Abstract
Microglia develop an inflammatory phenotype during normal aging. The mechanism by which this occurs is not well understood, but may be related to impairments in several key immunoregulatory systems. Here we show that miR-29a and miR-29b, two immunoregulatory micro-RNAs, were increased in the brain of aged BALB/c mice compared to adults. Insulin-like growth factor-1 (IGF-1) and fractalkine ligand (CX3CL1) are negative modulators of microglial activation and were identified as targets of miR-29a and miR-29b by luciferase assay and primary microglia transfection. Indeed, higher expression of miR-29b in the brain of aged mice was associated with reduced mRNA levels of IGF-1 and CX3CL1. Parallel to these results in mice, miR-29a and miR-29b were also markedly increased in cortical brain tissue of older individuals (mean 77 yrs) compared to middle-aged adults (mean 45 yrs). Moreover, increased expression of miR-29b in human cortical tissue was negatively correlated with IGF-1 and CX3CL1 expression. Collectively these data indicate that an age-associated increase in miR-29 corresponded with the reduction of two important regulators of microglia, IGF-1 and CX3CL1.
Keywords: microglia, miR-29a, miR-29b, aging, neuroinflammation, fractalkine, insulin-like growth factor
1. Introduction
An increase in the inflammatory potential of the brain is a normal consequence of aging. For example, inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 are increased in the brain of aged mice (Godbout et al., 2005; Njie et al., 2012; Ye and Johnson, 1999). Microglia are resident innate immune cells of central nervous system (CNS) and contribute to the increased level of pro-inflammatory cytokine expression in the aged brain (Corona et al., 2012). In support of this notion, several studies indicate that microglia from aged rodents have a “primed” phenotype with increased expression of major histocompatibility complex (MHC)II (Frank et al., 2006; Henry et al., 2009). This is important because primed (MHCII+) microglia produce exaggerated levels of IL-1β in the brain of aged mice after a peripheral immune challenge (Henry et al., 2009). Increased IL-1β production is associated with dendritic atrophy, acute cognitive impairment, and prolonged sickness and depressive-like complications (Chen et al., 2008; Godbout et al., 2005; Godbout et al., 2008; Richwine et al., 2008). While the cause of microglial priming with age is unclear, several studies indicate that a decrease in microglial regulatory systems may be involved. For instance, brain aging is associated with reduced expression of several mediators of microglial regulation including antiinflammatory cytokines (e.g., IL-10, IL-4) (Maher et al., 2005; Ye and Johnson, 2001a), neuronal-derived proteins (CD200, CX3CL1) (Jurgens and Johnson, 2012; Lyons et al., 2007; Wynne et al., 2010), and growth factors (IGF-1, NGF) (Deak and Sonntag, 2012; Larkfors et al., 1987; Sonntag et al., 2005b).
In rodent models of aging, fractalkine (CX3CL1) is an integral modulator of microglia activation that is decreased in the brain with age (Bachstetter et al., 2011; Deak and Sonntag, 2012; Lyons et al., 2009; Wynne et al., 2010). Within the brain, CX3CL1 is a chemokine constitutively expressed by neurons that binds to the fractalkine receptor (CX3CR1) on microglia (Harrison et al., 1998). Thus, CX3CL1-CX3CR1 binding creates a unique regulatory relationship between neurons and microglia (Cardona et al., 2008). Disruption of this CX3CL1-CX3CR1 signaling pathway by loss of either CX3CL1 or CX3CR1 allows for inflammatory-induced activation of microglia (Cardona et al., 2006; Lyons et al., 2009; Wynne et al., 2010). For example, an age-associated reduction in CX3CL1 corresponds with an increased number of primed/MHCII+ microglia (Bachstetter et al., 2011), increased IL-1β production in the hippocampus (Lyons et al., 2009), and increased reactivity of microglia to a secondary immune challenge (Wynne et al., 2010). This increased microglial activity has functional consequences in reduced learning and memory (Rogers et al., 2011), reduced neurogenesis (Bachstetter et al., 2011), and increased depressive-like behavior (Godbout et al., 2008). Moreover, central infusion of CX3CL1 reduced the primed MHCII+ microglia profile in the brain of aged rats (Bachstetter et al., 2011; Lyons et al., 2009). The idea that CX3CL1 is important in modulating microglia responses within the brain is also supported by studies using mice deficient in the fractalkine receptor (CX3CR1KO). CX3CR1KO mice have a hyper-reactive microglial response to an inflammatory challenge (Cardona et al., 2006) that results in amplified pro-inflammatory cytokine production, prolonged sickness behavior, and the development of depressive-like behavior (Corona et al., 2010). Therefore, CX3CL1-CX3CR1 interactions are critical in the modulation of microglial activation.
IGF-1 is another modulator of microglia activation that is reduced in aged brain. Classically, IGF-1 is a growth factor that increases neuroprotection (Sonntag et al., 2005a; Sonntag et al., 2005b), neurogenesis (Llorens-Martin et al., 2009), long-term potentiation (Maher et al., 2006; Sonntag et al., 2005b), and dendritic growth and complexity (Niblock et al., 2000). IGF-1, however, can also modulate immune function. For example, IGF-1 reduces inflammatory cytokine responses in the brain (O'Connor et al., 2008) and ameliorates LPS-induced sickness behaviors (Dantzer et al., 1999) that are primarily driven by microglia-dependent production of IL-1β and TNF-α (Dantzer et al., 2008). Moreover, central injection of a viral vector that upregulated IGF-1 in a mouse model of amyotrophic lateral sclerosis (ALS) reduced microglial secretion of TNF-α and nitric oxide (NO) (Dodge et al., 2008). Thus, age-related reduction in IGF-1 may also contribute to the enhanced inflammatory profile of microglia in the aged brain.
We hypothesize that the reduction of multiple microglial regulatory pathways with age, including CX3CL1 and IGF-1, indicate that there is loss of a global regulator of gene and protein expression. One possibility is that micro-RNA (miRNA) regulation is altered in the aged brain. miRNAs are small (19–24 nucleotides in length) non-coding RNAs that reduce post-transcriptional gene expression by binding to complementary target regions on mRNA to inhibit translation or promote mRNA degradation (Ambros, 2004; Bartel, 2004). miRNAs provide global regulation of gene expression and influence inflammatory processes (Baltimore et al., 2008; O'Connell et al., 2010). Target prediction algorithms of CX3CL1 and IGF-1 revealed that both of these genes are potentially regulated by the miR-29 cluster. We have previously shown that the miR-29a/b cluster is upregulated in immune cells during the course of chronic inflammation, including multiple sclerosis (MS) and the animal model of MS, experimental autoimmune encephalomyelitis (EAE) (Smith et al., 2012). Moreover, miRNAs in the miR-29 cluster were increased in the liver and muscle in a rodent model of accelerated aging (Zmpste24-null mice) (Ugalde et al., 2011). Thus, increased expression of miR-29 in the more inflammatory aged brain may contribute to the progression of microglial dysregulation and hyperactivity.
The purpose of this study was to determine the degree to which immunomodulatory miRNAs were altered in the aged brain and investigate their potential influence on microglial regulatory systems. Here, we show that miR-29a and miR-29b were increased in the brain of both aged mice and older humans. Moreover, this increase in miR-29a and miR-29b expression was associated with the down-regulation of specific CNS targets involved in the modulation of microglial activation, IGF-1 and CX3CL1. Indeed, increased expression of miR-29b in the brains of older humans significantly and negatively correlated with IGF-1 and CX3CL1 expression. We interpret these results to suggest that age-associated increases in miR-29 in the brain suppress multiple factors, including IGF-1 and CX3CL1, contributing to the development of an inflammatory profile of microglia in the aged brain.
2. Methods
2.1. Mice
Adult (2–3 mo) male BALB/c mice were obtained from a breeding colony kept in barrier-reared conditions in a specific pathogen-free facility at the Ohio State University. Aged (18–20 mo) male BALB/c mice were obtained from the National Institute on Aging specific pathogen-free colony (maintained at Charles River Laboratories, Inc., MA). Aged mice were allowed one week to acclimate to the facility prior to experimentation. All mice were individually housed in polypropylene cages and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Postmortem human brain tissue
Postmortem human brain tissue was obtained from the Harvard Brain Tissue Resource Center (Belmont, MA, supported by the PHS grant R24 MH068855) (10 samples) and the Human Brain and Spinal Fluid Resource Center (VA West Los Angeles Healthcare Center, Los Angeles, CA which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society and Department of Veterans Affairs) (17 samples). The tissue consisted of eleven adult (14 – 55 years old with a mean age of 45 years old) and sixteen aged (58 – 91 years old with a mean age of 73 years old) brain samples (Table 1). The differentiation between “Adult” or “Aged” brain tissue was 57 yrs (<57 = Adult; >57 = Aged). Tissue sections were collected from the normal appearing white matter or postcentral parietal area (Brodmann 3, 1, 2, 5) and all samples were immediately flash frozen in liquid nitrogen (−196° C) after collection, shipped on dry ice, and stored at −80° C until use. All brains were designated healthy and non-CNS diseased controls with the exception of four samples obtained from epileptic patients (3 adult, 1 aged).
