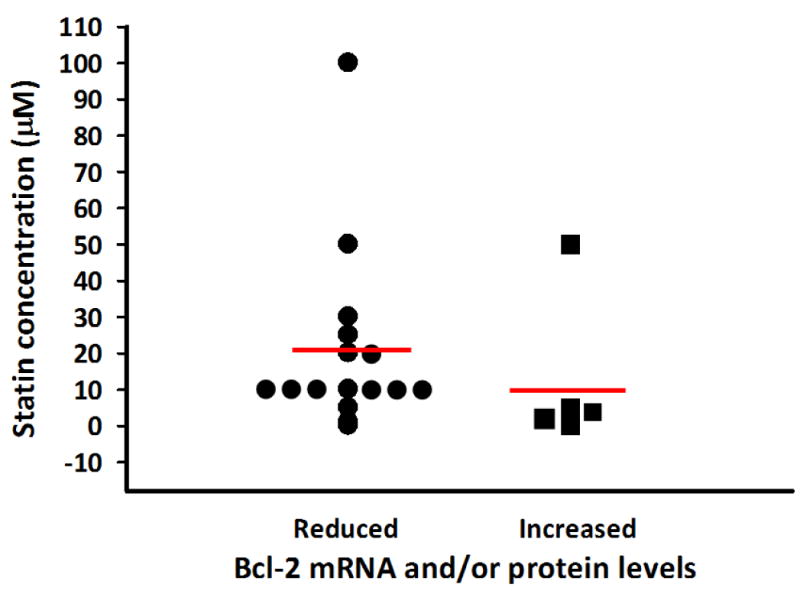
Studies reporting a reduction in Bcl-2 levels:23-25,28,30,31,33,34,36,37,52,60-66. Studies reporting an increase in Bcl-2 levels: 29, 40, 48-51. Red lines represent means of each group.
Mechanisms of Statin-Induced Changes in Bcl-2
Statins reduce cholesterol by reducing the production of mevalonate and upregulate the Low density lipoprotein receptor producing an increase in the removal of LDL from blood. Mevalonate is not only the precursor of cholesterol but it is the precursor of the two isoprenoids FPP and GGPP. FPP is a midpoint precursor of cholesterol and the direct precursor of GGPP. Both FPP and GGPP prenylate small GTPases such as the Rho, Ras, and Rab family of proteins whose coordinated activity is critical for cell structure/function. Simvastatin reduces FPP and GGPP levels [53] and it has been proposed that the beneficial effects of statins may be due to a reduction in prenylation of specific proteins [3;8;54-56]. How such changes in the mevalonate pathway would cause changes in Bcl-2 levels is unclear. Bcl-2 gene expression has been found to be activated by the transcription factor NF-κB [57]. Simvastatin at a high concentration (50 μM) inhibited TNF-alpha induced NF-κB activation which was associated with a reduction in Bcl-2 protein levels in human myeloid KBM-5 cells [26]. In the same study however, it was noted that simvastatin alone had no effect on NF-κB activation.
There is evidence that endothelin-1 (ET-1) can increase Bcl-2 abundance via the transcription factor nuclear factor of activated thymocytes (NFATc) [58]. We found that simvastatin increased ET-1 gene expression whose product is the precursor of the ET-1 protein [40]. The hypothesis was tested that simvastatin stimulation of Bcl-2 involves up-regulation of ET-1 and binding of NFATc to Bcl-2 promoter sites in SH-SY5Y human neuroblastoma cells [59]. Simvastatin increased both intracellular and secreted ET-1 protein levels. Exogenous ET-1 increased Bcl-2 protein abundance, which was inhibited by ET-1 receptor antagonists. Simvastatin increased translocation of NFATc3 to the nucleus while reducing nuclear NFATc1 and having no effect on NFATc4. The Bcl-2 promoter has multiple NFAT binding sites [58], and we found that treatment of cells with simvastatin stimulated binding of NFATc3 to the Bcl-2 promoter. This study was the first to directly identify a transcriptional mechanism for regulation of statin-induced changes in Bcl-2 protein levels. These results do not preclude other mechanisms and the role of protein prenylation in Bcl-2 regulation remains unknown. Also, further study is needed on how statins alter levels of other Bcl-2 family members.