Table 1.
Postmortem brain tissue samples.
| Human Sample Number | Age | Sex | Description | Tissue |
|---|---|---|---|---|
| AN08855 | 64 | M | HC | BA 3,1,2,5 |
| AN10180 | 73 | M | HC | BA 3,1,2,5 |
| AN04932 | 55 | F | HC | BA 3,1,2,5 |
| AN01234 | 85 | M | HC | BA 3,1,2,5 |
| AN07334 | 47 | M | HC | BA 3,1,2,5 |
| AN19092 | 46 | M | HC | BA 3,1,2,5 |
| AN16467 | 79 | M | HC | BA 3,1,2,5 |
| AN06400 | 91 | F | HC | BA 3,1,2,5 |
| AN16017 | 55 | M | HC | BA 3,1,2,5 |
| AN09007 | 52 | F | HC | BA 3,1,2,5 |
| HSB3603 | 74 | F | HC | Sect 8 NAWM |
| HSB3590 | 75 | M | HC | Sect 6 NAWM |
| HSB3602 | 66 | M | HC | Sect 6 NAWM |
| HSB3531 | 74 | M | HC | Sect 7 NAWM |
| HSB3589 | 53 | M | HC | Sect 3 NAWM |
| HSB3543 | 73 | F | HC | Sect 7 NAWM |
| HSB3540 | 68 | M | HC | Sect 7 NAWM |
| HSB3529 | 58 | M | HC | Sect 8 NAWM |
| HSB951 | 45 | M | EP | Sect 4 NAWM |
| HSB968 | 66 | F | EP | Sect 7 NAWM |
| HSB891 | 14 | M | EP | Sect 4 NAWM |
| HSB516 | 22 | F | EP | Sect 3 NAWM |
| 1B1 | 62 | M | HC | NAWM |
| 2B2 | 82 | M | HC | NAWM |
| 3B1 | 48 | M | HC | NAWM |
| 3175 | 54 | F | HC | NAWM |
| 2519 | 80 | M | HC | NAWM |
The sample number, age, sex, description, and tissue section are listed for each sample used. The tissue consisted of eleven adult (14 – 55 years old with a mean age of 45 years old) and sixteen aged (58 – 91 years old with a mean age of 73 years old) brain samples.
Abbreviations: BA = Brodmann’s Area; EP = Epileptic; HC = non-CNS disease control; NAWM = normal appearing white matter.
2.3. Microglial isolation
An enriched population of microglia was isolated from whole brain homogenates of mice as previously described (Wynne 2010, Fenn 2012). In brief, brains were homogenized in PBS through a 70 µm nylon cell strainer. Resulting homogenates were centrifuged and cell pellets were re-suspended in 70% isotonic Percoll. A discontinuous Percoll density gradient was layered, centrifuged, and enriched microglia were collected from the interphase between the 70% and 50% Percoll layers. Microglia were washed and re-suspended in PBS. Each brain extraction yielded approximately 3 × 105 viable cells. We have previously characterized these cells enriched (∼85%) microglia (CD11b+/CD45low ) (Henry et al., 2009).
2.4. miRNA/mRNA isolation and real-time PCR
miRNA and mRNA were isolated from enriched microglia using either a miRNA isolation kit (AM1561; Ambion, Austin, TX) or a PrepEase mRNA kit (USB, CA). From the coronal brain section, both miRNA and mRNA were isolated using the Tri Reagent protocol (Sigma, MO). miRNA was reverse transcribed to cDNA using miRNA specific primers from Taqman® (Applied Biosystems; Foster, CA) for each miRNA of interest (e.g., miR-29a, miR-29b, etc). mRNA was reverse transcribed to cDNA using the high capacity protocol (Applied Biosystems; Foster, CA) according to manufacturer instructions.
Real-time (RT)-PCR was performed for both miRNA and mRNA using the Applied Biosystems (Foster, CA) Taqman® Gene Expression assay as previously described (Wohleb et al., 2011). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as the relative expression across both groups or fold difference from control. To reduce inter-test variability, relative expression and fold change for miRNA and mRNA expression was evaluated after each individual experiment and values were then combined.
2.5. Luciferase assay
The 3’ untranslated region (3’UTR) segments containing the target sites for miR-29a and miR-29b from the murine IGF-1 and CX3CL1 gene were amplified from genomic DNA isolated from the brains of BALB/c mice (Table 2), and inserted into the PGL3 control vector (Promega) using the XBA1 site immediately downstream from the luciferase stop codon. HEK-293 cells were transfected with 800 ng firefly luciferase vector, 100 ng Renilla luciferase control vector, and 200 nM of precursor miR-29a, miR-29b, or scrambled oligonucleotides (negative control precursor; Ambion) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Cells were lysed in Passive Lysis Buffer and assayed in duplicate using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity as measured by a Veritas microplate luminometer (Promega) and was then calculated relative to the scrambled control in each independent replicate.
Table 2.
Murine 3’ UTR sequences for luciferase assay.
| Murine gene UTR | UTR sequence |
|---|---|
| mCX3CL1_UTR_F | AATTTCTAGATGCCTGTCCCCCTGACCTCC |
| mCX3CL1_UTR_R | AATTTCTAGACCGAGTGGGGACTGGACCCT |
| mIGF1_UTR_F1 | AATTTCTAGAACGTACCTGACTCCATCTGTGGCA |
| mIGF1_UTR_R1 | AATTTCTAGAGGCTCCAGGCTTTCGTTTGTTGT |
| mIGF1_UTR_F2 | AATTTCTAGAGCACTTGGGAGGATGCGCAGA |
| mIGF1_UTR_R2 | AATTTCTAGAGTGGTGGCTAGGGTGGTGGC |
cDNA from the brain sections of BALB/c mice was used to clone the UTR sequences for CX3CL1 and IGF-1. Sequences were placed into a vector downstream of the luciferase stop codon and transfected into HEK-293 cells for the luciferase assay. The forward and reverse sequences used for each UTR site investigated are shown.
2.6. Primary cultures and miR Transfection
Primary microglia cultures were established from neonatal mice (P0-P3) as previously described (Godbout et al., 2004). After 10–12 d in culture primary microglia were shaken at 180 rpm for 3.5 h and plated at 300,000 cells / well in a 12-well plate and used the following day for transfection.
Transfections were performed using the TransIT-TKO Transfection Reagent according to manufacturer’s protocol with minor modifications (Mirus Bio LLC, WI). In brief, primary microglia cells were maintained in 500 µL of 20% growth medium (DMEM supplemented with 20% FBS, sodium bicarbonate 3.7 g/L, 200 mM glutamine, 100 U/ml penicillin G, 100 Ag/mL streptomycin, 50 Ag/mL gentamicin) and medium was changed just prior to transfection. 100 µL of the transfection complexes (100 µL serum-free medium + 2.5 µL TransIT-TKO Reagent + siRNA [50 nM final concentration]) was added to the appropriate wells. siRNAs included miR-29b and miR-542-5p (Thermo Fisher Scientific, MA). For controls, 100 µL of serum-free medium + 2.5 µL TransIT-TKO was added. Primary microglia were incubated with transfection complexes at 37° C / 5% CO22 for 24 h. After 24 h supernatant was aspirated and 500 µL of fresh growth medium was added.
2.7 IGF-1 ELISA Assay
Twenty-four h after transfection, primary microglia were treated with vehicle (0.1% BSA) or 5 ng/mL recombinant IL-4 (R&D Systems, MN). After 24 or 48 h supernatants were collected and stored at −80° C until use. IGF-1 ELISA was performed on undiluted supernatants according to the manufacture’s protocol (Abcam, MA). Detection limits were 50 pg/mL + 5 pg/mL.
2.8. Statistical Analysis
To ensure a normal distribution, data were subjected to the Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). To determine significant main effects and interactions between main factors, data were analyzed with a one-way (i.e. Age) ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. Correlation analyses were performed by calculating the Pearson correlation coefficient (R) and determining p-value based on sample size (t = R/(√(1-R2)/n-2)). All data are expressed as treatment means ± standard error of the mean (SEM). Values were considered significant at p-values < 0.05 and a tendency at p-values < 0.1. Statistics are represented as F(degrees of freedom, sample size-1) = f-value, p-value.
3. Results
3.1. Increased expression of miR-146a, miR-155, miR-29a, and miR-29b in the brain of aged mice
miRNAs are important immunoregulatory factors (Baltimore et al., 2008; O'Connell et al., 2010) and aging is associated with an increase in brain inflammation (Fenn et al., 2012; Godbout et al., 2005; Henry et al., 2009). Thus, we sought to determine the extent to which miRNA expression was altered in the brain with normal aging. Expression of miRNAs associated with modulating inflammation (i.e., miR-146a, miR-155, miR-124, miR-29a, miR-29b, and miR-29c) was determined in a 1 mm coronal brain section (−0.5 Bregma) from adult (3–4 mo) and aged (20 mo) BALB/c mice. This 1 mm section was selected because it contains cortex and striatum to provide a cortical representation of alterations in miRNA or mRNA levels. Fig.1 shows that miR-146a (Fig.1A, F(1,23)=2.33, p=0.1) and miR-155 (Fig.1B, F(1,21)=2.66, p=0.1) tended to be increased in the brain with age, but miR-124 was unchanged (Fig. 1C). Moreover, there were significant alterations in the miR-29 cluster in the brain with age. For instance, miR-29a was increased with age (Fig.1D, F(1,31)=22.97, p<0.0001), miR-29b tended to be increased with age (Fig.1E, F(1,34)=2.71, p=0.1) and miR-29c was reduced with age (Fig.1F, F(1,21)=7.78, p<0.02). Overall, miRNAs known to modulate inflammatory responses (miR-146a, miR-155, miR-29a, and miR-29b) were increased in the brain of aged BALB/c mice.
Figure 1. Increased expression of miR-146a, miR-155, miR-29a, and miR-29b in the brain of aged mice.
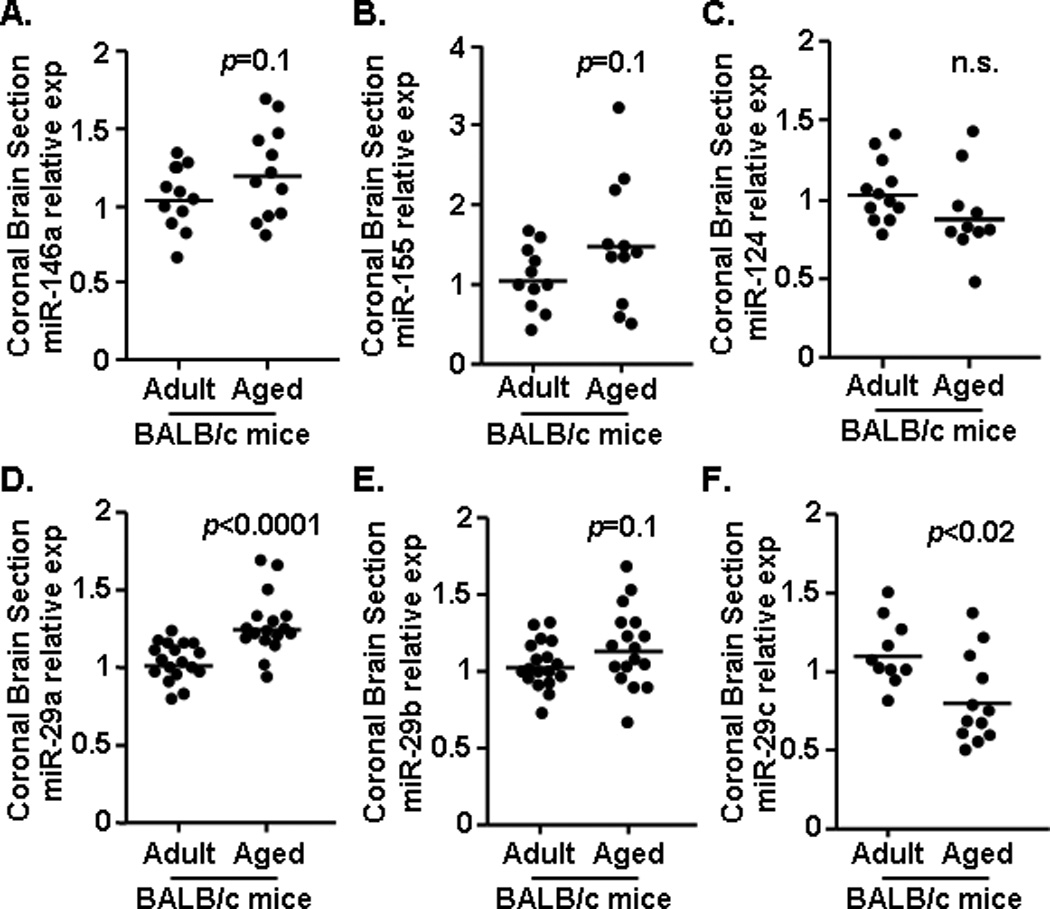
A 1 mm coronal brain section (−0.5 Bregma) was collected from adult (n=12–17) and aged (n=12–18) mice and levels of A) miR-146a, B) miR-155, C) miR-124 D) miR-29a, E) miR-29b, and F) miR-29c were determined. Samples represent three independent experiments and are presented as fold change compared to Adult. Horizontal bars represent the mean with each dot depicting a separate sample.
3.2. Increased expression of miR-155, miR-29a, and miR-29b in microglia of aged mice
Because microglia develop a more inflammatory and primed phenotype with age, these same miRNAs were determined in enriched CD11b cells (∼85% microglia) that were collected by Percoll gradient separation from whole brain homogenates (Fenn et al., 2012). Fig.2A & B shows that both miR-29a (Fig.2A, F(1,20)=8.51, p<0.009) and miR-29b (Fig.2B, F(1,19)=8.09, p<0.02) were increased in the microglia of aged mice compared to adult controls. Fig.2C shows the average relative expression of miR-146a, miR-155, miR-29c, and miR-124 in microglia of adult and aged mice. These data indicate that miR-155 tended to be increased in the microglia of aged mice (F(1,6)=3.06, p=0.1), but that no other miRNA was altered in microglia of aged mice compared to adults. Taken together these data indicate that miR-29a, miR-29b, and miR-155 were increased in microglia of aged BALB/c mice.
Figure 2. Increased expression of miR-155, miR-29a, and miR-29b in the microglia of aged mice.