Summary
There is evidence that statins may be efficacious in treating certain types of cancers by acting on Bcl-2 family members and increasing apoptosis and cell death. Equally compelling are studies showing that statins reduce apoptosis and increase Bcl-2. Much, but not all of the evidence supporting a pro-apoptotic effect of statins is based on data in cancer cell lines and the use of relatively high drug concentrations. Studies indicating an anti-apoptotic effect of statins are fewer in number, and generally used low drug concentrations and normal cells. Several questions remain unanswered regarding statin effects on apoptosis, cell death/protection and Bcl-2 family members. There has not been a comprehensive examination of differences in cell types, malignant versus non-malignant in response to statins or for that matter comparisons across different normal cells types (e.g., neurons, astrocytes, endothelial cells etc). The clinical use of statins for the treatment of cardiovascular disease began in the 1970's. Much more work is needed to determine if statins have efficacy in non-cardiovascular diseases such as different cancers and neurodegenerative diseases.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health AG-23524, AG-18357 and the Department of Veterans Affairs.
Reference List
- 1.Taylor F, Ward K, Moore THM, Burke M, Smith GD, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2012;1:2–8. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner N, Nickenig G, Laufs U. Pleiotropic effects of HMG-CoA reductase inhibitors. Basic Res Cardiol. 2002;97:105–116. doi: 10.1007/s003950200000. [DOI] [PubMed] [Google Scholar]
- 3.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2004;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong WWL, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 5.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. The Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 6.Elewa HF, El-Remessy AB, Somanath PR, Fagan SC. Pharmacotherapy. 2010;30:169–176. doi: 10.1592/phco.30.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Eckert GP, Muller WE, Wood WG. Cholesterol-lowering drugs and Alzheimer's disease. Future Lipidol. 2007;2:423–432. [Google Scholar]
- 8.Wood WG, Eckert GP, Igbavboa U, Muller WE. Statins and neuroprotection: A prescription to move the field forward. Ann NY Acad Sci. 2010;1197:1–8. doi: 10.1111/j.1749-6632.2009.05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal N, Houle AG, Melnykovych G. Comparison of dexamethasone and lovastatin (mevinolin) as growth inhibitors in cultures of T-cell derived human acute leukemia lines (CEM) Leuk Res. 1989;13:875–882. doi: 10.1016/0145-2126(89)90040-4. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Sala D, Mollinedo F. Inhibition of isoprenoid biosynthesis induces apoptosis in human promyelocytic HL-60 cells. Biochem Biophys Res Commun. 1994;199:1209–1215. doi: 10.1006/bbrc.1994.1359. [DOI] [PubMed] [Google Scholar]
- 11.Jones KD, Couldwell WT, Hinton DR, Su Y, He S, Anker L, Law RE. Lovastatin induces growth inhibition and apoptosis in human malignant glioma cells. Biochem Biophys Res Commun. 1994;205:1681–1687. doi: 10.1006/bbrc.1994.2861. [DOI] [PubMed] [Google Scholar]
- 12.Chang MY, Jan MS, Won SJ, Liu HS. Ha-rasVal12 oncogene increases susceptibility of NIH/3T3 cells to lovastatin. Biochem Biophys Res Commun. 1998;248:62–68. doi: 10.1006/bbrc.1998.8911. [DOI] [PubMed] [Google Scholar]
- 13.Cerezo-Guisado MI, Alvarez-Barrientos A, Argent R, Garcìa-Marín LJ, Bragado MJ, Lorenzo MJ. c-Jun N-terminal protein kinase signaling pathway mediates lovastatin-induced rat brain neuroblast apoptosis. Biochim Biophys Acta. 2007;1771:164–176. doi: 10.1016/j.bbalip.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barett's esophageal adenocarcinoma cells. Am J Gastroenterol. 2008;103:825–837. doi: 10.1111/j.1572-0241.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- 15.Chao DT, Korsmeyer SJ. Bcl-2 family: Regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by Bcl-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16:647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Walensky LD. Bcl-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 19.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DCS. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 20.Youle RJ. Cellular demolition and the rules of engagement. Science. 2007;315:776–778. doi: 10.1126/science.1138870. [DOI] [PubMed] [Google Scholar]