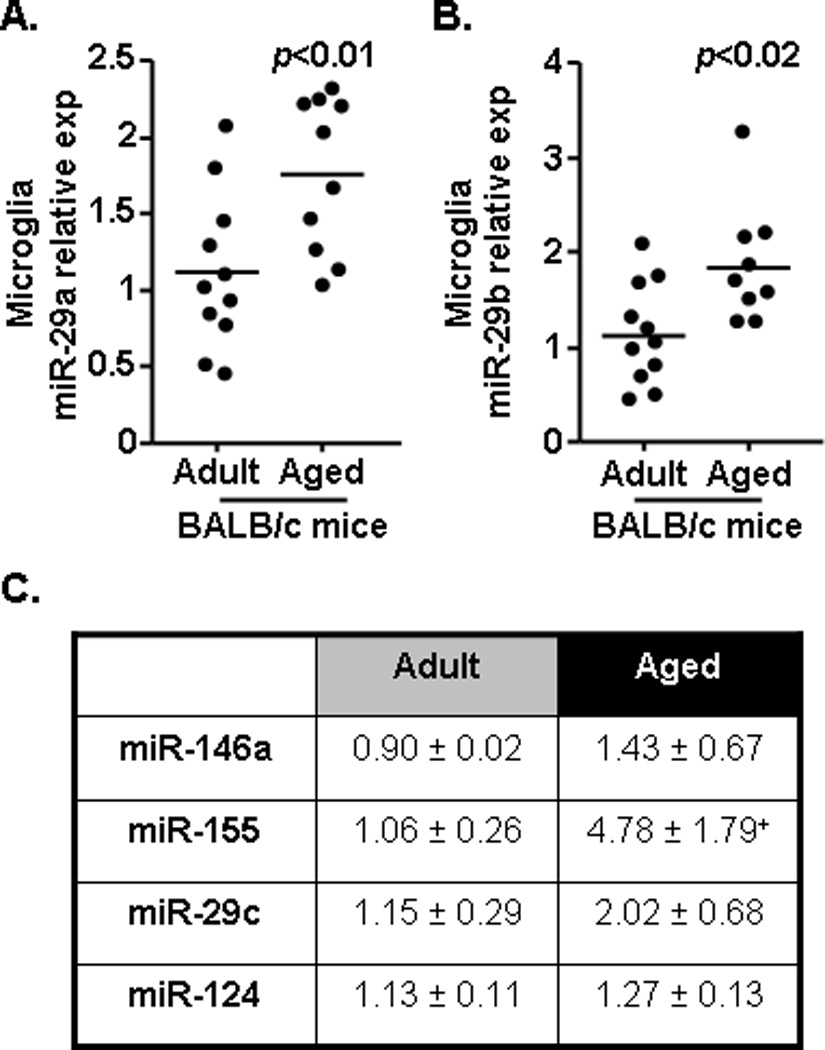
Microglia were isolated from the remainder of the brain tissue collected in Figure 1 and the expression of A) miR-29a and B) miR-29b were determined (n=10–11). C) In a subset of the adult (n=5–10) and aged (n=4–10) mice, miR-146a, miR-155, miR-29c, and miR-124 were determined in microglia and the mean value for each miRNA is shown. Samples represent three sets of independent experiments and are presented as fold change compared to Adult. Horizontal bars represent the mean with each dot depicting a separate sample. Means with + have a tendency (p=0.1) to be different from Adult controls.
3.3. Peripheral injection with LPS increased miR-29b expression in the brain
Our previous studies show that a peripheral injection with lipopolysaccharide (LPS) resulted in microglial activation with increased IL-1β production in the brain after 4 h (Fenn et al., 2012; Henry et al., 2009). Our current results indicate that miR-29a and miR-29b were increased in the aged brain (Figs.1&2), but the degree to which this related to increased inflammatory potential in the aged brain was unclear. Therefore, we next assessed the degree to which miR-29a and miR-29b were increased in the brain following a peripheral injection of LPS. Adult mice were injected i.p. with LPS (0.33 mg/kg), a 1 mm coronal section was collected after 4 h, and expression of IL-1β mRNA, miR-29a, and miR-29b were determined. As expected, LPS injection increased IL-1β mRNA expression in the brain (Fig.3A, F(1,17)=61.49, p<0.0001). miR-29a was unaffected by LPS (Fig.3B) and miR-29b was increased by LPS (Fig.3C, F(1,13)=5.85, p<0.04). These results indicate that brain inflammation associated with a peripheral LPS injection corresponded with increased expression of miR-29b, but not miR-29a.
Figure 3. Peripheral injection with LPS increased miR-29b expression in the brain.
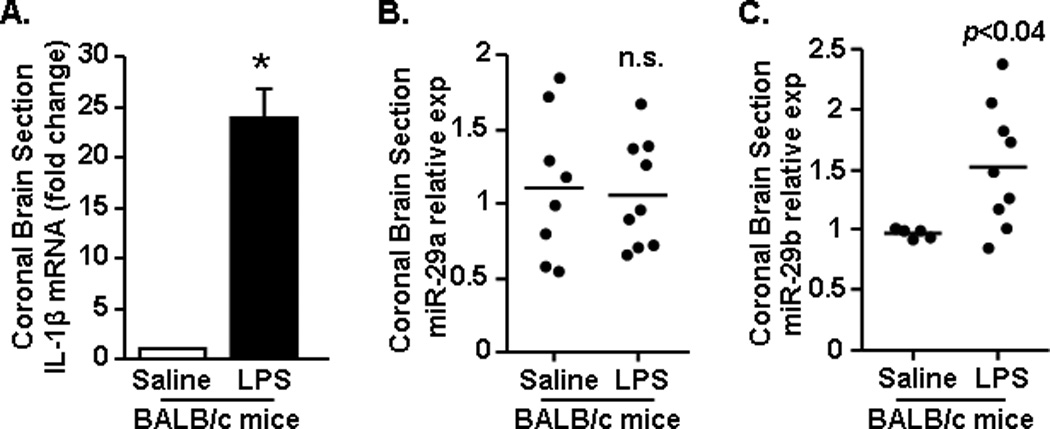
BALB/c mice received an intraperitoneal (i.p.) injection of saline or LPS (0.33 mg/kg) (n=9). After 4 h a 1 mm coronal brain section (−0.5 Bregma) was collected and A) IL-1β mRNA expression, along with expression of B) miR-29a, and C) miR-29b was determined. Results represent two independent experiments and are presented as fold change from Saline. Means with * are significantly (p<0.0001) different from Saline controls.
3.4. IGF-1 and CX3CL1 were targets of miR29a/b mediated suppression
Because miR-29a and miR-29b were increased in the aged brain (Figs.1&2) we sought to identify targets that: 1) are regulated by miR-29a/b and 2) promote increased microglia reactivity when suppressed. Using the online TargetScan program (www.targetscan.org, release 6.2, MIT, Cambridge, MA) insulin-like growth factor (IGF)-1 and fractalkine ligand (CX3CL1) were identified as potential targets of miR-29a and miR-29b (Fig. 4A). For example, TargetScan analysis of the possible targets for miR-29a and miR-29b showed that IGF-1 was a strongly projected target with a Total Context score below −0.3 and two conserved sites, while CX3CL1 was a projected target with a Total Context score of −0.09 and one conserved site. IGF-1 and CX3CL1 are relevant because both modulate the activation of microglia (Dodge et al., 2008; Lyons et al., 2009) and are influenced by normal aging (Bachstetter et al., 2011; Deak and Sonntag, 2012; Wynne et al., 2010).
Figure 4. miR-29a and miR-29b suppressed the expression of IGF-1 and CX3CL1.

A) TargetScan analysis of the possible targets for miR-29a and miR-29b showed that IGF-1 was a strongly projected target and CX3CL1 was a projected target. B) HEK-293 cells were transfected with a firefly luciferase vector and the 3’UTR segments containing the target sites for miR-29a and miR-29b for murine IGF-1 and CX3CL1 immediately downstream from the luciferase stop codon. Cells were co-transfected with miR-29a or miR-29b. The ratio of firefly to renilla luciferase activity was normalized to the control miRNA within each experimental replicate. Values represent the normalized luciferase activity of the constructs. C) A 1 mm coronal brain section was collected from adult (n=16–18) and aged (n=18) BALB/c mice and mRNA levels of IGF-1 and D) CX3CL1 were determined. Results represent two (B) or three (C,D) independent experiments and are presented as fold change compared to Control or Adult. Bars represent the mean ± SEM. Means with * are significantly (p<0.05) different and means with + tend (p=0.08) to be different from Control.
While TargetScan predicts the likelihood that a miRNA binds to the 3'UTR of a target gene, it is important to validate these predictions. To confirm that IGF-1 and CX3CL1 were targets of miR-29a and miR-29b, the 3'UTRs of murine IGF-1 or CX3CL1 were cloned downstream of the firefly luciferase gene. In these assays, if the transected miRNA binds the 3'UTR of the target gene then luciferase expression is suppressed. HEK-293 cells were co-transfected with miR-29a, miR-29b, or a negative control miRNA together with the firefly luciferase-IGF-1 or CX3CL1 3’UTR construct. Fig.4B shows that miR-29a and miR-29b repressed luciferase activity of the IGF-1 3’UTR more than 50% at two independent sites (p<0.005 for all). Luciferase activity of the constructs containing the CX3CL1 3’UTR was reduced by 30% by miR-29a (p=0.08), but was not reduced by miR-29b. These results indicate that miR-29a and miR-29b directly target the 3'UTR of IGF-1 and that miR-29a targets the 3'UTR of CX3CL1.
Next, mRNA expression of IGF-1 and CX3CL1 was determined in the brains of adult and aged mice. Corresponding with increased miR-29a and miR-29b (Figs.1&2), mRNA levels of IGF-1 (Fig.4C, F(1,34)=20.36, p<0.0001) and CX3CL1 (Fig.4D, F(1,35)=6.21, p<0.02) were significantly reduced with age. Taken together, these data indicate that the age-associated increase in brain levels of miR-29a and miR-29b coincided with the reduction of two confirmed targets, IGF-1 and CX3CL1.