- 21.Buemi M, Allegra A, Senatore M, Marino D, Medici MA, Aloisi C, DiPasquale G, Corica F. Pro-apoptotic effect of fluvastatin on human smooth muscle cells. Eur J Pharmacol. 1999;370:201–203. doi: 10.1016/s0014-2999(99)00122-3. [DOI] [PubMed] [Google Scholar]
- 22.Link A, Selejan S, Hewera L, Walter F, Nickenig G, Böhm M. Rosuvastatin induces apoptosis in CD4(+)CD28 (null) T cells in patients with acute coronary syndromes. Clin Res Cardiol. 2011;100:147–158. doi: 10.1007/s00392-010-0225-8. [DOI] [PubMed] [Google Scholar]
- 23.Jánosi J, Sebestyén A, Bocsi J, Barna G, Nagy K, Vályi-Nagy I, Kopper L. Mevastatin-induced apoptosis and growth suppression in U266 myeloma cells. Anticancer Res. 2004;24:1817–1822. [PubMed] [Google Scholar]
- 24.Garcia-Román N, Alvarez AM, Toro MJ, Lorenzo MJ. Lovastatin induces apoptosis of spontaneously immortalized rat brain neuroblasts: involvement of nonsterol isoprenoid biosynthesis inhibition. Mol Cell Neurosci. 2001;17:329–341. doi: 10.1006/mcne.2000.0904. [DOI] [PubMed] [Google Scholar]
- 25.Blanco-Colio LM, Justo P, Daehn I, Lorz C, Ortiz A, Egido J. Bcl-xl overexpression protects from apoptosis induced by HMG-CoA reductase inhibitors in murine tubular cells. Kidney Int. 2003;64:181–191. doi: 10.1046/j.1523-1755.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–2516. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 27.Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC, Song IS. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int J Cancer. 2008;123:951–957. doi: 10.1002/ijc.23593. [DOI] [PubMed] [Google Scholar]
- 28.Goc A, Kochuparambil ST, Al-Husein B, Al-Azayzih A, Mohammad S, Somanath PR. Simultaneous modulation of the intrinsic and extrinsic pathways by simvastatin in mediating prostate cancer cell apoptosis. BMC Cancer. 2012;12:409. doi: 10.1186/1471-2407-12-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, Cortright RN, Bamman MM, Neufer PD. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med. 2012;52:198–207. doi: 10.1016/j.freeradbiomed.2011.10.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco-Colio LM, Villa A, Ortego M, Hernández-Presa MA, Pascual A, Plaza JJ, Egido J. 3-hydroxy-3-methyl-glutaryl conenzyme A reductase inhibitors, atorvastatin and simvastatin, induce apoptosis of vascular smooth muscle cells by downregulation of Bcl-2 expression and Rho A prenylation. Atherosclerosis. 2002;161:17–26. doi: 10.1016/s0021-9150(01)00613-x. [DOI] [PubMed] [Google Scholar]
- 31.van de Donk NW, Kamphuis MM, Lokhorst HM, Bloem AC. The cholesterol lowering drug lovastatin induces cell death in myeloma plasma cells. Leukemia. 2002;16:1362–1371. doi: 10.1038/sj.leu.2402501. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Z, Zheng X, Lytle RA, Higashikubo R, Rich KM. Lovastatin-induced up-regulation of the BH3-only protein, Bim, and cell death in glioblastoma cells. J Neurochem. 2004;89:168–178. doi: 10.1111/j.1471-4159.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- 33.Samson KT, Minoguchi K, Tanaka A, Oda N, Yoke T, Okada S, Yamamoto Y, Watanabe Y, Yamamoto M, Ohta S, Adachi M. Effect of fluvastatin on apoptosis in human CD4+ T cells. Cell Immunol. 2005;235:136–144. doi: 10.1016/j.cellimm.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Fromigué O, Haÿ E, Modrowski D, Bouvet S, Jacquel A, Auberger P, Marie PJ. RhoA GTPase inactivation by statins induces osterosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ. 2006;13:1845–1856. doi: 10.1038/sj.cdd.4401873. [DOI] [PubMed] [Google Scholar]
- 35.Demyanets S, Kaun C, Pfaffenberger S, Hohensinner PJ, Rega G, Pammer J, Maurer G, Huber K, Wojta J. Hydroxymethylglutaryl-coenzyme A reductase inhibitors induce apoptosis in human cardiac myocytes in vitro. Biochem Pharmacol. 2006;71:1324–1330. doi: 10.1016/j.bcp.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Aberg M, Wickström M, Siegbahn A. Simvastatin induces apoptosis in human breast cancer cells in a NKkappaB-dependent manner and abolishes the anti-apoptotic signaling of TF/FVIIa and TF/FVIIa. Thromb Res. 2008;122:191–202. doi: 10.1016/j.thromres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Tu YS, Kang XL, Zhou JG, Lv XF, Tang YB, Guan YY. Involvement of Chk1-Cdc25A-cyclin A/CDK2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells. Eur J Pharmacol. 2013;670:356–364. doi: 10.1016/j.ejphar.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- 39.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 40.Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, Schubert-Zsilavecz M, Karas M, Muller WE, Wood WG. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther. 2005;312:786–793. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 41.Franke C, Nöldner M, Abdel-Kader R, Johnson-Anuna LN, Wood WG, Muller WE, Eckert GP. Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis. 2007;25:438–445. doi: 10.1016/j.nbd.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Patassini S, Giampà C, Martorana A, Bernardi G, Fusco FR. Effects of simvastatin on neuroprotection and modulation of Bcl-2 and BAX in the rat quinolinic acid model of Huntington's disease. Neurosci Res. 2008;448:166–169. doi: 10.1016/j.neulet.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Malik S, Sharma AK, Bharti S, Nepal S, Bhatia J, Nag TC, Narang R, Arya DS. In vivo cardioprotection by pitavastatin from ischemic-reperfusion injury through suppression of IKK/NF-κB and upregulation of pAkt-e-NOS. J Cardiovasc Pharmacol. 2011;58:199–206. doi: 10.1097/FJC.0b013e31822002a6. [DOI] [PubMed] [Google Scholar]
- 44.Rajtík T, Carnická S, Szobi A, Mesárošová L, Mát'uš M, Svec P, Ravingerová T, Adameová A. Pleiotropic effects of simvastatin are associated with mitigation of apoptotic component of cell death upon lethal myocardial reperfusion-induced injury. Physiol Res. 2012;61(S2):S33–S41. doi: 10.33549/physiolres.932420. [DOI] [PubMed] [Google Scholar]
- 45.Qin W, Lu Y, Zhan C, Shen T, Dou L, Man Y, Wang S, Xiao C, Bian Y, Li J. Simvastatin suppresses apoptosis in vulnerable atherosclerotic plaques through regulating the expression of p(53), Bcl-2 and Bcl-xL. Cardiovasc Drugs Ther. 2012;26:23–30. doi: 10.1007/s10557-011-6347-z. [DOI] [PubMed] [Google Scholar]
- 46.Doyon M, Hale TM, Huot-Marchand JE, Wu R, de Champlain J, DeBlois D. Does atorvastatin induce aortic smooth muscle cell apoptosis in vivo? Vascul Pharmacol. 2011;54:5–12. doi: 10.1016/j.vph.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Muller WE, Wood WG. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 48.Xu SZ, Zhong W, Watson NM, Dickerson E, Wake JD, Lindow SW, Newton CJ, Atkin SL. Fluvastatin reduces oxidative damage in human vacular endothelial cells by upregulating Bcl-2. J Thromb Haemost. 2008;6:692–700. doi: 10.1111/j.1538-7836.2008.02913.x. [DOI] [PubMed] [Google Scholar]
- 49.Dong Q, Yang Y, Song L, Qian H, Xu Z. Atorvastatin prevents mesenchymal stem cells from hypoxia and serum-free injury through activating AMP-activated protein kinase. Int J Cardiol. 2011;153:311–316. doi: 10.1016/j.ijcard.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Zhao XH, Xu ZR, Zhang Q, Yang YM. Simvastatin protects human osteosarcoma cells from oxidative stress-induced apoptosis through mitochondrial-mediated signaling. Mol Med Report. 2012;5:483–488. doi: 10.3892/mmr.2011.641. [DOI] [PubMed] [Google Scholar]
- 51.Lemoine S, Allouche S, Coulbault L, Cornet V, Massetti M, Galera P, Géard JL, Hanouz JL. Mechanisms involved in cardioprotective effects of pravastatin administered during reoxygenation in human myocardium in vitro. Anesthesiology. 2012;116:824–833. doi: 10.1097/ALN.0b013e31824be77c. [DOI] [PubMed] [Google Scholar]