3.5. IGF-1 was decreased in the brain after peripheral injection with LPS and suppressed by miR29b in microglia
We show that LPS injection increased miR-29b expression in the brain, but had no effect on miR-29a (Fig.3B&C). In addition, IGF-1 was a confirmed target of miR-29b as transfection with miR-29b repressed luciferase activity of the IGF-1 3’UTR (Fig.4B). Therefore, to continue to investigate the relationship between increased miR-29a/b and reduced expression of its predicted targets, mRNA expression of IGF-1 and CX3CL1 in the brain of mice injected with saline or LPS was determined. Fig.5A shows that IGF-1 mRNA was significantly reduced in the brain of mice injected with LPS compared to saline controls (p<0.02). CX3CL1 mRNA expression, however, remained unchanged after LPS injection (Fig.5B). These data are consistent with previous data that LPS resulted in increased expression of miR-29b, but not miR-29a (Fig.3C), and that IGF-1, but not CX3CL1, is a target of miR-29b (Fig.4B). Overall, these data indicate that the LPS-induced miR-29b expression in the brain corresponded with reduced mRNA expression of IGF-1.
Figure 5. IGF-1 was decreased in the brain after peripheral injection with LPS and suppressed by miR29b in microglia.
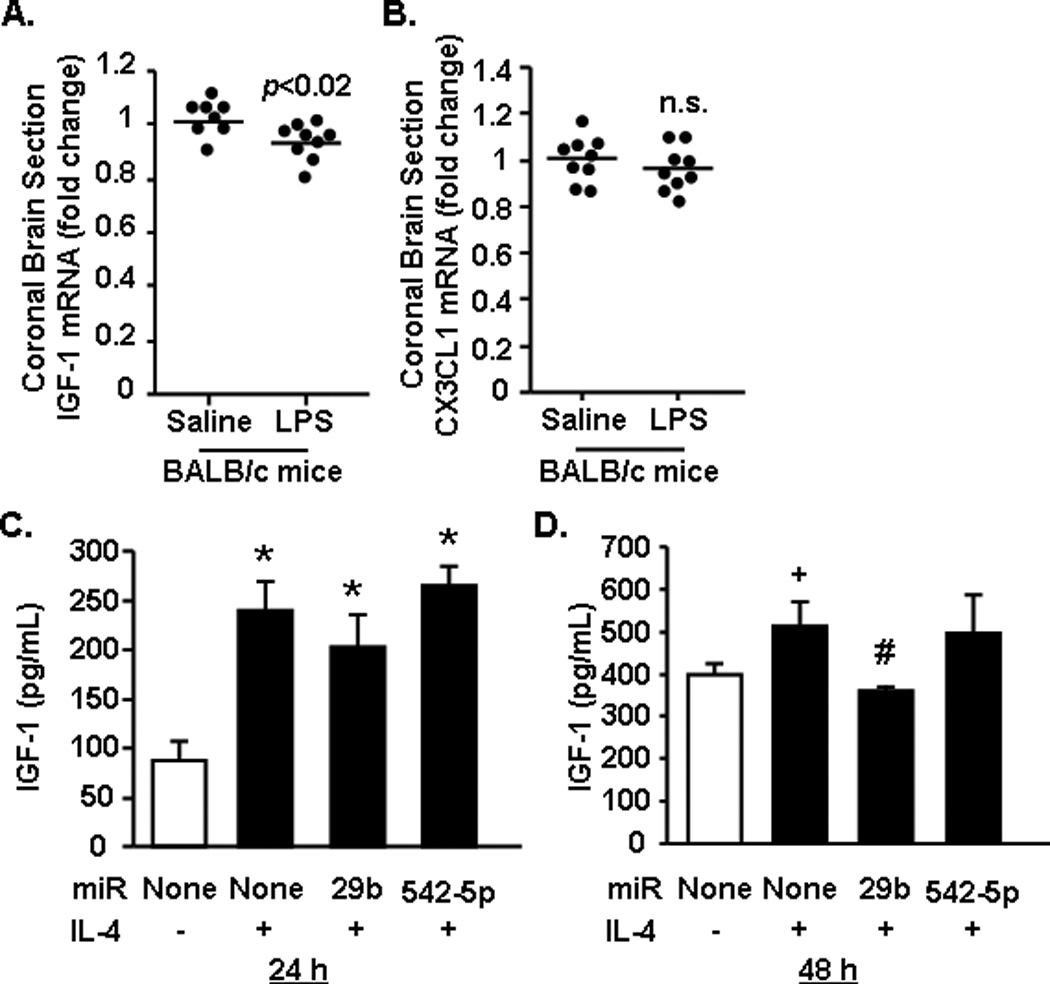
BALB/c mice received an intraperitoneal (i.p.) injection of saline or LPS (n=9). After 4 h a 1 mm coronal brain section (-0.5 Bregma) was collected and A) IGF-1 and B) CX3CL1 mRNA expression was determined. In a separate set of studies, primary microglia cultures were established and then transfected with miR-29b or a control miR (n=2–3). Protein levels of IGF-1 were determined C) 24 or D) 48 h after IL-4 stimulation. Results represent two independent experiments and are presented as fold change compared to Control or Adult. Bars represent the mean ± SEM. Means with * are significantly (p<0.05) different and means with + tend (p=0.1) to be different from Control. Means with # tend (p=0.08) to be different from Control-IL-4.
Next, the ability of miR-29b to suppress IGF-1 expression specifically in microglia was assessed. In these experiments, primary microglia were untransfected or transfected with either miR-29b or the non-specific miR-542-5p. Microglia were stimulated with interleukin (IL)-4, an inducer of IGF-1 (Zhao et al., 2006), and IGF-1 protein concentration was determined 24 and 48 h later. Fig.5C&D show that IL-4 increased IGF-1 protein secretion by microglia 24 h and 48 h later (main effect of IL-4: F(1,15)=14.52, p<0.003). Transfection with miR-29b reduced IL-4 induced IGF-1 protein levels in primary microglia by 15% at 24 h and by 30% at 48 h (p=0.08). Thus, by 48 h levels of IGF-1 retuned to baseline in the microglia transected with miR-29b. The non-specific miR control (542-5p) had no effect on IL-4-induced IGF-1 levels in microglia at either time point. Taken together, these data indicate that miR-29b directly suppresses IGF-1 production by microglia, as predicted by target scan analysis and luciferase assay.
3.6. miR-29a and miR-29b were increased in the brain of aged humans
To determine the degree to which increased miR-29a and miR-29b levels were relevant to human brain aging, cortical brain tissue was obtained from brain bank donor adults and older individuals (Harvard Brain Tissue Resource Center and the Human Brain and Spinal Fluid Resource Center). The tissue consisted of eleven adult (14 – 55 years old with a mean age of 45 ± 4.2 years old) and sixteen aged (58 – 91 years old with a mean age of 73 ± 2.2 years old) brain samples (Table 1).
Expression of miR-29a tended to be increased in the brain of older individuals compared to middle aged controls (Fig.6A, F(1,24)=2.87, p=0.1), and miR-29a expression was positively correlated with advancing age (Fig.6B, R=0.41, p=0.07). Similarly, miR-29b expression was significantly increased in the brains of older individuals compared to the middle aged controls (Fig.6C, F(1,24)=5.28, p<0.04), and miR29b expression was positively correlated with increasing age (Fig.6D, R=0.45, p<0.04). Taken together, these data indicate that miR-29a and miR-29b are increased in the brains of older individuals and increase as a function of advancing age.
Figure 6. miR-29a and miR-29b were increased in the brains of aged humans.