- 52.Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, Carteni M, Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of Bcl-2 gene expression. Int J Oncol. 2012;40:935–941. doi: 10.3892/ijo.2011.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Muller WE, Wood WG. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis. 2009;35:251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mcfarlane SI, Muniyappa R, Francisco R, Sowers JR. Pleiotropic effects of statins: Lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87:1451–1458. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- 55.Eckert GP, Wood WG, Muller WE. Statins: Drugs for Alzheimer's Disease? J Neural Transm. 2005;112:1057–1071. doi: 10.1007/s00702-004-0273-1. [DOI] [PubMed] [Google Scholar]
- 56.Li L, Zhang W, Cheng S, Cao D. Isoprenoids and related pharmacological interventions: Potential application in Alzheimer's disease. Mol Neurobiol. 2012;46:64–77. doi: 10.1007/s12035-012-8253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville MP, Bours V. NF-κB2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349–1356. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- 58.Kawamura T, Ono K, Morimoto T, Akao M, Iwai-Kanai E, Wada H, Sowa N, Kita T, Hasegawa K. Endothelin-1-dependent nuclear factor of activated T lymphocyte signaling associates with transcriptional coactivator p300 in the activation of the B cell leukemia-2 promoter in cardiac myocytes. Circ Res. 2004;94:1492–1499. doi: 10.1161/01.RES.0000129701.14494.52. [DOI] [PubMed] [Google Scholar]
- 59.Butterick TA, Igbavboa U, Eckert GP, Sun GY, Weisman GA, Muller WE, Wood WG. Simvastatin stimulates production of the antiapoptotic protein Bcl-2 via endothelin-1 and NFATc3 in SH-SY5Y cells. Mol Neurobiol. 2010;41:384–391. doi: 10.1007/s12035-010-8122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park WH, Lee YY, Kim ES, Seol JG, Jung CW, Lee CC, Kim BK. Lovastatin-induced inhibition of HL-60 cell proliferation via cell cycle arrest and apoptosis. Anticancer Res. 1999;19:3133–3140. [PubMed] [Google Scholar]
- 61.Igarashi M, Yamaguchi H, Hirata A, Tsuchiya H, Ohnuma H, Tominaga M, Daimon M, Kato T. Mechanisms of inhibitory effects of cerivastatin on rat vascular smooth muscle cell growth. J Cardiovasc Pharmacol. 2002;40:277–287. doi: 10.1097/00005344-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Mück AO, Seeger H, Wallwiener D. Class-specific pro-apoptotic effects of statins on human vascular endothelial cells. Z Kardiol. 2004;93:398–402. doi: 10.1007/s00392-004-0081-5. [DOI] [PubMed] [Google Scholar]
- 63.Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol. 2004;67:2175–2186. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 64.Mück AO, Seeger H, Wallwiener D. Inhibitory effect of statins on the proliferation of human breast cancer cells. Int J Clin Pharmacol Ther. 2004;42:695–700. doi: 10.5414/cpp42695. [DOI] [PubMed] [Google Scholar]
- 65.Konturek PC, Burnat G, Hahn EG. Inhibition of Barret's adenocarcinoma cell growth by simvastatin: involvement of COX-2 and apoptosis-related proteins. J Physiol Pharmacol. 2007;58:141–148. [PubMed] [Google Scholar]
- 66.Zhang W, Wu J, Zhou L, Xie HY, Zheng SS. Fluvastatin, a lipophilic statin, induces apoptosis in human hepatocellular carcinoma cells through mitochondria-operated pathway. Indian J Exp Biol. 2010;48:1167–1174. [PubMed] [Google Scholar]
- 67.Kaneko R, Tsuji N, Asanuma K, Tanabe H, Kobayashi D, Watanabe N. Survivin down-regulation plays a crucial role in 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor-induced apoptosis in cancer. J Biol Chem. 2007;282:19273–19281. doi: 10.1074/jbc.M610350200. [DOI] [PubMed] [Google Scholar]
- 68.Gauthaman K, Manasi N, Bongso A. Statins inhibit the growth of variant human embryonic stem cells and cancer cells in virro but not normal human embryonic stem cells. Br J Pharmacol. 2009;157:962–973. doi: 10.1111/j.1476-5381.2009.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SK, Kim YC, Song SB, Kim YS. Stabilization and translocation of p53 to mitochondria is linked to Bax translocation to mitochondria in simvastatin-induced apoptosis. Biochem Biophys Res Commun. 2010;391:1592–1597. doi: 10.1016/j.bbrc.2009.12.077. [DOI] [PubMed] [Google Scholar]