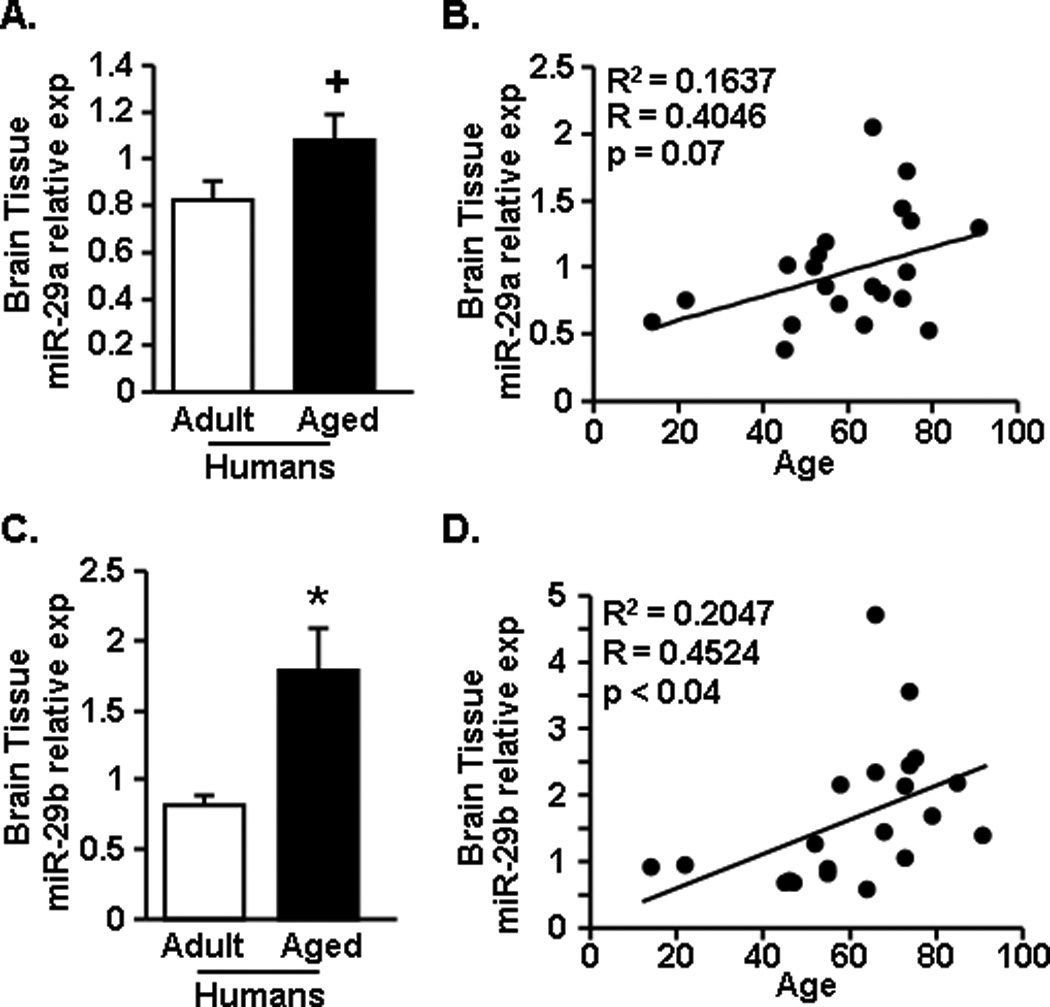
Postmortem brain tissue was acquired for both adult (n=11) and aged (n=16) age groups and A) miR-29a and C) miR-29b expression was determined. In addition, the correlation between the individual’s age and B) miR-29a and D) miR-29b expression are shown. Bars represent the mean ± SEM. Relative expression is compared to the average comparative Ct for all samples. Means with * are significantly (p<0.04) different and means with + tend (p<0.08) different from Adult controls.
3.7. The age-associated increase in brain levels of miR-29a and miR-29b was negatively correlated with reduced expression of CX3CL1 and IGF-1
Using the same post-mortem brain tissue as above, mRNA expression of IL-1β, IGF-1, and CX3CL1 was determined. Fig.7A shows that IL-1β mRNA levels appeared to increase in the brain with age, but this increase in older individuals was not statistically different from middle aged controls (Fig.7A). Consistent with the increase in miR-29a and miR-29b (Fig.7), there was a robust reduction in the mRNA levels of IGF-1 (Fig.7B, F(1,18)=14.17, p<0.002) and CX3CL1 (Fig.7C, F(1,20)=5.41, p<0.04) in the brains of older individuals compared to controls. To begin to address the relationship between increased miR-29a and miR-29b and reduced IGF-1 and CX3CL1 expression, a correlation analysis was performed. Fig.7D shows that increased miR-29a expression negatively correlated with reduced expression of IGF-1 (R=−0.42, p<0.05). Increased miR-29a, however, was not correlated with a reduction in CX3CL1 expression (Fig. 7E). Similar to miR-29a, Fig. 7F shows that increased miR-29b expression negatively correlated with reduced IGF-1 mRNA expression (R=−0.74, p<0.0002). In contrast to miR-29a, increased miR-29b expression also negatively correlated with reduced CX3CL1 mRNA expression (Fig.7G, R=−0.64, p<0.002). Taken together, age-associated increases in miR-29b correlated with reduced expression of both IGF-1 and CX3CL1 in the brains of older individuals.
Figure 7. The aged-associated increase in brain levels of miR-29a and miR-29b was negatively correlated with reduced expression of CX3CL1 and IGF-1.
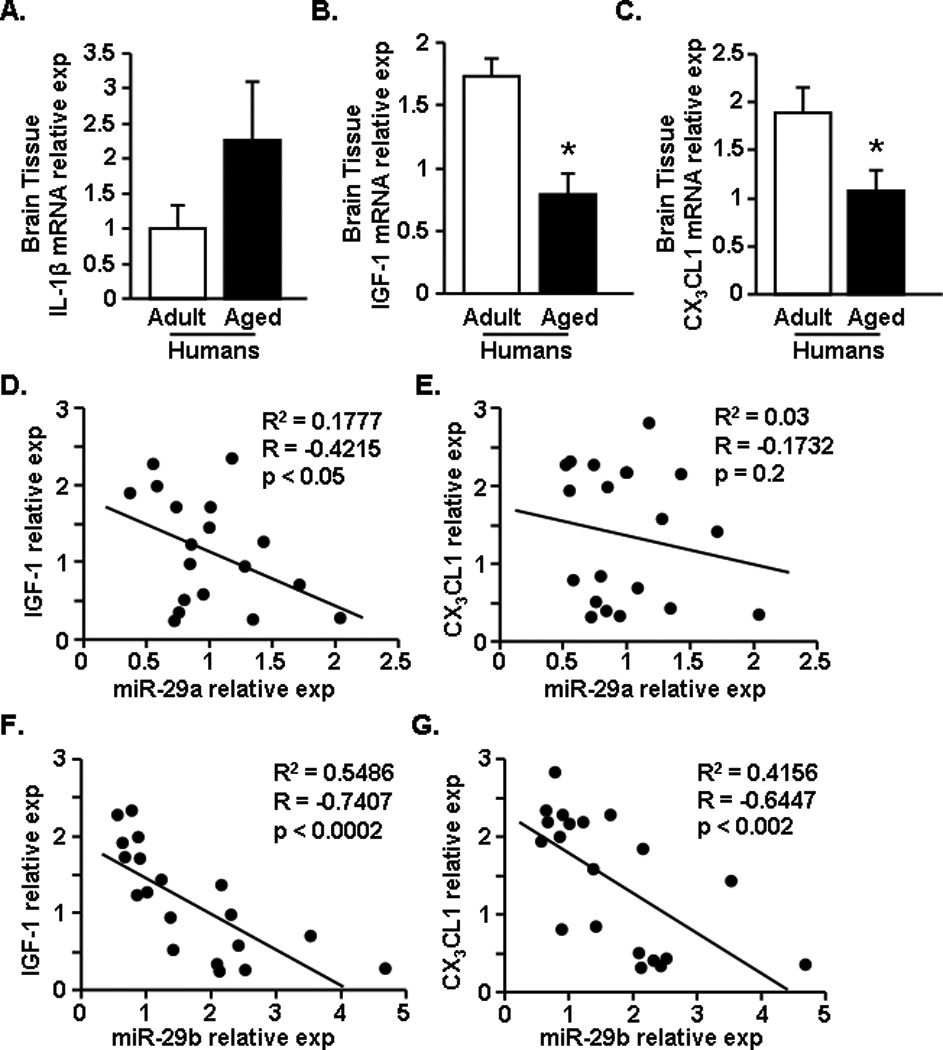
Postmortem brain tissue was acquired for both adult (n=9) and aged (n=13) age groups. From the human brain tissue mRNA expression of A) IL-1β, B) IGF-1, and C) CX3CL1 was determined. In addition, a correlation plot was created for IGF-1 and CX3CL1 compared to miR-29a or miR-29b levels determined in Fig.5. Plots depict expression levels of miR-29a versus D) IGF-1 and E) CX3CL1 in the human brain tissue. In addition, levels of miR-29b versus F) IGF-1 and G) CX3CL1 are shown. Bars represent the mean ± SEM. Relative expression is compared to the average comparative Ct for all samples. Means with * are significantly (p<0.04) different from Adult controls.
4. Discussion
We have previously reported that microglia from aged mice have a primed (MHCII+) phenotype (Henry et al., 2009; Wynne et al., 2010). This is important because a primed microglial profile leads to an exaggerated inflammatory response following a central or peripheral immune challenge (Fenn et al., 2012; Henry et al., 2009; Huang et al., 2008; Wynne et al., 2010). The mechanisms underlying this age-associated increase in brain inflammation may be related to deficient regulation of microglia by anti-inflammatory mediators, including IGF-1 and CX3CL1. Here we present novel evidence that the age-associated reduction in IGF-1 and CX3CL1 expression in the brain corresponded with increased expression of the miR-29a/b cluster. Moreover, the miR-29a/b cluster was found to target IGF-1 and CX3CL1 by luciferase assay and transfection studies confirmed that miR-29b attenuated IGF-1 protein expression in primary microglia. Parallel to the results in the brain of aged mice, miR-29a and miR-29b were increased in the brains of older humans and these increases were correlated with reductions in IGF-1 and CX3CL1 mRNA expression. Collectively, the results of this study indicate that the miR-29a/b cluster is increased in the brain with age corresponding in the reduced expression of two key mediators of microglial regulation.
One important finding of this study was that the expression of several miRNAs was increased in the brain of aged mice compared to adults. While there are numerous identified miRNAs, we focused on immunomodulatory miRNAs including miR-146a, miR-155, and the miR-29 cluster (Steiner et al., 2011; Taganov et al., 2006; Tili et al., 2007). For instance, our data indicate that miR-146a tended to be increased in the brain of aged BALB/c mice compared to adults. This is consistent with a previous report using aged C57BL/6J mice (Li et al., 2011). In addition, increased miR-146a levels support the notion of a more inflammatory CNS environment with age as miR-146a is increased by inflammatory-induced NF-κB (Labbaye and Testa, 2012). Indeed, several studies in aged rodents indicate that NF-κB nuclear binding and subsequent gene expression are increased in the aged brain (Kim et al., 2000; Korhonen et al., 1997; Ye and Johnson, 2001b). Similar to the results with miR-146a, miR-155 was also increased in the brain of aged mice. Higher miR-155 expression was also detected specifically in microglia. Previous work denotes that the expression of miR-155 is increased by c-Jun N-terminal kinase (JNK) (O'Connell et al., 2007) and functions to suppress genes involved in toll like receptor signaling (e.g., fas associated protein with death domain and IKKε), but also to increase the production of TNF-α (Sonkoly et al., 2008). Thus, higher miR-155 in microglia is relevant as microglia from aged mice show higher production of TNF-α (Njie et al., 2012). Overall, an increase in both miR-146a and miR-155 is indicative of an increased inflammatory state within the aged brain.
Our results indicate that miR-29a and miR-29b are increased in the brain with age. A previous study showed that miR-29 expression gradually increased in the human brain from birth until puberty, but no further elevation was noted with age (Somel et al., 2010). Nonetheless, in animal models, miR-29a and miR-29b were increased in the liver and lung of aged mice and in the Zmpste24-null mouse model of accelerating aging (Ugalde et al., 2011). Moreover, miR-29b was decreased in these same tissues in a rodent model of delayed aging (Bates et al., 2010). Here, our data support that miR-29a and miR-29b are increased in the brain as a function of age. It is important to highlight that this age-associated increase in miR-29a and miR-29b expression was reflected in microglia. Microglia are the primary immune cell within the CNS and, thus, increases in immunoregulatory miRNAs likely contribute to microglial dysregulation and an enhanced inflammatory profile in the aged brain. It is also important to mention that miR-29c is part of the miR-29 cluster, but was reduced in the brain of aged mice. This is similar to our previous report showing that the miR-29a/b cluster was elevated in memory T-cells of patients with MS, but expression of the miR-29b/c cluster was unchanged (Smith et al., 2012). Differential regulation of the miR-29 isoforms is related to the finding that miR-29a/b cluster is on chromosome 7 and the miR-29b/c cluster is on chromosome 1.
A key finding of this study was that increased miR-29a and miR-29b in the rodent brain was paralleled in the cortical brain tissue of human subjects. Therefore, these data support the relevance of using rodent models to study brain aging. In addition, brain tissue used was from adult (mean 45 yrs) and aged (mean 77 yrs) individuals who did not have a neurodegenerative disease. This is relevant to point out because a previous study indicates that miR-29a/b were decreased in the brain of aged patients with Alzheimer’s disease (Hebert et al., 2008). miR-29 was found to target BACE1 expression in vitro and indeed, patients with sporadic Alzheimer’s disease that exhibited reduced levels of miR-29a and miR-29b had abnormally high BACE1 expression (Hebert et al., 2008). In the current study, however, miR-29a and miR-29b were elevated in the brain of older individuals compared to adults, and increased expression of miR-29a and miR-29b was positively correlated with advancing age. Reductions in miR-29a/b may, therefore, indicate a dysfunctional response to increased neuroinflammatory load and a predisposition to Alzheimer’s disease (Blasko et al., 2004), whereas increased miR-29a/b would suggest a normal response to increased inflammation.
Multiple studies indicate brain aging is associated with increased DNA damage, oxidative stress, and inflammation (e.g., elevated NF-κB, IL-1β, TNF-α). Several of these factors have been reported to increase miR-29 expression in the periphery and in vitro (Smith et al., 2012; Ugalde et al., 2011). Although the function of the miR-29a/b cluster is to provide negative feedback and reduce inflammation (Ma et al., 2011; Smith et al., 2012; Steiner et al., 2011), this miR-mediated response does not appear to be effective in the aged brain. We have demonstrated a similar relationship with miR-29a/b expression and its target IFNγ in MS patients in which T-cells from MS patients had higher levels of IFNγ concordant with higher expression of miR-29b (Smith et al., 2012). A possible explanation for this relationship in the aged brain is that miR-29-dependent repression of key immuno-regulatory genes, including IGF-1 and CX3CL1, may potentiate microglial priming and contribute to increased inflammatory load. A similar relationship also exists between inflammatory induced IL-10 in the aged brain. Indeed, inflammatory activation of aged microglia promotes exaggerated expression of inflammatory (e.g., IL-1β) and anti-inflammatory (e.g., IL-10) cytokines (Henry et al., 2009). Despite this exaggerated increase in anti-inflammatory mediators, heightened inflammation persists. The cause for this is unknown, but contributes to the idea that negative regulatory systems within the aged brain are impaired on multiple levels.
Our results showing age-associated reductions in brain levels of IGF-1 and CX3CL1 mRNA are consistent with previous studies investigating IGF-1 (Deak and Sonntag, 2012; Llorens-Martin et al., 2009; O'Connor et al., 2008; Sonntag et al., 2005b) and CX3CL1 with age (Lyons et al., 2009; Wynne et al., 2010). Here we extend these previous findings to show that the reductions of IGF-1 and CX3CL1 were significantly correlated with an increase in the miR-29a/b cluster in microglia. These correlations are consistent with the evidence that both of these genes are confirmed targets of the miR-29a/b cluster by luciferase assay. Moreover, transfection with miR-29b reduced IGF-1 protein secretion specifically in microglia. Of note, even if miR-29a/b was only increased within microglia, it could still influence CX3CL1 expression by neurons through the transfer of miRNAs in exosomes (Valadi et al., 2007). Nonetheless, one discrepancy between the luciferase studies and human brain correlations regarding CX3CL1 was that luciferase assay confirmed CX3CL1 as a target of miR-29a suppression, but CX3CL1 was not negatively correlated with miR-29a in the brain of aged humans. This could be explained by the actions of miR-29a on CX3CL1, which may be to inhibit translation rather than cause mRNA degradation (Valencia-Sanchez et al., 2006). Another unexpected finding was that although miR-29b did not repress CX3CL1 expression in the luciferase experiments, miR-29b negatively correlated with CX3CL1 expression in human cortical tissue. A potential explanation for these results is that murine sequences, rather than human sequences, were used for the target UTR sites and thus target sites may differ in humans. Alternatively, miR-29b-promoted reductions in IGF-1 could indirectly contribute to CX3CL1 reductions. Localized IGF-1 release by microglia prevents neuronal apoptosis (Galli et al., 1995) and sustains neurogenesis (Choi et al., 2008; Lichtenwalner et al., 2001), primarily under conditions of inflammation (Ekdahl et al., 2009). Thus, reduced neuronal support by IGF-1 may contribute to reduced CX3CL1 levels as CX3CL1 is primarily released by healthy neurons in the brain (Cardona et al., 2006). Indeed, IGF-1 significantly and negatively correlated with CX3CL1 in the current study (data not shown).
Importantly, the data obtained using human cortical tissue indicates that the increase in miR-29b with age correlated with the reduction of both IGF-1 and CX3CL1. The relevance of miR-29b in the targeting of IGF-1 and CX3CL1 in the aged is consistent with its induction by inflammatory stimuli (Smith et al., 2012). For example, our data indicate that a peripheral challenge with LPS increased IL-1β and miR-29b expression in the brain, but did not affect the expression miR-29a. Moreover, there was a 2-fold increase in IL-1β expression in the brain of the older individuals compared to the middle aged controls. Therefore, we interpret these data to indicate that age-associated increases in pro-inflammatory cytokines results in increased expression of miR-29b in rodents and humans. Of note, miR-29a was not increased in the brain after a peripheral injection of LPS, but was increased in the brain of aged mice and humans. One possibility for the age-related increase in miR-29a is increased DNA damage in the aged brain. Indeed, miR-29a is preferentially induced by DNA damage in a p53-dependent manner independent of an inflammatory response (Ugalde et al., 2011). Taken together, these findings support our hypothesis that the reduction of CX3CL1 and IGF-1 result from an increase in miR-29 dependent regulation.
In conclusion, miRNA-mediated immune regulation in the brain is altered as a function of normal aging. While increased miR-29a/b expression with age may represent a homeostatic negative feedback response to increased inflammatory potential within the aged brain, a consequence is the suppression of IGF-1 and CX3CL1. Down-regulation of IGF-1 and CX3CL1 contribute to an increased inflammatory profile of microglia in the aged brain. These findings are important because a heightened inflammatory profile in aged microglia may also lead to other impairments including reduced neuronal plasticity, cognitive impairment, and hyperactivity of microglia following central or peripheral immune challenges. Therefore, understanding how miRNAs regulate microglial activation is critical in identifying novel therapies to reduce microglial priming with age.
Acknowledgements
We thank Todd Shawler and Yan Huang for their technical assistance. This research was supported by an NIH grant R01-AG-033028 to J.P.G.., NIH grant R21-AI-092417 to C.C.W., and NIH grant R01-NS-067441 to A.E.L. In addition, A.M.F. was supported by a Howard Hughes Medical Institute (HHMI) Med into Grad Scholarship and K.M.S. was supported by a T32 training grant GM068412.
All procedures using mice were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee. All postmortem brain tissue was obtained through the appropriate application process and approval of the Harvard Brain Tissue Resource Center (Belmont, MA, supported by the PHS grant R24 MH068855) and the Human Brain and Spinal Fluid Resource Center (VA West Los Angeles Healthcare Center, Los Angeles, CA which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society and Department of Veterans Affairs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors of this manuscript declare that there are no actual or potential conflicts of interest. The authors affirm that there are no financial, personal or other relationships with other people or organizations that have inappropriately influenced or biased their research.
Data being submitted in the present manuscript have not been previously published and have not been submitted elsewhere. Moreover, these data will not be submitted elsewhere while under consideration at Neurobiology of Aging.
All authors have reviewed the contents of this manuscript and have approved the validity of the data and the context of data presentation.
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32(11):2030. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9(8):839. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010;9(1):1. doi: 10.1111/j.1474-9726.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3(4):169. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J Leukoc Biol. 2008;84(3):587–594. doi: 10.1189/jlb.1107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan J, Sparkman N, Godbout J, Freund G, Johnson R. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-S, Cho H-Y, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56(7):791. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona A, Fenn A, Godbout J. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharm. 2012;7(1):7. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Corona A, Huang Y, O'Connor J, Dantzer R, Kelley K, Popovich P, Godbout J. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflamm. 2010;7(1):93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Gheusi G, Johnson RW, Kelley KW. Central administration of insulin-like growth factor-1 inhibits lipopolysaccharide-induced sickness behavior in mice. NeuroReport. 1999;10(2):289–292. doi: 10.1097/00001756-199902050-00015. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor J, Freund G, Johnson R, Kelley K. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67(6):611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, Roskelley EM, Treleaven CM, Rizo L, Martin H, Kim SH, Kaspar R, Taksir TV, Griffiths DA, Cheng SH, Shihabuddin LS, Kaspar BK. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16(6):1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26(5):766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Barrientos R, Biedenkapp J, Rudy J, Watkins L, Maier S. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G. Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci. 1995;15(2):1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout J, Berg B, Kelley K, Johnson R. [alpha]-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149(1-2):101. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Godbout J, Chen J, Abraham J, Richwine A, Berg B, Kelley K, Johnson R. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;10:1329. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout J, Moreau M, Lestage J, Chen J, Sparkman N, O'Connor J, Castanon N, Kelley K, Dantzer R, Johnson R. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacol. 2008;33(10):2341. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. P Natl Acad Sci USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1[beta] and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23(3):309. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry C, Dantzer R, Johnson R, Godbout J. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29(11):1744. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol. 2012;233(1):40. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-J, Kim K-W, Yu B-P, Chung H-Y. The effect of age on cyclooxygenase-2 gene expression: NF-kB activation and IkBa degradation. Free Radical Bio Med. 2000;28(5):683. doi: 10.1016/s0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- Korhonen P, Helenius M, Salminen A. Age-related changes in the regulation of transcription factor NF-kB in rat brain. Neurosci Lett. 1997;225(1):61. doi: 10.1016/s0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- Labbaye C, Testa U. The emerging role of miR-146a in the control of hematopoiesis, immune function and cancer. J Clin Hematol Oncol. 2012;5(1):13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkfors L, Ebendal T, Whittemore SR, Persson H, Hoffer B, Olson L. Decreased level of nerve growth factor (NGF) and its messenger RNA in the aged rat brain. Mol Brain Res. 1987;3(1):55. doi: 10.1016/0169-328x(87)90044-1. [DOI] [PubMed] [Google Scholar]
- Li N, Bates DJ, An J, Terry DA, Wang E. Up-regulation of key microRNAs, and inverse down-regulation of their predicted oxidative phosphorylation target genes, during aging in mouse brain. Neurobiol Aging. 2011;32(5):944. doi: 10.1016/j.neurobiolaging.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15(2):134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer E, Crotty S, Nolan Y, Mills K, Lynch M. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27(31):8309. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110(5):1547. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12(9):1547. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Yvonne, Lynch Marina A. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:12. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96(6):1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Brunso-Bechtold JK, Riddle DR. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci. 2000;20(11):4165–4176. doi: 10.1523/JNEUROSCI.20-11-04165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie e.G, Boelen E, Stassen FR, Steinbusch HWM, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33(1):195.e1. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. P Natl Acad Sci USA. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252(1-2):91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrino. 2008;33(10):1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31(45):16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Guerau-de-Arellano M, Costinean S, Williams JL, Bottoni A, Mavrikis Cox G, Satoskar AR, Croce CM, Racke MK, Lovett-Racke AE, Whitacre CC. miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. J Immunol. 2012;189(4):1567–1576. doi: 10.4049/jimmunol.1103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel M, Guo S, Fu N, Yan Z, Hu HY, Xu Y, Yuan Y, Ning Z, Hu Y, Menzel C, Hu H, Lachmann M, Zeng R, Chen W, Khaitovich P. MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res. 2010;20(9):1207–1218. doi: 10.1101/gr.106849.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18(2):131. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005a;146(7):2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005b;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M, Blelloch R, Ansel KM. MicroRNA-29 regulates T-box transcription factors and interferon-gamma production in helper T cells. Immunity. 2011;35(2):169–181. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- Ugalde AP, Ramsay AJ, de la Rosa J, Varela I, Marino G, Cadinanos J, Lu J, Freije JMP, Lopez-Otin C. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J. 2011;30(11):2219. doi: 10.1038/emboj.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(515):524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Wynne A, Henry C, Huang Y, Cleland A, Godbout J. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24(7):1190. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S-M, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93(1-2):139. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye S-M, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulat. 2001a;9(4):183. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Ye S-M, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kB. J Neuroimmunol. 2001b;117(1-2):87. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99(4):1176. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]


